Characteristics of Hydrothermal Carbonization Hydrochar Derived from Cattle Manure
Abstract
1. Introduction
2. Materials and Methods
2.1. Cattle Manure
2.2. Hydrothermal Carbonization
2.3. TG Run for Pyrolysis Analysis
2.4. Non-Isothermal Kinetics
3. Results
3.1. Characteristics of Hydrothermal Carbonization Hydrochars with Reaction Temperature
3.2. Thermogravimetric Analysis of Cattle Manure and Hydrochars
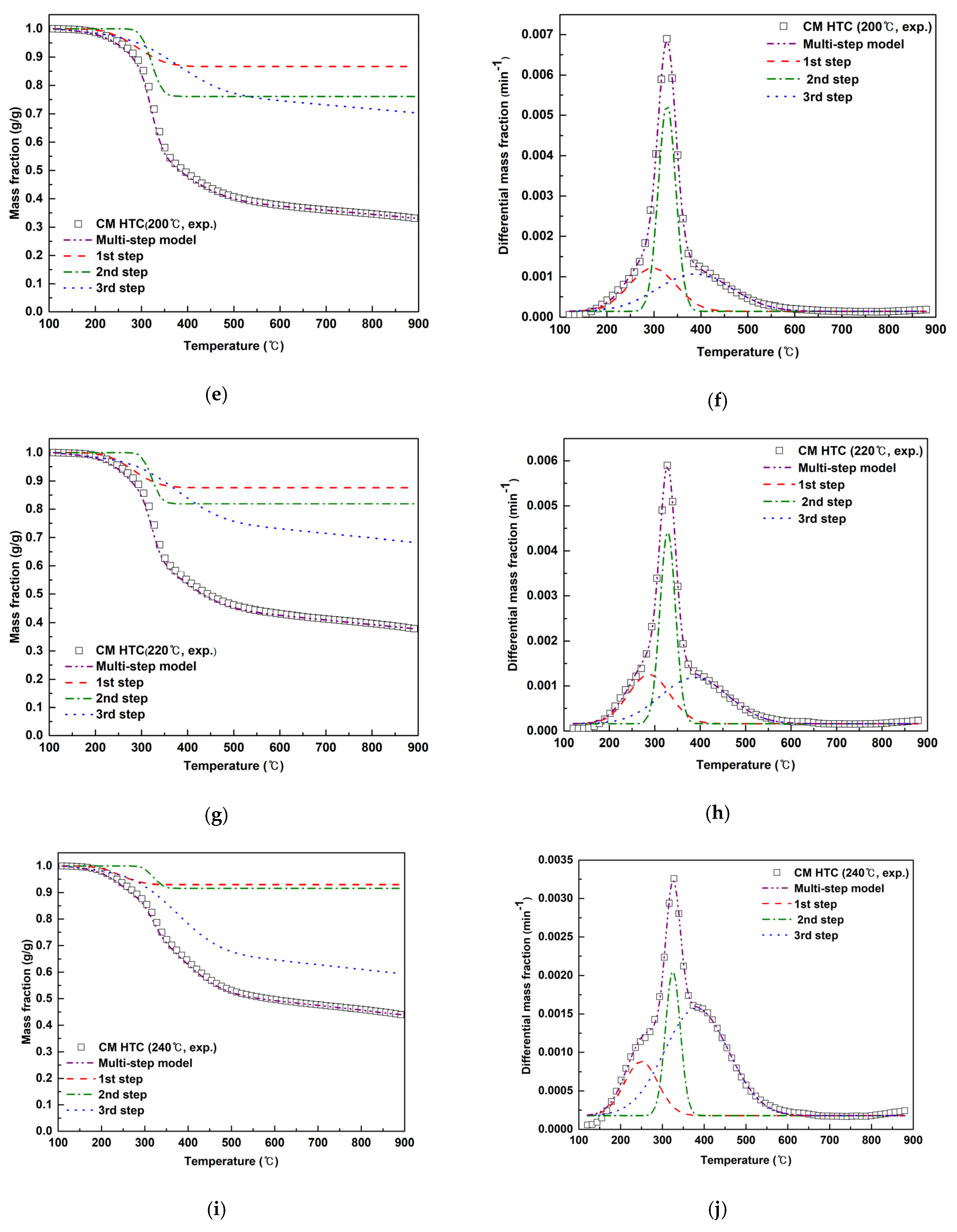
3.3. Effect of HTC Reaction Temperature for the Fuel Quality of Hydrochars
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Biogas Research South Korea: Application Study of Biogas Process for Public Treatment of Cattle Dung. 2019. Available online: http://www.me.go.kr/synap/synapView.jsp;jsessionid=sTRU0hZmRxLigv11bcrOe3HbXDAbR7Ub5kAafhJwhsSgoZQQU7Dmkf21tYB1DN9k.meweb2vhost_servlet_engine1?fileId=155773&fileSeq=2 (accessed on 2 October 2022).
