Abstract
In an era where sustainability is becoming the main driving force for research and development, supercritical fluids-based techniques are presented as a very efficient alternative technology to conventional extraction, purification, and recrystallization processes. Supercritical antisolvent (SAS) precipitation is a novel technique that can replace liquid antisolvent precipitation techniques. Additionally, through the optimization of precipitation operating conditions, morphology, particle size, and particle size distribution of nanoparticles can be controlled. As an antisolvent, supercritical carbon dioxide (scCO2) is far more sustainable than its conventional liquid counterparts; not only does it have a critical point (304 K and 73.8 bar) on its phase diagram that allows for the precipitation processes to be developed so close to room temperature, but also its recovery and, consequently, the precipitated solute purification stage is considerably simpler. This technique can be used efficiently for preparing nanocatalysts to be used in biodiesel production processes.
1. Introduction
Ever since Baron Charles de la Tour first theorized supercritical fluids (SCFs) in 1822 [1,2,3], several research studies have been executed regarding its applications, resulting in several technologies such as supercritical fluid extraction, supercritical drying, supercritical dyeing, and supercritical fluid chromatography. [1] SCFs are characterized by having both their temperature and pressure values higher than their critical point, where a significant difference between liquid and gas does not exist [2,4].
As these operating conditions exceed the critical point, a clear interface between the liquid and gas phases tends to disappear and, thus, becomes a mixed gas, as shown in Figure 1. This mixed gas has properties inherited from both gaseous and liquid states, namely: low viscosity, high density, high diffusivity, non-existing surface tension, good fluidity, heat and mass transfer characteristics, as well as an adjustable solvent selectivity [1,5,6,7,8,9,10]. The particular combination of these properties is appropriate for creating procedures for recovering, purifying, and extracting fine chemicals and pharmaceuticals and also for creating new products that can not be produced by applying more traditional processes [2,3].
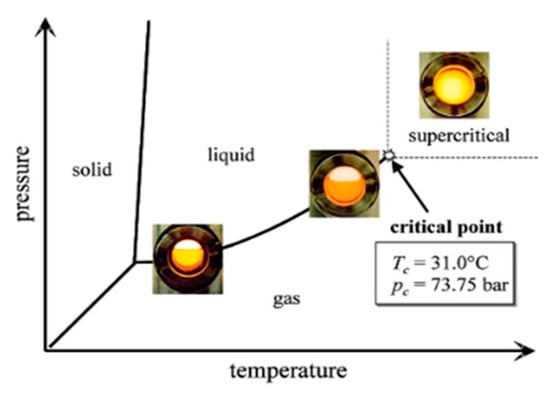
Figure 1.
A brilliant orange CO2-philic complex of rhodium was added in order to obtain better contrast and to demonstrate the solvent behavior of the liquid and the supercritical phase in the CO2 phase diagram, which is illustrated with figures of the transition from the liquid/gas region to the supercritical region [11].
Supercritical carbon dioxide (scCO2) and supercritical water have been recognized as green solvents for the future, mostly due to their ecological benefits, as they are nontoxic, noncarcinogenic, non-mutagenic, nonflammable, and thermodynamically stable. Supercritical carbon dioxide (scCO2), when compared to supercritical water, exists at temperatures above 647 K and pressures over 221 bar, and it is more easily accessible by having a critical temperature (TC) of 304 K and a critical pressure (PC) of 73.8 bar, as shown in Figure 1. Thus, this considerably low critical temperature allows processes to be developed very near to ambient temperature [3,12,13,14,15].
2. Preparation of Nanomaterials Using Supercritical CO2
Nanoscale materials, often known as nanomaterials, are defined by the International Union of Pure and Applied Chemistry (IUPAC) as having organized components with at least one dimension less than 100 nm [16]. New nanomaterials, including nanoclays, nanofibers, nanoporous materials, carbon nanotubes, nanocomposites, and nanoparticles, have recently been used for several different applications [17].
