Studies on Methane Gas Hydrate Formation Kinetics Enhanced by Isopentane and Sodium Dodecyl Sulfate Promoters for Seawater Desalination
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
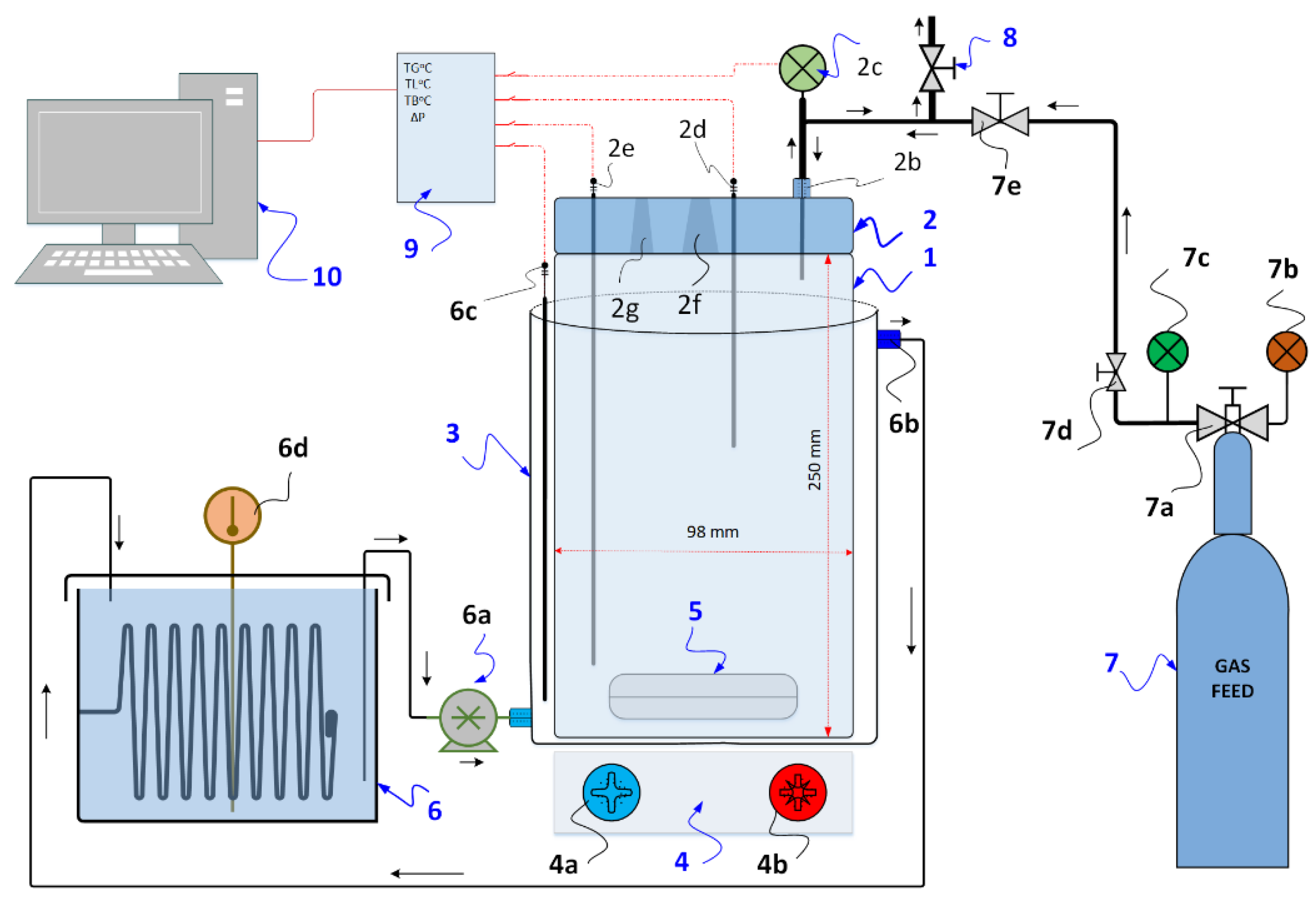
2.2. Experimental Setup
2.3. Methodology
2.3.1. Preparation of Solutions
2.3.2. Experimental Procedure
2.3.3. Calculation of Kinetic Parameters of Hydrate Formation
3. Results
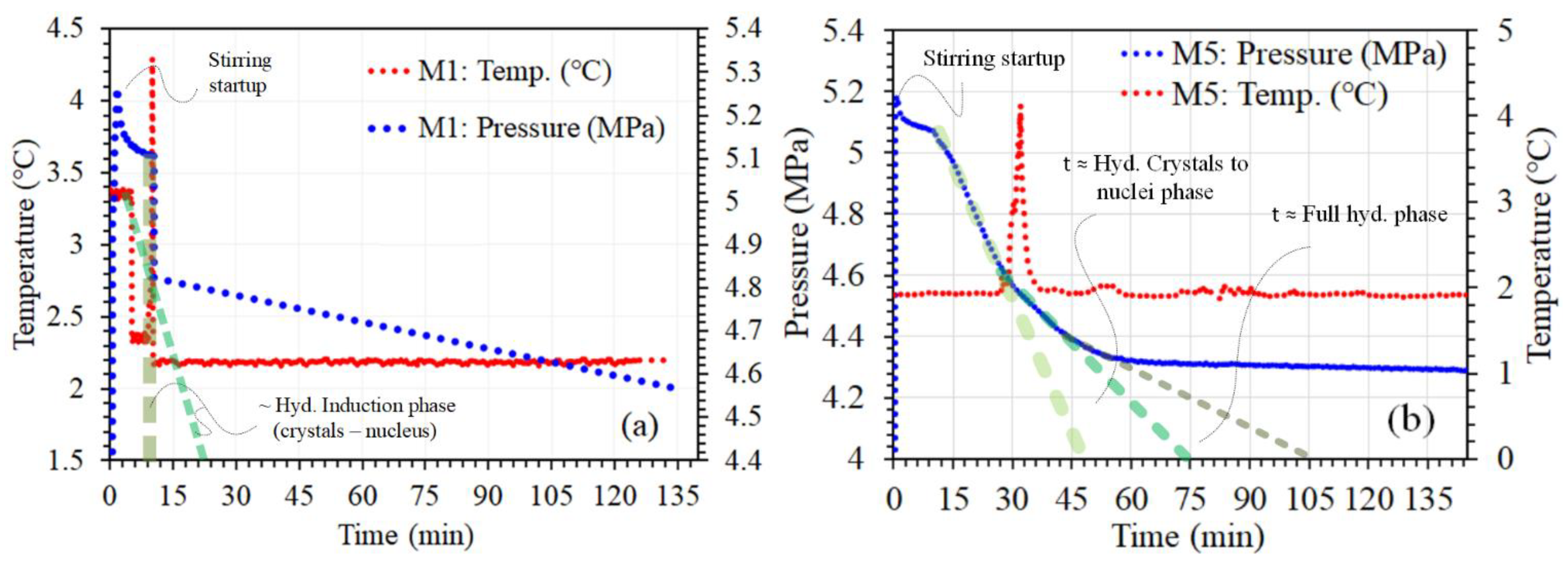
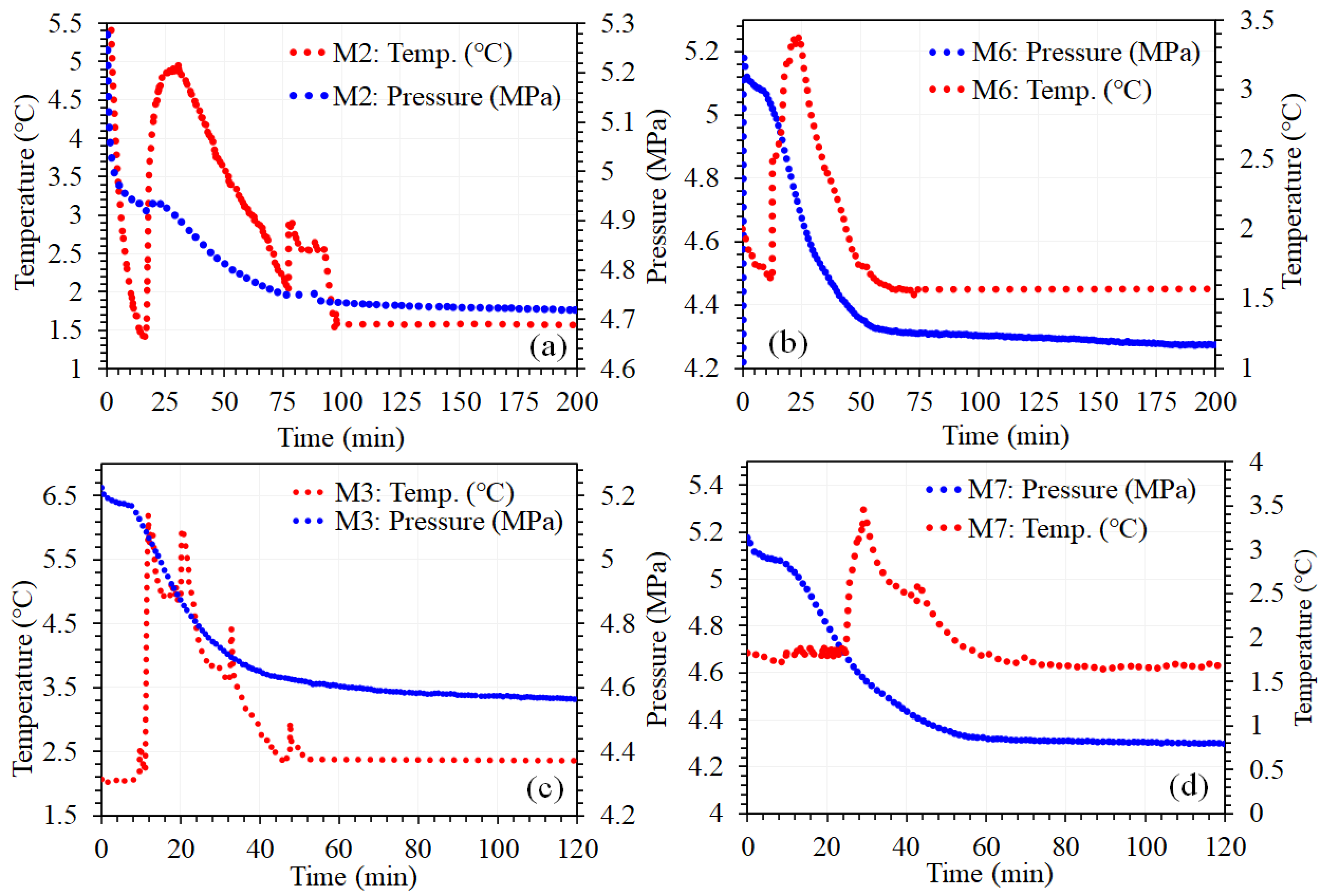
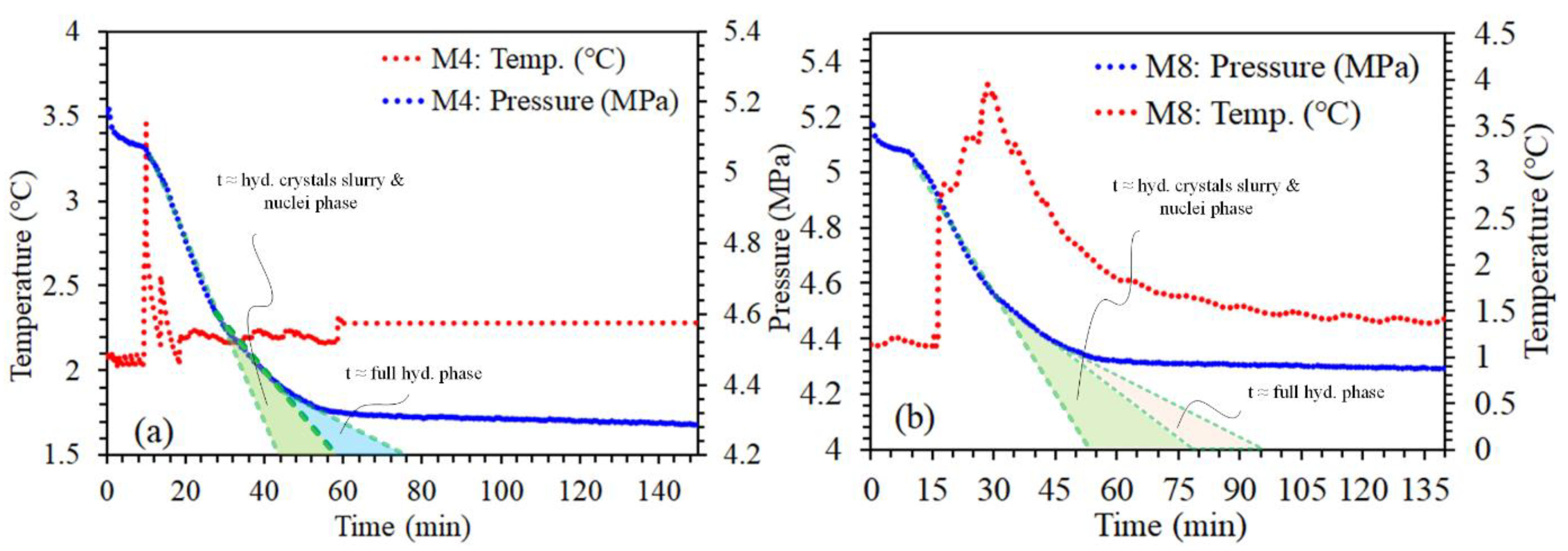
3.1. Comparison of P-T Profile Kinetics of CH₄ Gas Hydrate and Their Importance for Desalination
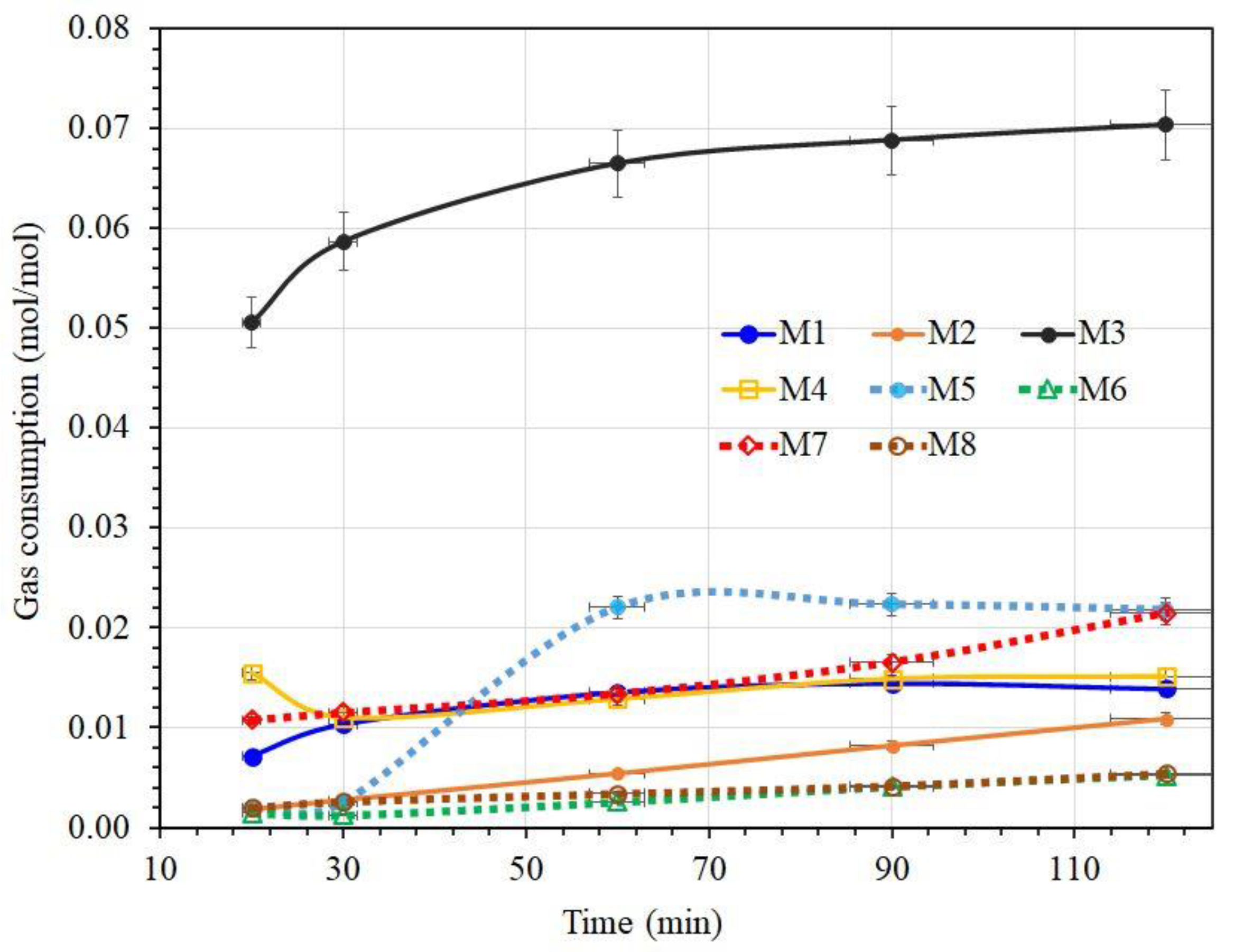
3.2. CH₄ Gas Uptake (Moles) and Consumption (mol/mol) in Two Different Kinds of Water with and without Promoters
3.3. Effects of Promoters and Their Blends on CH4 Hydrate Induction Time for Desalination
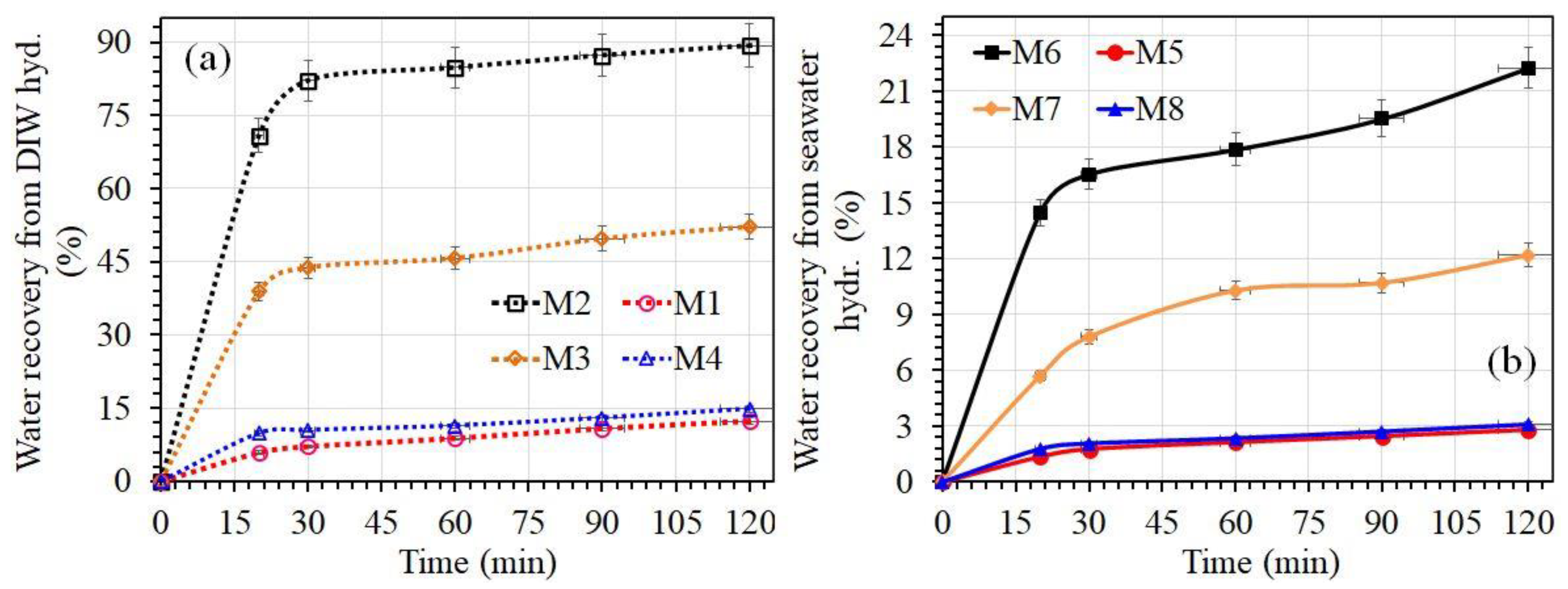
3.4. Comparison of Water Recovery from Two Different CH₄ Gas Hydrates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hassanpouryouzband, A.; Joonaki, E.; Farahani, M.V.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; et al. Gas hydrates in sustainable chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.; Nambiar, A.; He, T.; Karimi, I.A.; Lee, J.D.; Englezos, P.; Linga, P. A Review of Clathrate Hydrate Based Desalination To Strengthen Energy–Water Nexus. ACS Sustain. Chem. Eng. 2018, 6, 8093–8107. [Google Scholar] [CrossRef]
- Schicks, J.M. Gas hydrates in nature and in the laboratory: Necessary requirements for formation and properties of the resulting hydrate phase. ChemTexts 2022, 8, 13. [Google Scholar] [CrossRef]