- Lee, J.H.; Febrisiantosa, A. Improvement of nitrogen balance (land budget) in South Korea in terms of livestock manure: A review. IOP Conf. Ser. Earth Environ. Sci. 2020, 462, 012011. [Google Scholar] [CrossRef]
- Kim, J.-H.; Nam, J.; Yoo, S.-H. Public acceptance of a large-scale offshore wind power project in South Korea. Mar. Policy 2020, 120, 104141. [Google Scholar] [CrossRef]
- Lee, S.; Yu, B.; Ju, S.; Kang, Y.; Jung, G. Characteristics of solid fuel from cattle manure. New Renew. Energy 2016, 12, 64–69. [Google Scholar] [CrossRef]
- Szymajda, A.; Łaska, G.; Joka, M. Assessment of cow dung pellets as a renewable solid fuel in direct combustion technologies. Energies 2021, 14, 1192. [Google Scholar] [CrossRef]
- Marin-Batista, J.D.; Villamil, J.A.; Qaramaleki, S.V.; Coronella, C.J.; Mohedano, A.F.; de La Rubia, M.A. Energy valorization of cow manure by hydrothermal carbonization and anaerobic digestion. Renew. Energy 2020, 160, 623–632. [Google Scholar] [CrossRef]
- Maj, I.; Kalisz, S.; Ciukaj, S. Properties of Animal-Origin Ash—A Valuable Material for Circular Economy. Energies 2022, 15, 1274. [Google Scholar]
- Kirtania, K. Thermochemical Conversion Processes for Waste Biorefinery. In Waste Biorefinery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 129–156. [Google Scholar]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Escala, M.; Zumbuhl, T.; Koller, C.; Junge, R.; Krebs, R. Hydrothermal carbonization as an energy-efficient alternative to established drying technologies for sewage sludge: A feasibility study on a laboratory scale. Energy Fuels 2013, 27, 454–460. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Ferreiro, A.I.; Giudicianni, P.; Grottola, C.M.; Rabaçal, M.; Costa, M.; Ragucci, R. Unresolved issues on the kinetic modeling of pyrolysis of woody and nonwoody biomass fuels. Energy Fuels 2017, 31, 4035–4044. [Google Scholar] [CrossRef]
