Hierarchical Porous Activated Carbon-Supported Ruthenium Catalysts for Catalytic Cleavage of Lignin Model Compounds
Abstract
1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Characterization
2.3. Lignin Model Compound Conversion
3. Results and Discussion
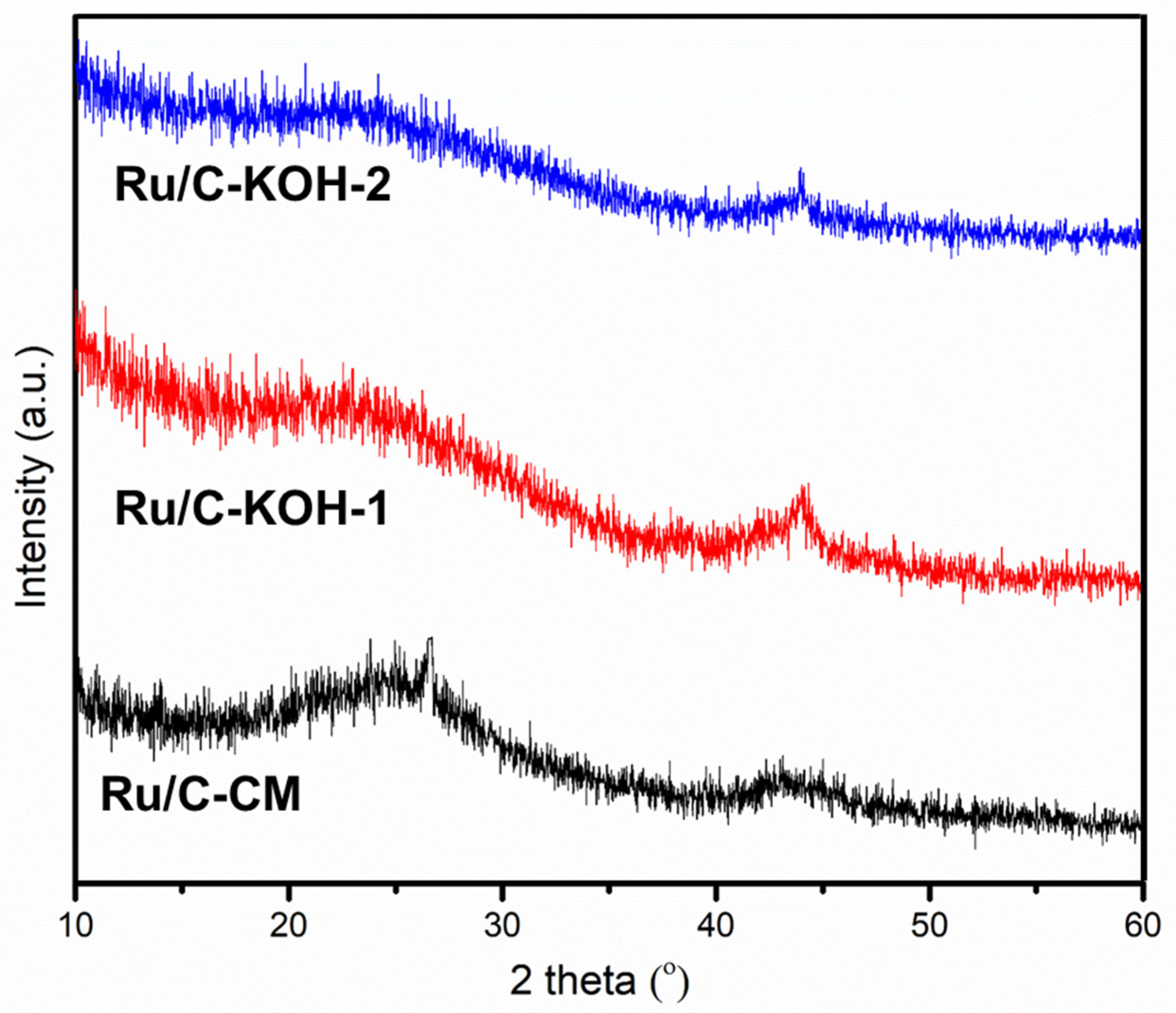
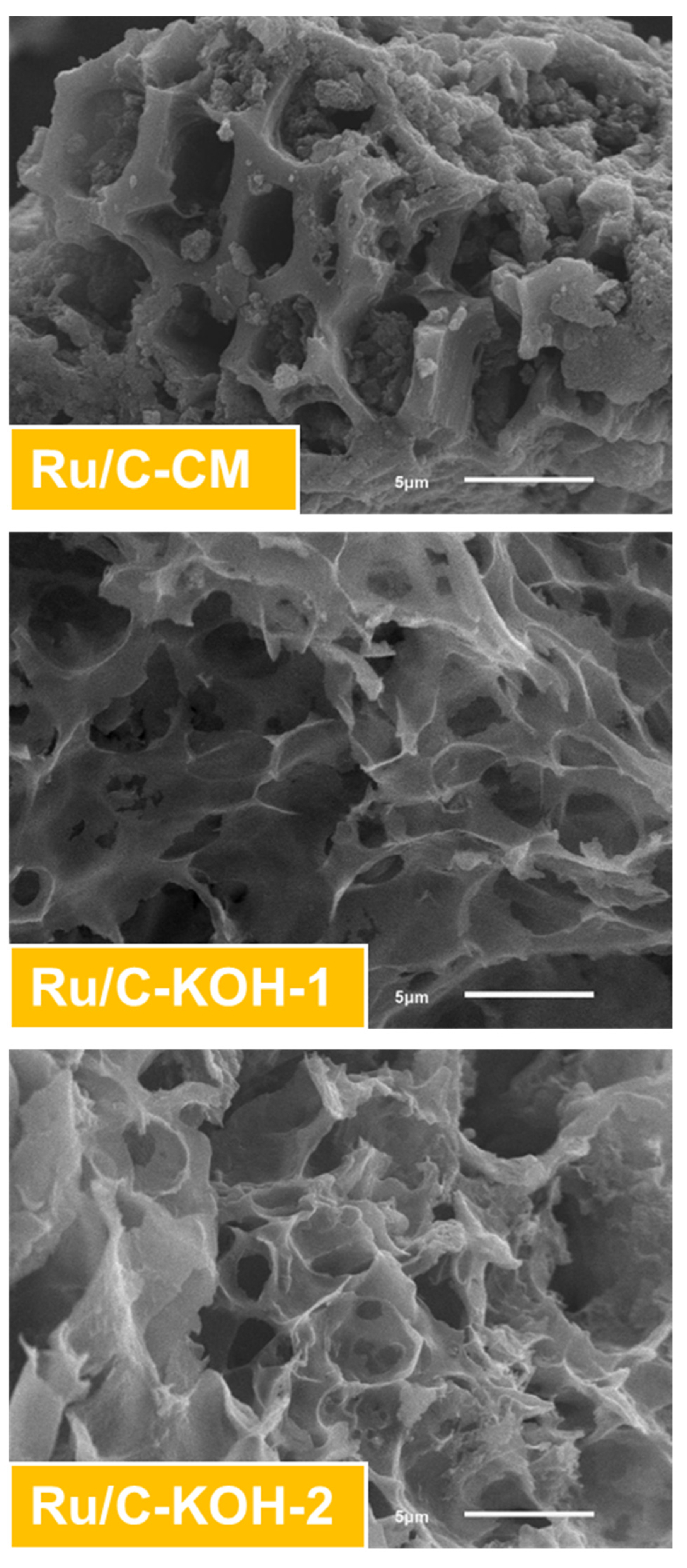
3.1. Characterizations of the Investigated Catalysts
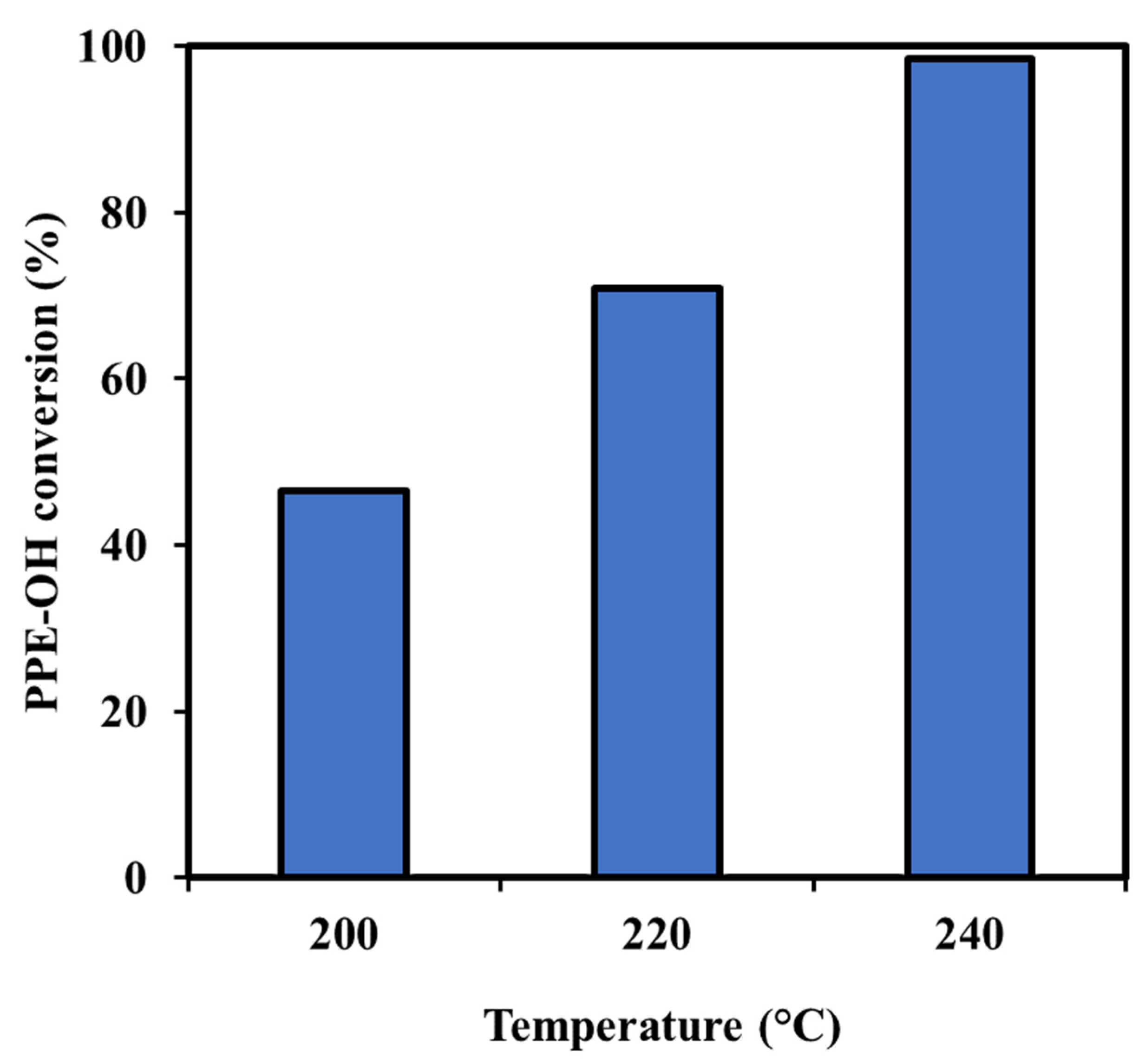
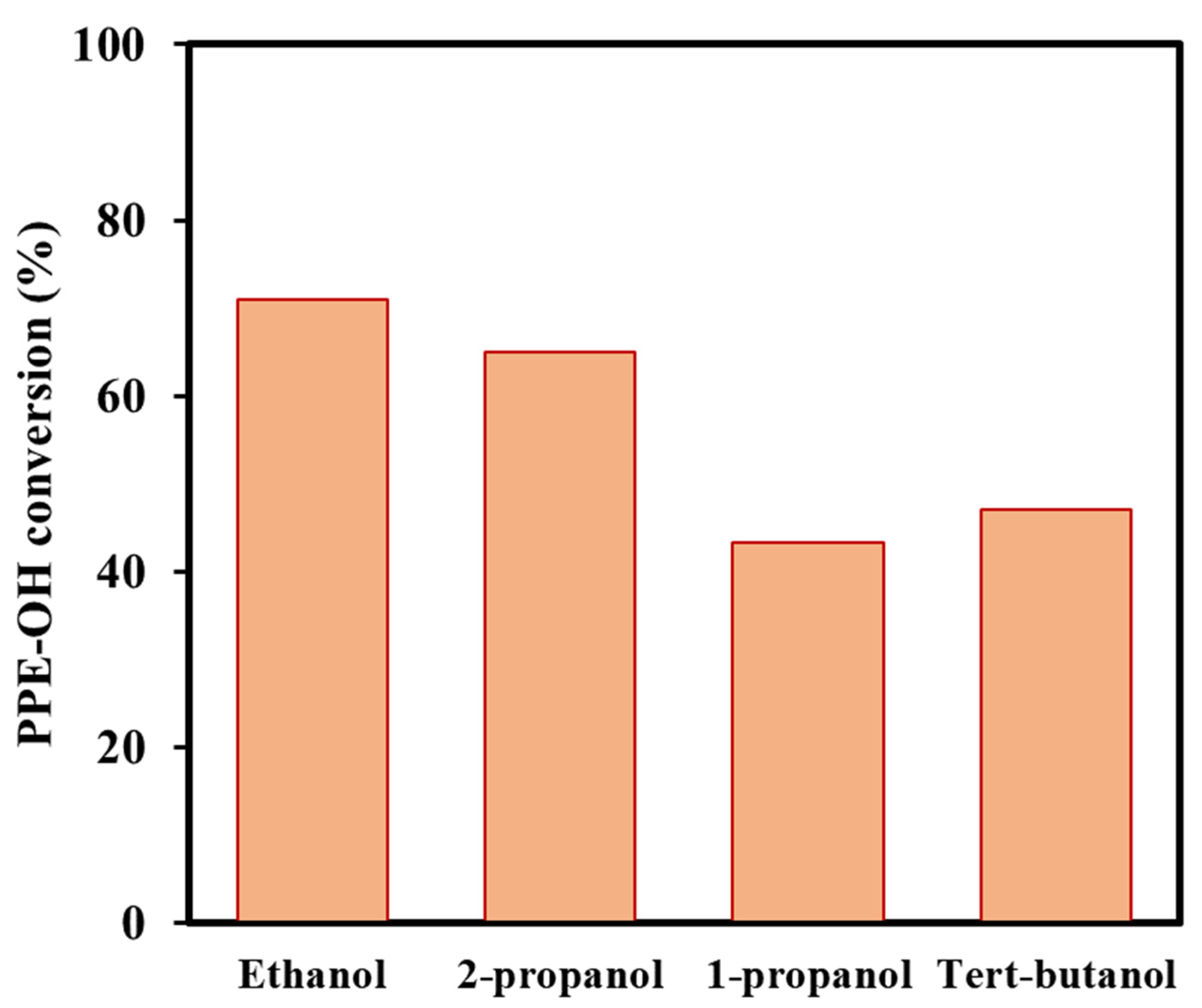
3.2. Conversion of Lignin Model Compounds Using the Investigated Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huong, P.T.; Jitae, K.; Al Tahtamouni, T.M.; Le Minh Tri, N.; Kim, H.-H.; Cho, K.H.; Lee, C. Novel activation of peroxymonosulfate by biochar derived from rice husk toward oxidation of organic contaminants in wastewater. J. Water Process Eng. 2020, 33, 101037. [Google Scholar] [CrossRef]
- Doan, T.T.; Henry-des-Tureaux, T.; Rumpel, C.; Janeau, J.-L.; Jouquet, P. Impact of compost, vermicompost and biochar on soil fertility, maize yield and soil erosion in Northern Vietnam: A three year mesocosm experiment. Sci. Total Environ. 2015, 514, 147–154. [Google Scholar] [CrossRef]
- Thang, P.Q.; Jitae, K.; Giang, B.L.; Viet, N.M.; Huong, P.T. Potential application of chicken manure biochar towards toxic phenol and 2,4-dinitrophenol in wastewaters. J. Environ. Manag. 2019, 251, 109556. [Google Scholar] [CrossRef]