- Lal, B.; Nallakukkala, S. Gas Hydrate in Water Treatment: Technological, Economic, and Industrial Aspects; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Xia, Z.; Zhao, Q.; Chen, Z.; Li, X.; Zhang, Y.; Xu, C.; Yan, K. Review of methods and applications for promoting gas hydrate formation process. J. Nat. Gas Sci. Eng. 2022, 101, 104528. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Chen, F.-L.; Yu, S.-J.; Wang, F. Biopromoters for Gas Hydrate Formation: A Mini Review of Current Status. Front. Chem. 2020, 8, 514. [Google Scholar] [CrossRef] [PubMed]
- Chong, Z.R.; He, T.; Babu, P.; Zheng, J.-N.; Linga, P. Economic evaluation of energy efficient hydrate based desalination utilizing cold energy from liquefied natural gas (LNG). Desalination 2019, 463, 69–80. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, Q.; Mu, C. Influence of temperature on methane hydrate formation. Sci. Rep. 2017, 7, 7904. [Google Scholar] [CrossRef]
- Sloan, E.D. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef]
- Pandey, J.S.; Daas, Y.J.; von Solms, N. Insights into Kinetics of Methane Hydrate Formation in the Presence of Surfactants. Processes 2019, 7, 598. [Google Scholar] [CrossRef]
- Kumar, A.; Bhattacharjee, G.; Kulkarni, B.D.; Kumar, R. Role of Surfactants in Promoting Gas Hydrate Formation. Ind. Eng. Chem. Res. 2015, 54, 12217–12232. [Google Scholar] [CrossRef]
- Lv, X.; Jing, S.; Zhao, D.; Lu, D.; Liu, Y.; Ma, Q.; Song, S.; Zhou, S. Study on the kinetic characteristics of gas hydrate in the dioctyl sodium sulfosuccinate system. RSC Adv. 2021, 11, 39108–39117. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.; Sandoval, J.F.; Ortiz, N.; Ovalle, S.; Beltran, J.G. Mechanisms, Growth Rates and Morphologies of Gas Hydrates of Carbon Dioxide, Methane and Their Mixtures. Methane 2022, 1, 2. [Google Scholar] [CrossRef]
