Sustainable Strategies for the Conversion of Lignocellulosic Materials into Biohydrogen: Challenges and Solutions toward Carbon Neutrality
Abstract
1. Introduction
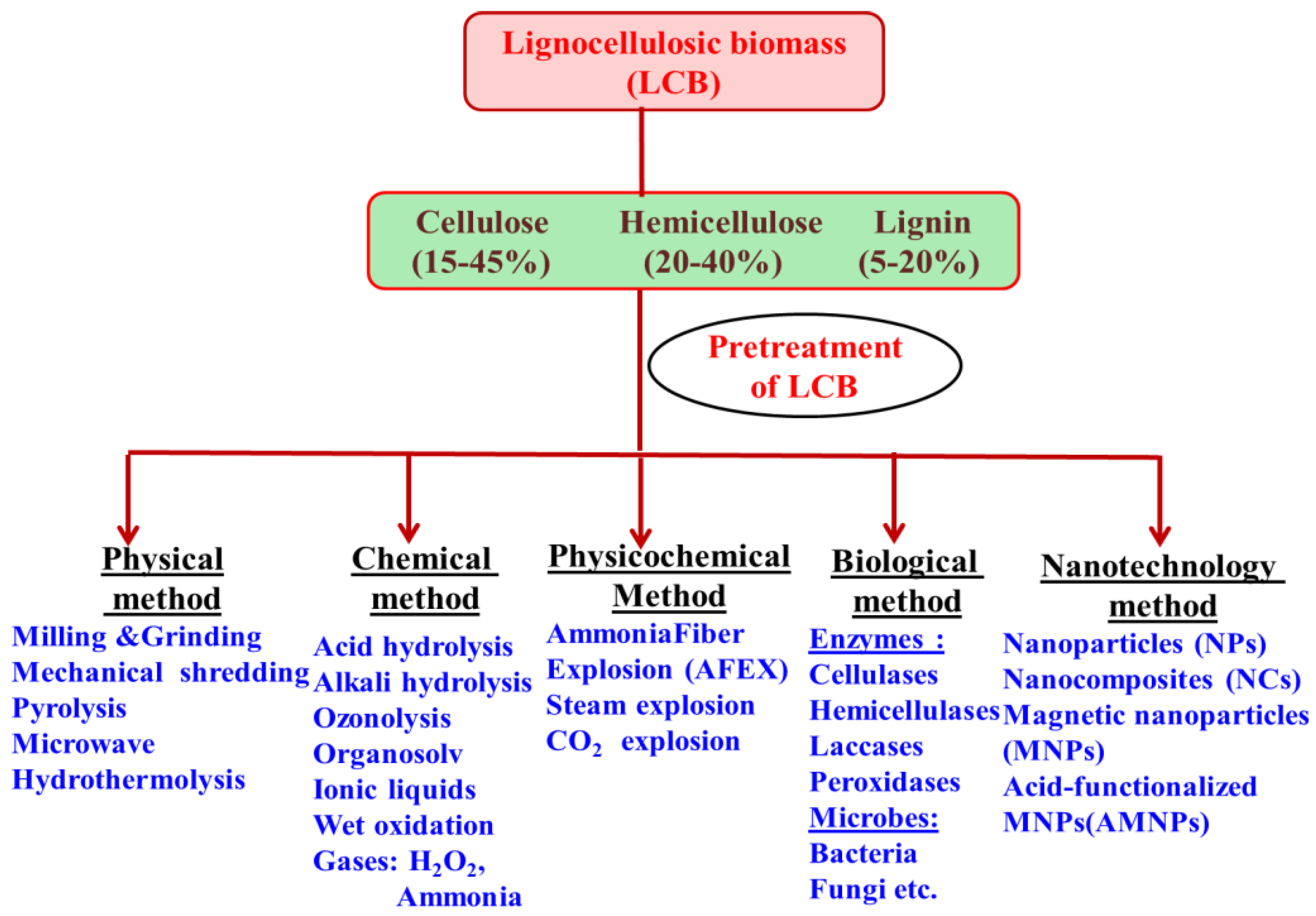
2. Pretreatment of Lignocellulosic Biomass
2.1. LCB Pretreatment by Physical Process
2.2. LCB Pretreatment by Chemical Process
2.3. LCB Pretreatment by Physico-Chemical Process
2.4. LCB Pretreatment by Biological Process
2.5. LCB Pretreatment Using Nanotechnology
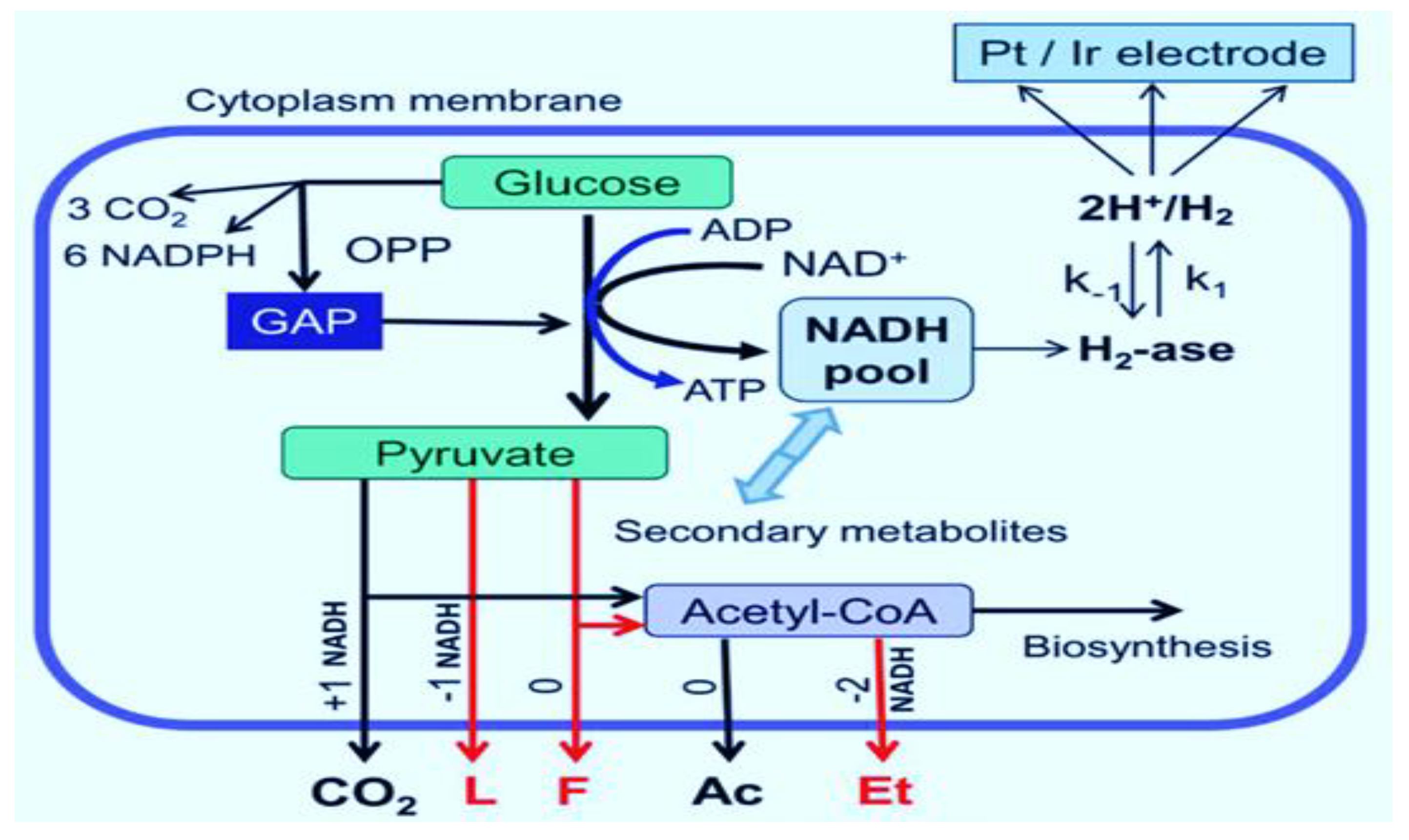
3. Biohydrogen Production by Fermentation
Role of Nanotechnology in Hydrogen Production
4. Future Perspectives and Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nong, G.; Chen, Y.; Li, M.; Zhou, Z. Generation of hydrogen free radicals from water for fuels by electric field induction. Energy Convers. Manag. 2015, 105, 545–551. [Google Scholar] [CrossRef]
- Akia, M.; Yazdani, F.; Motaee, E.; Han, D.; Arandiyan, H. A review on conversion of biomass to biofuel by nanocatalysts. Biofuel Res. J. 2014, 1, 16–25. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Bakonyi, P.; Kim, S.H.; Kobayashi, T.; Xu, K.Q.; Lakner, G.; Tóth, G.; Nemestóthy, N.; Bélafi-Bakó, K. A critical review on issues and overcoming strategies for the enhancement of dark fermentative hydrogen production in continuous systems. Int. J. Hydrogen Energy 2016, 41, 3820–3836. [Google Scholar] [CrossRef]
- Moreno, J.; Dufour, J. Life cycle assessment of hydrogen production from biomass gasification. Evaluation of different Spanish feedstocks. Int. J. Hydrogen Energy 2013, 38, 7616–7622. [Google Scholar] [CrossRef]
- Goryunov, A.G.; Goryunova, N.N.; Ogunlana, A.O.; Manenti, F. Production of energy from biomass: Near or distant future prospects? Chem. Eng. Trans. 2016, 52, 1219–1224. [Google Scholar]
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sevda, S.; Abu Reesh, I.M.; Vanbroekhoven, K.; Rathore, D.; Pant, D. Biohydrogen production from lignocellulosic biomass: Technology and sustainability. Energies 2015, 8, 13062–13080. [Google Scholar] [CrossRef]
- Shanmugam, S.; Ngo, H.H.; Wu, Y.R. Advanced CRISPR/Cas-based genome editing tools for microbial biofuels production: A review. Renew. Energy 2020, 149, 1107–1119. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.-H.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts—A critical review. Bioresour. Technol. 2012, 344, 126195. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Biswas, J.K.; da Silva, S.S. Emerging role of nanobiocatalysts in hydrolysis of lignocellulosic biomass leading to sustainable bioethanol production. Catal. Rev. 2019, 61, 1–26. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gaikwad, S.; Dussán, K.J.; da Silva, S.S. Role of nanoparticles in enzymatic hydrolysis of lignocellulose in ethanol. In Nanotechnology for Bioenergy and Biofuel Production, 2nd ed.; Springer: Cham, Switzerland; Berlin, Germany, 2017; pp. 153–171. [Google Scholar]
