Optimization of Energy Production from Two-Stage Mesophilic–Thermophilic Anaerobic Digestion of Cheese Whey Using a Response Surface Methodology Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
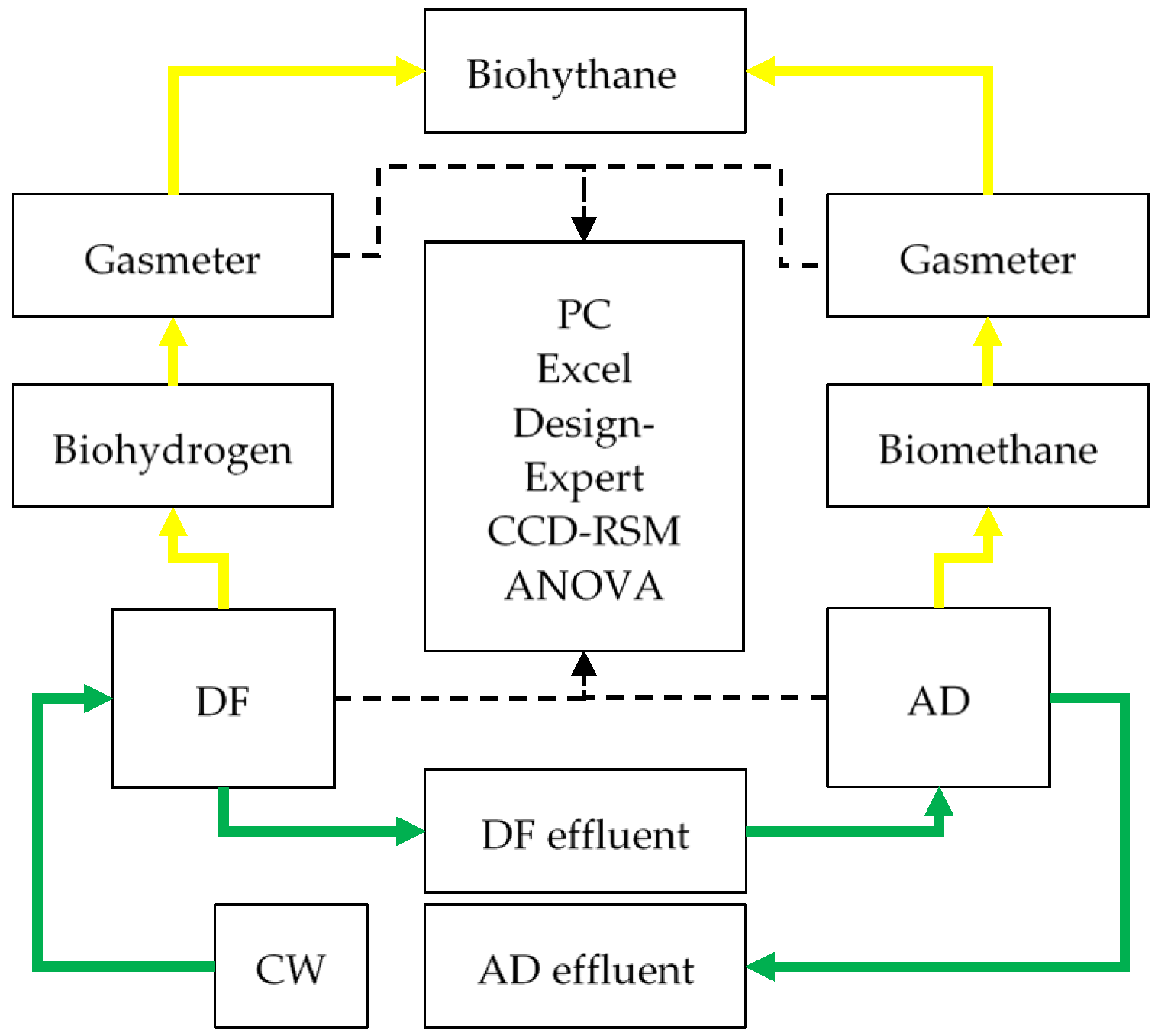
2.2. Description of the Laboratory Setup
2.3. Operation of the Laboratory Setup: Dark Fermentation (DF) and Anaerobic Digestion (AD)
2.4. Analytical Methods
