Hydrogen Production System through Dimethyl Ether Autothermal Reforming, Based on Model Predictive Control
Abstract
1. Introduction
2. Mathematical Modelling and Control Method
2.1. ATR Reformer Model
2.2. State Space Equations Applied in the MPC
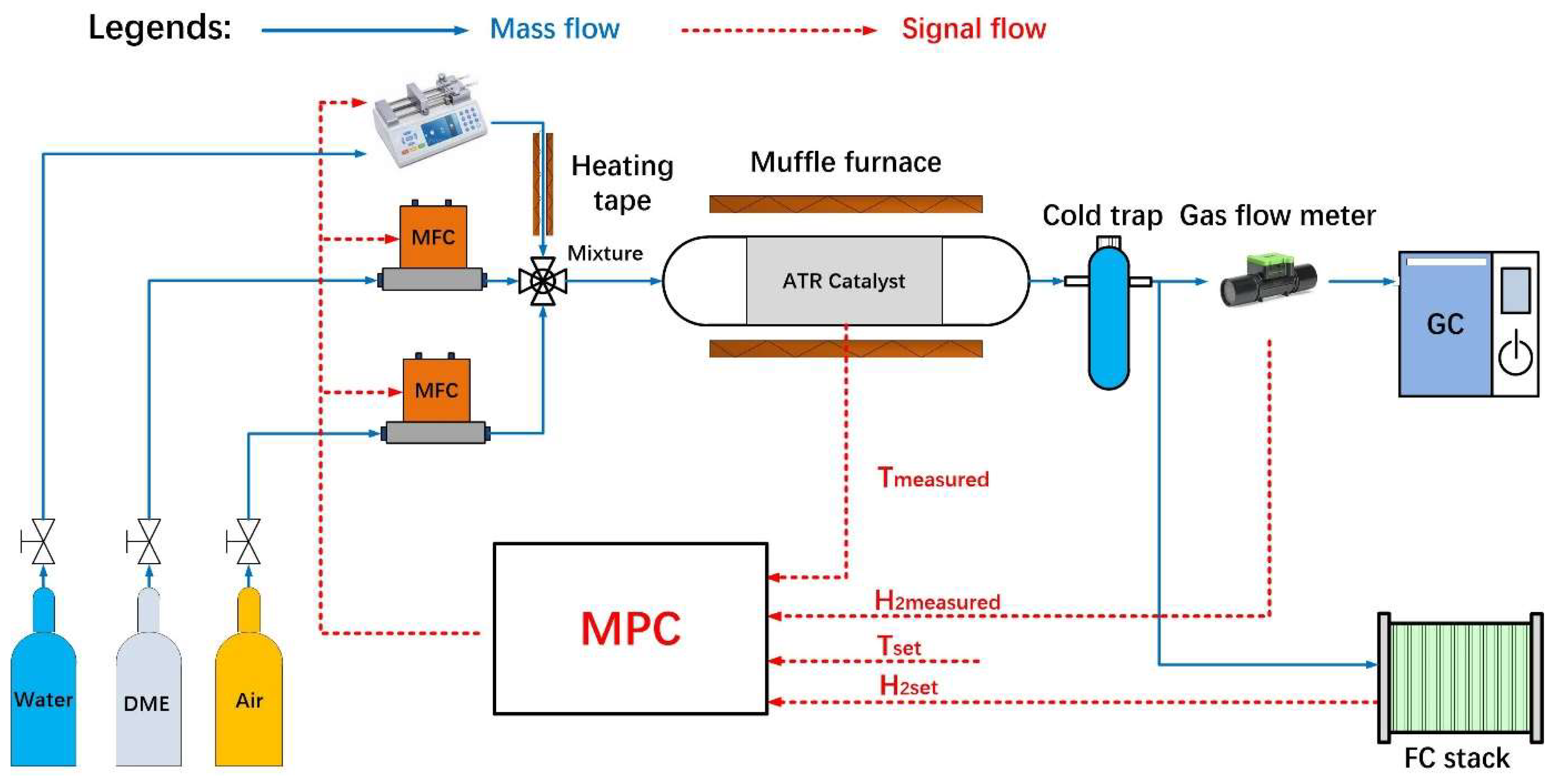
3. Experiment Apparatus
3.1. Catalyst Preparation
3.2. MPC Controller Design
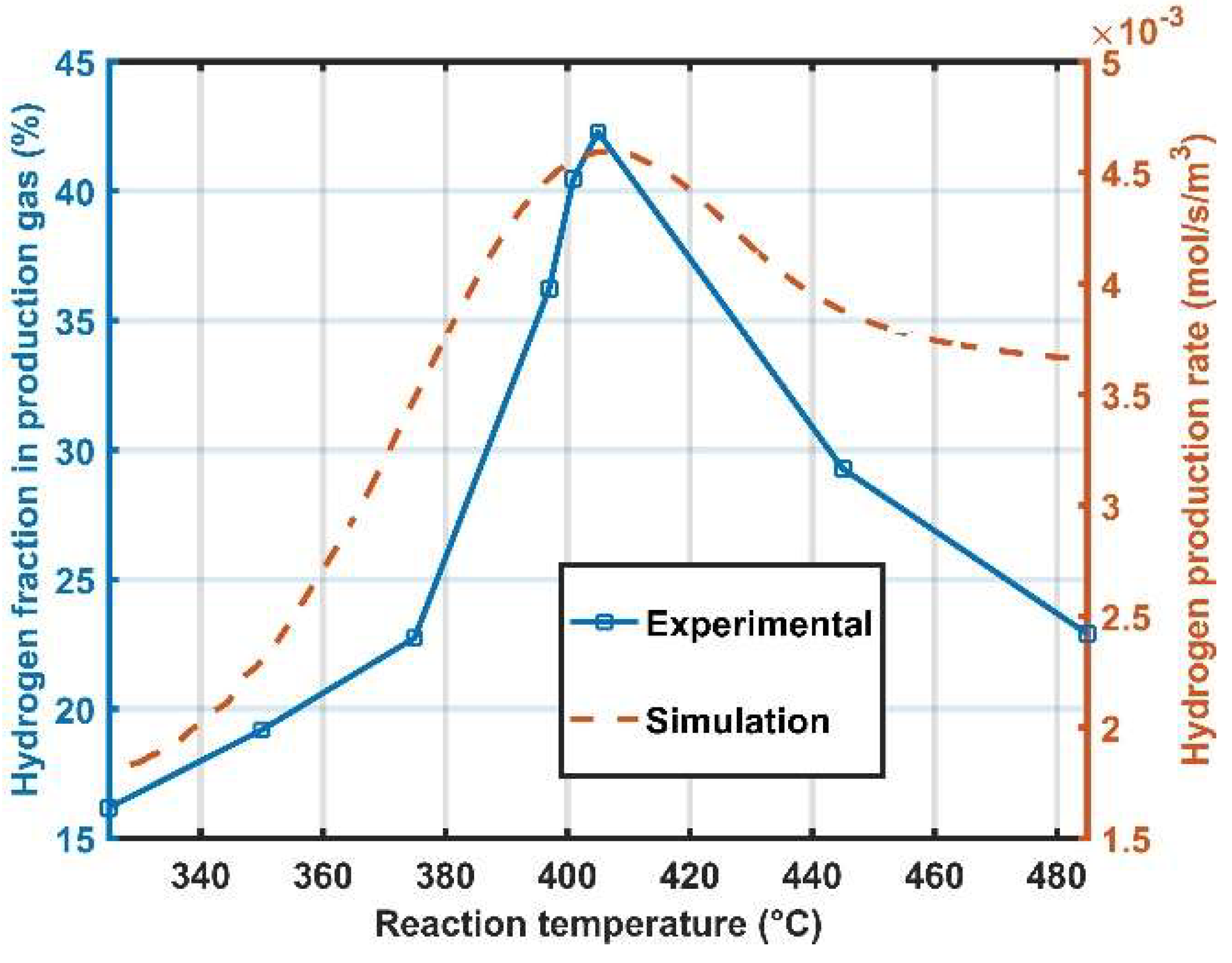
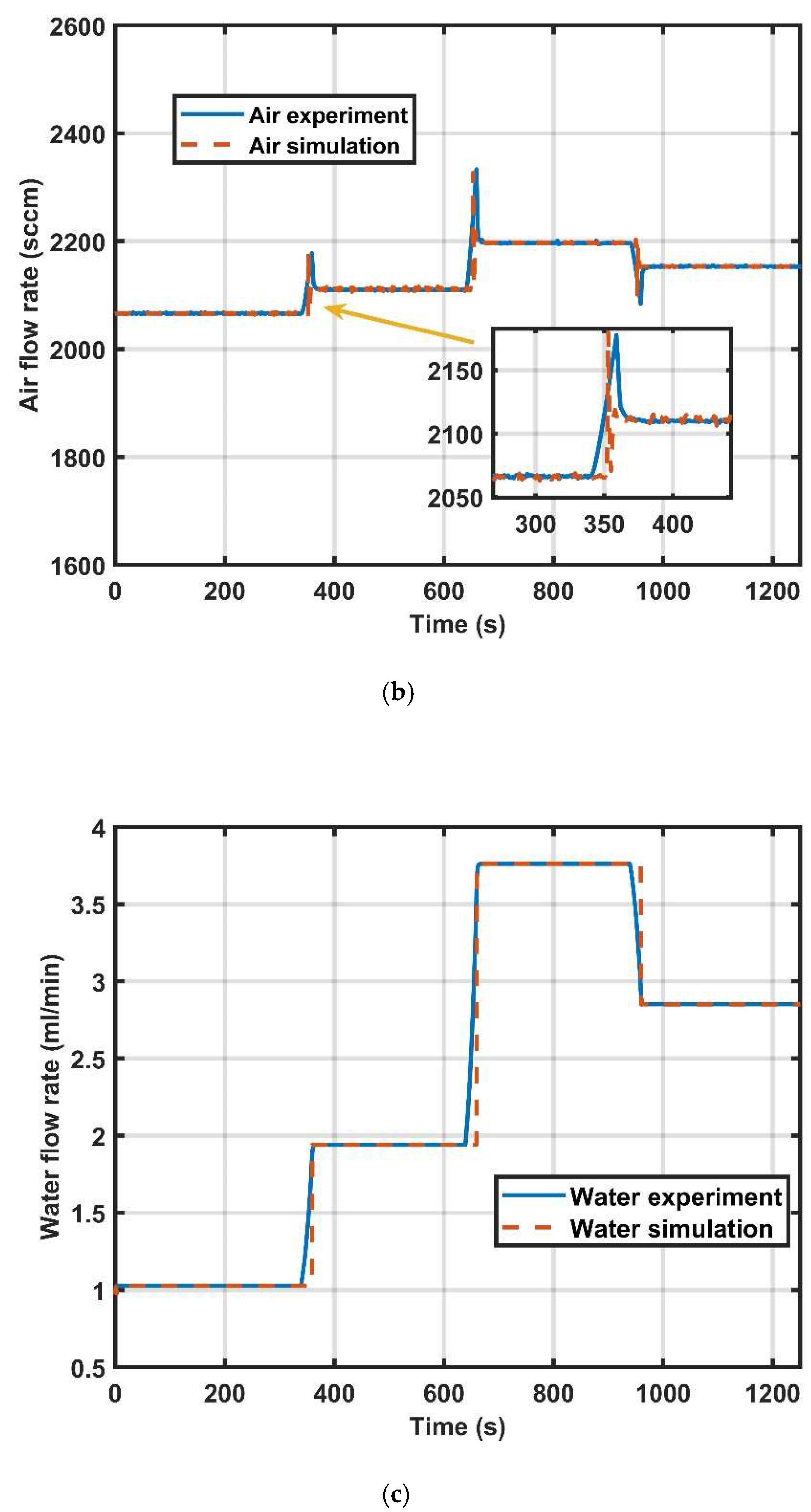
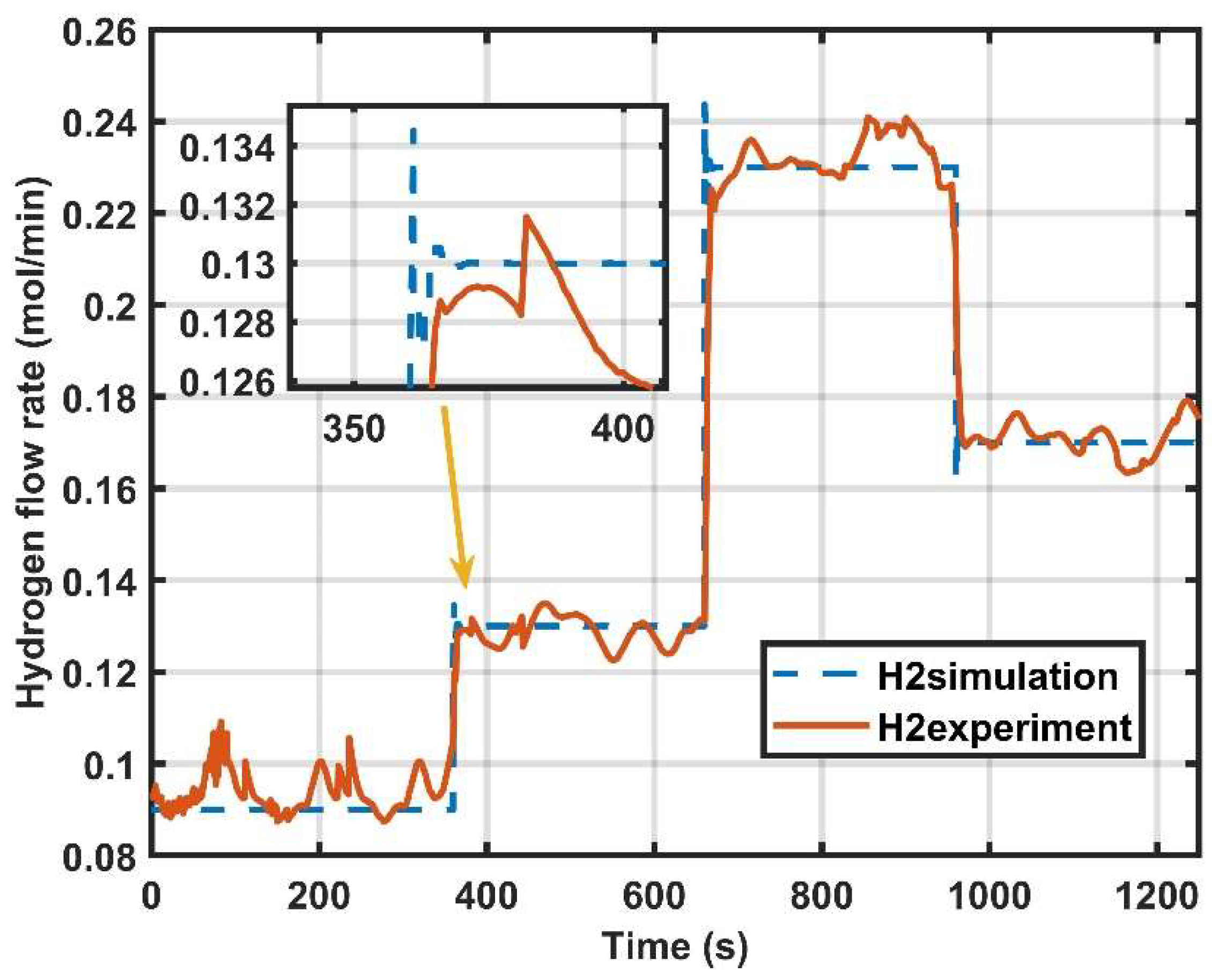
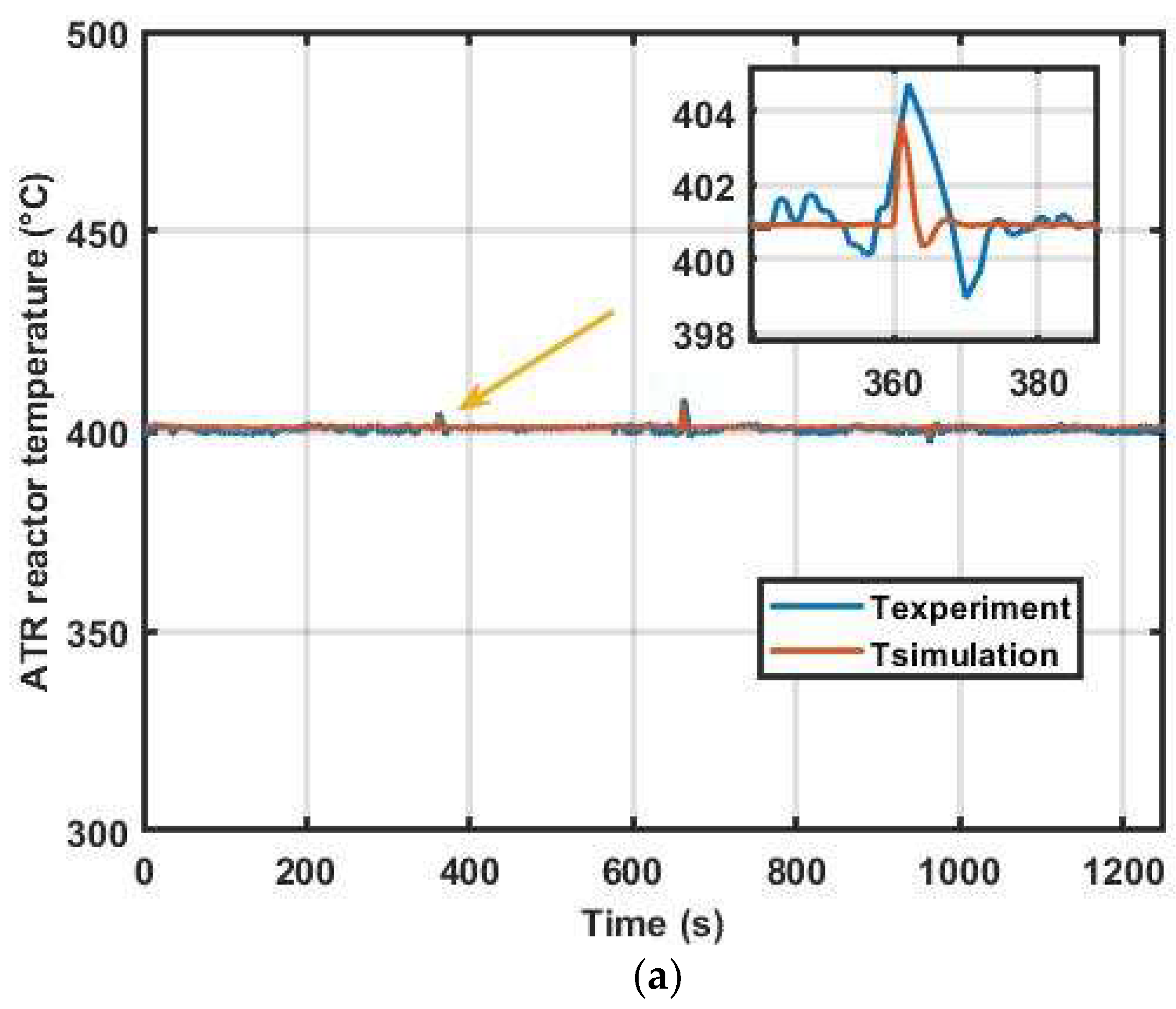
4. Result Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Tan, J.; Yang, W.; Li, Y.; Wang, C. Better electrochemical performance of PEMFC under a novel pneumatic clamping mechanism. Energy 2021, 229, 120796. [Google Scholar] [CrossRef]
- Chiu, W.C.; Hou, S.S.; Chen, C.Y.; Lai, W.H.; Horng, R.F. Hydrogen-rich gas with low-level CO produced with autothermal methanol reforming providing a real-time supply used to drive a kW-scale PEMFC system. Energy 2022, 239, 122267. [Google Scholar] [CrossRef]
- Yang, B.; Li, D.; Zeng, C.; Chen, Y.; Guo, Z.; Wang, J.; Shu, H.; Yu, T.; Zhu, J. Parameter extraction of PEMFC via Bayesian regularization neural network based meta-heuristic algorithms. Energy 2021, 228, 120592. [Google Scholar] [CrossRef]
- Karayel, G.K.; Javani, N.; Dincer, I. Effective use of geothermal energy for hydrogen production, A comprehensive application. Energy 2022, 249, 123597. [Google Scholar] [CrossRef]
- Kafetzis, A.; Ziogou, C.; Papadopoulou, S.; Voutetakis, S.; Seferlis, P. Nonlinear Model Predictive Control of an Autonomous Power System Based on Hydrocarbon Reforming and High Temperature Fuel Cell. Energies 2021, 14, 1371. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Q.; Ou, S.; Liu, J. Factor decomposition and the decoupling effect of carbon emissions in China’s manufacturing high-emission subsectors. Energy 2022, 248, 123568. [Google Scholar] [CrossRef]