- Peña, L.; Ikenberry, M.; Hohn, K.L.; Wang, D. Acid-functionalized nanoparticles for pre-treatment of wheat straw. J. Biomater. Nanobiotechnol. 2012, 3, 342. [Google Scholar]
- Singhvi, M.S.; Kim, B.S. Current developments in lignocellulosic biomass conversion into biofuels using nanobiotechology approach. Energies 2020, 13, 5300. [Google Scholar] [CrossRef]
- Asadi, N.; Zilouei, H. Optimization of organosolv pretreatment of rice straw for enhanced biohydrogen production using Enterobacter aerogenes. Bioresour. Technol. 2017, 227, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, J.; Feng, J.; Wang, S.; Guo, L.; Wang, Y.; Lee, Y.Y.; Taylor, S.; McDonald, T.; Wang, Y. Enhancement of acid re-assimilation and biosolvent production in Clostridium saccharoperbutylacetonicum through metabolic engineering for efficient biofuel production from lignocellulosic biomass. Bioresour. Technol. 2019, 281, 217–225. [Google Scholar] [CrossRef]
- Dharmaraja, J.; Shobana, S.; Arvindnarayan, S.; Vadivel, M.; Atabani, A.E.; Pugazhendhi, A.; Kumar, G. Biobutanol from lignocellulosic biomass: Bioprocess strategies. In Lignocellulosic Biomass to Liquid Biofuels, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–193. [Google Scholar]
- Chandel, H.; Kumar, P.; Chandel, A.K.; Verma, M.L. Biotechnological advances in biomass pretreatment for bio-renewable production through nanotechnological intervention. Biomass Convers. Biorefinery 2022, 2022, 1–23. [Google Scholar] [CrossRef]
- Singh, H.; Tomar, S.; Qureshi, K.A.; Jaremko, M.; Rai, P.K. Recent advances in biomass pretreatment technologies for biohydrogen production. Energies 2022, 15, 999. [Google Scholar] [CrossRef]
- Karimi, K.; Taherzadeh, M.J. A critical review of analytical methods in pretreatment of lignocelluloses: Composition, imaging, and crystallinity. Bioresour. Technol. 2016, 200, 1008–1018. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Badiei, M.; Asim, N.; Jahim, J.M.; Sopian, K. Comparison of chemical pretreatment methods for cellulosic biomass. APCBEE Procedia 2014, 9, 170–174. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef]