2.5. Design of Experiment Using RSM
3. Results
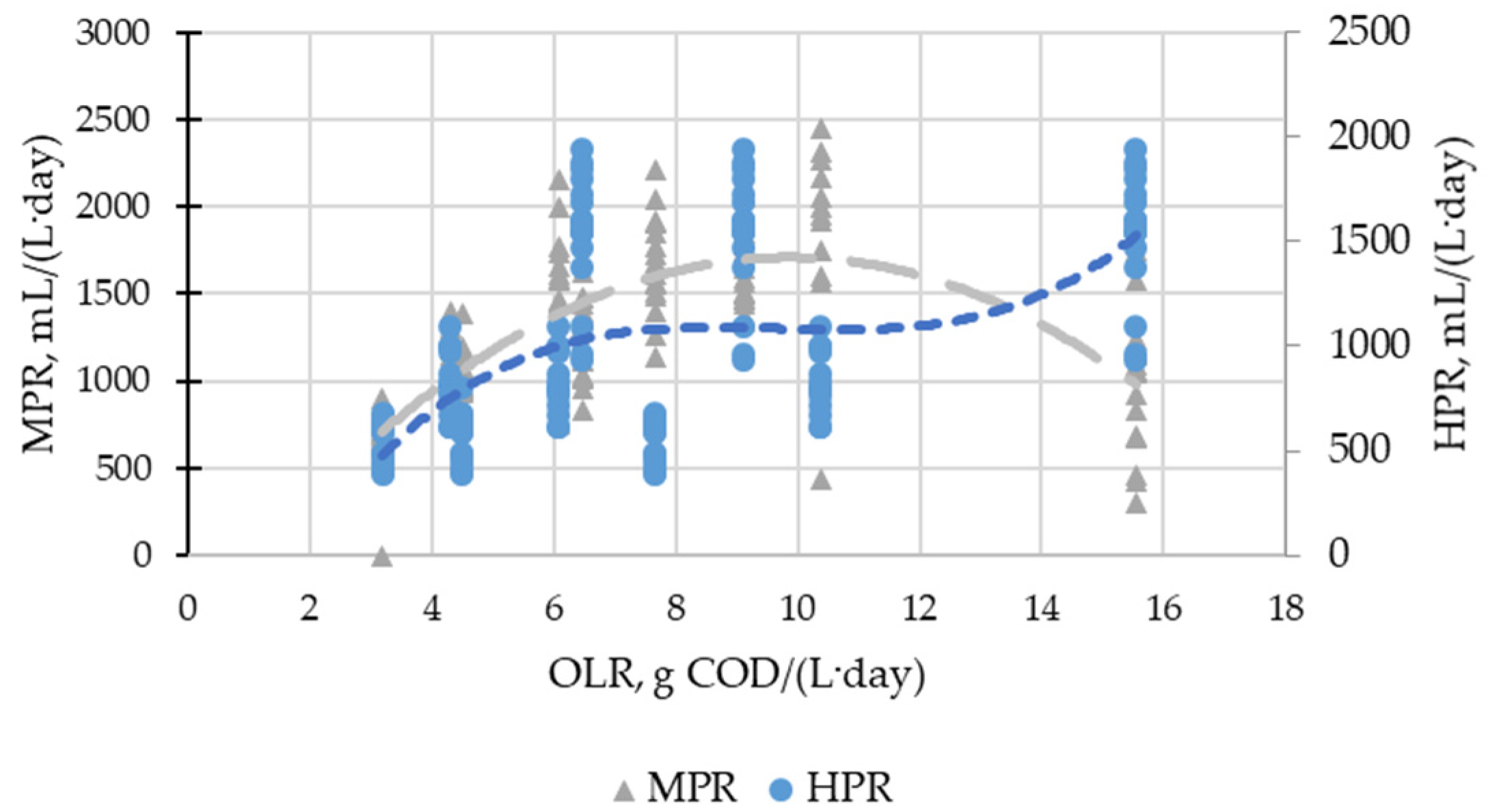
3.1. Biogas Production
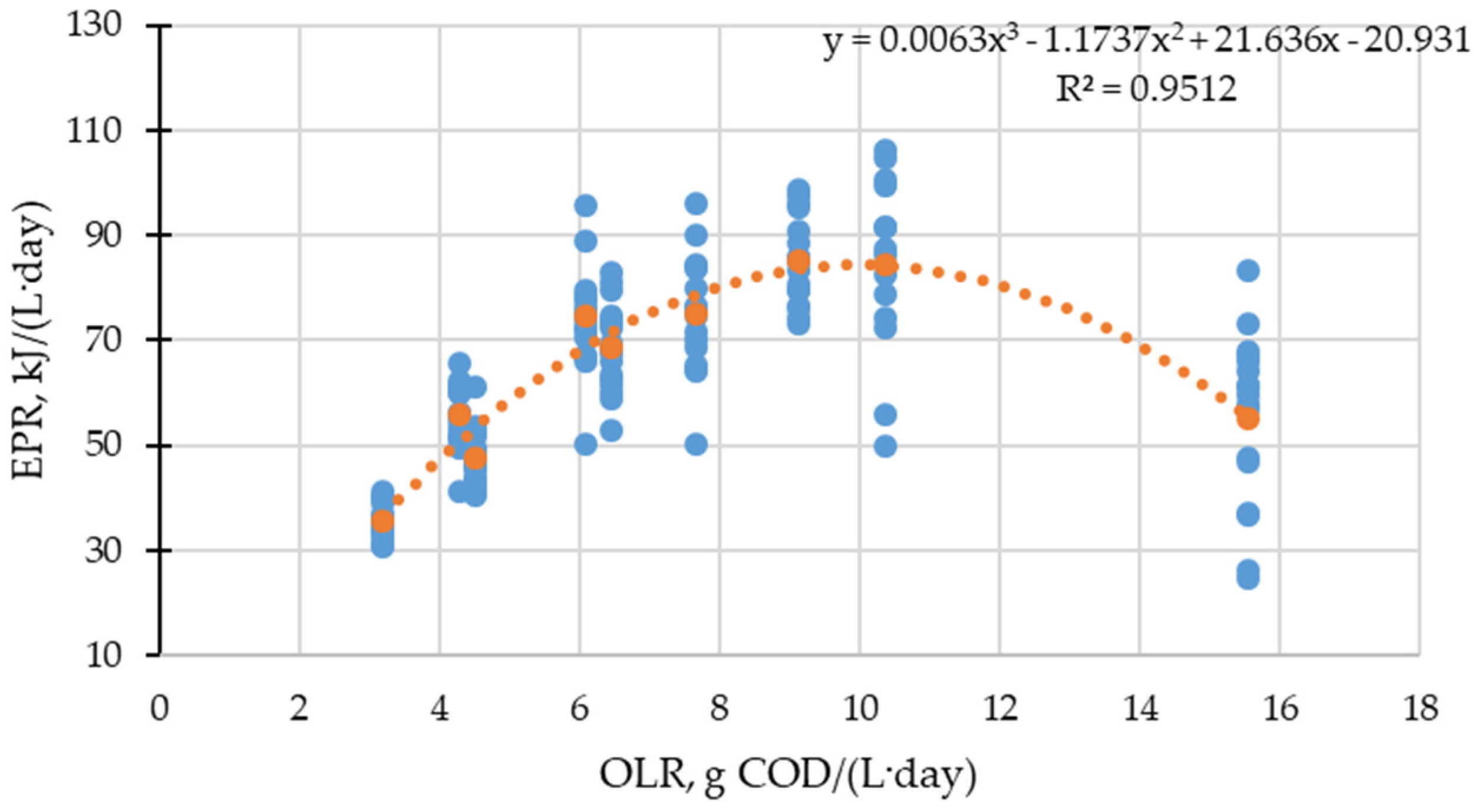
3.2. Energy Recovery
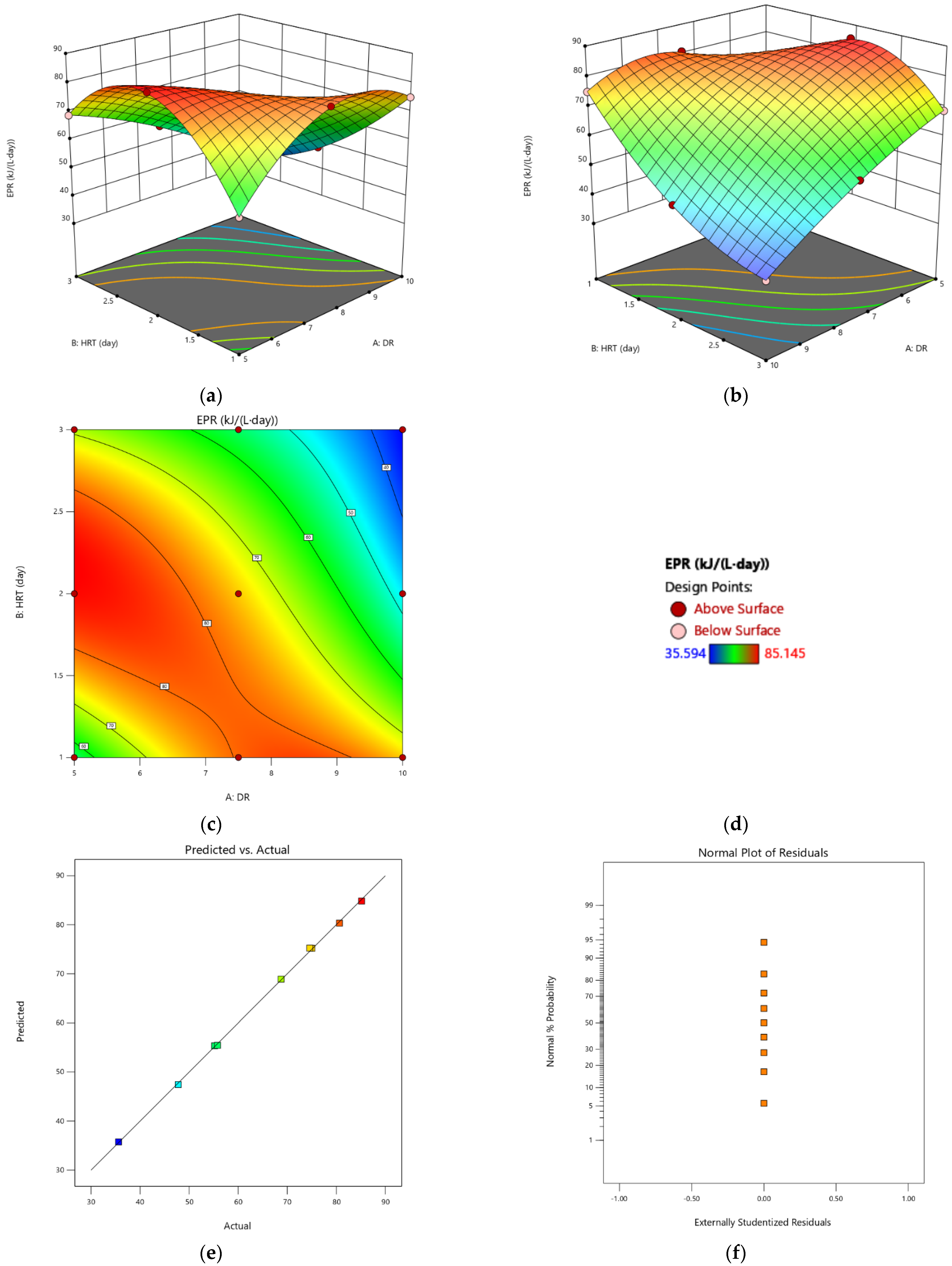
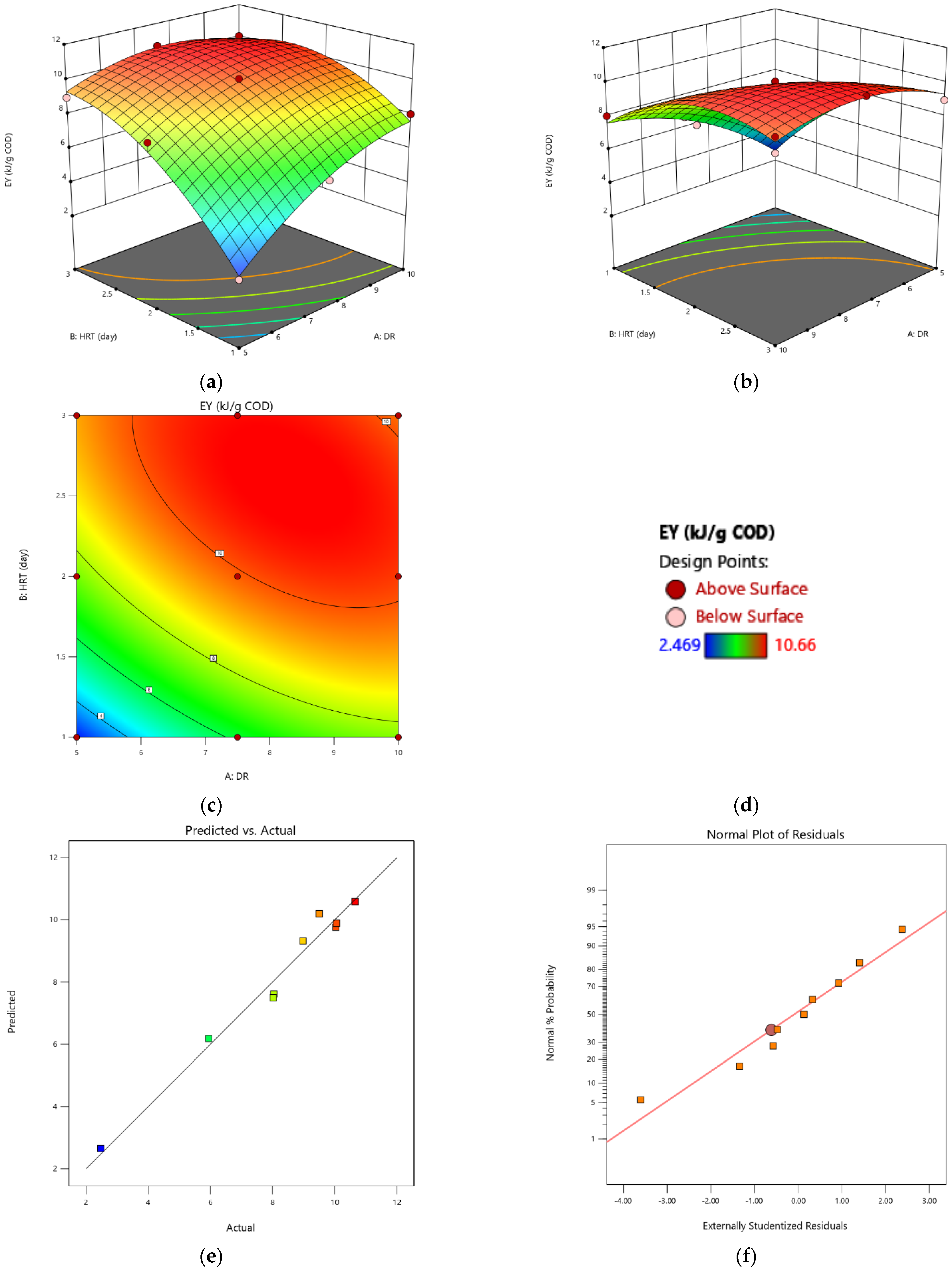
3.3. Optimization of Energy Recovery of a Two-Stage AD of CW Using RSM
4. Discussion
4.1. Energy Recovery
4.2. Two-Stage AD Energy Recovery Optimization Using RSM
5. Conclusions