- Rabaçal, M.; Costa, M.; Vascellarib, M.; Hasseb, C. Kinetic modelling of sawdust and beech wood pyrolysis in drop tube reactors using advanced predictive models. Chem. Eng. 2014, 37, 79–84. [Google Scholar]
- Varhegyi, G.; Antal, M.J., Jr.; Jakab, E.; Szabóa, P. Kinetic modeling of biomass pyrolysis. J. Anal. Appl. Pyrolysis 1997, 42, 73–87. [Google Scholar]
- Wu, K.; Gao, Y.; Zhu, G.; Zhu, J.; Yuan, Q.; Chen, Y.; Cai, M.; Feng, L. Characterization of dairy manure hydrochar and aqueous phase products generated by hydrothermal carbonization at different temperatures. J. Anal. Appl. Pyrolysis 2017, 127, 335–342. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Pandey, D.S.; Kwapinski, W.; Leahy, J.J. Hydrothermal carbonisation of poultry litter: Effects of treatment temperature and residence time on yields and chemical properties of hydrochars. Bioresour. Technol. 2016, 216, 373–380. [Google Scholar]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal carbonization of biomass for energy and crop production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar]
- Titirici, M.-M.; Antonietti, M. Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chem. Soc. Rev. 2010, 39, 103–116. [Google Scholar] [CrossRef]
- Rillig, M.C.; Wagner, M.; Salem, M.; Antunes, P.M.; George, C.; Ramke, H.G.; Titirici, M.M.; Antonietti, M. Material derived from hydrothermal carbonization: Effects on plant growth and arbuscular mycorrhiza. Appl. Soil Ecol. 2010, 45, 238–242. [Google Scholar] [CrossRef]
- Reza, M.T.; Wirth, B.; Lüder, U.; Werner, M. Behavior of selected hydrolyzed and dehydrated products during hydrothermal carbonization of biomass. Bioresour. Technol. 2014, 169, 352–361. [Google Scholar]
- Reza, M.T.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J. Hydrothermal carbonization of loblolly pine: Reaction chemistry and water balance. Biomass Convers. Biorefinery 2014, 4, 311–321. [Google Scholar] [CrossRef]