- Sahoo, D.; Ummalyma, S.B.; Okram, A.K.; Pandey, A.; Sankar, M.; Sukumaran, R.K. Effect of dilute acid pretreatment of wild rice grass (Zizania latifolia) from Loktak Lake for enzymatic hydrolysis. Bioresour. Technol. 2018, 253, 252–255. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Yuan, Z.; Wen, Y.; Li, G. Production of bioethanol and value added compounds from wheat straw through combined alkaline/alkaline-peroxide pretreatment. Bioresour. Technol. 2018, 259, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.A.; Tabassum, R. Enhancing biogas production from lime soaked corn cob residue. Int. J. Renew. Energy Res. 2018, 8, 761–766. [Google Scholar]
- Shen, J.; Zhao, C.; Liu, G.; Chen, C. Enhancing the performance on anaerobic digestion of vinegar residue by sodium hydroxide pretreatment. Waste Biomass Valorization 2017, 8, 1119–1126. [Google Scholar] [CrossRef]
- Khan, M.U.; Usman, M.; Ashraf, M.A.; Dutta, N.; Luo, G.; Zhang, S. A review of recent advancements in pretreatment techniques of lignocellulosic materials for biogas production: Opportunities and imitations. Chem. Eng. J. Adv. 2022, 10, 100263. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K.; Zilouei, H. Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour. Technol. 2014, 152, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Tong, W.; Chen, J.; Wu, S.; Jin, Y.; Hu, J.; Song, K. Organosolv pretreatment assisted by carbocation scavenger to mitigate surface barrier effect of lignin for improving biomass saccharification and utilization. Biotechnol. Biofuels 2021, 14, 136. [Google Scholar] [CrossRef]
- Silverstein, R.A.; Chen, Y.; Sharma-Shivappa, R.R.; Boyette, M.D.; Osborne, J. A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour. Technol. 2007, 98, 3000–3011. [Google Scholar] [CrossRef] [PubMed]
- García-Cubero, M.T.; González-Benito, G.; Indacoechea, I.; Coca, M.; Bolado, S. Effect of ozonolysis pretreatment on enzymatic digestibility of wheat and rye straw. Bioresour. Technol. 2009, 100, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment—A review. Renew. Sustain. Energy Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Das, L.; Achinivu, E.C.; Barcelos, C.A.; Sundstrom, E.; Amer, B.; Baidoo, E.E.K.; Simmons, B.A.; Sun, N.; Gladden, J.M. Deconstruction of woody biomass via protic and aprotic ionic liquid pretreatment for ethanol production. ACS Sustain. Chem. Eng. 2021, 9, 4422–4432. [Google Scholar] [CrossRef]
- Rahim, A.H.A.; Man, Z.; Sarwono, A.; Muhammad, N.; Khan, A.S.; Hamzah, W.S.W.; Yunus, N.M.; Elsheikh, Y.A. Probe sonication assisted ionic liquid pretreatment for rapid dissolution of lignocellulosic biomass. Cellulose 2020, 27, 2135–2148. [Google Scholar] [CrossRef]