- Mohammadi, A.; Cowie, A.L.; Anh Mai, T.L.; Brandão, M.; Anaya de la Rosa, R.; Kristiansen, P.; Joseph, S. Climate-change and health effects of using rice husk for biochar-compost: Comparing three pyrolysis systems. J. Clean. Prod. 2017, 162, 260–272. [Google Scholar] [CrossRef]
- Cuong, D.V.; Liu, N.-L.; Nguyen, V.A.; Hou, C.-H. Meso/micropore-controlled hierarchical porous carbon derived from activated biochar as a high-performance adsorbent for copper removal. Sci. Total Environ. 2019, 692, 844–853. [Google Scholar] [CrossRef]
- Khataee, A.; Kalderis, D.; Gholami, P.; Fazli, A.; Moschogiannaki, M.; Binas, V.; Lykaki, M.; Konsolakis, M. Cu2O-CuO@biochar composite: Synthesis, characterization and its efficient photocatalytic performance. Appl. Surf. Sci. 2019, 498, 143846. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Feng, H.; Zou, J.; Wang, J.; Feng, C.; Zhu, X.; Ouyang, X.; et al. Hierarchical porous biochar from shrimp shell for persulfate activation: A two-electron transfer path and key impact factors. Appl. Catal. B 2020, 260, 118160. [Google Scholar] [CrossRef]
- Seafood Exports Far Exceed the Target. 2021. Available online: https://thuysanvietnam.com.vn/nam-2021-xuat-khau-thuy-san-vuot-xa-muc-tieu/ (accessed on 18 August 2022).