- For the energy production rate, the values A, B, AB, A2 and AB2 (A was the dilution rate of the COD and B was the HRT) were significant model terms with an R2 value of 99%. The surface plot showed a nonlinear decrease in the EPR for an increase in the DR or HRT.
- For the energy yield, the values A, B, AB and B2 (A were the dilution rate of the COD and B was the HRT) were significant model terms with an R2 value of 97%. The surface plot showed a nonlinear increase in the EY for an increase in the DR or HRT.
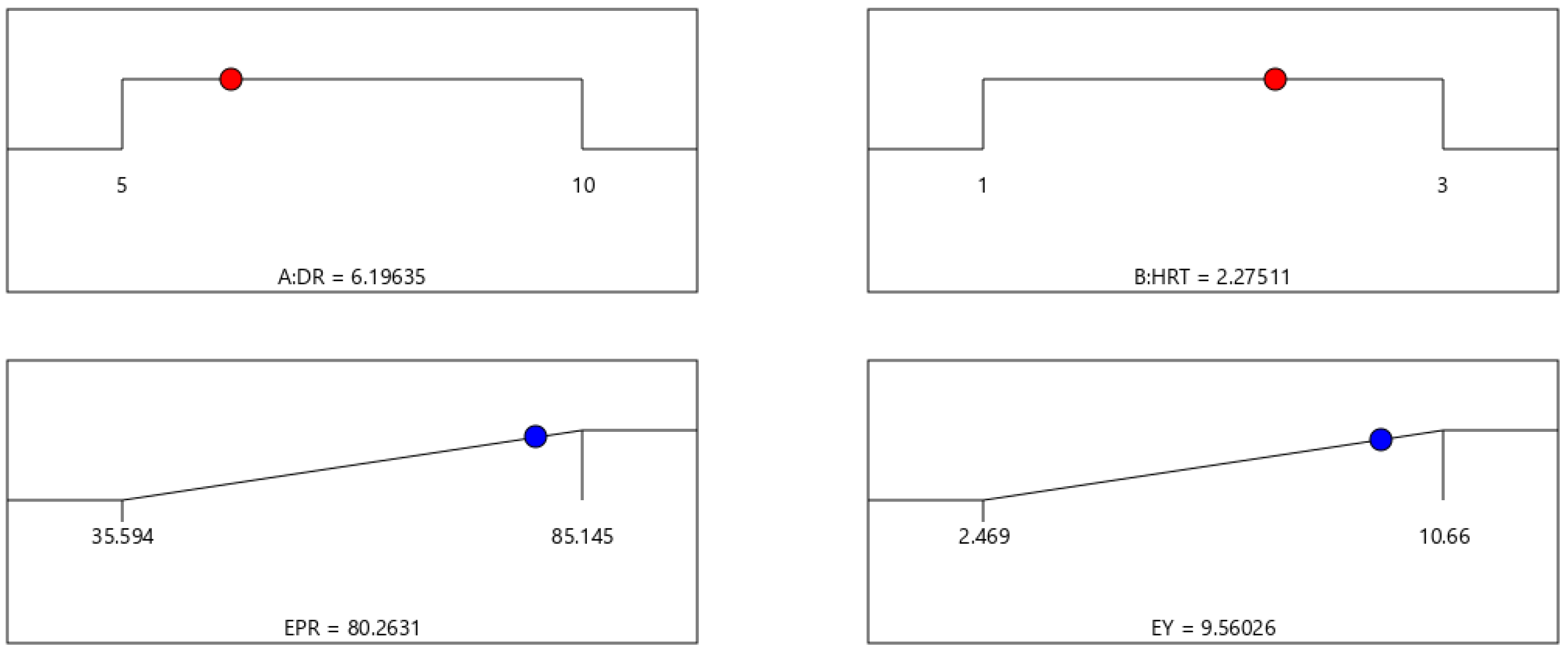
- The desirability approach produced a combined optimum condition as follows: a DR of 6.2 and a HRT of 2.28, which allowed us to achieve both a maximum EPR and EY (80.263 kJ/(L·day) and 9.56 kJ/g COD, respectively), with an overall desirability value of 0.883.