- Zhang, Y.; Lynd, L.R. A functionally based model for hydrolysis of cellulose by fungal cellulase. Biotechnol. Bioeng. 2006, 694, 888–898. [Google Scholar] [CrossRef]
- Quesada, J.; Rubio, M.; Gómez, D. Ozonation of lignin rich solid fractions from corn stalks. J. Wood Chem. Technol. 1999, 19, 115–137. [Google Scholar] [CrossRef]
- Mosier, N.S.; Hendrickson, R.; Brewer, M.; Ho, N.; Sedlak, M.; Dreshel, R.; Ladisch, M.R. Industrial scale-up of pH-controlled liquid hot water pretreatment of corn fiber for fuel ethanol production. Appl. Biochem. Biotechnol. 2005, 125, 77–97. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladish, M.R.; Lee, Y.Y. Coordinated development of leading biomass pretreatment technologies. Bioresour. Technol. 2005, 96, 1959–1966. [Google Scholar] [CrossRef]
- Zhang, Y.H.P. Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J. Ind. Microbiol. Biotechnol. 2008, 35, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Zwart, R.W.; Boerrigter, H.; van der Drift, A. The impact of biomass pretreatment on the feasibility of overseas biomass conversion to Fischer-Tropsch products. Energy Fuels 2006, 20, 2192–2197. [Google Scholar] [CrossRef]
- Anu, K.A.; Rapoport, A.; Kunze, G.; Kumar, S.; Singh, D.; Singh, B. Multifarious pretreatment strategies for the lignocellulosic substrates for the generation of renewable and sustainable biofuels: A review. Renew. Energy 2020, 160, 1228–1252. [Google Scholar] [CrossRef]
- Banoth, C.; Sunkar, B.; Tondamanati, P.R.; Bhukya, B. Improved physicochemical pretreatment and enzymatic hydrolysis of rice straw for bioethanol production by yeast fermentation. 3 Biotech 2017, 7, 334. [Google Scholar] [CrossRef] [PubMed]
- Barbanera, M.; Buratti, C.; Cotana, F.; Foschini, D.; Lascaro, E. Effect of steam explosion pretreatment on sugar production by enzymatic hydrolysis of olive tree pruning. Energy Procedia 2015, 81, 146–154. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.; Kumar, G.; Vickram, A.S.; Mohan, M.; Singhania, R.R.; Patel, A.K.; Dong, C.-D.; Anbarasu, K.; Thanigaivel, S.; Ponnusamy, V.K. Nanotechnology-assisted production of value-added biopotent energy-yielding products from lignocellulosic biomass refinery—A review. Bioresour. Technol. 2022, 344, 126171. [Google Scholar] [CrossRef] [PubMed]
- Suhara, H.; Kodama, S.; Kamei, I.; Maekawa, N.; Meguro, S. Screening of selective lignin-degrading basidiomycetes and biological pretreatment for enzymatic hydrolysis of bamboo culms. Int. Biodeterior. Biodegrad. 2012, 75, 176–180. [Google Scholar] [CrossRef]