- Vietnam Shrimp Exports in 2021 “Overcome the Storm” to the Finish Line Smoothly. Available online: https://vasep.com.vn/san-pham-xuat-khau/tom/xuat-nhap-khau/xuat-khau-tom-viet-nam-nam-2021-vuot-bao-ve-dich-suon-se-23692.html (accessed on 18 August 2022).
- Zhao, C.; Huang, Y.; Zhao, C.; Shao, X.; Zhu, Z. Rose-derived 3D carbon nanosheets for high cyclability and extended voltage supercapacitors. Electrochim. Acta 2018, 291, 287–296. [Google Scholar] [CrossRef]
- Wang, H.-m.; Miao, R.-r.; Yang, Y.; Qiao, Y.-h.; Zhang, Q.-f.; Li, C.-s.; Huang, J.-p. Study on the catalytic gasification of alkali lignin over Ru/C nanotubes in supercritical water. J. Fuel Chem. Technol. 2015, 43, 1195–1201. [Google Scholar] [CrossRef]
- Hu, L.; Wei, X.-Y.; Zong, Z.-M. Ru/Hβ catalyst prepared by the deposition-precipitation method for enhancing hydrodeoxygenation ability of guaiacol and lignin-derived bio-oil to produce hydrocarbons. J. Energy Inst. 2021, 97, 48–57. [Google Scholar] [CrossRef]
- Lin, F.; Ma, Y.; Sun, Y.; Zhao, K.; Gao, T.; Zhu, Y. Heterogeneous Ni–Ru/H-ZSM-5 one-pot catalytic conversion of lignin into monophenols. Renew. Energy 2021, 170, 1070–1080. [Google Scholar] [CrossRef]
- Diao, Z.-J.; Huang, L.-Q.; Chen, B.; Gao, T.; Cao, Z.-Z.; Ren, X.-D.; Zhao, S.-J.; Li, S. Amorphous Ni-Ru bimetallic phosphide composites as efficient catalysts for the hydrogenolysis of diphenyl ether and lignin. Fuel 2022, 324, 124489. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Z.; Zhu, X.; Hu, X.; Gholizadeh, M.; Sun, H.; Huang, Y.; Zhang, S.; Zhang, H. Hydrogenolysis of lignin to phenolic monomers over Ru based catalysts with different metal-support interactions: Effect of partial hydrogenation of C(sp2)-O/C. Fuel 2021, 302, 121184. [Google Scholar] [CrossRef]
- Kong, L.; Dai, L.; Wang, Y. Enhancing aromatic hydrocarbon formation via catalytic depolymerization of lignin waste over Ru/WOx/N-C catalyst. Fuel 2023, 332, 126263. [Google Scholar] [CrossRef]
- Verziu, M.; Tirsoaga, A.; Cojocaru, B.; Bucur, C.; Tudora, B.; Richel, A.; Aguedo, M.; Samikannu, A.; Mikkola, J.P. Hydrogenolysis of lignin over Ru-based catalysts: The role of the ruthenium in a lignin fragmentation process. Mol. Catal. 2018, 450, 65–76. [Google Scholar] [CrossRef]
- Bjelić, A.; Grilc, M.; Likozar, B. Catalytic hydrogenation and hydrodeoxygenation of lignin-derived model compound eugenol over Ru/C: Intrinsic microkinetics and transport phenomena. Chem. Eng. J. 2018, 333, 240–259. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, B.; Shu, R.; Zhong, Z.; Tian, Z.; Wang, C.; Chen, Y. Synergistic effect of active metal–acid sites on hydrodeoxygenation of lignin-derived phenolic compounds under mild conditions using Ru/C-HPW catalyst. Fuel 2022, 319, 123617. [Google Scholar] [CrossRef]