The most used methods to produce nanomaterials are gas condensation, vacuum evaporation and deposition, precipitation, impregnation, chemical vapor deposition, nano-grinding, calcination-hydration-dehydration, and sol–gel techniques [1,18,19,20]. Liquid anti-solvent processes are also used in the industry, based on the miscibility between two solvents. The solute to be micronized has to be soluble in the first solvent, but not soluble in the anti-solvent. Therefore, by adding the anti-solvent, the formation of a solution between the two liquids and the supersaturation is induced, and subsequent precipitation of the solute occurs [21]. This traditional micronization technique usually produces wide particle size ranges and products with an uneven morphology. The elimination of liquid solvent residues is also a matter of concern [21,22]. These limitations can be particularly pertinent for some industrial applications, such as the production of pharmaceutical compounds [23].
Due to the features of supercritical fluids (SCFs) that have been previously described, approaches based on SCFs have been suggested as an alternative to traditional procedures [5]. By adjusting the operational parameters like the temperature, pressure, and solvent flow rate, supercritical fluids like scCO2 can produce nanoparticles. It is also feasible to modify the particle size as well as the morphology of nanoparticles by utilizing the unique properties of supercritical solvents [1]. The earliest evidence of supercritical fluids being used for particle formation upon their depressurization was found in 1879 by Hannay and Hogart [24,25]. Surprisingly, the first patent for the rapid expansion of supercritical expansion solutions by valves or other spraying devices, or Rapid Expansion of Supercritical Solutions (RESS), was not published until 1986, more than a century after the invention was made [25]. Since CO2 has a low critical pressure, and particularly temperature, in addition to being abundant and reasonably non-expensive, it has several technological advantages: used CO2 can be easily collected and reutilized [26], and different processes have been developed for that purpose.
Recently, new proposals and developments have been made concerning various micronization technologies aiming to benefit from the peculiarities of fluids at supercritical conditions: new approaches for particle formation methods focused on the use of scCO2 have started to be designed, developed, and tested, such as solvent (RESS), antisolvent (supercritical antisolvent (SAS)), aerosol solvent extraction system (ASES), precipitation with compressed antisolvent (PCA), gaseous antisolvent (GAS), supercritical antisolvent using enhanced mass transfer (SAS-EM), solution enhanced dispersion by supercritical fluids (SEDS), suspension-enhanced dispersion by supercritical fluids (SpEDS)), co-solute (particle formation from gas-saturated solutions (PGSS)) and co-solvent [25,27,28,29]. A conceptual picture of the various scCO2-based particle production processes is shown in Figure 2.
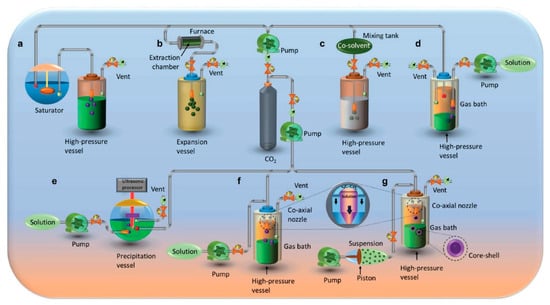
Figure 2.
Conceptually distinct particle precipitation mechanisms are represented using scCO2: (a) Formation of particles from gas-saturated solutions (PGSS). (b) Expansion (rapid) of supercritical solutions (RESS). (c) Expansion (rapid) of a supercritical solution into a liquid solvent (RESOLV). (d) Precipitation using a compressed anti-solvent (PCA)/Aerosol solvent extraction system (ASES). (e) Supercritical antisolvent with enhanced mass transfer (SAS-EM). (f) Solution-enhanced dispersion by supercritical fluids (SEDS). (g) Suspension-enhanced dispersion by supercritical fluids (SpEDS) [28].