- Wang, J.; Qian, Y.; Li, L.; Qiu, X. Atomic force microscopy and molecular dynamics simulations for study of lignin solution self-assembly mechanisms in organic–aqueous solvent mixtures. Chem. Sus. Chem. 2020, 13, 4420–4427. [Google Scholar] [CrossRef]
- Khalid, M.J.; Waqas, A.; Nawaz, I. Synergistic effect of alkaline pretreatment and magnetite nanoparticle application on biogas production from rice straw. Bioresour. Technol. 2015, 275, 288–296. [Google Scholar] [CrossRef]
- Soltani, R.D.C.; Khataee, A.; Koolivand, A. Kinetic, isotherm, and thermodynamic studies for removal of direct red 12b using nanostructured biosilica incorporated into calcium alginate matrix. Environ. Prog. Sustain. Energy. 2015, 34, 1435–1443. [Google Scholar] [CrossRef]
- Solomon, B.O.; Zeng, A.P.; Biebl, H.; Schlieker, H.; Posten, C.; Deckwer, W.D. Comparison of the energetic efficiencies of hydrogen and oxychemicals formation in Klebsiella pneumoniae and Clostridium butyricum during anaerobic growth on glycerol. J. Biotechnol. 1995, 39, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lay, C.H.; Sen, B.; Chu, C.Y.; Kumar, G.; Chen, C.C.; Chang, J.S. Fermentative hydrogen production from wastewaters: A review and prognosis. Int. J. Hydrogen Energy 2012, 37, 15632–15642. [Google Scholar] [CrossRef]
- Bunker, C.E.; Smith, M.J. Nanoparticles for hydrogen generation. J. Mater. Chem. 2005, 21, 12173–12180. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Improving mechanisms of biohydrogen production from grass using zero-valent iron nanoparticles. Bioresour. Technol. 2018, 266, 413–420. [Google Scholar] [CrossRef]
- Mohanraj, S.; Anbalagan, K.; Kodhaiyolii, S.; Pugalenthi, V. Comparative evaluation of fermentative hydrogen production using Enterobacter cloacae and mixed culture: Effect of Pd (II) ion and phytogenic palladium nanoparticles. J. Biotechnol. 2014, 192, 87–95. [Google Scholar] [CrossRef]
- Malik, S.N.; Pugalenthi, V.; Vaidya, A.N.; Ghosh, P.C.; Mudliar, S.N. Kinetics of Nano-catalysed dark fermentative hydrogen production from distillery wastewater. Energy Procedia 2014, 54, 417–430. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Enhancement effect of hematite and nickel nanoparticles on biohydrogen production from dairy wastewater. Int. J. Hydrogen Energy 2015, 40, 4502–4511. [Google Scholar] [CrossRef]
- Han, H.; Cui, M.; Wei, L.; Yang, H.; Shen, J. Enhancement effect of hematite nanoparticles on fermentative hydrogen production. Bioresour. Technol. 2011, 102, 7903–7909. [Google Scholar] [CrossRef] [PubMed]
