Abstract
An important feature of this research is the investigation of the de-alloyed catalysts based on the nanoparticles with a simple structure (alloy) and a complex structure (gradient). The resulting samples exhibit the 2–4 times higher mass activity in the ORR compared with the commercial Pt/C. The novelty of this study is due to the application of the express-electrochemical experiment to register the trend of changes in the ORR activity caused by rearranging the structure of bimetallic nanoparticles. The state-of-the-art protocol makes it possible to establish the dependence of properties of the de-alloyed catalysts on the nanoparticles’ structure obtained at the stage of the material’s synthesis. The study shows the possibility of determining the rate of the ongoing reorganization of bimetallic nanoparticles with different architectures. The PtCu/C electrocatalysts for proton-exchange membrane fuel cells presented in this work are commercially promising in terms of both the high functional characteristics and the production by facile one-pot methods.
1. Introduction
One of the key strands of the research which is concerned with increasing the activity and the stability of the electrocatalysts for proton-exchange membrane fuel cells (PEMFCs) is to obtain the materials based on platinum alloyed with d-metals (Ni, Co, Cu, etc.) as well as to study their structure and behavior [1,2,3,4]. There are several basic approaches to synthesizing the bimetallic catalysts [3,5]. The simplest one to implement is the simultaneous reduction of metal precursors to obtain the bimetallic nanoparticles (NPs) with the “solid solution” structure [3,6]. Experimental studies [5,7] in combination with theoretical ones [8] show that the activity of the Pt-based alloys’ NPs in the oxygen reduction reaction (ORR) is determined by the interaction force of the oxygen–metal bond. This force depends on the position of the Pt d-band center relative to the Fermi level [1,6,9,10]. The changeover to multistage synthesis methods to obtain the catalysts based on the bimetallic NPs of a more complex structure (“core-shell” [3,5,10], “gradient” [11,12,13] structures) is connected with i) the search for an optimal architecture of NPs and ii) the attempt to prevent atoms of the alloying component from the selective dissolution by forming the platinum shell on the surface of NPs [2,10,14,15]. Notably, the latest works demonstrate that with the formation of a great number of bimetallic NPs on the support surface, the desired architecture cannot be provided for all the NPs [16]. Moreover, the platinum shell does not normally ensure the entire protection of the component, which constitutes the core, from the dissolution during the operation of the catalyst [6,10].
The preparation of the bimetallic electrocatalysts for use in the membrane electrode assemblies (MEAs) is typically conducted by the acid treatment of the materials followed by obtaining the de-alloyed NPs [5,15,17]. When obtaining these catalysts, the primary bimetallic NPs are transformed into the secondary structures characterized by the prevailing segregation of platinum atoms in their surface layer [14]. These materials contain the NPs which are banded by the alloying component although they exhibit high ORR activity [7,18] and high stability during long-term stress tests [7]. For example, Strasser et al. reported that the de-alloyed PtCu NPs showed the higher activity in the ORR compared with Pt/C due to the compressive strain of the shell enriched with platinum. The formation of this shell is caused by the dissolution of atoms of the non-noble metal from the alloy’s surface layer during the electrochemical cycling (the electrochemical de-alloying) of the material [16,19]. It should be noted that the composition of the catalysts or rather the NPs, which are acidly treated or electrochemically activated, normally differs significantly from the initial one and is often close to Pt3M/C [7].
In reference [20], we answered the question if the optimal initial composition of bimetallic NPs needs searching, given its inevitable change during the pretreatment or the initial stage of the catalyst’s operation. We have shown that the initial content of the alloying component affects the ORR activity of the PtCu/C catalysts. At the same time, the question if the initial structure of bimetallic NPs affects characteristics of their de-alloyed analogues, which are obtained by the acid treatment, remains unanswered [20].
Despite the absence of univocal answers to this question, it is evident that to control the structure of the de-alloyed NPs, the correlation between the characteristics and the architecture of initial materials and their analogues, which are obtained after the pretreatment, needs understanding. The theoretical selection of optimal low-platinum catalytic systems for PEMFCs is yet not possible [1]. In this regard, the urgent task is a systematical study of the effect of the bimetallic particles’ initial structure on functional characteristics of the de-alloyed electrocatalysts.
In spite of the focused research, the peculiarities of the correlation between structures and features of bimetallic NPs established for model systems are not always applicable to foresee the electrochemical behavior of real catalytic materials, which contain a lot of heterogeneous NPs [21].
In reference [22], we proposed an original approach to studying the electrochemical behavior of bimetallic catalysts in the ORR. This approach is sensitive to peculiarities of the NPs’ surface rearrangement. The recently published studies show that changes in the fine structure of NPs can be captured in situ by electrochemical methods [22,23,24]. That being the case, the analysis of the electrochemical behavior of catalytic electrodes can partially replace rather complex and hardly accessible research methods, such as synchrotron research methods [23,24,25,26,27], ISP-mass with a flow cell [16,28,29], and in situ HR TEM [19,30,31].
This study is based on the hypothesis that the initial architecture of bimetallic NPs could significantly affect functional characteristics of the de-alloyed catalysts with a close composition. In particular, in many works, the characteristics of catalysts are considered only in the initial state or only in the de-alloyed state. We will try to show, for the catalysts based on de-alloyed nanoparticles, that it is important to consider both the initial and final characteristics. Regarding these data together, one can see that the initial structure of nanoparticles is affected by the properties of the de-alloyed system subsequently. This approach can be used to create a number of other ORR electrocatalysts based on PtM nanoparticles.
The purpose of this work is to study the effect of electrochemical and acid treatment of PtCu/C catalysts on their structure and ORR activity in order to improve their functional properties. The novelty of this study is due to the application of the express-electrochemical experiment to register the trend of changes in the ORR activity by rearranging the structure of bimetallic NPs.
2. Materials and Methods
The detailed research scheme, including the preparation of the materials and the study of structural and electrochemical characteristics of the catalysts, is shown in Figure 1.