3. Supercritical Antisolvent (SAS) as a Micronization Technique
Supercritical antisolvent precipitation (SAS), is a novel, ecologically benign method of creating nanomaterials that may be used as a substitute for liquid solvent precipitation since it is far more efficient. scCO2 has been extensively used for producing a variety of materials, such as polymers, biopolymers, superconductors, explosives, colouring agents, active pharmaceutical ingredients (APIs), and catalysts, using an antisolvent for the controlled precipitation of solids dissolved in conventional solvent, if the processed compounds do not dissolve in the supercritical medium [5,23,30,31,32,33,34]. Under the right process conditions, these compounds dissolve into an organic liquid miscible with the supercritical antisolvent [32]. SAS combines the benefits of the sol–gel process with the use of a supercritical CO2 antisolvent to create highly porous nanoparticles. The sol–gel technique is used in the SAS process and takes place in a supercritical atmosphere, allowing for quick precursor hydrolysis and quick condensation. This rapid method is, then, connected to a simple purification phase [26]. The dissolution of the solvent and, also, of the supercritical fluid occurs very fast when the solution is put into contact with scCO2 because of very high mass transfer rates, ensuring for the creation of micro- and nanoparticles within the solute precipitation. The process is carried out in either a one-phase supercritical manner or a two-phase gas-liquid approach, with particle production taking place in both cases, depending on operating variables such as pressure and temperature. As a result, the SAS process, based on the characteristics of supercritical fluids, is a viable micronization method [31,35,36].
Additionally, the size and morphology of the resulting solids precipitated by SAS technologies are usually correlated with the system solvent/antisolvent high-pressure VLEs (vapour-liquid equilibrium), or the position of the SAS operating point around the critical point of the mixture (MCP) [7,29]. For instance, if the process is being conducted as a single-phase method with no interface between the solution and the antisolvent, this indicates that micronization is occurring at supercritical conditions, i.e., above the MCP, and the very fast diffusion of scCO2 into the liquid solvent causes its expansion, thus producing the solute’s supersaturation and resulting in forming nanoparticle morphologies that are not typically achieved by traditional catalyst preparation methods [26,33]. This phenomenon, adding to the quasi-zero surface tension of scCO2, allows for obtaining particles of smaller size and having a narrow particle size distribution (PSD) with the complete elimination of the solvents, when compared to the traditional micronization techniques [23,31,36,37]; or, if the process shows a two-phase mixing, the micronization is occurring at subcritical operation conditions, i.e., in the biphasic region below the MCP, resulting in the production of microparticles [25,29].
The success of SAS precipitation techniques is strongly dependant on the affinity between the solvent and the supercritical antisolvent, i.e., the solubility of the liquid solvent in the supercritical CO2 and the quick gas-like diffusion of the scCO2 in the solvent [22,29], as shown in Figure 3. Several organic liquids that are completely miscible with scCO2 under process conditions have been used, such as acetic acid, acetone, chloroform, dichloromethane, dimethyl formamide, dimethylsulfoxide, ethanol, ethyl acetate, formic acid, isopropanol, methanol, N-methyl pyrrolidone, and tetrahydrofuran. In some other cases, mixtures of two of the indicated solvents have also been used [25,34].
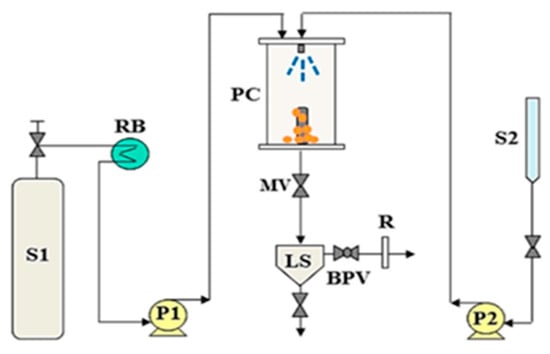
Figure 3.
Schematic diagram of the supercritical antisolvent (SAS) apparatus, composed of: BPV: Back-pressure valve; LS: Liquid separator; MV: Micrometric valve; P1, P2: Pumps; PC: precipitation chamber; RB: Refrigeration bath; S1: CO2 supply; S2: Liquid solution supply; R: Rotameter [22].
