Evaluation of Pyrolysis Reactivity, Kinetics, and Gasification Reactivity of Corn Cobs after Torrefaction Pretreatment
Abstract
1. Introduction
2. Experimental Section
2.1. Torrefaction Experiment
2.2. Pyrolysis and Gasification Experiment
3. Results and Discussion
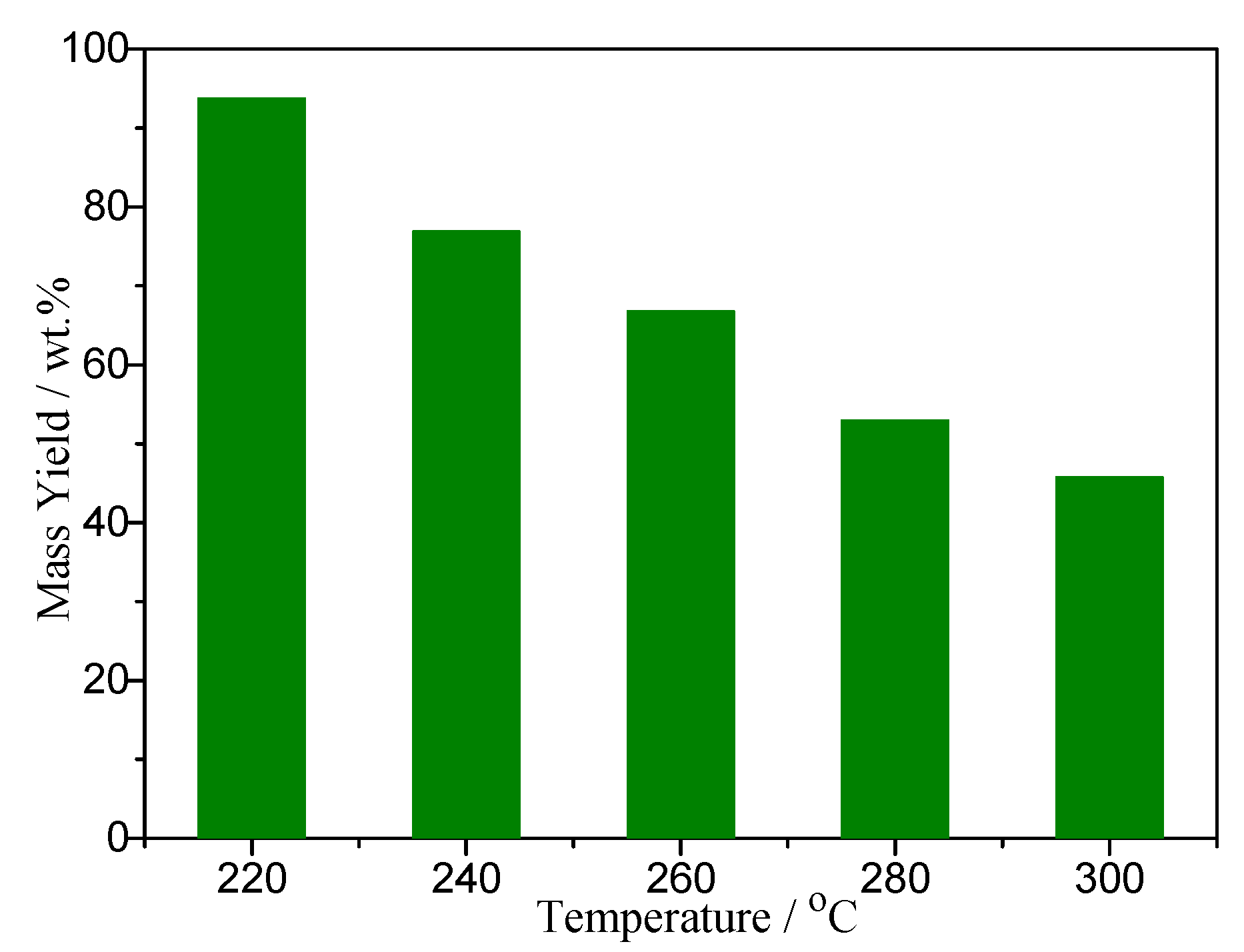
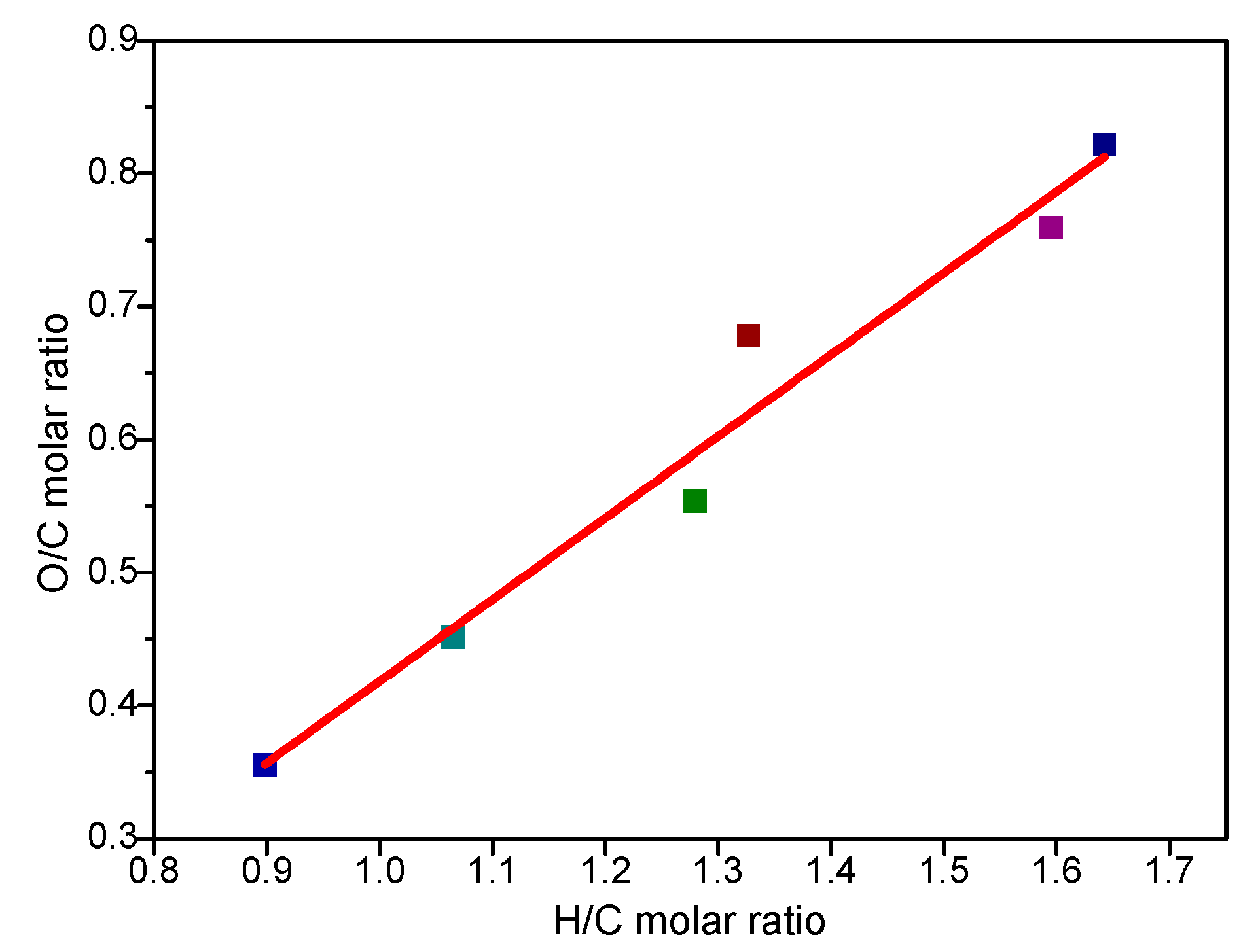
3.1. The Effect of Torrefaction Temperature on the Mass Yields and Elemental Composition of Torrefied Corn Cobs
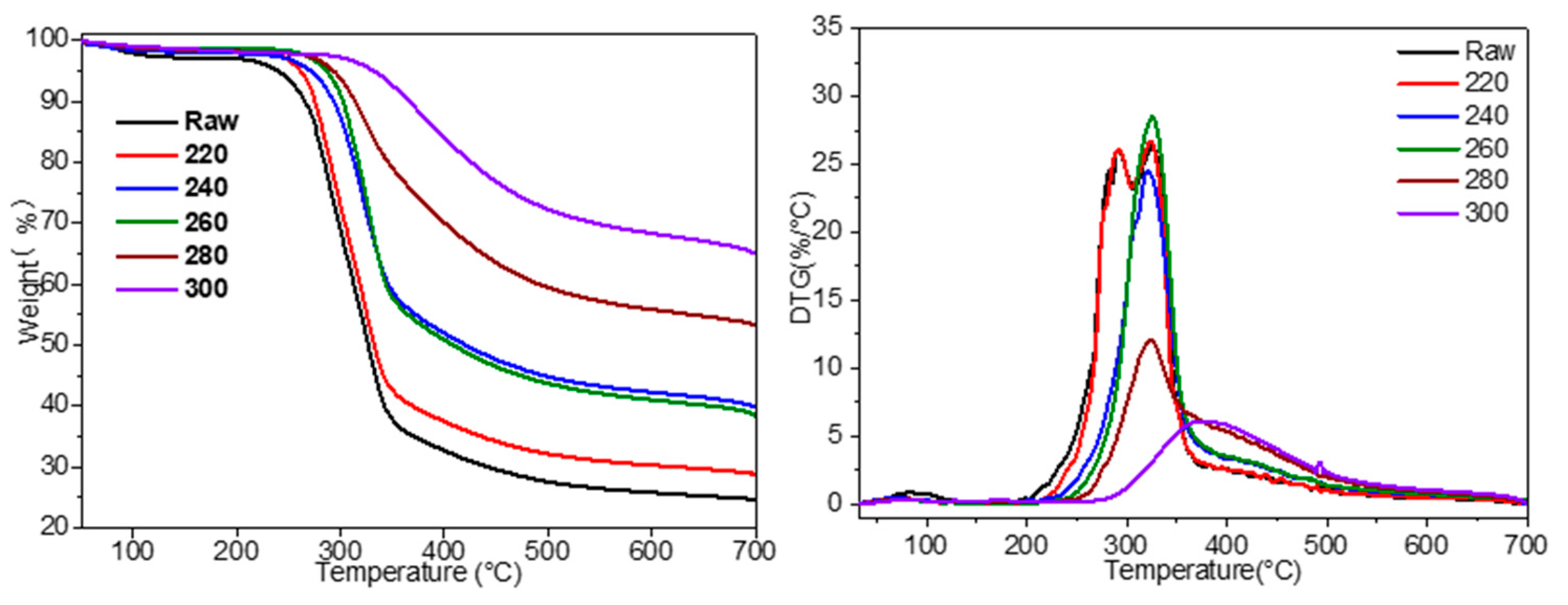
3.2. The Effect of Torrefaction Temperature on Pyrolysis Reactivity and Kinetics of Corn cobs
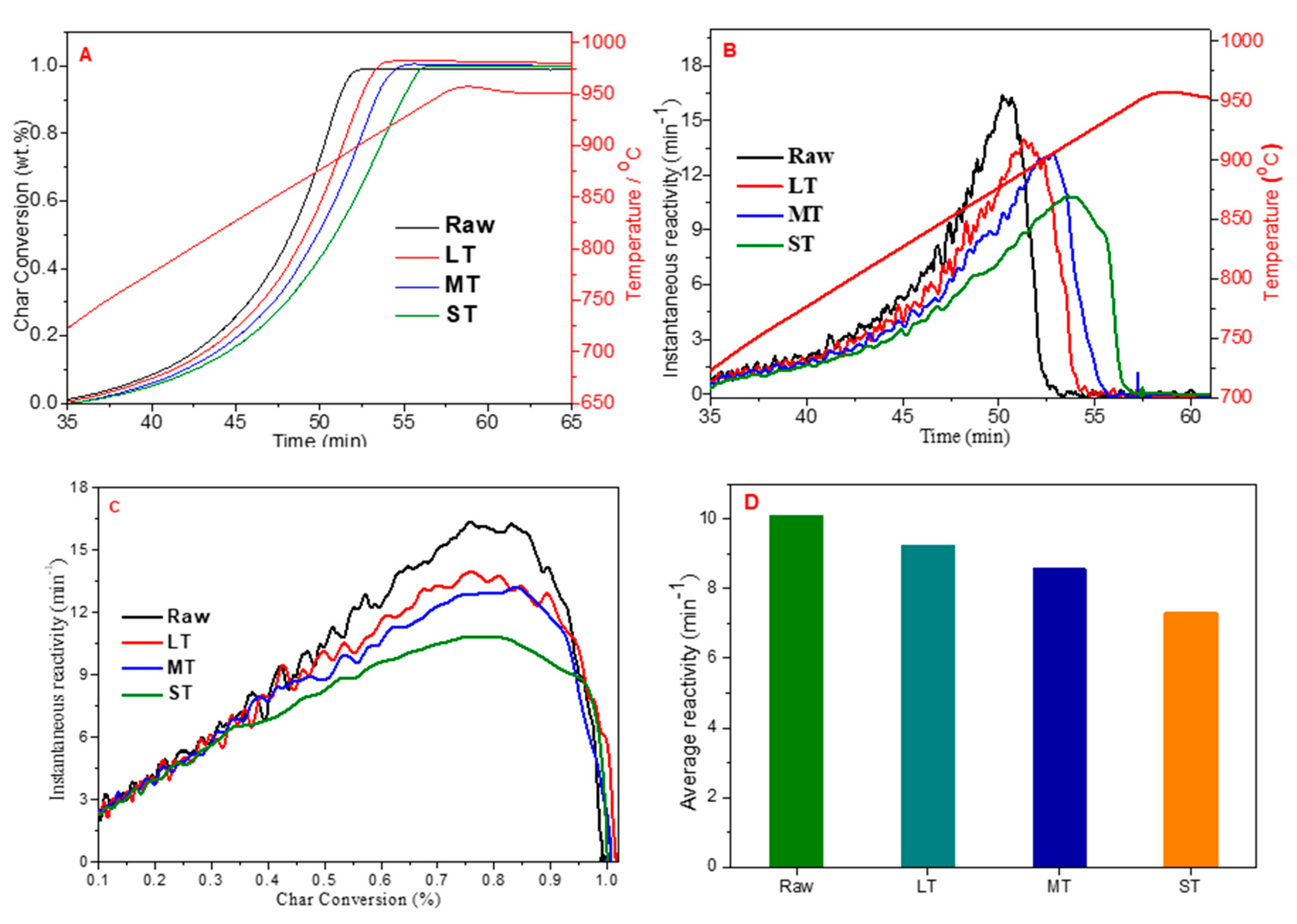
3.3. The Effect of Torrefaction Severity on Char Gasification Reactivity of Corn cobs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Sulaiman, C.; Abdul-Rahim, A.; Ofozor, C.A. Does wood biomass energy use reduce CO2 emissions in European Union member countries? Evidence from 27 members. J. Clean. Prod. 2020, 253, 119996. [Google Scholar] [CrossRef]
- Zuo, H.; Zeng, K.; Zhong, D.; Li, J.; Qiu, Y.; Xu, H.; Flamant, G.; Yang, H.; Chen, H. Multi-dimensional shrinkage models developed by phase field method for gasification of carbonaceous feedstock in packed-bed solar reactor. Fuel 2023, 331, 125749. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, S.; Cheng, X.; Qian, L.; Barati, B.; Gong, X.; Cao, B.; Yuan, C. Study on two-step hydrothermal liquefaction of macroalgae for improving bio-oil. Bioresour. Technol. 2021, 319, 124176. [Google Scholar] [CrossRef]