- Takach, M.; Sarajlić, M.; Peters, D.; Kroener, M.; Schuldt, F.; Maydell, K. Review of Hydrogen Production Techniques from Water Using Renewable Energy Sources and Its Storage in Salt Caverns. Energies 2022, 15, 1415. [Google Scholar] [CrossRef]
- Sharma, P.; Sahoo, B.B. Precise prediction of performance and emission of a waste derived Biogas–Biodiesel powered Dual–Fuel engine using modern ensemble Boosted regression Tree: A critique to Artificial neural network. Fuel 2022, 321, 124131. [Google Scholar] [CrossRef]
- Shafiei, E.; Davidsdottir, B.; Leaver, J.; Stefansson, H.; Asgeirsson, E.I. Energy, economic, and mitigation cost implications of transition toward a carbon-neutral transport sector, A simulation-based comparison between hydrogen and electricity. J. Clean. Prod. 2017, 141, 237–247. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy, An overview. Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Techno-economic analysis of a stand-alone hybrid renewable energy system with hydrogen production and storage options. Int. J. Hydrog. Energy 2015, 40, 7652–7664. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Review and evaluation of hydrogen production options for better environment. J. Clean. Prod. 2019, 218, 835–849. [Google Scholar] [CrossRef]
- Ahmed, A.; Al-Amin, A.Q.; Ambrose, A.F.; Saidur, R. Hydrogen fuel and transport system, A sustainable and environmental future. Int. J. Hydrog. Energy 2016, 41, 1369–1380. [Google Scholar] [CrossRef]