The common and universal mode of operation of a semi-continuous SAS apparatus comprises the utilisation of a high-pressure diaphragm pump with a cooling system for the pumping head to supply supercritical CO2 into the precipitation vase at a constant flow rate until the appropriate pressure is achieved. To achieve steady-state fluid phase composition conditions during solute precipitation, a high-pressure pump then injects pure solvent through the nozzle into the precipitation chamber. A stainless-steel frit is generally located at the bottom of the chamber to collect the solute precipitate formed due to the solvent supersaturation, enabling the solvent/scCO2 mixture to pass through and then be recovered and separated further downstream, where the solvent is collected in a second vessel. A dry test meter and a rotameter are frequently employed to gauge the CO2 flow rate as well as the total amount of antisolvent supplied at the exit of this second vessel. The solution created by the liquid solubilized in the supercritical antisolvent is removed as supercritical CO2, which will flow through the chamber in order to wash it. When the solvent condensates during the depressurization process, it has the potential to solubilize or alter the precipitates if a final purge with pure CO2 is eliminated. The precipitator is then depressurized to atmospheric pressure after this washing step, allowing for the collection of the precipitated powder [7,22,25].
As stated before, numerous materials have been produced using scCO2 as an antisolvent to perform the controlled precipitation of particles dissolved in more traditional solvents.
Ha et al. (2020) created nanoparticles of pure trans-resveratrol without the addition of any sugars, polymers, or surfactants using supercritical antisolvent (SAS) technology. The preferred solvents were combinations of dichloromethane and alcohol (methanol or ethanol). Using an ethanol/dichloromethane combination at a 25%/75% (w/w) ratio, the SAS approach enabled the production of trans-resveratrol nanoparticles having a mean particle size of 0.17 m. The trans-resveratrol nanoparticles produced by SAS displayed enhanced oral bioavailability when compared to other microparticles of varied sizes manufactured by two other milling processes [38].
Hariyanto et al. (2021) used the supercritical antisolvent (SAS) procedure to produce micronized ecamsule powder (having dimensions in the range 1.8–2.8 µm) using scCO2 as antisolvent. The effect of different operational parameters, such as pressure, temperature, flow rate of ecamsule solution, and solute (ecamsule) feed concentration on the particle size and size distribution, as well as properties of ecamsule were evaluated. The authors obtained a yield of micronized (1.8 ± 0.8 µm) ecamsule particles of about 91% at 323 K, 14 MPa, and a solution flow rate of 1 mL min−1 for a solute feed concentration of 5% wt. Compared to previous drying processes, the microparticles had a reduced crystalline structure and improved chemical characteristics [39].
Kim et al. (2008) successfully used the supercritical antisolvent (SAS) technique to produce amorphous atorvastatin calcium nanoparticles. The dissolution rates of amorphous calcium atorvastatin nanoparticles were much higher than those of the identical unprocessed drug due to an increase in intrinsic dissolving rate and a decrease in particle size, which, in turn, improved the specific surface area. Following oral administration of the nanoparticles to rats, the researchers also observed a noticeably increased rate of medicine absorption [40].
Reverchon et al. (1999) used supercritical antisolvent (SAS) for producing zinc acetate to assess the viability of using this method to create controlled-size nanoparticles of catalyst precursors. It was possible to create zinc acetate nanoparticles as small as 30 nm and with a mean particle size distribution of 50 nm. Depending on the liquid solution’s concentration, the generated nanoparticles displayed a range of porosities. Using N2 adsorption, BET surface areas were measured, ranging up to 175 m2/g [41].
Nobre et al. (2020) investigated the precipitation of calcium acetate made from eggshells using the SAS method. The experimental parameters such as pressure, temperature, liquid solution concentration, and injection flow rate were reported by the authors along with a study of precipitation efficiency as a function of these parameters. In contrast to metallic acetates described in the literature, the produced particles had a distinct morphology and exhibited a rod-like structure. Furthermore, FTIR and thermogravimetric analyses proved that the precipitated calcium acetate had a better purity than the initial acetate made from the eggshells [42]. Santos et al. [43,44] also used this technique to obtain nano-structured calcium oxide catalysts that can be used in the obtaining of biodiesel via transesterification, with certain advantages over other heterogeneous catalysts.