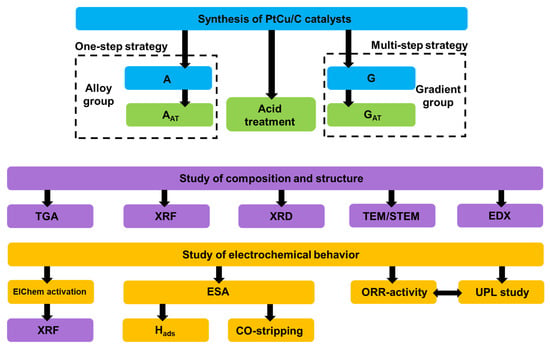
Figure 1.
The scheme of research stages.
2.1. The Synthesis of the PtCu/C Catalyst with the “Alloy” Nanoparticle Structure
The catalyst was obtained by the liquid-phase synthesis in H2O-ethylene glycol (EG) by the combined reduction of platinum and copper precursors on the carbon support. The resulting sample is labeled as A.
2.2. The Synthesis of the PtCu/C Catalyst with the “Gradient” Nanoparticle Structure
Copper and platinum were deposited onto the carbon support containing ultrasmall platinum nanoparticles (~2 nm) in three successive stages from the solutions of CuSO4 and H2PtCl6. The concentration of Pt (IV) in the mixture was increased at each stage (patent No. RU2778126C1). The resulting sample is labeled as G.
2.3. The Preparation of the De-Alloyed Catalysts
The obtained PtCu/C materials were subjected to the 3-h treatment with 1 M HNO3 with stirring at the temperature of 23–25 °C to remove copper atoms from surface layers of NPs. The resulting samples are further labeled as AAT and GAT, respectively.
2.4. Methods to Study the Composition and the Structure of the Catalysts
The composition and structure of the catalysts were studied by thermogravimetry (TGA), X-ray fluorescence (XRF), X-ray diffraction (XRD), energy dispersive X-ray spectroscopy (EDX), transmission electron microscopy (TEM), and scanning transmission electron microscopy (STEM).
2.5. Electrochemical Methods
The electrochemical behavior of the catalysts was studied in a three-electrode cell on a rotating disk electrode.
The electrochemically active surface area (ESA) was determined in the stationary state according to the results of cyclic voltammetry after catalysts activation for 100 voltammetric cycles. The activity in the oxygen reduction reaction was evaluated by linear voltammetry at various electrode rotation speeds. The mass and specific reaction currents were determined using the Koutecky–Levich equation.
The method of preparing catalytic ink, as well as more detailed descriptions of the electrochemical experiment, are presented in Supplementary Materials.
The main performance characteristics are presented in comparison with the commercial Pt/C analogue (labeled as JM20) with a close loading of platinum (Johnson Matthey Corp., London, United Kingdom).
3. Results and Discussion
The effect of the acid treatment on the PtCu/C catalysts with a close Pt loading and a different NPs’ structure (solid solution A and gradient G) was studied. Sample A was obtained by the co-reduction of platinum and copper precursors with sodium borohydride during one stage. Sample G was obtained by the multistage method of the stepwise reduction of precursors on platinum nuclei (patent No. RU2778126C1). The “as-prepared” materials were acidly treated followed by obtaining the de-alloyed structures (samples AAT and GAT, respectively). The commercial Pt/C analogue with a close Pt mass fraction (HiSPEC3000, Johnson Matthey) was studied as a reference sample. This sample is hereafter referred to as JM20.
The Pt mass fraction in the obtained PtCu/C samples is about 20% (Table 1). The content of copper in the G sample is rather low compared with the A sample, which is due to the peculiarities of the multistage synthesis method. One cannot also exclude the galvanic substitution of a portion of copper for platinum during the synthesis [32,33]. Although the platinum–copper ratio in the A and G samples varies, the obtained de-alloyed materials (AAT and GAT) have the same composition (Table 1).

Table 1.
The composition and structural parameters of the obtained PtCu/C catalysts and the commercial Pt/C analogue.
The X-ray diffraction patterns of the obtained samples demonstrate the broad reflex equal to about 25° θ typical for the Vulcan XC72 carbon support (Figure 2) and corresponding to the graphite phase (002) [11]. The platinum reflections (111) and (200) are shifted to the high-angle region 2θ compared with pure platinum (sample JM20), which indicates the alloy formation of Pt and Cu. The X-ray diffraction patterns of the AAT and GAT samples show the shift of maxima (111) to the low-angle region compared with the diffractograms of the A and G samples due to the decrease in the copper content after the acid treatment (Table 1, Figure 2). It is worth noting that the absence of the reflections typical for the copper or its oxide phase in the X-ray diffraction patterns does not mean their actual absence since the reflections can be present in the X-ray amorphous form for these materials [34]. The average crystallite size of the metal phase for the obtained samples does not exceed 3 nm. These results may be slightly undervalued due to the fact that the structural inhomogeneity of bimetallic particles can make a contribution to the broadening of maxima in the X-ray diffraction patterns [35]. Among other things, for the materials with the gradient structure of NPs, the X-ray diffraction pattern can be the result of the reflections of several phases with a different composition overlapping over one another, thus hampering a precise calculation of the average crystallite size [36]. A slight increase in the average crystallite size is observed after the acid treatment of the A and G materials [24] (Table 1).
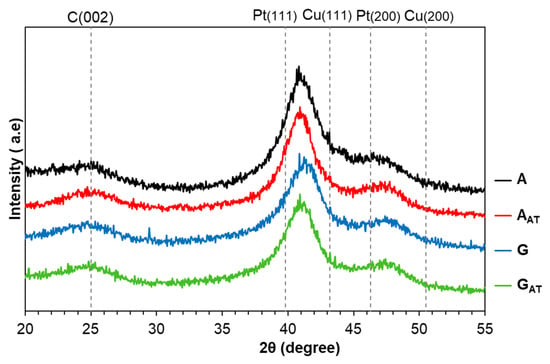
Figure 2.
The XRD pattern of the obtained PtCu/C materials.