- Jiang, W.; Cao, J.-P.; Zhu, C.; Xie, J.-X.; Zhao, L.; Zhang, C.; Zhao, X.-Y.; Zhao, Y.-P.; Bai, H.-C. Selective hydrogenolysis of C-O bonds in lignin and its model compounds over a high-performance Ru/AC catalyst under mild conditions. Chem. Eng. Sci. 2022, 253, 117554. [Google Scholar] [CrossRef]
- Jiang, W.; Cao, J.-P.; Zhao, X.-Y.; Xie, T.; Zhu, C.; Xie, J.-X.; Zhao, L.; Zhao, M.; Zhao, Y.-P.; Zhang, J.-L. Highly selective aromatic ring hydrogenation of lignin-derived compounds over macroporous Ru/Nb2O5 with the lost acidity at room temperature. Fuel 2020, 282, 118869. [Google Scholar] [CrossRef]
- Cao, J.-P.; Xie, T.; Zhao, X.-Y.; Zhu, C.; Jiang, W.; Zhao, M.; Zhao, Y.-P.; Wei, X.-Y. Selective cleavage of ether C-O bond in lignin-derived compounds over Ru system under different H-sources. Fuel 2021, 284, 119027. [Google Scholar] [CrossRef]
- Hossain, M.A.; Phung, T.K.; Rahaman, M.S.; Tulaphol, S.; Jasinski, J.B.; Sathitsuksanoh, N. Catalytic cleavage of the β-O-4 aryl ether bonds of lignin model compounds by Ru/C catalyst. Appl. Catal. A 2019, 582, 117100. [Google Scholar] [CrossRef]
- Bhushan Tewari, B.; Ostwald Thornton, C. Use of basic Methylene Blue Dye for specific surface area measurement of metal hexacyanoferrate (II) complexes. Rev. Soc. Quím. Perú 2010, 76, 330–335. [Google Scholar]