4. Conclusions
Supercritical antisolvent (SAS) precipitation using supercritical CO2 allows for more efficient production of nanomaterials than liquid solvent precipitation, due to being faster, allowing for control of the nanomaterial’s particle size as well as particle size distribution through fine-tuning of the precipitation operating conditions, and having an easier purification stage. All these advantages, together with the fact that supercritical CO2 is considered a green solvent for the future due to its ecological benefits when compared to conventional solvents, make this a very desirable precipitation process.
Author Contributions
Conceptualization, J.G. and J.P.; investigation S.S.; writing—original draft preparation S.S.; writing—review and editing, J.G. and J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Su, W.; Zhang, H.; Xing, Y.; Li, X.; Wang, J.; Cai, C. A Bibliometric Analysis and Review of Supercritical Fluids for the Synthesis of Nanomaterials. Nanomaterials 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Knez; Markočič, E.; Leitgeb, M.; Primožič, M.; Knez Hrnčič, M.; Škerget, M. Industrial Applications of Supercritical Fluids: A Review. Energy 2014, 77, 235–243. [Google Scholar] [CrossRef]
- Knez, Ž.; Pantić, M.; Cör, D.; Novak, Z.; Knez Hrnčič, M. Are Supercritical Fluids Solvents for the Future? Chem. Eng. Process. Process Intensif. 2019, 141, 107532. [Google Scholar] [CrossRef]
- Ahangari, H.; King, J.W.; Ehsani, A.; Yousefi, M. Supercritical Fluid Extraction of Seed Oils–A Short Review of Current Trends. Trends Food Sci. Technol. 2021, 111, 249–260. [Google Scholar] [CrossRef]
- Prosapio, V.; De Marco, I.; Scognamiglio, M.; Reverchon, E. Folic Acid–PVP Nanostructured Composite Microparticles by Supercritical Antisolvent Precipitation. Chem. Eng. J. 2015, 277, 286–294. [Google Scholar] [CrossRef]
- Skouta, R. Selective Chemical Reactions in Supercritical Carbon Dioxide, Water, and Ionic Liquids. Green Chem. Lett. Rev. 2009, 2, 121–156. [Google Scholar] [CrossRef]
- Liu, H.; Chen, B.Q.; Pan, Y.J.; Fu, C.P.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Role of Supercritical Carbon Dioxide (ScCO2) in Fabrication of Inorganic-Based Materials: A Green and Unique Route. Sci. Technol. Adv. Mater. 2021, 22, 695–717. [Google Scholar] [CrossRef]
- Li, K.; Xu, Z. A Review of Current Progress of Supercritical Fluid Technologies for E-Waste Treatment. J. Clean. Prod. 2019, 227, 794–809. [Google Scholar] [CrossRef]
- West, C. Recent Trends in Chiral Supercritical Fluid Chromatography. TrAC Trends Anal. Chem. 2019, 120, 115648. [Google Scholar] [CrossRef]
- Brunner, G. Applications of Supercritical Fluids. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 321–342. [Google Scholar] [CrossRef]
- Leitner, W. Supercritical Carbon Dioxide as a Green Reaction Medium for Catalysis. Acc. Chem. Res. 2002, 35, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Noyori, R. Supercritical Fluids: Introduction. Chem. Rev. 1999, 99, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Heinonen, S.; Levänen, E. Applications of Supercritical Carbon Dioxide in Materials Processing and Synthesis. RSC Adv. 2014, 4, 61137–61152. [Google Scholar] [CrossRef]
- Soh, S.H.; Lee, L.Y. Microencapsulation and Nanoencapsulation Using Supercritical Fluid (SCF) Techniques. Pharmaceutics 2019, 11, 21. [Google Scholar] [CrossRef]