The micrographs of transmission electron microscopy (TEM) for the studied PtCu/C materials demonstrate the presence of nanosized metal particles on the carbon support surface (Figure 3). By results of the TEM analysis of local regions, the A sample obtained during one stage is observed to be characterized by a more uniform distribution of NPs over the support surface and a narrower size distribution compared with the gradient sample obtained during several stages (Figure 3a–c,g–i). On the other hand, the average size of NPs for the A sample obtained during one stage is significantly greater than that for the gradient one obtained during several stages. This has to do with the use of the ultra-small platinum NPs, which are deposited on the carbon support during the first stage of the gradient synthesis, as nuclei of the subsequent phase formation [12]. The formation of the particles of a different size and the formation of agglomerates are known to be typical for multistage synthesis methods. After the chemical treatment of the A sample, the average size of NPs decreases from 5.1 to 2.9 nm due to the growing proportion of the particles from 2.5 to 3.0 nm in size (Figure 3d–f). The similar feature cannot be established for the gradient sample, which indirectly testifies to high robustness of the NPs with the gradient microstructure against the acid treatment. Notably, when calculating the average size of NPs for all the materials, only individual particles were taken into account whereas the proportion of agglomerates and the size of their particles were not estimated.
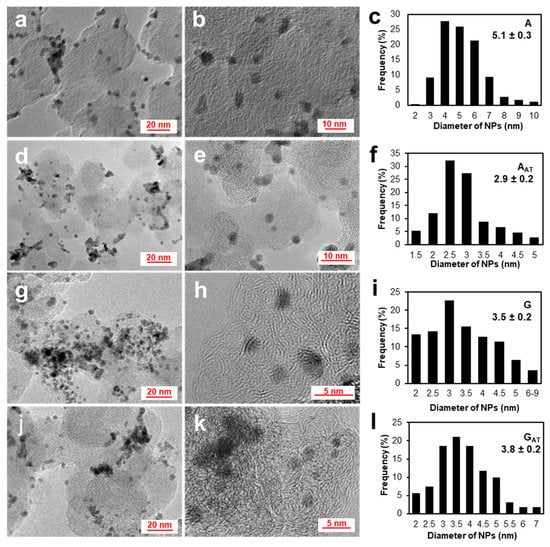
Figure 3.
The TEM micrographs of the PtCu/C sample: A (a,b), AAT (d,e), G (g,h), GAT (j,k), and the histograms of the size distribution of NPs in the corresponding materials (c,f,i,l).
Figures S1–S3 demonstrate the results of the elemental mapping of the catalysts’ surface fragments and the line scanning of NPs for the “as-prepared” and de-alloyed PtCu/C samples. The overlapping of the copper and platinum atoms’ localization regions indicates that the NPs are bimetallic in all the studied samples. At the same time, the acid treatment of the A catalyst with the NPs of the alloy microstructure leads to the formation of the secondary Cu-core–Pt-shell microstructure (the histograms in Figure S1). The NPs in the G sample obtain the core-shell architecture in both the “as-prepared” and de-alloyed states (the histograms in Figure S2). The presence of the platinum nucleus in the core of the studied NP is indicated through the pronounced exceedances of the platinum concentration with respect to the copper concentration in the center of the NP (the first histogram in Figures S2 and S3).
According to the results of thermogravimetry and differential scanning calorimetry (TG&DSC), the intense oxidation of the PtCu/C materials starts at higher temperatures than the oxidation of the Pt/C material with a close platinum mass fraction (Figure S4). In this regard, the oxidation kinetics of the A and G samples is markedly different. The oxidation of the G and GAT materials proceeds similarly and starts at the temperature of about 320 °C (Figure S4a). The first weakly pronounced heat release peak on the DSC curve is observed in the temperature range from 410 to 420 °C, as with the oxidation of the Pt/C sample (Figure S4b). The second intense heat release maximum on the DSC curves of the G and GAT samples is observed at approximately 440 °C. The thermogram of the A sample is shifted to the high-temperature region (Figure S4a), as a result of which the heat release maximum is observed at approximately 480 °C (Figure S4b). The AAT sample obtained after the acid treatment of the A sample begins to oxidize intensively a little earlier (Figure S4a). As a result, the corresponding heat release maximum on the DSC curve is observed at 465–470 °C. Therefore, the carbon oxidation is easiest to occur (at lower temperatures) at the border with the NPs of pure platinum, which oxidize this process effectively. The G and GAT materials appear to obtain a small number of the pure platinum particles, which failed to become centers of the nucleation during the synthesis of the gradient PtCu NPs. Their presence causes the appearance of the weakly pronounced peak on the DSC curves for these materials at 410–420 °C. The Cu-core–Pt-shell NPs in the G material are already formed during the synthesis. This architecture is sure to be much more pronounced for the de-alloyed NPs in the GAT sample. Nevertheless, the presence of copper atoms in NPs slightly inhibits the oxidation of these samples compared with the Pt/C material. With respect to the used synthesis technique and according to the results of the elemental analysis (Table 1), the surface concentration of copper is certain to be highest for NPs in the A sample. This is what inhibits its oxidation. The surface layer of NPs in the AAT sample is enriched with platinum compared with the A sample. Therefore, the former is oxidized faster and more easily than the initial material in the “as-prepared” state. The distribution of copper atoms in NPs of the de-alloyed AAT and GAT samples can be different, which is one reason why the kinetics of their oxidation varies. It should be pointed out that the temperature range of the A material’s combustion is much narrower than that of the G material. According to the data from DSC, a wider combustion range of the G material may be connected with a broader distribution of NPs in size and composition compared with the A material [37].
The initial samples (A, G) and the samples obtained after the acid treatment (AAT, GAT) were electrochemically activated by setting 100 current-voltage cycles in the potential range from 0.04 to 1.0 V (Figure S5). During the activation of the PtM/C catalysts, the standardization of the electrode surface was observed, which was due to leaching the alloying component from the bimetallic NPs as well as cleaning the NPs’ surface off any impurities. According to the results of total reflection X-ray fluorescence (TXRF), after the electrochemical activation, the composition of both the initial samples proved to be close to PtCu0.3 (Table 2). The composition of the de-alloyed samples was almost unchanged after the electrochemical activation (Table 2). A noteworthy detail is that achieving the constant composition (in the realm of Pt3Cu) (Table 1 and Table 2, Figure S6) is certain to be compliant with the components’ parting limit, by reaching which the de-alloying fails to proceed or is significantly inhibited [38].

Table 2.
The composition and electrochemical characteristics of the activated PtCu/C catalysts.