- Hou, T.F.; Shanmugasundaram, A.; Hassan, M.A.; Johar, M.A.; Ryu, S.W.; Lee, D.W. ZnO/Cu2O-decorated rGO, heterojunction photoelectrode with improved solar water splitting performance. Int. J. Hydrog. Energy 2019, 44, 19177–19192. [Google Scholar] [CrossRef]
- Bellotti, D.; Rivarolo, M.; Magistri, L.; Massardo, A.F. Feasibility study of methanol production plant from hydrogen and captured carbon dioxide. J. CO2 Util. 2017, 21, 132–138. [Google Scholar] [CrossRef]
- Zou, W.J.; Shen, K.Y.; Jung, S.; Kim, Y.B. Application of thermoelectric devices in performance optimization of a domestic PEMFC-based CHP system. Energy 2021, 229, 120698. [Google Scholar] [CrossRef]
- Ou, K.; Wang, Y.X.; Li, Z.Z.; Shen, Y.D.; Xuan, D.J. Feedforward fuzzy-PID control for air flow regulation of PEM fuel cell system. Int. J. Hydrog. Energy 2015, 40, 11686–11695. [Google Scholar] [CrossRef]
- Bianchini, M.; Alayo, N.; Soler, L.; Salleras, M.; Fonseca, L.; Llorca, J.; Tarancón, A. Standalone micro-reformer for on-demand hydrogen production from dimethyl ether. J. Power Sources 2021, 506, 230241. [Google Scholar] [CrossRef]
- Brown, L.F. A comparative study of fuels for on-board hydrogen production for fuel-cell-powered automobiles. Int. J. Hydrog. Energy 2001, 26, 381–397. [Google Scholar] [CrossRef]
- Faungnawakij, K.; Tanaka, Y.; Shimoda, N.; Fukunaga, T.; Kikuchi, R.; Eguchi, K. Hydrogen production from dimethyl ether steam reforming over composite catalysts of copper ferrite spinel and alumina. Appl. Catal. B-Environ. 2007, 74, 144–151. [Google Scholar] [CrossRef]
- Choi, S.; Bae, J. Autothermal reforming of dimethyl ether with CGO-based precious metal catalysts. J. Power Sources 2016, 307, 351–357. [Google Scholar] [CrossRef]