- Fakkaew, K. Evaluation of Hydrothermal Carbonization Reactions for Faecal Sludge Treatment and Hydrochar Production; Asian Institute of Technology School of Environment, Resources and Development Thailand: Bangkok, Thailand, 2016. [Google Scholar]
- Brown, A.E.; Adams, J.M.; Grasham, O.R.; Camargo-Valero, M.A.; Ross, A.B. An Assessment of Different Integration Strategies of Hydrothermal Carbonisation and Anaerobic Digestion of Water Hyacinth. Energies 2020, 13, 5983. [Google Scholar]
- Chen, C.; Liang, W.; Fan, F.; Wang, C. The Effect of Temperature on the Properties of Hydrochars Obtained by Hydrothermal Carbonization of Waste Camellia oleifera Shells. ACS Omega 2021, 6, 16546–16552. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Lee, J.-Y.; Mao, X.; Ye, L.; Xu, W.; Ning, X.; Zhang, N.; Teng, H.; Wang, C. Hydrothermal carbonization of maize straw for hydrochar production and its injection for blast furnace. Appl. Energy 2020, 266, 114818. [Google Scholar]
- Krysanova, K.; Krylova, A.; Zaichenko, V. Properties of biochar obtained by hydrothermal carbonization and torrefaction of peat. Fuel 2019, 256, 115929. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, Y.; Meng, F.; Zhang, Y.; Liu, Z.; Zhang, W.; Xue, G. Preparation and characterization of hydrochar from waste eucalyptus bark by hydrothermal carbonization. Energy 2016, 97, 238–245. [Google Scholar] [CrossRef]
- Fan, F.; Zheng, Y.; Huang, Y.; Lu, Y.; Wang, Z.; Chen, B.; Zheng, Z. Preparation and characterization of biochars from waste Camellia oleifera shells by different thermochemical processes. Energy Fuels 2017, 31, 8146–8151. [Google Scholar] [CrossRef]
- Zhao, Y.; Su, R.; Zhang, W.; Yao, G.L.; Chen, J. Antibacterial activity of tea saponin from Camellia oleifera shell by novel extraction method. Ind. Crops Prod. 2020, 153, 112604. [Google Scholar] [CrossRef]