- Jafari, O.; Zilouei, H. Enhanced biohydrogen and subsequent biomethane production from sugarcane bagasse using nano-titanium dioxide pretreatment. Bioresour. Technol. 2016, 2014, 670–678. [Google Scholar] [CrossRef]
- Mullai, P.; Yogeswari, M.K.; Sridevi, K. Optimisation and enhancement of biohydrogen production using nickel nanoparticles—A novel approach. Bioresour. Technol. 2013, 141, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Beckers, L.; Hiligsmann, S.; Lambert, S.D.; Heinrichs, B.; Thonart, P. Improving effect of metal and oxide nanoparticles encapsulated in porous silica on fermentative biohydrogen production by Clostridium butyricum. Bioresour. Technol. 2013, 133, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.F.; Li, S.; Zhang, W.X. Renewable hydrogen generation by bimetallic zero valent iron nanoparticles. Chem. Eng. J. 2011, 170, 562–567. [Google Scholar] [CrossRef]
- Mishra, P.; Thakur, S.; Mahapatra, D.M.; Wahid, Z.A.; Liu, H.; Singh, L. Impacts of nano-metal oxides on hydrogen production in anaerobic digestion of palm oil mill effluent—A novel approach. Int. J. Hydrogen Energy 2018, 43, 2666–2676. [Google Scholar] [CrossRef]
- Reddy, K.; Nasr, M.; Kumari, S.; Kumar, S.; Gupta, S.K.; Enitan, A.M.; Bux, F. Biohydrogen production from sugarcane bagasse hydrolysate: Effects of pH, S/X, Fe2+, and magnetite nanoparticles. Environ. Sci. Pollut. Res. 2017, 24, 8790–8804. [Google Scholar] [CrossRef] [PubMed]
- Taherdanak, M.; Zilouei, H.; Karimi, K. The effects of FeO and NiO nanoparticles versus Fe2+ and Ni2+ ions on dark hydrogen fermentation. Int. J. Hydrogen Energy 2016, 41, 167–173. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Ding, L.; Song, W.; Liu, M.; Zhou, J.; Cen, K. Enhanced dark hydrogen fermentation by addition of ferric oxide nanoparticles using Enterobacter aerogenes. Bioresour. Technol. 2016, 207, 213–219. [Google Scholar] [CrossRef]
- Engliman, N.S.; Abdul, P.M.; Wu, S.Y.; Jahim, J.M. Influence of iron (II) oxide nanoparticle on biohydrogen production in thermophilic mixed fermentation. Int. J. Hydrogen Energy 2017, 42, 27482–27493. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, C.; Zhang, H.; Wang, Z.; Zhang, J.; Song, M. Ferric oxide/carbon nanoparticles enhanced bio-hydrogen production from glucose. Int. J. Hydrogen Energy 2018, 43, 8729–8738. [Google Scholar] [CrossRef]