The cyclic voltammograms (CVs) of the catalysts after the standardization have the appearance typical for the Pt-based electrode and include the hydrogen (~0.04–0.35 V), double-layered (~0.35–0.65 V), and oxygen (~0.65–1.0 V) regions (Figure 4a). The ESA increases in the order AAT = A < G < GAT < JM20 (Table 2). In the study concerned with the PtCu/C catalysts, Gatalo et al. emphasize that the estimation of the ESA through the electrochemical adsorption/desorption of the atomic hydrogen gives overestimated values [39]. Nevertheless, the ESA estimated by the CO desorption method (Figure 4b) for the studied samples almost coincides with that assessed through the adsorption/desorption of hydrogen. In this regard, the coincidence of the ESAH and ESACO values is due to the fact that the surface of NPs after the activation is enriched with platinum and contains almost no copper atoms. This proves the fact that the obtained samples have been successfully activated. It is also worth mentioning that the lower ESA values of the platinum-copper catalysts compared with the Pt/C ones are characteristic in this sense. The ESAH and ESACO values for the catalysts obtained by other researchers are presented in Table S1.
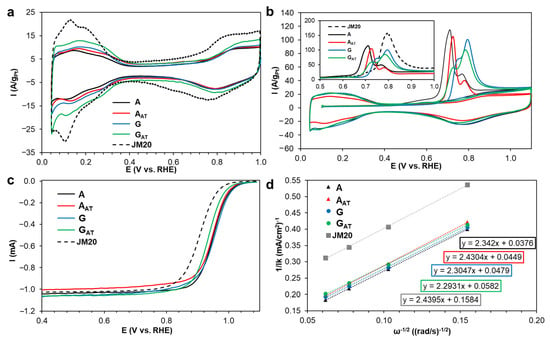
Figure 4.
The cyclic voltammograms, the potential sweep rate is 20 mV s−1 (a), CO stripping, the potential sweep rate is 40 mV s−1 (b) of the samples activated in the potential range of 0.04–1.00 V, under the Ar atmosphere. The linear voltammograms of the oxygen electroreduction in the studied catalysts at the disk rotation speed of 1600 rpm, the potential sweep rate is 20 mV s−1, under the O2 atmosphere (c). 0.1 M HClO4. The dependence 1/j of ω−1/2 at the potential of 0.90 V (d).
The CVs corresponding to the process of the CO desorption in the studied materials are presented in Figure 4b. In all the cases, the oxidation peak between 0.6 and 1.0 V is compliant with removing the CO monolayer, which is preliminarily adsorbed, from the surface of NPs. A significant shift in the potential of the beginning of the CO oxidation to the low-potential region is typical for the A sample (0.65 V). At the same time, for all the bimetallic catalysts, the CO oxidation starts at lower potentials compared with Pt/C, which is due to the weakening of the Pt–CO bond caused by the effect of alloying the platinum with copper [40,41,42]. The study [23] concerned with the PtNi/C materials shows that a difference in the potential of the beginning of the CO oxidation for the bimetallic catalysts with a various structure of NPs depends on a degree of the surface distortion of NPs.
The multiple CO electrooxidation peaks are observed for all the bimetallic samples (Figure 4b). A series of questions are raised in the literature regarding the nature of multiple peaks during the CO stripping analysis [42,43,44,45]. It is shown that the presence of multiple peaks of the CO oxidation may be caused by a difference in facets of Pt, an agglomeration of particles, a wide dispersion of NPs in size, and a difference in composition of their surface. Since the synthesized bimetallic catalysts obtain the NPs with various sizes and compositions, to divide the effect of these factors on the CO desorption does not seem possible. It is worth noting that the appearance of the CO oxidation peaks for all the groups of the synthesized materials (alloy, A and AAT, and gradient, G and GAT) coincides, which testifies to differences in the obtained structures. Meanwhile, the peculiarities of the CO desorption peaks typical for the initial samples are still present after the acid treatment.
The ORR activity of the obtained catalysts was estimated at different rotation speeds of the rotating disk electrode (RDE). The linear sweep voltammograms (LSVs) of the oxygen electroreduction are presented in Figure 4c. The number of the electrons participating in the electroreduction of the oxygen molecule for all the studied samples is close to 4. According to the results of the calculation carried out with the Koutecky–Levich equation (Figure 4d) [46], at the potential of 0.90 V, the samples can be arranged in the following order with the increase in kinetic currents: JM20 << GAT < G < AAT < A. Several researchers link the activity value in the ORR of the bimetallic catalysts to thickness of the Pt shell of NPs [10,47,48].
The work based on theoretical calculations [21] shows that thickness of the Pt shell of bimetallic NPs is related to the degree of the surface strain, which, in turn, correlates with the position of the shell metal’s d-band center (and adsorbate binding strength). The shell thickness provides a facile way to tune the strength of surface–adsorbate interactions [21]. The examples of this influence are given in references [25,49]. According to these works, when Pt NPs catalyze the ORR, the compression of Pt–Pt bonds correlates with the d-band down-shift, which weakens the bonding between Pt and oxygenated species and increases the catalytic activity. In regard to the gradient group samples obtained by the multistage method, the decrease in the ORR activity compared with the alloy group ones is connected with a high proportion of the NPs with a thick Pt shell as well as with the decrease in the ligand effect (Figure 5). In the alloy group catalysts, the higher amount of copper appears to be located in near-surface layers. Therefore, they have a stronger promoting effect on the catalytic activity compared with the gradient group ones.

Figure 5.
The histograms of mass and specific activities for the obtained PtCu/C catalysts and the commercial Pt/C analogue.
As shown in the previous study [20], the ORR activity of the bimetallic catalysts with the structure of alloy NPs is significantly affected by the initial content of copper. At the ratio of PtCux (where X ≥ 1.1) in the “as-prepared” state, the samples exhibit the ORR activity of at least 800 A/g (Pt) [20]. In the current study, the A sample, which is similar in structure, also shows the increased catalytic activity.
The acid treatment of the bimetallic samples leads to the removal of the copper from the surface of NPs. The decrease in the mass activity is significant for the A catalyst: from 827 A/gPt (sample A) to 643 A/gPt (sample AAT) (Figure 5). Therefore, the decrease in the activity after the acid treatment for the alloy group samples is 22%. Additionally, it is only 9.8% for the gradient group catalysts.