- Sharma, R.; Jasrotia, K.; Singh, N.; Ghosh, P.; Srivastava, S.; Sharma, N.R.; Singh, J.; Kanwar, R.; Kumar, A. A comprehensive review on hydrothermal carbonization of biomass and its applications. Chem. Afr. 2020, 3, 1–19. [Google Scholar] [CrossRef]
- Sheng, C.; Azevedo, J.L.T. Estimating the higher heating value of biomass fuels from basic analysis data. Biomass Bioenergy 2005, 28, 499–507. [Google Scholar]
- Yuan, X.; He, T.; Cao, H.; Yuan, Q. Cattle manure pyrolysis process: Kinetic and thermodynamic analysis with isoconversional methods. Renew. Energy 2017, 107, 489–496. [Google Scholar]
- Chen, S.; Wen, Z.; Liao, W.; Liu, C.; Kincaid, R.L.; Harrison, J.H.; Elliott, D.C.; Brown, M.D.; Stevens, D.J. Studies into using manure in a biorefinery concept. Appl. Biochem. Biotechnol. 2005, 124, 999–1015. [Google Scholar]
- Langone, M.; Soldano, M.; Fabbri, C.; Pirozzi, F.; Andreottola, G. Anaerobic digestion of cattle manure influenced by swirling jet induced hydrodynamic cavitation. Appl. Biochem. Biotechnol. 2018, 184, 1200–1218. [Google Scholar]
- Keiller, B.G.; Muhlack, R.; Burton, R.A.; van Eyk, P.J. Biochemical Compositional Analysis and Kinetic Modeling of Hydrothermal Carbonization of Australian Saltbush. Energy Fuels 2019, 33, 12469–12479. [Google Scholar]
- Kruse, A.; Funke, A.; Titirici, M.M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef]
- Lucian, M.; Volpe, M.; Fiori, L. Hydrothermal carbonization kinetics of lignocellulosic agro-wastes: Experimental data and modeling. Energies 2019, 12, 516. [Google Scholar] [CrossRef]
- Cao, H.; Xin, Y.; Wang, D.; Yuan, Q. Pyrolysis characteristics of cattle manures using a discrete distributed activation energy model. Bioresour. Technol. 2014, 172, 219–225. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Hayhurst, A.N. The kinetics of the pyrolysis or devolatilisation of sewage sludge and other solid fuels. Combust. Flame 2013, 160, 138–144. [Google Scholar] [CrossRef]