- Chen, J.M. Carbon neutrality: Toward a sustainable future. Innovation 2021, 2, 100127. [Google Scholar] [CrossRef]
- Xu, K.; Li, J.; Zeng, K.; Zhong, D.; Peng, J.; Qiu, Y.; Flamant, G.; Yang, H.; Chen, H. The characteristics and evolution of nitrogen in bio-oil from microalgae pyrolysis in molten salt. Fuel 2023, 331, 125903. [Google Scholar] [CrossRef]
- Wang, S.; Mukhambet, Y.; Esakkimuthu, S.; Abomohra, A.E.L.F. Integrated microalgal biorefinery—Routes, energy, economic and environmental perspectives. J. Clean. Prod. 2022, 348, 131245. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Lin, Y.-Y.; Chu, Y.-S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.; Ho, S.-H.; Culaba, A.B. Progress in biomass torrefaction: Principles, applications and challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Huang, M.; Ma, Z.; Zhou, B.; Yang, Y.; Chen, D. Enhancement of the production of bio-aromatics from renewable lignin by combined approach of torrefaction deoxygenation pretreatment and shape selective catalytic fast pyrolysis using metal modified zeolites. Bioresour. Technol. 2020, 301, 122754. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, Z.; Chang, S.; Huang, Z.; Zhao, K.; Wei, G.; He, F.; Li, H. Comparison of the effect of wet and dry torrefaction on chemical structure and pyrolysis behavior of corncobs. Bioresour. Technol. 