All the obtained PtCu/C materials are characterized by the higher activity in the ORR compared with the commercial Pt/C catalyst. Meanwhile, the most active A sample has the mass activity 3.5 times higher and the specific activity 6 times higher than JM20. Interestingly, the ORR activity values established by DOE were achieved during the study of the PtCu/C catalysts [50].
The results of the physicochemical methods to examine the NPs’ composition/microstructure of the obtained PtCu/C materials were expanded with a detailed electrochemical study using the technique proposed in reference [22] (Figure 6). The ORR activity of the obtained catalysts was estimated in the course of the seven-stage experiment. The electrode underwent the activation at every step in the range of potentials from 0.04 V to the upper potential limit (UPL). The UPL value was changed from 0.9 to 1.2 V every 0.05 V. Next, the mass activity values were determined, and the corresponding markers were plotted on the graph of dependence of Imass on the UPL. Therefore, seven markers corresponding to seven stages of the experiment were obtained for each sample. The linear slope coefficients are presented on the graph for each Imass–UPL dependence (Figure 6).
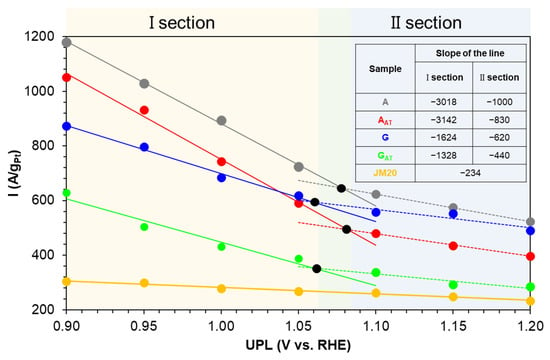
Figure 6.
The dependence of the UPL on the mass activity. The color of the markers in the figure corresponds to the color of the material in the table (inset).
Two characteristic sections are observed on the graph of the Imass–UPL dependence for all the bimetallic materials. The first section (section I) corresponds to four initial values of the potentials. This section is characterized by a quick decrease in the activity, as also shown by the linear slope coefficients. The section is compliant with a more intense rearrangement of the NPs’ microstructure. The second section (a dashed line) is characterized by a weaker decrease in the mass activity of the catalysts whereas the potential grows. The most significant changes in the NPs’ structure are assumed to occur when the catalysts are activated to the UPL value equal to 1.06–1.08 V, which corresponds to the salient point of the PtCu/C materials’ Imass–UPL dependences.
It is noteworthy that in the Imass–UPL diagram, the activity markers for the alloy group samples are located higher than those for the gradient group ones. This is due to the higher ORR activity of the catalysts based on the solid solution NPs compared with the gradient ones. Nevertheless, the different sensitivity of the activity to the values of the upper potential of the activation in two sections of the dependence is typical for both the alloy and gradient materials (Figure 6). At the same time, the gradient samples are characterized by a less sharp decrease in the activity in the first section of the dependence compared with the alloy ones, which indirectly testifies to the higher stability of the gradient catalysts.
Therefore, the results indicate that (i) the de-alloyed catalysts obtained after the acid treatment exhibit the electrochemical behavior similar to that of the initial catalysts (in the “as-prepared” state); (ii) despite the similar composition and the formation of the secondary core-shell microstructure after the acid treatment (the de-alloying), the PtCu NPs “remember” their primary microstructure and save the peculiarities of the electrochemical behavior caused by it. The ORR, which is a structure-sensitive reaction, allows for the estimation of the differences in the materials’ behavior caused by the rearrangement of NPs as a result of the de-alloying and the electrochemical activation.
4. Conclusions
Two groups of the high-performance bimetallic electrocatalysts for the ORR, alloy, and gradient ones were obtained and studied in this work. Although this does not result directly from the article, the acid treatment is a necessary stage to obtain commercially promising catalysts for PEMFCs. The obtained de-alloyed PtCux-y/C catalysts are significantly superior to the commercial Pt/C analogue in terms of the ORR activity.
The results of the study show that the catalysts based on the de-alloyed NPs with a various structure can be characterized by the notably different activity in the ORR in spite of the similar composition and the same method of the acid treatment. Therefore, it may be concluded that there is a presence of the “memory effect”. The de-alloyed NPs “remember” their primary microstructure and demonstrate the electrochemical behavior similar to that of the initial analogues. The conducted experiment testifies to the fact that the study of the electrochemical behavior of the bimetallic systems based on the NPs with a different microstructure needs pursuing over time to understand peculiarities of structural rearrangements and to search for highly active catalysts based on the de-alloyed NPs.
Despite the large number of works on the high activity of bimetallic catalysts based on nanoparticles with a complex architecture, we recommend obtaining catalysts based on nanoparticles with a simple alloy structure. In addition, the optimal range of potentials during electrochemical treatment to obtain the most efficient catalysts was established. Acid treatment, as a necessary and important stage in the preparation of a catalyst, will make it possible to prepare active de-alloyed materials characterized by a stable composition and high functional characteristics in the ORR.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en15249643/s1. TEM and STEM images of catalysts, mapping, and line profiles of nanoparticles. Results of thermogravimetric analysis. Voltammograms of catalysts electrochemical activation. Histograms of changes in the compositions of catalysts. Comparison table of the ESA values of PtCu catalysts presented in publications. References [51,52] are cited in the supplementary materials.
Author Contributions
Investigation, A.S.P. and I.V.P.; writing—original draft preparation, A.S.P., A.A.A. and S.V.B.; conceptualization, A.S.P. and A.A.A.; writing—review and editing, V.E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation (State Assignment in the Field of Scientific Activity No. 0852-2020-0019).