- Nath, D.; Manhar, A.K.; Gupta, K.; Saikia, D.; Das, S.K.; Mandal, M. Phytosynthesized iron nanoparticles: Effects on fermentative hydrogen production by Enterobacter cloacae DH-89. Bull. Mater. Sci 2015, 38, 1533–1538. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.E.; Kumar, G.; Yang, Y.H. Renewable biohydrogen production from lignocellulosic biomass using fermentation and integration of systems with other energy generation technologies. Sci. Total Environ. 2021, 765, 144429. [Google Scholar] [CrossRef] [PubMed]
- Wimonsong, P.; Nitisoravut, R. Biohydrogen enhancement using highly porous activated carbon. Energy Fuels 2014, 28, 4554–4559. [Google Scholar] [CrossRef]
- Wimonsong, P.; Nitisoravut, R. Comparison of different catalyst for fermentative hydrogen production. J. Clean Energy Technol. 2015, 3, 128–131. [Google Scholar] [CrossRef]
- Singhvi, M.; Kim, B.S. Green hydrogen production through consolidated bioprocessing of lignocellulosic biomass using nanobiotechnology approach. Bioresour. Technol. 2022, 365, 128108. [Google Scholar] [CrossRef] [PubMed]
- Singhvi, M.; Maharjan, A.; Thapa, A.; Jun, H.B.; Kim, B.S. Nanoparticle-associated single step hydrogen fermentation for the conversion of starch potato waste biomass by thermophilic Parageobacillus thermoglucosidasius. Bioresour. Technol. 2021, 337, 125490. [Google Scholar] [CrossRef]

| Pretreatment Process | Advantages | Limitations and Disadvantages | References |
|---|---|---|---|
| Ionic liquids (ILs) | Environmentally friendly Nonderivatizing, nonvolatile, thermostable single component solvent for cellulose with potential applications incellulose fractionation and dissolution | High cost Poor biodegradability Toxic to micro-organisms | [40] |
| Ozonolysis | Reduces lignin content Does not produce toxic residues | Large amount of ozone required Expensive | [41] |
| Acid hydrolysis | Hydrolyzes hemicellulose to xylose and other sugars to alter lignin structure | High cost Equipment corrosion Formation of toxic substances | [42] |
| Alkaline hydrolysis | Removes hemicellulose and lignin Increases accessible surface area | Long residence times required Irrecoverable salts formed and incorporated into biomass | [43] |
| Organosolv | Organosolv lignin is sulfur free with high purity and low molecular weight Can be used as fuel to power pretreatment plant or further purified to obtain high quality lignin, which is used as a substitute for polymeric materials Very effective for the pretreatment of high-lignin lignocellulose materials | Solvents need to be drained from the reactor, evaporated, condensed, and recycled High cost Generation of compounds inhibitory to micro-organisms | [44] |