- Tartakovsky, L.; Mosyak, A.; Zvirin, Y. Energy analysis of ethanol steam reforming for hybrid electric vehicle. Int. J. Energy Res. 2013, 37, 259–267. [Google Scholar] [CrossRef]
- Malik, F.R.; Kim, Y.B. Autothermal reforming of n-hexadecane over Rh catalyst to produce syngas in microchannel reactor using finite element method. Int. J. Energy Res. 2019, 43, 779–790. [Google Scholar] [CrossRef]
- Nwosu, C.; Ayodele, O.; Ibrahim, H. Optimization of hydrogen production via catalytic autothermal reforming of crude glycerol using response surface methodology and artificial neural network. Int. J. Energy Res. 2021, 45, 18999–19013. [Google Scholar] [CrossRef]
- Teng, C.Y.; Chiu, S.J. Hydrogen production by partial oxidation of ethanol over Pt/CNT catalysts. Int. J. Energy Res. 2013, 37, 1689–1698. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, Z.; Yang, J.; Wang, Q. Effect of diameter distribution of particles on methane steam reforming in multi-channel grille-sphere composite packed bed. Energy Conv. Manag. 2022, 265, 115764. [Google Scholar] [CrossRef]
- Cherif, A.; Nebbali, R.; Sen, F.; Sheffield, J.W.; Doner, N.; Nasseri, L. Modeling and simulation of steam methane reforming and methane combustion over continuous and segmented catalyst beds in autothermal reactor. Int. J. Hydrog. Energy 2022, 47, 9127–9138. [Google Scholar] [CrossRef]
- Faungnawakij, K.; Viriya-Empikul, N.; Tanthapanichakoon, W. Evaluation of the thermodynamic equilibrium of the autothermal reforming of dimethyl ether. Int. J. Hydrog. Energy 2011, 36, 5865–5874. [Google Scholar] [CrossRef]
- Wu, Z.; Aguirre, A.; Tran, A.; Durand, H.; Ni, D.; Christofides, P.D. Model predictive control of a steam methane reforming reactor described by a computational fluid dynamics model. Ind. Eng. Chem. Res. 2017, 56, 6002–6011. [Google Scholar] [CrossRef]