- Lane, M.K.M.; Zimmerman, J.B. Controlling Metal Oxide Nanoparticle Size and Shape with Supercritical Fluid Synthesis. Green Chem. 2019, 21, 3769–3781. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for Biorelated Polymers and Applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Banković–Ilić, I.B.; Miladinović, M.R.; Stamenković, O.S.; Veljković, V.B. Application of Nano CaO–Based Catalysts in Biodiesel Synthesis. Renew. Sustain. Energy Rev. 2017, 72, 746–760. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent Advancement in Biodiesel Production Methodologies Using Various Feedstock: A Review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Rajput, N. Methods of Preparation of Nanoparticles-A Review. Int. J. Adv. Eng. Technol. 2015, 7, 1806–1811. [Google Scholar] [CrossRef]
- Santos, S.; Puna, J.; Gomes, J.; Marchetti, J. A Review on the Use of Bio/Nanostructured Heterogeneous Catalysts in Biodiesel Production. In Nano-and Biocatalysts for Biodiesel Production; Wiley: Hoboken, NJ, USA, 2021; pp. 59–91. [Google Scholar]
- Reverchon, E. Supercritical Antisolvent Precipitation of Micro- and Nano-Particles. J. Supercrit. Fluids 1999, 15, 1–21. [Google Scholar] [CrossRef]
- Franco, P.; Marco, I. De Supercritical Antisolvent Process for Pharmaceutical Applications: A Review. Processes 2020, 8, 938. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G. Production of Antibiotic Micro- and Nano-Particles by Supercritical Antisolvent Precipitation. Powder Technol. 1999, 106, 23–29. [Google Scholar] [CrossRef]
- Majerik, V.; Charbit, G.; Badens, E.; Horváth, G.; Szokonya, L.; Bosc, N.; Teillaud, E. Bioavailability Enhancement of an Active Substance by Supercritical Antisolvent Precipitation. J. Supercrit. Fluids 2007, 40, 101–110. [Google Scholar] [CrossRef]
- Padrela, L.; Rodrigues, M.A.; Duarte, A.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. Supercritical Carbon Dioxide-Based Technologies for the Production of Drug Nanoparticles/Nanocrystals–A Comprehensive Review. Adv. Drug Deliv. Rev. 2018, 131, 22–78. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.P.; Winkler, M.E.G.; Giufrida, W.M.; Cardozo-Filho, L.; Alonso, C.G.; Lopes, J.B.O.; Rubira, A.F.; Silva, R. Effect of Phase Composition on the Photocatalytic Activity of Titanium Dioxide Obtained from Supercritical Antisolvent. J. Colloid Interface Sci. 2019, 535, 245–254. [Google Scholar] [CrossRef]
- De Marco, I. The Supercritical Antisolvent Precipitation from a Sustainable Perspective: A Life Cycle Assessment. J. CO2 Util. 2022, 55, 101808. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhang, Y.S.; Wang, S.-B.; Lee, C.-H.; Chen, A.-Z. Supercritical Fluid Technology: An Emphasis on Drug Delivery and Related Biomedical Applications. Adv. Healthc. Mater. 2017, 16, 1700433. [Google Scholar] [CrossRef]
- Franco, P.; De Marco, I. Nanoparticles and Nanocrystals by Supercritical CO2-Assisted Techniques for Pharmaceutical Applications: A Review. Appl. Sci. 2021, 11, 1476. [Google Scholar] [CrossRef]
- Tenorio, A.; Gordillo, D.M.; Pereyra, M.C.; Martínez de la Ossa, E.J. Relative Importance of the Operating Conditions Involved in the Formation of Nanoparticles of Ampicillin by Supercritical Antisolvent Precipitation. Ind. Eng. Chem. Res. 2006, 46, 114–123. [Google Scholar] [CrossRef]