- Chernov, A.A.; Pil’Nik, A.A.; Elistratov, D.S.; Mezentsev, I.V.; Meleshkin, A.V.; Bartashevich, M.; Vlasenko, M. New hydrate formation methods in a liquid-gas medium. Sci. Rep. 2017, 7, 40809. [Google Scholar] [CrossRef]
- de Deugd, R.M.; Jager, M.D.; Arons, J.D.S. Mixed hydrates of methane and water-soluble hydrocarbons modeling of empirical results. AIChE J. 2001, 47, 693–704. [Google Scholar] [CrossRef]
- Ganji, H.; Manteghian, M.; Zadeh, K.S.; Omidkhah, M.; Mofrad, H.R. Effect of different surfactants on methane hydrate formation rate, stability and storage capacity. Fuel 2007, 86, 434–441. [Google Scholar] [CrossRef]
- Khurana, M.; Yin, Z.; Linga, P. A Review of Clathrate Hydrate Nucleation. ACS Sustain. Chem. Eng. 2017, 5, 11176–11203. [Google Scholar] [CrossRef]
- Lin, W.; Chen, G.-J.; Sun, C.-Y.; Guo, X.-Q.; Wu, Z.-K.; Liang, M.-Y.; Chen, L.-T.; Yang, L.-Y. Effect of surfactant on the formation and dissociation kinetic behavior of methane hydrate. Chem. Eng. Sci. 2004, 59, 4449–4455. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, S.; Lee, J. Does SDS micellize under methane hydrate-forming conditions below the normal Krafft point? J. Colloid Interface Sci. 2007, 315, 313–318. [Google Scholar] [CrossRef]
- Gootam, D.; Gaikwad, N.; Kumar, R.; Kaisare, N. Modeling Growth Kinetics of Methane Hydrate in Stirred Tank Batch Reactors. ACS Eng. Au 2021, 1, 148–159. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. Medium pressure hydrate based gas separation (HBGS) process for pre-combustion capture of carbon dioxide employing a novel fixed bed reactor. Int. J. Greenh. Gas Control 2013, 17, 206–214. [Google Scholar] [CrossRef]