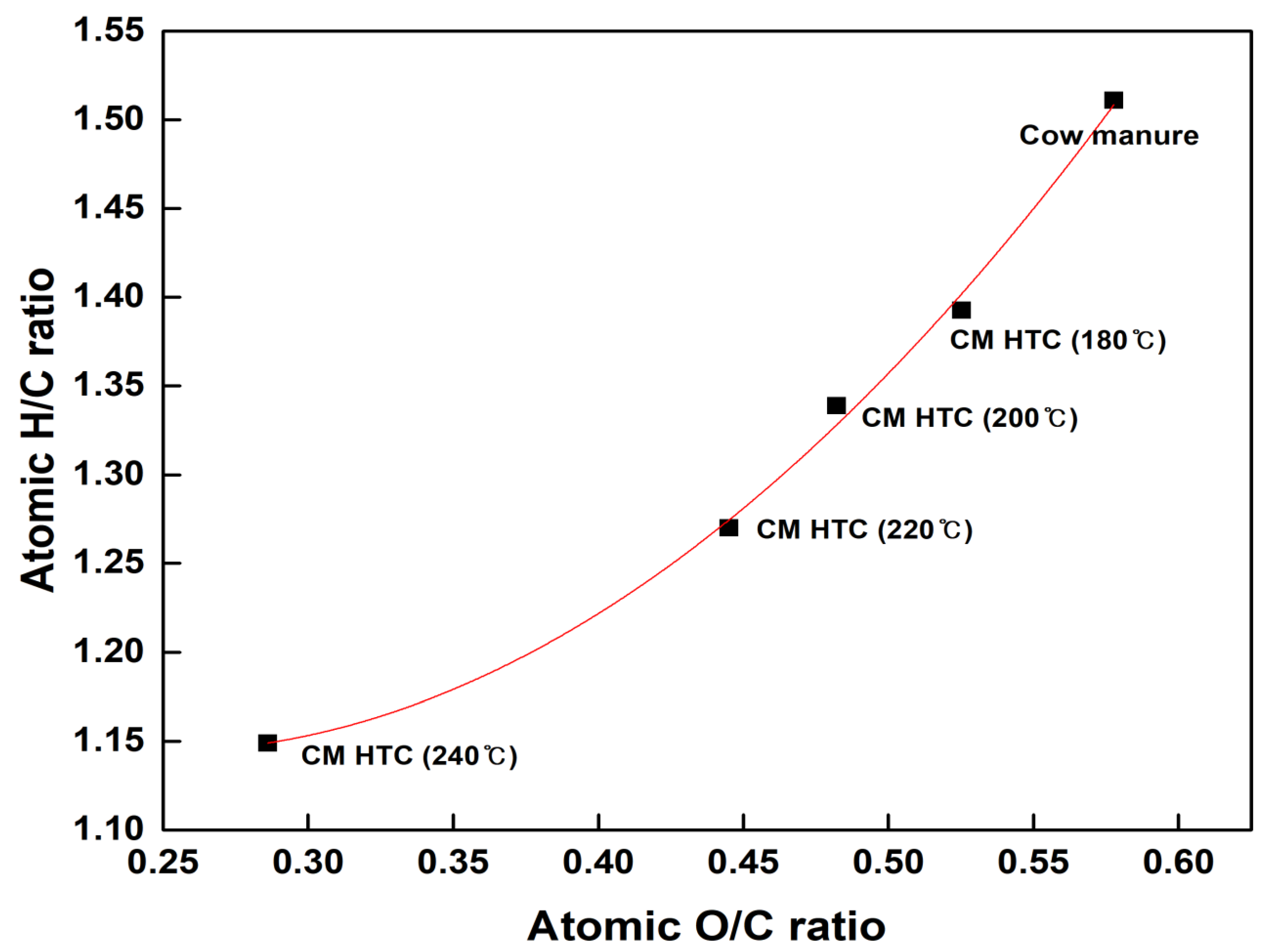

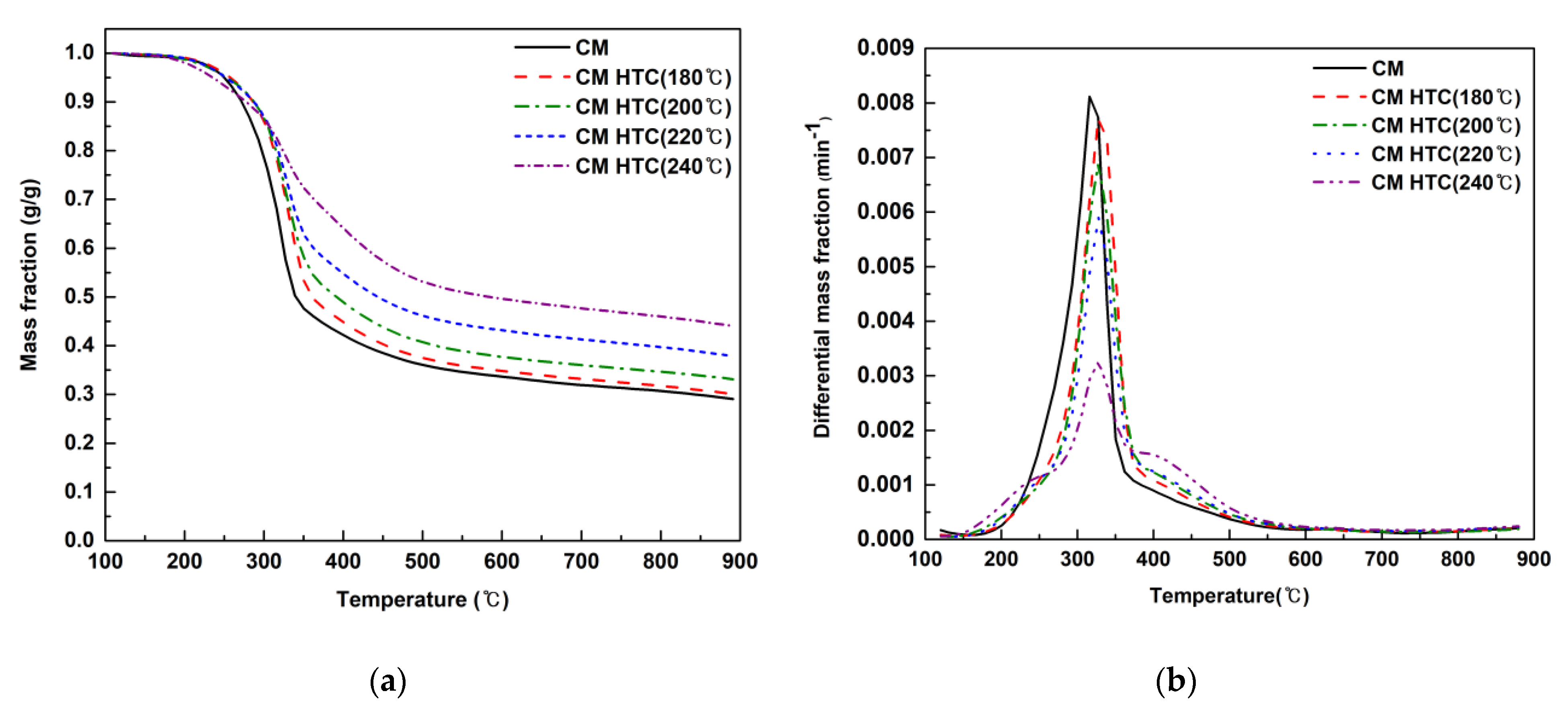

| Descriptions | Cattle Manure (Raw) | CM Hydrochar (180 °C) | CM Hydrochar (200 °C) | CM Hydrochar (220 °C) | CM Hydrochar (240 °C) |
|---|---|---|---|---|---|
| Proximate analysis (wt %, d.b. 1) | |||||
| Moisture | 0.50 | 0.17 | 0.17 | 0.15 | 0.13 |
| Volatile matter | 69.30 | 66.37 | 64.31 | 63.79 | 48.84 |
| Fixed carbon | 19.80 | 22.73 | 24.73 | 24.81 | 29.05 |
| Ash | 10.40 | 10.73 | 10.79 | 11.24 | 21.98 |
| Ultimate analysis (wt %, d.b. 1) | |||||
| Carbon | 44.65 | 48.34 | 50.01 | 52.18 | 55.13 |
| Hydrogen | 5.62 | 5.61 | 5.52 | 5.52 | 5.28 |
| Oxygen | 34.39 | 33.85 | 32.15 | 30.96 | 21.03 |
| Nitrogen | 2.39 | 2.09 | 2.90 | 2.43 | 3.05 |
| Sulfur | 0.23 | 0.00 | 0.00 | 0.00 | 0.00 |
| Calorific value (MJ/kg, d.b. 1) | |||||
| HHV | 18.91 | 20.13 | 22.34 | 22.76 | 24.14 |
| Sample | Regime | Ti (°C) | Tpk (°C) | Tf (°C) | Rpk (°C) | Mf (g/g) |
|---|---|---|---|---|---|---|
| Cattle manure | 1st | 201 | 293 | 385 | 3.25 × 10−3 | 0.75 |
| 2nd | 270 | 327 | 362 | 5.04 × 10−3 | 0.81 | |
| 3rd | 109 | 373 | 615 | 9.14 × 10−4 | 0.72 | |
| CM ydrochar (180 °C) | 1st | 201 | 293 | 396 | 1.64 × 10−3 | 0.86 |
| 2nd | 270 | 327 | 396 | 5.82 × 10−3 | 0.74 | |
| 3rd | 120 | 385 | 615 | 1.05 × 10−3 | 0.69 | |
| CM hydrochar (200 °C) | 1st | 201 | 293 | 465 | 1.07 × 10−3 | 0.87 |
| 2nd | 270 | 327 | 396 | 5.11 × 10−3 | 0.76 | |
| 3rd | 155 | 385 | 626 | 1.07 × 10−3 | 0.7 | |
| CM hydrochar (220 °C) | 1st | 132 | 281 | 465 | 1.09 × 10−3 | 0.88 |
| 2nd | 258 | 328 | 396 | 4.25 × 10−3 | 0.81 | |
| 3rd | 189 | 385 | 638 | 1.19 × 10−3 | 0.68 | |
| CM hydrochar (240 °C) | 1st | 135 | 247 | 385 | 7.03 × 10−4 | 0.92 |
| 2nd | 265 | 328 | 396 | 1.85 × 10−3 | 0.91 | |
| 3rd | 178 | 385 | 615 | 1.50 × 10−3 | 0.59 |
| Sample | Regime | n (-) | E (kJ/mol) | A (min−1) | r2 (-) | S (%) | yi (-) |