Author Contributions
Funding
Conflicts of Interest
References
- Slavov, A. Dairy Wastewaters–General Characteristics and Treatment Possibilities—A Review. Food Technol. Biotechnol. 2017, 55, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Farizoglu, B.; Keskinler, B.; Yildiz, E.; Nuhoglu, A. Simultaneous Removal of C, N, P from Cheese Whey by Jet Loop Membrane Bioreactor (JLMBR). J. Hazard. Mater. 2007, 146, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Aadil, R.M.; Ahmed, H.; Rahman, U.U.; Soares, B.C.V.; Souza, S.L.Q.; Pimentel, T.C.; Scudino, H.; Guimarães, J.T.; Esmerino, E.A.; et al. Treatment and Utilization of Dairy Industrial Waste: A Review. Trends Food Sci. Technol. 2019, 88, 361–372. [Google Scholar] [CrossRef]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese Whey Wastewater: Characterization and Treatment. Sci. Total Environ. 2013, 445–446, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Macwan, S.; Dabhi, B.; Parmar, S.; Aparnathi, K. Whey and Its Utilization. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 134–155. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L.H. Whey and Whey Products BT—Fundamentals of Cheese Science; Fox, P.F., Guinee, T.P., Cogan, T.M., McSweeney, P.L.H., Eds.; Springer US: Boston, MA, USA, 2017; pp. 755–769. [Google Scholar] [CrossRef]
- Escalante, H.; Castro, L.; Amaya, M.P.; Jaimes, L.; Jaimes-Estévez, J. Anaerobic Digestion of Cheese Whey: Energetic and Nutritional Potential for the Dairy Sector in Developing Countries. Waste Manag. 2018, 71, 711–718. [Google Scholar] [CrossRef]
- Imeni, S.M.; Pelaz, L.; Corchado-Lopo, C.; Maria Busquets, A.; Ponsá, S.; Colón, J. Techno-Economic Assessment of Anaerobic Co-Digestion of Livestock Manure and Cheese Whey (Cow, Goat & Sheep) at Small to Medium Dairy Farms. Bioresour. Technol. 2019, 291, 121872. [Google Scholar] [CrossRef]
- Kim, I.S.; Hwang, M.H.; Jang, N.J.; Hyun, S.H.; Lee, S.T. Effect of Low PH on the Activity of Hydrogen Utilizing Methanogen in Bio-Hydrogen Process. Int. J. Hydrogen Energy 2004, 29, 1133–1140. [Google Scholar] [CrossRef]
- Fontana, A.; Campanaro, S.; Treu, L.; Kougias, P.; Cappa, F.; Morelli, L.; Angelidaki, I. Performance and Genome-Centric Metagenomics of Thermophilic Single and Two-Stage Anaerobic Digesters Treating Cheese Wastes. Water Res. 2018, 134, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Nozhevnikova, A.N.; Russkova, Y.I.; Litti, Y.V.; Parshina, S.N.; Zhuravleva, E.A.; Nikitina, A.A. Syntrophy and Interspecies Electron Transfer in Methanogenic Microbial Communities. Microbiology 2020, 89, 129–147. [Google Scholar] [CrossRef]
- Kovalev, A.A.; Kovalev, D.A.; Nozhevnikova, A.N.; Zhuravleva, E.A.; Katraeva, I.V.; Grigoriev, V.S.; Litti, Y.V. Effect of Low Digestate Recirculation Ratio on Biofuel and Bioenergy Recovery in a Two-Stage Anaerobic Digestion Process. Int. J. Hydrogen Energy 2021, 46, 39688–39699. [Google Scholar] [CrossRef]
- De Gioannis, G.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D. Energy Recovery from One- and Two-Stage Anaerobic Digestion of Food Waste. Waste Manag. 2017, 68, 595–602. [Google Scholar] [CrossRef]
- Fu, S.-F.; Liu, R.; Sun, W.-X.; Zhu, R.; Zou, H.; Zheng, Y.; Wang, Z.-Y. Enhancing Energy Recovery from Corn Straw via Two-Stage Anaerobic Digestion with Stepwise Microaerobic Hydrogen Fermentation and Methanogenesis. J. Clean. Prod. 2020, 247, 119651. [Google Scholar] [CrossRef]
- Tunçay, E.G.; Erguder, T.H.; Eroğlu, İ.; Gündüz, U. Dark Fermentative Hydrogen Production from Sucrose and Molasses. Int. J. Energy Res. 2017, 41, 1891–1902. [Google Scholar] [CrossRef]
- Cheng, J.; Lin, R.; Ding, L.; Song, W.; Li, Y.; Zhou, J.; Cen, K. Fermentative Hydrogen and Methane Cogeneration from Cassava Residues: Effect of Pretreatment on Structural Characterization and Fermentation Performance. Bioresour. Technol. 2015, 179, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Bakonyi, P.; Sivagurunathan, P.; Kim, S.-H.; Nemestóthy, N.; Bélafi-Bakó, K. Lignocellulose Biohydrogen: Practical Challenges and Recent Progress. Renew. Sustain. Energy Rev. 2015, 44, 728–737. [Google Scholar] [CrossRef]