2015, 176, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Li, C.; Yang, Y.; Liu, X.; Zhao, C.; Chen, D. New sight on the lignin torrefaction pretreatment: Relevance between the evolution of chemical structure and the properties of torrefied gaseous, liquid, and solid products. Bioresour. Technol. 2019, 288, 121528. [Google Scholar] [CrossRef] [PubMed]
- Ru, B.; Wang, S.; Dai, G.; Zhang, L. Effect of Torrefaction on Biomass Physicochemical Characteristics and the Resulting Pyrolysis Behavior. Energy Fuels 2015, 29, 5865–5874. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Ru, B.; Zhao, Y.; Wang, X.; Zhou, J.; Luo, Z.; Cen, K. Effects of torrefaction on hemicellulose structural characteristics and pyrolysis behaviors. Bioresour. Technol. 2016, 218, 1106–1114. [Google Scholar] [CrossRef]
- Zheng, A.; Jiang, L.; Zhao, Z.; Huang, Z.; Zhao, K.; Wei, G.; Wang, X.; He, F.; Li, H. Impact of Torrefaction on the Chemical Structure and Catalytic Fast Pyrolysis Behavior of Hemicellulose, Lignin, and Cellulose. Energy Fuels 2015, 29, 8027–8034. [Google Scholar] [CrossRef]
- Chen, W.-H.; Hsu, H.-C.; Lu, K.-M.; Lee, W.-J.; Lin, T.-C. Thermal pretreatment of wood (Lauan) block by torrefaction and its influence on the properties of the biomass. Energy 2011, 36, 3012–3021. [Google Scholar] [CrossRef]