- Wang, H.S.; Chang, C.P.; Huang, Y.J.; Su, Y.C.; Tseng, F.G. A high-yield and ultra-low-temperature methanol reformer integratable with phosphoric acid fuel cell (PAFC). Energy 2017, 133, 1142–1152. [Google Scholar] [CrossRef]
- Pantoleontos, G.; Kikkinides, E.S.; Georgiadis, M.C. A heterogeneous dynamic model for the simulation and optimisation of the steam methane reforming reactor. Int. J. Hydrog. Energy 2012, 37, 16346–16358. [Google Scholar] [CrossRef]
- Ubago-Pérez, R.; Carrasco-Marín, F.; Moreno-Castilla, C. Methanol partial oxidation on carbon-supported Pt and Pd catalysts. Catal. Today 2007, 123, 158–163. [Google Scholar] [CrossRef]
- Dolanc, G.; Pregelj, B.; Petrovčič, J.; Pasel, J.; Kolb, G. Control of autothermal reforming reactor of diesel fuel. J. Power Sources 2016, 313, 223–232. [Google Scholar] [CrossRef]
- Khalid, M.; Savkin, A.V. A model predictive control approach to the problem of wind power smoothing with controlled battery storage. Renew. Energy 2010, 35, 1520–1526. [Google Scholar] [CrossRef]
- Kyriakides, S.; Seferlis, P.; Voutetakis, S.; Papadopoulou, S. Model predictive control for hydrogen production in a membrane methane steam reforming reactor. Chem. Eng. Trans. 2016, 52, 991–996. [Google Scholar]
- Paliwal, N.K.; Singh, A.K.; Singh, N.K. Short-term optimal energy management in stand-alone microgrid with battery energy storage. Arch. Electr. Eng. 2018, 67, 499–513. [Google Scholar]
- Hu, Y.; Chmielewski, D.J. Nonlinear multivariable predictive control of an autothermal reforming reactor for fuel cell applications. In Proceedings of the 2009 American Control Conference, St. Louis, MO, USA, 10–12 June 2009; pp. 659–664. [Google Scholar]