Acknowledgments
The authors are grateful to Nikulin A.Yu. for the assistance in the XRD pattern registration and to the Shared Use Center “High-Resolution Transmission Electron Microscopy” (SFedU) for conducting the TEM studies. The authors appreciate the support by LLC “PROMETHEUS R&D” (Rostov-on-Don) and LLC “Systems for Microscopy and Analysis” (Skolkovo, Moscow) for conducting the TEM and EDX studies. The authors are grateful to Maltsev A.V. for the support in translation and editing processes and the assistance in communication with the editorial board.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cruz-Martínez, H.; Rojas-Chávez, H.; Matadamas-Ortiz, P.T.; Ortiz-Herrera, J.C.; López-Chávez, E.; Solorza-Feria, O.; Medina, D.I. Current Progress of Pt-Based ORR Electrocatalysts for PEMFCs: An Integrated View Combining Theory and Experiment. Mater. Today Phys. 2021, 19, 100406. [Google Scholar] [CrossRef]
- Wang, Y.J.; Long, W.; Wang, L.; Yuan, R.; Ignaszak, A.; Fang, B.; Wilkinson, D.P. Unlocking the Door to Highly Active ORR Catalysts for PEMFC Applications: Polyhedron-Engineered Pt-Based Nanocrystals. Energy Environ. Sci. 2018, 11, 258–275. [Google Scholar] [CrossRef]
- Mukherjee, P.; Kakade, B.; Swami, A. Current Trends in Platinum-Based Ternary Alloys as Promising Electrocatalysts for the Oxygen Reduction Reaction: A Mini Review. Energy Fuels 2022, 36, 2306–2322. [Google Scholar] [CrossRef]
- Kostuch, A.; Rutkowska, I.A.; Dembinska, B.; Wadas, A.; Negro, E.; Vezzù, K.; Di Noto, V.; Kulesza, P.J. Enhancement of Activity and Development of Low Pt Content Electrocatalysts for Oxygen Reduction Reaction in Acid Media. Molecules 2021, 26, 5147. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, D.; Zhang, Q.; Sun, Y.; Zhang, S.; Du, F.; Jin, X. Synthesis of PtCu–Based Nanocatalysts: Fundamentals and Emerging Challenges in Energy Conversion. J. Energy Chem. 2022, 64, 583–606. [Google Scholar] [CrossRef]
- Wang, H.; Lin, R.; Cai, X.; Liu, S.; Zhong, D.; Hao, Z. Transition Metal Dissolution Control in Pt-Alloy Catalyst Layers for Low Pt-Loaded PEMFCs for Improving Mass Transfer. Int. J. Heat Mass Transf. 2021, 178, 121615. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, H.; Zhong, H.; Abdelhafiz, A.; Zeng, J. De-Alloyed PtCu/C Catalysts with Enhanced Electrocatalytic Performance for the Oxygen Reduction Reaction. Nanoscale 2021, 13, 13896–13904. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of Platinum and Early Transition Metals as Oxygen Reduction Electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef]
- Kong, F.; Ren, Z.; Norouzi Banis, M.; Du, L.; Zhou, X.; Chen, G.; Zhang, L.; Li, J.; Wang, S.; Li, M.; et al. Active and Stable Pt-Ni Alloy Octahedra Catalyst for Oxygen Reduction via Near-Surface Atomical Engineering. ACS Catal. 2020, 10, 4205–4214. [Google Scholar] [CrossRef]
- Zhao, X.; Sasaki, K. Advanced Pt-Based Core-Shell Electrocatalysts for Fuel Cell Cathodes. Acc. Chem. Res. 2022, 55, 1226–1236. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Guterman, V.E.; Belenov, S.V.; Menshikov, V.S.; Tabachkova, N.Y.; Safronenko, O.I.; Moguchikh, E.A. Pt/C Electrocatalysts Based on the Nanoparticles with the Gradient Structure. Int. J. Hydrog. Energy 2018, 43, 3676–3687. [Google Scholar] [CrossRef]
- Pavlets, A.S.; Alekseenko, A.A.; Tabachkova, N.Y.; Safronenko, O.I.; Nikulin, A.Y.; Alekseenko, D.V.; Guterman, V.E. A Novel Strategy for the Synthesis of Pt–Cu Uneven Nanoparticles as an Efficient Electrocatalyst toward Oxygen Reduction. Int. J. Hydrog. Energy 2021, 46, 5355–5368. [Google Scholar] [CrossRef]
- Lyu, X.; Jia, Y.; Mao, X.; Li, D.; Li, G.; Zhuang, L.; Wang, X.; Yang, D.; Wang, Q.; Du, A.; et al. Gradient-Concentration Design of Stable Core–Shell Nanostructure for Acidic Oxygen Reduction Electrocatalysis. Adv. Mater. 2020, 32, 2003493. [Google Scholar] [CrossRef]
- Sohn, Y.; Park, J.H.; Kim, P.; Joo, J.B. Dealloyed PtCu Catalyst as an Efficient Electrocatalyst in Oxygen Reduction Reaction. Curr. Appl. Phys. 2015, 15, 993–999. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, W.; Lin, A.; Cheng, D. Low Pt-Content Ternary PtNiCu Nanoparticles with Hollow Interiors and Accessible Surfaces as Enhanced Multifunctional Electrocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 9600–9608. [Google Scholar] [CrossRef]
- Moriau, L.J.; Hrnjić, A.; Pavlišič, A.; Kamšek, A.R.; Petek, U.; Ruiz-Zepeda, F.; Šala, M.; Pavko, L.; Šelih, V.S.; Bele, M.; et al. Resolving the Nanoparticles’ Structure-Property Relationships at the Atomic Level: A Study of Pt-Based Electrocatalysts. iScience 2021, 24, 102102. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, A.; Luo, L.; Wang, C.; Yang, F.; Wei, G.; Xia, G.; Yin, J.; Zhang, J. The Asymmetric Effects of Cu2+ Contamination in a Proton Exchange Membrane Fuel Cell (PEMFC). Fuel Cells 2020, 20, 196–202. [Google Scholar] [CrossRef]