- Park, J.; Meng, J.; Lim, K.H.; Rojas, O.; Park, S. Transformation of lignocellulosic biomass during torrefaction. J. Anal. Appl. Pyrolysis 2013, 100, 199–206. [Google Scholar] [CrossRef]
- Chen, W.-H.; Cheng, C.-L.; Show, P.-L.; Ong, H.C. Torrefaction performance prediction approached by torrefaction severity factor. Fuel 2019, 251, 126–135. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, Z.; Chang, S.; Huang, Z.; Wang, X.; He, F.; Li, H. Effect of torrefaction on structure and fast pyrolysis behavior of corncobs. Bioresour. Technol. 2013, 128, 370–377. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, Z.; Chang, S.; Huang, Z.; He, F.; Li, H. Effect of Torrefaction Temperature on Product Distribution from Two-Staged Pyrolysis of Biomass. Energy Fuels 2012, 26, 2968–2974. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, J.; Liu, B.; Mei, Q.; Luo, Z. Influence of torrefaction pretreatment on biomass gasification technology. Chin. Sci. Bull. 2011, 56, 1449–1456. [Google Scholar] [CrossRef]
- Cui, C.; Xia, S.; Wang, C.; Kang, S.; Zheng, A.; Zhao, Z.; Li, H. Beyond pretreatment: Oriented valorization of biomass into platform chemicals via torrefaction mediated by acid-base balance. Fuel 2022, 317, 123411. [Google Scholar] [CrossRef]