| Pyrolysis | Produces gas and liquid products | High temperature Ash production | [45] |
| Sr. No. | Nanoparticles | Microbial Strains | Substrates | Improvement in Hydrogen Production (%) | References |
|---|---|---|---|---|---|
| 1. | NiO, | Bacillus anthracis | Palm oil mill effluent | 151 | [68] |
| CoO | 167 | ||||
| 2. | FeO | Enterobacter sp., Clostridium sp. | Grass | 73.1 | [59] |
| 3. | Fe3O4 | Anaerobic sludge | Sugarcane bagasse | 69 | [69] |
| 4. | FeNPs | Mesophilic culture | Starch | 200 | [70] |
| 5. | Fe2O3 | Enterobacter aerogenes | Cassava starch | 92 | [71] |
| 6. | NiO | Anaerobic sludge containing H2 producing bacteria | Molasses waste | 24 | [62] |
| Fe2O3 | 43 | ||||
| 7. | TiO2 | Anaerobic sludge | Sugarcane bagasse | 127 | [64] |
| 8. | Fe2O3 | Thermophillic anaerobic mixed culture | Glucose | 53.6 | [72] |
| 9. | Fe2O3-Fe3O4/carbon nanocomposite | Anaerobic mixed bacteria | Glucose | 33.7 | [73] |
| 10. | FeNPs | Enterobacter cloacae | Glucose | 130 | [74] |
| 11. | Nano activated carbon | Anaerobic sludge | Sucrose | 70 | [75] |
| 12. | Nano activated carbon | Anaerobic sludge | Sucrose | 70 | [76] |
| 13. | Pd, Ag, Cu, and Fe encapsulated SiO2 NPs | Clostridium butyricum | Glucose | 38 | [66] |
| 14. | Caron nanotube | Anaerobic sludge | Glucose | 50 | [20] |
| 15. | Activated carbon | Anaerobic sludge | Sucrose | 62.5 | [77] |
| 16. | CeFe3O4 | Clostridium cellulovorans | Corn cob | 149 | [78] |
| 17. | Fe3O4 | Parageobacillus thermoglucosidasius | Potato peel | 315 | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singhvi, M.; Zinjarde, S.; Kim, B.-S. Sustainable Strategies for the Conversion of Lignocellulosic Materials into Biohydrogen: Challenges and Solutions toward Carbon Neutrality. Energies 2022, 15, 8987. https://doi.org/10.3390/en15238987
Singhvi M, Zinjarde S, Kim B-S. Sustainable Strategies for the Conversion of Lignocellulosic Materials into Biohydrogen: Challenges and Solutions toward Carbon Neutrality. Energies. 2022; 15(23):8987. https://doi.org/10.3390/en15238987
Chicago/Turabian StyleSinghvi, Mamata, Smita Zinjarde, and Beom-Soo Kim. 2022. "Sustainable Strategies for the Conversion of Lignocellulosic Materials into Biohydrogen: Challenges and Solutions toward Carbon Neutrality" Energies 15, no. 23: 8987. https://doi.org/10.3390/en15238987
APA StyleSinghvi, M., Zinjarde, S., & Kim, B.-S. (2022). Sustainable Strategies for the Conversion of Lignocellulosic Materials into Biohydrogen: Challenges and Solutions toward Carbon Neutrality. Energies, 15(23), 8987. https://doi.org/10.3390/en15238987