- Xiao, Z.; Jiang, Y.; Wu, H.; Zhong, H.; Song, H.; Abdelhafiz, A.; Zeng, J. De-Alloyed Ternary Electrocatalysts with High Activity and Stability for Oxygen Reduction Reaction. J. Alloy. Compd. 2021, 877, 160221. [Google Scholar] [CrossRef]
- Ruiz-Zepeda, F.; Gatalo, M.; Pavlišič, A.; Dražić, G.; Jovanovič, P.; Bele, M.; Gaberšček, M.; Hodnik, N. Atomically Resolved Anisotropic Electrochemical Shaping of Nano-Electrocatalyst. Nano Lett. 2019, 19, 4919–4927. [Google Scholar] [CrossRef]
- Pavlets, A.S.; Alekseenko, A.A.; Nikolskiy, A.V.; Kozakov, A.T.; Safronenko, O.I.; Pankov, I.V.; Guterman, V.E. Effect of the PtCu/C Electrocatalysts Initial Composition on Their Activity in the de-Alloyed State in the Oxygen Reduction Reaction. Int. J. Hydrog. Energy 2022, 47, 30460–30471. [Google Scholar] [CrossRef]
- Gamler, J.T.L.; Leonardi, A.; Sang, X.; Koczkur, K.M.; Unocic, R.R.; Engel, M.; Skrabalak, S.E. Effect of Lattice Mismatch and Shell Thickness on Strain in Core@shell Nanocrystals. Nanoscale Adv. 2020, 2, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Alekseenko, A.A.; Pavlets, A.S.; Belenov, S.V.; Safronenko, O.I.; Pankov, I.V.; Guterman, V.E. The Electrochemical Activation Mode as a Way to Exceptional ORR Performance of Nanostructured PtCu/C Materials. Appl. Surf. Sci. 2022, 595, 153533. [Google Scholar] [CrossRef]
- Chattot, R.; Martens, I.; Scohy, M.; Herranz, J.; Drnec, J.; Maillard, F.; Dubau, L. Disclosing Pt-Bimetallic Alloy Nanoparticle Surface Lattice Distortion with Electrochemical Probes. ACS Energy Lett. 2020, 5, 162–169. [Google Scholar] [CrossRef]
- Chattot, R.; Le Bacq, O.; Beermann, V.; Kühl, S.; Herranz, J.; Henning, S.; Kühn, L.; Asset, T.; Guétaz, L.; Renou, G.; et al. Surface Distortion as a Unifying Concept and Descriptor in Oxygen Reduction Reaction Electrocatalysis. Nat. Mater. 2018, 17, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Mukerjee, S.; Srinivasan, S.; Soriaga, M.P.; McBreen, J. Role of Structural and Electronic Properties of Pt and Pt Alloys on Electrocatalysis of Oxygen Reduction: An In Situ XANES and EXAFS Investigation. J. Electrochem. Soc. 1995, 142, 1409–1422. [Google Scholar] [CrossRef]
- Srabionyan, V.V.; Pryadchenko, V.V.; Kurzin, A.A.; Belenov, S.V.; Avakyan, L.A.; Guterman, V.E.; Bugaev, L.A. Atomic Structure of PtCu Nanoparticles in PtCu/C Catalysts from EXAFS Spectroscopy Data. Phys. Solid State 2016, 58, 752–762. [Google Scholar] [CrossRef]
- Sasaki, K.; Marinkovic, N.; Isaacs, H.S.; Adzic, R.R. Synchrotron-Based in Situ Characterization of Carbon-Supported Platinum and Platinum Monolayer Electrocatalysts. ACS Catal. 2016, 6, 69–76. [Google Scholar] [CrossRef]
- Gatalo, M.; Jovanovič, P.; Ruiz-Zepeda, F.; Pavlišič, A.; Robba, A.; Bale, M.; Dražić, G.; Gaberšček, M.; Hodnik, N. Insights into Electrochemical Dealloying of Cu out of Au-Doped Pt-Alloy Nanoparticles at the Sub-Nano-Scale. J. Electrochem. Sci. Eng. 2018, 8, 87–100. [Google Scholar] [CrossRef]
- Gatalo, M.; Jovanovič, P.; Petek, U.; Šala, M.; Šelih, V.S.; Ruiz-Zepeda, F.; Bele, M.; Hodnik, N.; Gaberšček, M. Comparison of Pt–Cu/C with Benchmark Pt–Co/C: Metal Dissolution and Their Surface Interactions. ACS Appl. Energy Mater. 2019, 2, 3131–3141. [Google Scholar] [CrossRef]
- Jeyabharathi, C.; Hodnik, N.; Baldizzone, C.; Meier, J.C.; Heggen, M.; Phani, K.L.N.; Bele, M.; Zorko, M.; Hocevar, S.; Mayrhofer, K.J.J. Time Evolution of the Stability and Oxygen Reduction Reaction Activity of PtCu/C Nanoparticles. ChemCatChem 2013, 5, 2627–2635. [Google Scholar] [CrossRef]
- Hodnik, N.; Jeyabharathi, C.; Meier, J.C.; Kostka, A.; Phani, K.L.; Rečnik, A.; Bele, M.; Hočevar, S.; Gaberšček, M.; Mayrhofer, K.J.J. Effect of Ordering of PtCu3 Nanoparticle Structure on the Activity and Stability for the Oxygen Reduction Reaction. Phys. Chem. Chem. Phys. 2014, 16, 13610–13615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, K.; Liu, Q.; Qin, J.; Jiang, Q.; Yang, B.; Yin, F.; Zhang, Y.; Ye, K.; Liu, Q.; et al. Ni2+-Directed Anisotropic Growth of PtCu Nested Skeleton Cubes Boosting Electroreduction of Oxygen. Adv. Sci. 2022, 9, 2104927. [Google Scholar] [CrossRef] [PubMed]
- Podlovchenko, B.I.; Zhumaev, U.E.; Maksimov, Y.M. Galvanic Displacement of Copper Adatoms on Platinum in PtCl42- Solutions. J. Electroanal. Chem. 2011, 651, 30–37. [Google Scholar] [CrossRef]
- Pryadchenko, V.V.; Srabionyan, V.V.; Kurzin, A.A.; Bulat, N.V.; Shemet, D.B.; Avakyan, L.A.; Belenov, S.V.; Volochaev, V.A.; Zizak, I.; Guterman, V.E.; et al. Bimetallic PtCu Core-Shell Nanoparticles in PtCu/C Electrocatalysts: Structural and Electrochemical Characterization. Appl. Catal. A Gen. 2016, 525, 226–236. [Google Scholar] [CrossRef]