- Smith, J.; Van Ness, H.; Abbot, M. Introduction to Chemical Engineering Thermodynamics, 6th ed.; McGraw Hill: New York, NY, USA, 2001. [Google Scholar]
- Ripmeester, J.A. Hydrate Research-From Correlations to a Knowledge-based Discipline: The Importance of Structure. Ann. N. Y. Acad. Sci. 2006, 912, 1–16. [Google Scholar] [CrossRef]
- Zhong, D.-L.; Ding, K.; Yan, J.; Yang, C.; Sun, D.-J. Influence of Cyclopentane and SDS on Methane Separation from Coal Mine Gas by Hydrate Crystallization. Energy Fuels 2013, 27, 7252–7258. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, B.-Y.; Wu, Q.; Linga, P. Kinetic Evaluation of Cyclopentane as a Promoter for CO2 Capture via a Clathrate Process Employing Different Contact Modes. ACS Sustain. Chem. Eng. 2018, 6, 11913–11921. [Google Scholar] [CrossRef]
- Lv, Q.N.; Song, Y.C.; Li, X.S. Kinetic Study on the Process of Cyclopentane plus Methane Hydrate Formation in NaCl Solution. Energy Fuels 2016, 30, 1310–1316. [Google Scholar]
- Lekvama, K.; Bishnoit, P.R. Dissolution of methane in water at low temperatures and intermediate pressures. Fluid Phase Equilibria 1997, 131, 297–309. [Google Scholar] [CrossRef]
- Ahmed, I.; Bamaga, O.; Albeirutty, M.H.; Abulkhair, H.; Alsaiari, A.; Organji, H.; Linga, P. Significance of Low Stirring Modes on the Kinetics of Methane Hydrate Formation. Energy Fuels 2022, 36, 7676–7686. [Google Scholar] [CrossRef]
- Oua, W.; Lu, W.; Ninga, F.; Wu, X. Measurement of methane solubility in pure water in equilibrium with hydrate by using high–pressure optical capillary cell. Mar. Chem. 2019, 212, 74–82. [Google Scholar] [CrossRef]