- Bolzonella, D.; Battista, F.; Cavinato, C.; Gottardo, M.; Micolucci, F.; Lyberatos, G.; Pavan, P. Recent Developments in Biohythane Production from Household Food Wastes: A Review. Bioresour. Technol. 2018, 257, 311–319. [Google Scholar] [CrossRef]
- Hans, M.; Kumar, S. Biohythane Production in Two-Stage Anaerobic Digestion System. Int. J. Hydrogen Energy 2019, 44, 17363–17380. [Google Scholar] [CrossRef]
- Alexandropoulou, M.; Antonopoulou, G.; Trably, E.; Carrere, H.; Lyberatos, G. Continuous Biohydrogen Production from a Food Industry Waste: Influence of Operational Parameters and Microbial Community Analysis. J. Clean. Prod. 2018, 174, 1054–1063. [Google Scholar] [CrossRef]
- Alibardi, L.; Cossu, R. Effects of Carbohydrate, Protein and Lipid Content of Organic Waste on Hydrogen Production and Fermentation Products. Waste Manag. 2016, 47, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Distefano, T.; Palomar, A. Effect of Anaerobic Reactor Process Configuration on Useful Energy Production. Water Res. 2010, 44, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Reyes, J.; Cortés-Carmona, M.A.; Bárcenas-Ruiz, C.D.; Razo-Flores, E. Cell Wash-out Enrichment Increases the Stability and Performance of Biohydrogen Producing Packed-Bed Reactors and the Community Transition along the Operation Time. Renew. Energy 2016, 97, 266–273. [Google Scholar] [CrossRef]
- Venetsaneas, N.; Antonopoulou, G.; Stamatelatou, K.; Kornaros, M.; Lyberatos, G. Using Cheese Whey for Hydrogen and Methane Generation in a Two-Stage Continuous Process with Alternative PH Controlling Approaches. Bioresour. Technol. 2009, 100, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Kisielewska, M.; Wysocka, I.; Rynkiewicz, M. Continuous Biohydrogen and Biomethane Production from Whey Permeate in a Two-Stage Fermentation Process. Environ. Prog. Sustain. Energy 2013, 33, 1411–1418. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Kornaros, M. Effect of Hydraulic Retention Time (HRT) on the Anaerobic Co-Digestion of Agro-Industrial Wastes in a Two-Stage CSTR System. Bioresour. Technol. 2014, 167, 407–415. [Google Scholar] [CrossRef]
- Zhang, H.; Bruns, M.A.; Logan, B.E. Biological Hydrogen Production by Clostridium Acetobutylicum in an Unsaturated Flow Reactor. Water Res. 2006, 40, 728–734. [Google Scholar] [CrossRef]
- Antonopoulou, G.; Stamatelatou, K.; Venetsaneas, N.; Kornaros, M.; Lyberatos, G. Biohydrogen and Methane Production from Cheese Whey in a Two-Stage Anaerobic Process. Ind. Eng. Chem. Res. 2008, 47, 5227–5233. [Google Scholar] [CrossRef]
- Naghavi, R.; Abdoli, M.A.; Karbassi, A.; Adl, M. Determining the Appropriate Mixing Ratio in a Multi-Substrate Anaerobic Digestion of Organic Solid Wastes Employing Taguchi Method. J. Environ. Health Sci. Eng. 2022, 20, 545–554. [Google Scholar] [CrossRef]
- Kainthola, J.; Kalamdhad, A.S.; Goud, V.V. Optimization of Methane Production during Anaerobic Co-Digestion of Rice Straw and Hydrilla Verticillata Using Response Surface Methodology. Fuel 2019, 235, 92–99. [Google Scholar] [CrossRef]
- Yılmaz, Ş.; Şahan, T. Improved Anaerobic Digestion Activity of Poultry Dung with Pumice-Supported Trace Elements: Focus on the Statistical Optimization Approach. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Chai, W.S.; Lay, C.-H.; Chen, C.-C.; Lee, C.-Y.; Show, P.-L. Optimization of Hydrolysis-Acidogenesis Phase of Swine Manure for Biogas Production Using Two-Stage Anaerobic Fermentation. Processes 2021, 9, 1324. [Google Scholar] [CrossRef]
- Mikheeva, E.R.; Katraeva, I.V.; Kovalev, A.A.; Kovalev, D.A.; Nozhevnikova, A.N.; Panchenko, V.; Fiore, U.; Litti, Y.V. The Start-Up of Continuous Biohydrogen Production from Cheese Whey: Comparison of Inoculum Pretreatment Methods and Reactors with Moving and Fixed Polyurethane Carriers. Appl. Sci. 2021, 11, 510. [Google Scholar] [CrossRef]