- Wang, J.; Chen, H.; Tian, Y.; Yao, M.; Li, Y. Thermodynamic analysis of hydrogen production for fuel cells from oxidative steam reforming of methanol. Fuel 2012, 97, 805–811. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Q.; Flytzani-Stephanopoulos, M. Low-temperature water-gas shift reaction over Cu-and Ni-loaded cerium oxide catalysts. Appl. Catal. B-Environ. 2000, 27, 179–191. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies–A review. Int. J. Hydrog. Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Malik, F.R.; Jung, S.; Kim, Y.B. Hydrogen production and temperature control for DME autothermal reforming process. Energy 2022, 239, 121980. [Google Scholar] [CrossRef]
- Ouzounidou, M.; Ipsakis, D.; Voutetakis, S.; Papadopoulou, S.; Seferlis, P. A combined methanol autothermal steam reforming and PEM fuel cell pilot plant unit: Experimental and simulation studies. Energy 2009, 34, 1733–1743. [Google Scholar] [CrossRef]
- Ipsakis, D.; Voutetakis, S.; Papadopoulou, S.; Seferlis, P. Optimal operability by design in a methanol reforming-PEM fuel cell autonomous power system. Int. J. Hydrog. Energy 2012, 37, 16697–16710. [Google Scholar] [CrossRef]
- Nilsson, M.; Jozsa, P.; Pettersson, L.J. Evaluation of Pd-based catalysts and the influence of operating conditions for autothermal reforming of dimethyl ether. Appl. Catal. B-Environ. 2007, 76, 42–50. [Google Scholar] [CrossRef]
- Wang, J.; Wei, S.; Wang, Q.; Sundén, B. Transient numerical modeling and model predictive control of an industrial-scale steam methane reforming reactor. Int. J. Hydrog. Energy 2021, 46, 15241–15256. [Google Scholar] [CrossRef]
- Zecevic, N.; Bolf, N. Advanced operation of the steam methane reformer by using gain-scheduled model predictive control. Ind. Eng. Chem. Res. 2020, 59, 3458–3474. [Google Scholar] [CrossRef]