- Mahdi Pourmortazavi, S.; Somayyeh Hajimirsadeghi, S. Application of Supercritical Carbon Dioxide in Energetic Materials Processes: A Review. Ind. Eng. Chem. Res. 2005, 44, 6523–6533. [Google Scholar] [CrossRef]
- Reverchon, E.; Della Porta, G.; Di Trolio, A.; Pace, S. Supercritical Antisolvent Precipitation of Nanoparticles of Superconductor Precursors. Ind. Eng. Chem. Res. 1998, 37, 952–958. [Google Scholar] [CrossRef]
- Tang, Z.R.; Edwards, J.K.; Bartley, J.K.; Taylor, S.H.; Carley, A.F.; Herzing, A.A.; Kiely, C.J.; Hutchings, G.J. Nanocrystalline Cerium Oxide Produced by Supercritical Antisolvent Precipitation as a Support for High-Activity Gold Catalysts. J. Catal. 2007, 249, 208–219. [Google Scholar] [CrossRef]
- Reverchon, E.; Adami, R.; Caputo, G.; De Marco, I. Spherical Microparticles Production by Supercritical Antisolvent Precipitation: Interpretation of Results. J. Supercrit. Fluids 2008, 47, 70–84. [Google Scholar] [CrossRef]
- Vorobei, A.M.; Parenago, O.O. Using Supercritical Fluid Technologies to Prepare Micro- and Nanoparticles. Russ. J. Phys. Chem. A 2021, 95, 407–417. [Google Scholar] [CrossRef]
- Nerome, H.; Machmudah, S.; Wahyudiono; Fukuzato, R.; Higashiura, T.; Youn, Y.S.; Lee, Y.W.; Goto, M. Nanoparticle Formation of Lycopene/β-Cyclodextrin Inclusion Complex Using Supercritical Antisolvent Precipitation. J. Supercrit. Fluids 2013, 83, 97–103. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; De Marco, I.; Vaiano, V. Zinc Oxide Nanoparticles Obtained by Supercritical Antisolvent Precipitation for the Photocatalytic Degradation of Crystal Violet Dye. Catalysts 2019, 9, 346. [Google Scholar] [CrossRef]
- Ha, E.-S.; Park, H.; Lee, S.-K.; Sim, W.-Y.; Jeong, J.-S.; Baek, I.-H.; Kim, M.-S. Pure Trans-Resveratrol Nanoparticles Prepared by a Supercritical Antisolvent Process Using Alcohol and Dichloromethane Mixtures: Effect of Particle Size on Dissolution and Bioavailability in Rats. Antioxidants 2020, 9, 342. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, P.; Myint, A.A.; Kim, J. Complete Drying and Micronization of Ecamsule Using Supercritical CO2 as the Antisolvent. J. Supercrit. Fluids 2021, 170, 105157. [Google Scholar] [CrossRef]
- Kim, M.-S.; Jin, S.-J.; Kim, J.-S.; Park, H.J.; Song, H.-S.; Neubert, R.H.H.; Hwang, S.-J. Preparation, Characterization and in Vivo Evaluation of Amorphous Atorvastatin Calcium Nanoparticles Using Supercritical Antisolvent (SAS) Process. Eur. J. Pharm. Biopharm. 2008, 69, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, E.; Della Porta, G.; Sannino, D.; Ciambelli, P. Supercritical Antisolvent Precipitation of Nanoparticles of a Zinc Oxide Precursor. Powder Technol. 1999, 102, 127–134. [Google Scholar] [CrossRef]
- Nobre, L.C.S.; Santos, S.; Palavra, A.M.F.; Calvete, M.J.F.; de Castro, C.A.N.; Nobre, B.P. Supercritical Antisolvent Precipitation of Calcium Acetate from Eggshells. J. Supercrit. Fluids 2020, 163, 104862. [Google Scholar] [CrossRef]
- Santos, S.; Nobre, L.; Gomes, J.; Puna, J.; Quinta-Ferreira, R.; Bordado, J. Soybean Oil Transesterification for Biodiesel Production with Micro-Structured Calcium Oxide (CaO) from Natural Waste Materials as a Heterogeneous Catalyst. Energies 2019, 12, 4670. [Google Scholar] [CrossRef]
- Santos, S.; Puna, J.; Gomes, J.; Quinta-Ferreira, R.; Bordado, J. Use of micro and nano-structured catalysts for biodiesel production: A review. Curr. Top. Catal. 2019, 14, 83–95. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).