- Ren, S.; Huang, F.; Zheng, J.; Chen, S.; Zhang, H. Ruthenium supported on nitrogen-doped ordered mesoporous carbon as highly active catalyst for NH3 decomposition to H2. Int. J. Hydrogen Energy 2017, 42, 5105–5113. [Google Scholar] [CrossRef]
- Huo, X.; Van Hoomissen, D.J.; Liu, J.; Vyas, S.; Strathmann, T.J. Hydrogenation of aqueous nitrate and nitrite with ruthenium catalysts. Appl. Catal. B 2017, 211, 188–198. [Google Scholar] [CrossRef]
- Li, X.; Guo, T.; Xia, Q.; Liu, X.; Wang, Y. One-Pot Catalytic Transformation of Lignocellulosic Biomass into Alkylcyclohexanes and Polyols. ACS Sustain. Chem. Eng. 2018, 6, 4390–4399. [Google Scholar] [CrossRef]
- Hossain, M.A.; Saelee, T.; Tulaphol, S.; Rahaman, M.S.; Phung, T.K.; Maihom, T.; Praserthdam, P.; Praserthdam, S.; Yelle, D.J.; Sathitsuksanoh, N. Catalytic hydrogenolysis of lignin into phenolics by internal hydrogen over Ru catalyst. ChemCatChem 2022, 14, e202200549. [Google Scholar] [CrossRef]
- Mukundan, S.; Beltramini, J.; Kumar, K.G.; Ravindran, D.S. Surface engineering of carbon supported CoMoS– an effective nanocatalyst for selective deoxygenation of lignin derived phenolics to arenes. Appl. Catal. A 2020, 606, 117811. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Wu, J.; Zhu, L.; Wang, S. Hydrodeoxygenation of lignin-derived phenolics to cycloalkanes over Ni–Co alloy coupled with oxophilic NbOx. Appl. Energy 2022, 328, 120199. [Google Scholar] [CrossRef]