- Ismagilov, I.Z.; Matus, E.V.; Kuznetsov, V.V.; Mota, N.; Navarro, R.M.; Kerzhentsev, M.A.; Ismagilov, Z.R.; Fierro, J.L.G. Nanoscale control during synthesis of Me/La2O3, Me/CexGd1−xOy and Me/CexZr1− xOy (Me = Ni, Pt, Pd, Rh) catalysts for autothermal reforming of methane. Catal. Today 2013, 210, 10–18. [Google Scholar] [CrossRef]
- Ledesma, C.; Llorca, J. CuZn/ZrO2 catalytic honeycombs for dimethyl ether steam reforming and autothermal reforming. Fuel 2013, 104, 711–716. [Google Scholar] [CrossRef]
- Nilsson, M.; Jansson, K.; Jozsa, P.; Pettersson, L.J. Catalytic properties of Pd supported on ZnO/ZnAl2O4/Al2O3 mixtures in dimethyl ether autothermal reforming. Appl. Catal. B-Environ. 2009, 86, 18–26. [Google Scholar] [CrossRef]
- Takeishi, K.; Akaike, Y. Hydrogen production by dimethyl ether steam reforming over copper alumina catalysts prepared using the sol–gel method. Appl. Catal. A-Gen. 2016, 510, 20–26. [Google Scholar] [CrossRef]






| Parameters | Annotation | Unit |
|---|---|---|
| ωi | Mass fraction of component j | - |
| Ac | Cross-sectional area of the channel | m2 |
| Rj | Overall reaction rate for component j | mol/s/m3 |
| ϵp | Catalyst porosity | - |
| τF | Effective transport factor | - |
| u | Fluid velocity | m/s |
| ρ | Fluid density | kg/m3 |
| Cp,s | Heat capacity of solid | J/K/kg |
| Cp,f | Heat capacity of gas | J/K/kg |
| q | Flux of heat | W/m2 |
| keff | Effective thermal conductivity | W/m/K |
| (ρCp)eff | Heat capacity per unit volume | J/m3/K |
| Q | Heat production rate per unit volume | W/m3 |
| Reactions | Enthalpy | Kinetics of the Reactions | |
|---|---|---|---|
| DME Hydrolysis | ∆H = 24 kJ/mol | ||
| MeOH SR | ∆H = 49 kJ/mol | ||