- Li, L.; Huang, Y.; Zhang, D.; Zheng, A.; Zhao, Z.; Xia, M.; Li, H. Uncovering Structure–Reactivity Relationships in Pyrolysis and Gasification of Biomass with Varying Severity of Torrefaction. ACS Sustain. Chem. Eng. 2018, 6, 6008–6017. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood. J. Anal. Appl. Pyrolysis 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Wannapeera, J.; Fungtammasan, B.; Worasuwannarak, N. Effects of temperature and holding time during torrefaction on the pyrolysis behaviors of woody biomass. J. Anal. Appl. Pyrolysis 2011, 92, 99–105. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, W.; Chen, W.-H.; Ho, S.-H.; Pétrissans, A.; Pétrissans, M. Effect of torrefaction on the structure and reactivity of rice straw as well as life cycle assessment of torrefaction process. Energy 2022, 240, 122470. [Google Scholar] [CrossRef]


| Feedstock | Raw | Torrefaction Temperature/°C | |||||
|---|---|---|---|---|---|---|---|
| 220 | 240 | 260 | 280 | 300 | |||
| Elemental analysis/wt.% | C | 44.70 | 46.5 | 49.45 | 54.02 | 58.86 | 64.21 |
| H | 6.12 | 6.181 | 5.468 | 5.76 | 5.222 | 4.807 | |
| N | 0.23 | 0.25 | 0.37 | 0.36 | 0.49 | 0.56 | |
| O | 48.95 | 47.07 | 44.71 | 39.86 | 35.43 | 30.42 | |
| H/C molar ratio | 1.64 | 1.60 | 1.33 | 1.28 | 1.06 | 0.90 | |
| O/C molar ratio | 0.82 | 0.76 | 0.68 | 0.55 | 0.45 | 0.36 | |
| HHV (MJ/kg) | 18.50 | 19.05 | 19.40 | 20.88 | 21.88 | 23.11 | |
| Energy densification ratio | 1.00 | 1.03 | 1.05 | 1.13 | 1.18 | 1.25 | |
| Energy yield (%) | 100 | 96.59 | 80.71 | 75.43 | 62.71 | 57.22 | |
| Feedstock | Raw | Torrefaction Temperature/°C | ||||