- Lekvama, K.; Ruoff, P. A reaction kinetic mechanism for methane hydrate formation in liquid water. J. Am. Chem. Soc. 1993, 115, 8565–8569. [Google Scholar] [CrossRef]
- Zatsepina, O.Y.; Buffett, B.A. Phase equilibrium of gas hydrate: Implications for the formation of hydrate in the deep seafloor. Geophys. Res. Lett. 1997, 24, 567–1570. [Google Scholar] [CrossRef]
- Zatsepina, O.Y.; Buffett, B.A. Thermodynamic conditions for the stability of gas hydrate in the seafloor. J. Geophys. Res. Earth Surf. 1998, 103, 24127–24139. [Google Scholar] [CrossRef]
- Servio, P.; Englezos, P. Measurement of Dissolved Methane in Water in Equilibrium with Its Hydrate. J. Chem. Eng. Data 2001, 47, 87–90. [Google Scholar] [CrossRef]
- Gambelli, A.; Tinivella, U.; Giovannetti, R.; Castellani, B.; Giustiniani, M.; Rossi, A.; Zannotti, M.; Rossi, F. Observation of the Main Natural Parameters Influencing the Formation of Gas Hydrates. Energies 2021, 14, 1803. [Google Scholar] [CrossRef]
- You, K.; Kneafsey, T.J.; Flemings, P.B.; Polito, P.; Bryant, S.L. Salinity-buffered methane hydrate formation and dissociation in gas-rich systems. J. Geophys. Res. Solid Earth 2015, 120, 643–661. [Google Scholar] [CrossRef]
- Nesterov, A.N.; Reshetnikov, A.M. Combined effect of NaCl and sodium dodecyl sulfate on the mechanism and kinetics of methane hydrate formation in an unstirred system. J. Nat. Gas Sci. Eng. 2022, 99, 104424. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Q.; Zhang, H. Effect of propane and NaCl-SDS solution on nucleation process of mine gas hydrate. J. Chem. 2017, 2017, 1059109. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, D.; Liu, Z.; Pan, Z. Influence of sodium chloride on the kinetics of methane hydrate formation in the presence of surfactant. J. Nat. Gas Sci. Eng. 2020, 83, 103622. [Google Scholar] [CrossRef]