- Bär, K.; Merkle, W.; Tuczinski, M.; Saravia, F.; Horn, H.; Ortloff, F.; Graf, F.; Lemmer, A.; Kolb, T. Development of an Innovative Two-Stage Fermentation Process for High-Calorific Biogas at Elevated Pressure. Biomass Bioenergy 2018, 115, 186–194. [Google Scholar] [CrossRef]
- Mikheeva, E.R.; Katraeva, I.V.; Vorozhtsov, D.L.; Kovalev, D.A.; Kovalev, A.A.; Grigoriev, V.S.; Litti, Y.V. Dark Fermentative Biohydrogen Production from Confectionery Wastewater in Continuous-Flow Reactors. Int. J. Hydrogen Energy 2022, 47, 22348–22358. [Google Scholar] [CrossRef]
- Mikheeva, E.R.; Katraeva, I.V.; Kovalev, A.A.; Kovalev, D.A.; Litti, Y.V. Effects of Pretreatment in a Vortex Layer Apparatus on the Properties of Confectionery Wastewater and Its Dark Fermentation. Int. J. Hydrogen Energy 2022, 47, 23165–23174. [Google Scholar] [CrossRef]
- Ghaleb, A.A.S.; Kutty, S.R.M.; Ho, Y.-C.; Jagaba, A.H.; Noor, A.; Al-Sabaeei, A.M.; Almahbashi, N.M.Y. Response Surface Methodology to Optimize Methane Production from Mesophilic Anaerobic Co-Digestion of Oily-Biological Sludge and Sugarcane Bagasse. Sustainability 2020, 12, 2116. [Google Scholar] [CrossRef]
- Kumari, S.; Das, D. Biohythane Production from Sugarcane Bagasse and Water Hyacinth: A Way towards Promising Green Energy Production. J. Clean. Prod. 2018, 207, 689–701. [Google Scholar] [CrossRef]
- Silva-Illanes, F.; Tapia-Venegas, E.; Schiappacasse, M.C.; Trably, E.; Ruiz-Filippi, G. Impact of Hydraulic Retention Time (HRT) and PH on Dark Fermentative Hydrogen Production from Glycerol. Energy 2017, 141, 358–367. [Google Scholar] [CrossRef]
- Vo, T.-P.; Lay, C.-H.; Lin, C.-Y. Effects of Hydraulic Retention Time on Biohythane Production via Single-Stage Anaerobic Fermentation in a Two-Compartment Bioreactor. Bioresour. Technol. 2019, 292, 121869. [Google Scholar] [CrossRef]
- Onu, C.E.; Nweke, C.N.; Nwabanne, J.T. Modeling of Thermo-Chemical Pretreatment of Yam Peel Substrate for Biogas Energy Production: RSM, ANN, and ANFIS Comparative Approach. Appl. Surf. Sci. Adv. 2022, 11, 100299. [Google Scholar] [CrossRef]
- Ibrahim, T.H.; Betiku, E.; Solomon, B.O.; Oyedele, J.O.; Dahunsi, S.O. Mathematical Modelling and Parametric Optimization of Biomethane Production with Response Surface Methodology: A Case of Cassava Vinasse from a Bioethanol Distillery. Renew. Energy 2022, 200, 395–404. [Google Scholar] [CrossRef]
- Ramos, L.R.; de Menezes, C.A.; Soares, L.A.; Sakamoto, I.K.; Varesche, M.B.A.; Silva, E.L. Controlling Methane and Hydrogen Production from Cheese Whey in an EGSB Reactor by Changing the HRT. Bioprocess Biosyst. Eng. 2020, 43, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.R.; Silva, E.L. Improving EGSB Reactor Performance for Simultaneous Bioenergy and Organic Acid Production from Cheese Whey via Continuous Biological H2 Production. Biotechnol. Lett. 2017, 39, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Lembo, G.; Rosa, S.; Mazzurco Miritana, V.; Marone, A.; Massini, G.; Fenice, M.; Signorini, A. Thermophilic Anaerobic Digestion of Second Cheese Whey: Microbial Community Response to H2 Addition in a Partially Immobilized Anaerobic Hybrid Reactor. Processes 2021, 9, 43. [Google Scholar] [CrossRef]
- Dareioti, M.A.; Vavouraki, A.I.; Tsigkou, K.; Kornaros, M. Assessment of Single- vs. Two-Stage Process for the Anaerobic Digestion of Liquid Cow Manure and Cheese Whey. Energies 2021, 14, 5423. [Google Scholar] [CrossRef]
- Castelló, E.; García y Santos, C.; Iglesias, T.; Paolino, G.; Wenzel, J.; Borzacconi, L.; Etchebehere, C. Feasibility of Biohydrogen Production from Cheese Whey Using a UASB Reactor: Links between Microbial Community and Reactor Performance. Int. J. Hydrogen Energy 2009, 34, 5674–5682. [Google Scholar] [CrossRef]
- Ghimire, A.; Luongo, V.; Frunzo, L.; Lens, P.N.L.; Pirozzi, F.; Esposito, G. Biohythane Production from Food Waste in a Two-Stage Process: Assessing the Energy Recovery Potential. Environ. Technol. 2022, 43, 2190–2196. [Google Scholar] [CrossRef]