|---|---|---|---|---|---|---|
| 220 | 240 | 260 | 280 | 300 | ||
| The yield of char at 700 °C | 24.6 | 28.8 | 40.0 | 38.3 | 53.2 | 65.1 |
| Dmax (%/min) | 26.2 | 26.6 | 24.5 | 28.5 | 12.2 | 6.1 |
| Tmax (°C) | 324.4 | 325.2 | 319.3 | 325.2 | 323.5 | 379.8 |
| Ti (°C) | 199.2 | 214.1 | 227.5 | 243.1 | 250.6 | 271.3 |
| CPI a (10−4%/(min·°C2)) | 3.23 | 3.68 | 4.18 | 5.34 | 2.59 | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, S.; Zheng, A.; Zhao, K.; Zhao, Z.; Li, H. Evaluation of Pyrolysis Reactivity, Kinetics, and Gasification Reactivity of Corn Cobs after Torrefaction Pretreatment. Energies 2022, 15, 9277. https://doi.org/10.3390/en15249277
Xia S, Zheng A, Zhao K, Zhao Z, Li H. Evaluation of Pyrolysis Reactivity, Kinetics, and Gasification Reactivity of Corn Cobs after Torrefaction Pretreatment. Energies. 2022; 15(24):9277. https://doi.org/10.3390/en15249277
Chicago/Turabian StyleXia, Shengpeng, Anqing Zheng, Kun Zhao, Zengli Zhao, and Haibin Li. 2022. "Evaluation of Pyrolysis Reactivity, Kinetics, and Gasification Reactivity of Corn Cobs after Torrefaction Pretreatment" Energies 15, no. 24: 9277. https://doi.org/10.3390/en15249277
APA StyleXia, S., Zheng, A., Zhao, K., Zhao, Z., & Li, H. (2022). Evaluation of Pyrolysis Reactivity, Kinetics, and Gasification Reactivity of Corn Cobs after Torrefaction Pretreatment. Energies, 15(24), 9277. https://doi.org/10.3390/en15249277