- Zhang, J.S.; Lee, A.S.; Lee, J.W. Kinetics of Methane Hydrate Formation from SDS Solution. Ind. Eng. Chem. Res. 2007, 46, 6353–6359. [Google Scholar] [CrossRef]
- Lo, C.; Zhang, J.S.; Couzis, A.; Somasundaran, P.; Lee, J.W. Adsorption of Cationic and Anionic Surfactants on Cyclopentane Hydrates. J. Phys. Chem. C 2010, 114, 13385–13389. [Google Scholar] [CrossRef]
- Muller-Bongartz, B.; Wildeman, T.R.; Sloan, E.D., Jr. A hypothesis for hydrate nucleation phenomena. In Proceedings of the Second International Offshore and Polar Engineering Conference, San Francisco, CA, USA, 14–19 June 1992. ISOPE-I-92-089. [Google Scholar]
- Sloan, E.D.; Fleyfel, F. A molecular mechanism for gas hydrate nucleation from ice. AIChE J. 1991, 37, 1281–1292. [Google Scholar] [CrossRef]
- Natarajan, V.; Bishnoi, P.; Kalogerakis, N. Induction phenomena in gas hydrate nucleation. Chem. Eng. Sci. 1994, 49, 2075–2087. [Google Scholar] [CrossRef]
- Ke, W.; Svartaas, T.M.; Chen, D. A review of gas hydrate nucleation theories and growth models. J. Nat. Gas Sci. Eng. 2018, 61, 169–196. [Google Scholar] [CrossRef]
- Zhong, Y.; Rogers, R. Surfactant effects on gas hydrate formation. Chem. Eng. Sci. 2000, 55, 4175–4187. [Google Scholar] [CrossRef]
- Chanakro, W.; Jaikwang, C.; Inkong, K.; Kulprathipanja, S.; Rangsunvigit, P. Comparative Study of Tetra-N-Butyl Ammonium Bromide and Cyclopentane on the Methane Hydrate Formation and Dissociation. Energies 2020, 13, 6518. [Google Scholar] [CrossRef]