- Avakyan, L.; Tolchina, D.; Barkovski, V.; Belenov, S.; Alekseenko, A.; Shaginyan, A.; Srabionyan, V.; Guterman, V.; Bugaev, L. Ultimate sensitivity of radial distribution functions to architecture of PtCu bimetallic nanoparticles. Comput. Mater. Sci. 2022, 208, 111326. [Google Scholar] [CrossRef]
- Editor, G.; Russell, A.; Otani, M.; Hamada, I.; Sugino, O.; Morikawa, Y.; Okamoto, Y.; Ikeshoji, T.; Wasileski, S.A.; Janik, M.J.; et al. Voltammetric Surface Dealloying of Pt Bimetallic Nanoparticles: An Experimental and DFT Computational Analysis. Phys. Chem. Chem. Phys. 2008, 10, 3670–3683. [Google Scholar] [CrossRef]
- Guterman, V.E.; Belenov, S.V.; Krikov, V.V.; Vysochina, L.L.; Yohannes, W.; Tabachkova, N.Y.; Balakshina, E.N. Reasons for the Differences in the Kinetics of Thermal Oxidation of the Support in Pt/C Electrocatalysts. J. Phys. Chem. C 2014, 118, 23835–23844. [Google Scholar] [CrossRef]
- Erlebacher, J. An Atomistic Description of Dealloying: Porosity Evolution, the Critical Potential, and Rate-Limiting Behavior. J. Electrochem. Soc. 2004, 151, C614. [Google Scholar] [CrossRef]
- Gatalo, M.; Moriau, L.; Petek, U.; Ruiz-Zepeda, F.; Šala, M.; Grom, M.; Galun, T.; Jovanovič, P.; Pavlišič, A.; Bele, M.; et al. CO-Assisted Ex-Situ Chemical Activation of Pt-Cu/C Oxygen Reduction Reaction Electrocatalyst. Electrochim. Acta 2019, 306, 377–386. [Google Scholar] [CrossRef]
- Caballero-Manrique, G.; Velázquez-Palenzuela, A.; Brillas, E.; Centellas, F.; Garrido, J.A.; Rodríguez, R.M.; Cabot, P.L. Electrochemical Synthesis and Characterization of Carbon-Supported Pt and Pt–Ru Nanoparticles with Cu Cores for CO and Methanol Oxidation in Polymer Electrolyte Fuel Cells. Int. J. Hydrog. Energy 2014, 39, 12859–12869. [Google Scholar] [CrossRef]
- Garcia-Cardona, J.; Sirés, I.; Alcaide, F.; Brillas, E.; Centellas, F.; Cabot, P.L. Electrochemical Performance of Carbon-Supported Pt(Cu) Electrocatalysts for Low-Temperature Fuel Cells. Int. J. Hydrog. Energy 2020, 45, 20582–20593. [Google Scholar] [CrossRef]
- Zamanzad Ghavidel, M.R.; Monteverde Videla, A.H.A.; Specchia, S.; Easton, E.B. The Relationship between the Structure and Ethanol Oxidation Activity of Pt-Cu/C Alloy Catalysts. Electrochim. Acta 2017, 230, 58–72. [Google Scholar] [CrossRef]
- Urchaga, P.; Baranton, S.; Coutanceau, C.; Jerkiewicz, G. Electro-Oxidation of CO Chem on Pt Nanosurfaces: Solution of the Peak Multiplicity Puzzle. Langmuir 2012, 28, 3658–3663. [Google Scholar] [CrossRef] [PubMed]
- Ciapina, E.G.; Santos, S.F.; Gonzalez, E.R. Electrochemical CO Stripping on Nanosized Pt Surfaces in Acid Media: A Review on the Issue of Peak Multiplicity. J. Electroanal. Chem. 2018, 815, 47–60. [Google Scholar] [CrossRef]
- Taylor, S.; Fabbri, E.; Levecque, P.; Schmidt, T.J.; Conrad, O. The Effect of Platinum Loading and Surface Morphology on Oxygen Reduction Activity. Electrocatalysis 2016, 7, 287–296. [Google Scholar] [CrossRef]
- Garsany, Y.; Ge, J.; St-Pierre, J.; Rocheleau, R.; Swider-Lyons, K.E. Analytical Procedure for Accurate Comparison of Rotating Disk Electrode Results for the Oxygen Reduction Activity of Pt/C. J. Electrochem. Soc. 2014, 161, F628–F640. [Google Scholar] [CrossRef]
- Weber, P.; Weber, D.J.; Dosche, C.; Oezaslan, M. Highly Durable Pt-Based Core-Shell Catalysts with Metallic and Oxidized Co Species for Boosting the Oxygen Reduction Reaction. ACS Catal. 2022, 12, 6394–6408. [Google Scholar] [CrossRef]
- Hashiguchi, Y.; Nakamura, I.; Honma, T.; Matsushita, T.; Murayama, H.; Tokunaga, M.; Choe, Y.-K.; Fujitani, T. Effects of Pt Shell Thickness on Oxygen Reduction Reaction Over Well-Defined Pd@Pt Core-Shell Model Surface. ChemPhysChem 2022. early view. [Google Scholar] [CrossRef]
- Gamler, J.T.L.; Ashberry, H.M.; Skrabalak, S.E.; Koczkur, K.M. Random Alloyed versus Intermetallic Nanoparticles: A Comparison of Electrocatalytic Performance. Adv. Mater. 2018, 30, 1801563. [Google Scholar] [CrossRef]
- DOE Technical Targets for Polymer Electrolyte Membrane Fuel Cell Components|Department of Energy. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-polymer-electrolyte-membrane-fuel-cell-components (accessed on 23 September 2022).
- Garsany, Y.; Singer, I.L.; Swider-Lyons, K.E. Impact of Film Drying Procedures on RDE Characterization of Pt/VC Electrocatalysts. J. Electroanal. Chem. 2011, 662, 396–406. [Google Scholar] [CrossRef]
- Shinozaki, K.; Zack, J.W.; Pylypenko, S.; Pivovar, B.S.; Kocha, S.S. Oxygen Reduction Reaction Measurements on Platinum Electrocatalysts Utilizing Rotating Disk Electrode Technique: II. Influence of Ink Formulation, Catalyst Layer Uniformity and Thickness. J. Electrochem. Soc. 2015, 162, F1384. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).