| Parameter | Reactor | Temperature, °C | Period #1 | Period #2 | Period #3 |
|---|---|---|---|---|---|
| HRT, day | RH | 37 ± 1 | 0.42 | ||
| R1 | 55 ± 1 | 3 | |||
| R2 | 2 | ||||
| R3 | 1 | ||||
| Dilution rate (DR), parts of water per part CW | - | - | 10 | 7.5 | 5 |
| COD of diluted CW, g/L | - | - | 10.9 | 14.53 | 21.8 |
| OLR, g COD/(L·day) | DR | HRT, day |
|---|---|---|
| 3.18 | 10 | 3 |
| 4.30 | 7.5 | 3 |
| 4.5 | 10 | 2 |
| 6.08 | 7.5 | 2 |
| 6.46 | 5 | 3 |
| 7.66 | 10 | 1 |
| 9.12 | 5 | 2 |
| 10.37 | 7.5 | 1 |
| 15.55 | 5 | 1 |
| Factor | Name | Low Level | High Level |
|---|---|---|---|
| A | DR | 5.00 | 10.00 |
| B | HRT | 1.00 | 3.00 |
| Std | Run | Factor 1 A:DR | Factor 2 B:HRT, day | Response 1 EPR, kJ/(L·day) | Response 2 EY, kJ/g COD |
|---|---|---|---|---|---|
| 7 | 1 | 7.5 | 1 | 80.635 | 5.943 |
| 1 | 2 | 5 | 1 | 55.193 | 2.469 |
| 4 | 3 | 10 | 3 | 35.594 | 10.037 |
| 2 | 4 | 10 | 1 | 75.023 | 8.043 |
| 6 | 5 | 10 | 2 | 47.77 | 9.505 |
| 3 | 6 | 5 | 3 | 68.739 | 8.983 |
| 5 | 7 | 5 | 2 | 85.145 | 8.025 |
| 9 | 8 | 7.5 | 2 | 74.583 | 10.064 |
| 8 | 9 | 7.5 | 3 | 55.778 | 10.66 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 2193.68 | 7 | 313.38 | 338.28 | 0.0418 | Significant |
| A-DR | 698.45 | 1 | 698.45 | 753.93 | 0.0232 | Significant |
| B-HRT | 308.94 | 1 | 308.94 | 333.48 | 0.0348 | Significant |
| AB | 701.59 | 1 | 701.59 | 757.32 | 0.0231 | Significant |
| A2 | 165.18 | 1 | 165.18 | 178.31 | 0.0476 | Significant |
| B2 | 107.72 | 1 | 107.72 | 116.28 | 0.0589 | |
| A2B | 47.33 | 1 | 47.33 | 51.09 | 0.0885 | |
| AB2 | 314.52 | 1 | 314.52 | 339.51 | 0.0345 | Significant |
| A3 | 0.0000 | 0 | ||||
| B3 | 0.0000 | 0 | ||||
| Residual | 0.9264 | 1 | 0.9264 | |||
| Cor Total | 2194.60 | 8 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 51.95 | 5 | 10.39 | 24.80 | 0.0121 | Significant |
| A-DR | 10.96 | 1 | 10.96 | 26.15 | 0.0145 | Significant |
| B-HRT | 29.15 | 1 | 29.15 | 69.57 | 0.0036 | Significant |
| AB | 5.11 | 1 | 5.11 | 12.19 | 0.0397 | Significant |
| A2 | 2.19 | 1 | 2.19 | 5.22 | 0.1066 | |
| B2 | 4.55 | 1 | 4.55 | 10.87 | 0.0459 | Significant |
| Residual | 1.26 | 3 | 0.4190 | |||
| Cor Total | 53.21 | 8 |
| HRT, day | Temperature, °C | OLR, g COD/(L·day) | EPR, kJ/(L·day) | EY, kJ/g COD | Note | Reference |
|---|---|---|---|---|---|---|
| 0.33–0.01 | 30 | 20–610 | 25.42–14.42 * | 8.98–1.46 * | [44] | |
| 0.17 | 30 | 135 -210 | 2.93–3.96 * | DF only | [45] | |
| 15 | 55 | 2.18–2.4 | - | 13.11 | AD only | [46] |
| 20 | 37 | 2.36–3.83 | 7.17–11.56 | AD only | [47] | |
| 0.5 | 30 | 20 | 1.56 | DF only | [48] | |
| 2.28 | 37/55 | 6.5 | 80.26 | 9.56 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalev, A.A.; Mikheeva, E.R.; Panchenko, V.; Katraeva, I.V.; Kovalev, D.A.; Zhuravleva, E.A.; Litti, Y.V. Optimization of Energy Production from Two-Stage Mesophilic–Thermophilic Anaerobic Digestion of Cheese Whey Using a Response Surface Methodology Approach. Energies 2022, 15, 8928. https://doi.org/10.3390/en15238928
Kovalev AA, Mikheeva ER, Panchenko V, Katraeva IV, Kovalev DA, Zhuravleva EA, Litti YV. Optimization of Energy Production from Two-Stage Mesophilic–Thermophilic Anaerobic Digestion of Cheese Whey Using a Response Surface Methodology Approach. Energies. 2022; 15(23):8928. https://doi.org/10.3390/en15238928
Chicago/Turabian StyleKovalev, Andrey A., Elza R. Mikheeva, Vladimir Panchenko, Inna V. Katraeva, Dmitriy A. Kovalev, Elena A. Zhuravleva, and Yuriy V. Litti. 2022. "Optimization of Energy Production from Two-Stage Mesophilic–Thermophilic Anaerobic Digestion of Cheese Whey Using a Response Surface Methodology Approach" Energies 15, no. 23: 8928. https://doi.org/10.3390/en15238928
APA StyleKovalev, A. A., Mikheeva, E. R., Panchenko, V., Katraeva, I. V., Kovalev, D. A., Zhuravleva, E. A., & Litti, Y. V. (2022). Optimization of Energy Production from Two-Stage Mesophilic–Thermophilic Anaerobic Digestion of Cheese Whey Using a Response Surface Methodology Approach. Energies, 15(23), 8928. https://doi.org/10.3390/en15238928