- Kutergin, O.B.; Mel’nikov, V.P.; Nesterov, A.N. Influence of surfactants on the mechanism and kinetics of the formation of gas hydrates. Dokl. Akad. Nauk SSSR 1992, 323, 549. [Google Scholar]
- Karaaslan, U.; Parlktuna, M. Surfactants as hydrate promoters? Energy Fuels 2000, 14, 1103. [Google Scholar] [CrossRef]
- Daimaru, T.; Yamasaki, A.; Yanagisawa, Y. Effect of surfactant carbon chain length on hydrate formation kinetics. J. Pet. Sci. Eng. 2007, 56, 89–96. [Google Scholar] [CrossRef]
- Link, D.D.; Ladner, E.P.; Elsen, H.A.; Taylor, C.E. Formation and dissociation studies for optimizing the uptake of methane by methane hydrates. Fluid Phase Equilibria 2003, 211, 1–10. [Google Scholar] [CrossRef]
- Di Profio, P.; Arca, S.; Germani, R.; Savelli, G. Novel Nanostructured Media for Gas Storage and Transport: Clathrate Hydrates of Methane and Hydrogen. J. Fuel Cell Sci. Technol. 2006, 4, 49–55. [Google Scholar] [CrossRef]

| Additives or Promoters | Components of Deionized Water (DIW) Solution | Components of Seawater (SW) Solution | |||||
|---|---|---|---|---|---|---|---|
| DIW | Promoters | Seawater | Promoters | ||||
| IP | 100 mL | 32.3 mL | 100 mL | 32.3 mL | |||
| SDS | 100 mL | 0.10 g | 100 mL | 0.10 g | |||
| IP + SDS | 100 mL | 32.3 mL + 0.10 g | 100 mL | 32.3 mL + 0.10 g | |||
| Codes of CH₄ hydrate formation experiments and corresponding liquid feed composition | |||||||
| M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 |
| DIW | DIW/IP | DIW/SDS | DIW/IP + SDS | SW | SW/IP | SW/SDS | SW/IP + SDS |
| Parameters for CH₄ Hydrate Formation | |||||||
| Gas pressures (MPa) | 5.2 to 5.3 ± 0.1 | ||||||
| Temperature (°C) | 2 ± 0.5 | ||||||
| Stirring (rpm) | 600 | ||||||
| Hydrate formation duration (min) | 150–400 | ||||||