| MeOH Decomposition | ∆H = 90.1 kJ/mol | ||
| Partial Oxidation | ∆H = −193 kJ/mol | ||
| CO Oxidation | ∆H = −283 kJ/mol | ||
| Water Gas Shift | ∆H = −41.1 kJ/mol | ||
| Parameters | Annotation | Value | Unit |
|---|---|---|---|
| kH | Pre-coefficient of Reaction i | 1163.232 | m3·s/kgcat |
| kSR | 154.7 | m6/(mol·s·kgcat) | |
| kD | 99.7 × 103 | mol/(s·kgcat) | |
| kPO | 400.5 | m6/(mol·s·kgcat) | |
| kCOX | 2.1 × 105 | m3/(s·kgcat) | |
| kWGS | 5000 | mol/(kgcat·s·Pa2) | |
| Ceq,WGS | 3.33 | - | |
| EH | Activation Energy of Reaction i | 22.237 | kJ/mol |
| ESR | 57.9 | ||
| ED | 110.8 | ||
| EPO | 54.9 | ||
| ECOX | 63.3 | ||
| EWGS | 100 | ||
| Eeq,WGS | 40 | ||
| R | Universal Gas Constant | 8.314 | J/K/mol |
| Concentration of Species i | - | mol/m3 | |
| T | Temperature of Reactor | - | K |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.-Q.; Jung, S.; Kim, Y.-B. Hydrogen Production System through Dimethyl Ether Autothermal Reforming, Based on Model Predictive Control. Energies 2022, 15, 9038. https://doi.org/10.3390/en15239038
Zhang T-Q, Jung S, Kim Y-B. Hydrogen Production System through Dimethyl Ether Autothermal Reforming, Based on Model Predictive Control. Energies. 2022; 15(23):9038. https://doi.org/10.3390/en15239038
Chicago/Turabian StyleZhang, Tie-Qing, Seunghun Jung, and Young-Bae Kim. 2022. "Hydrogen Production System through Dimethyl Ether Autothermal Reforming, Based on Model Predictive Control" Energies 15, no. 23: 9038. https://doi.org/10.3390/en15239038
APA StyleZhang, T.-Q., Jung, S., & Kim, Y.-B. (2022). Hydrogen Production System through Dimethyl Ether Autothermal Reforming, Based on Model Predictive Control. Energies, 15(23), 9038. https://doi.org/10.3390/en15239038