|---|---|---|---|---|---|---|---|
| Cattle manure | 1st | 1.4 | 123.72 | 1.06 × 1011 | 0.99 | 0.014 | 0.35 |
| 2nd | 1.4 | 332.85 | 2.08 × 1029 | 0.99 | 0.022 | 0.26 | |
| 3rd | 1.6 | 23.73 | 3.65 | 0.99 | 0.010 | 0.39 | |
| CM hydrochar (180 °C) | 1st | 1.4 | 112.87 | 7.84 × 109 | 0.99 | 0.006 | 0.20 |
| 2nd | 1.4 | 242.552 | 8.32 × 1020 | 0.99 | 0.013 | 0.40 | |
| 3rd | 1.6 | 25.96 | 6.09 | 0.98 | 0.008 | 0.43 | |
| CM hydrochar (200 °C) | 1st | 1.4 | 65.42 | 2.18 × 105 | 0.99 | 0.002 | 0.20 |
| 2nd | 1.6 | 249.04 | 4.8 × 1021 | 0.99 | 0.012 | 0.36 | |
| 3rd | 1.6 | 25.87 | 5.39 | 0.98 | 0.012 | 0.44 | |
| CM hydrochar (220 °C) | 1st | 1.2 | 63.73 | 1.66 × 105 | 0.99 | 0.004 | 0.20 |
| 2nd | 1.6 | 282.51 | 4.30 × 1024 | 0.99 | 0.012 | 0.30 | |
| 3rd | 1.8 | 28.52 | 10.95 | 0.97 | 0.015 | 0.51 | |
| CM hydrochar (240 °C) | 1st | 1.2 | 61.69 | 3.3 × 105 | 0.99 | 0.002 | 0.13 |
| 2nd | 1.6 | 261.45 | 8.08 × 1022 | 0.99 | 0.005 | 0.15 | |
| 3rd | 1.6 | 30.05 | 16.31 | 0.97 | 0.019 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, E.; Park, S.; Han, S.; Lee, E.; Kim, H. Characteristics of Hydrothermal Carbonization Hydrochar Derived from Cattle Manure. Energies 2022, 15, 9195. https://doi.org/10.3390/en15239195
Song E, Park S, Han S, Lee E, Kim H. Characteristics of Hydrothermal Carbonization Hydrochar Derived from Cattle Manure. Energies. 2022; 15(23):9195. https://doi.org/10.3390/en15239195
Chicago/Turabian StyleSong, Eunhye, Seyong Park, Seongkuk Han, Eusil Lee, and Ho Kim. 2022. "Characteristics of Hydrothermal Carbonization Hydrochar Derived from Cattle Manure" Energies 15, no. 23: 9195. https://doi.org/10.3390/en15239195
APA StyleSong, E., Park, S., Han, S., Lee, E., & Kim, H. (2022). Characteristics of Hydrothermal Carbonization Hydrochar Derived from Cattle Manure. Energies, 15(23), 9195. https://doi.org/10.3390/en15239195