| Exp. | Liquid Feed Components | Trial 1 | Trial 2 | Trial 3 | Average HIT |
|---|---|---|---|---|---|
| Code | (mL) | (min) | |||
| M1 | DW | 11.5 | 8.5 | 9.7 | |
| M2 | DW + A1 | 12 | 8.5 | 7.7 | |
| M3 | DW + A2 | 10.3 | 9.2 | 8.7 | |
| M4 | DW + A3 | 11 | 9 | 8.2 | |
| M5 | SW | 30.4 | 28.2 | 26 | |
| M6 | SW + A1 | 11 | 14 | 13 | |
| M7 | SW + A2 | 26.7 | 23 | 25 | |
| M8 | SW + A3 | 18.2 | 16.5 | 14.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bamaga, O.; Ahmed, I.; Wafiyah, A.M.; Albeirutty, M.; Abulkhair, H.; Shaiban, A.; Linga, P. Studies on Methane Gas Hydrate Formation Kinetics Enhanced by Isopentane and Sodium Dodecyl Sulfate Promoters for Seawater Desalination. Energies 2022, 15, 9652. https://doi.org/10.3390/en15249652
Bamaga O, Ahmed I, Wafiyah AM, Albeirutty M, Abulkhair H, Shaiban A, Linga P. Studies on Methane Gas Hydrate Formation Kinetics Enhanced by Isopentane and Sodium Dodecyl Sulfate Promoters for Seawater Desalination. Energies. 2022; 15(24):9652. https://doi.org/10.3390/en15249652
Chicago/Turabian StyleBamaga, Omar, Iqbal Ahmed, Asim M. Wafiyah, Mohammed Albeirutty, Hani Abulkhair, Amer Shaiban, and Praveen Linga. 2022. "Studies on Methane Gas Hydrate Formation Kinetics Enhanced by Isopentane and Sodium Dodecyl Sulfate Promoters for Seawater Desalination" Energies 15, no. 24: 9652. https://doi.org/10.3390/en15249652
APA StyleBamaga, O., Ahmed, I., Wafiyah, A. M., Albeirutty, M., Abulkhair, H., Shaiban, A., & Linga, P. (2022). Studies on Methane Gas Hydrate Formation Kinetics Enhanced by Isopentane and Sodium Dodecyl Sulfate Promoters for Seawater Desalination. Energies, 15(24), 9652. https://doi.org/10.3390/en15249652