- Wang, X.; Rinaldi, R. Solvent effects on the hydrogenolysis of diphenyl ether with Raney nickel and their implications for the conversion of lignin. ChemSusChem 2012, 5, 1455–1466. [Google Scholar] [CrossRef]

| Notation | % Ru | Source | Specific Surface Area (m2/g) | Preparation Method |
|---|---|---|---|---|
| Ru/C-CM | 5 | Alfa Aesar | ~597 | Commercial (reduced), used as received |
| Ru/C-KOH-1 | 5 | Homemade | ~1530 | 5% Ru on C-KOH-1, reduced at 350 °C for 5 h |
| Ru/C-KOH-2 | 5 | Homemade | ~1749 | 5% Ru on C-KOH-2, reduced at 350 °C for 5 h |
| Entry | Catalyst | Solvent | Temp. (°C) | Conversion (%) | 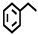 |  | 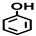 |  |  | 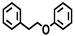 | 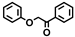 |  | Others |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ru/C-CM | Ethanol | 240 | 50.3 | 12.1 | 1.0 | 18.1 | 3.6 | - | 50.0 | 1.2 | - | 14.0 |
| 2 | Ru/C-KOH-1 | Ethanol | 240 | 89.2 | 18.7 | 6.3 | 19.3 | 1.7 | 1.3 | 48.2 | 1.2 | 2.4 | 0.9 |
| 3 | Ru/C-KOH-2 | Ethanol | 200 | 46.6 | 4.3 | 3.0 | 9.4 | 0.9 | 0.6 | 72.1 | 4.8 | 3.6 | 1.3 |
| 4 | Ru/C-KOH-2 | Ethanol | 220 | 70.9 | 4.4 | 8.2 | 16.4 | 0.7 | 2.6 | 52.2 | 6.4 | 6.2 | 2.9 |
| 5 | Ru/C-KOH-2 | Ethanol | 240 | 98.5 | 20.1 | 1.1 | 19.5 | 2.1 | - | 57.2 | - | - | - |
| 6 | Ru/C-KOH-2 | 1-propanol | 220 | 43.4 | 6.5 | 1.6 | 11.1 | 1.7 | - | 67.1 | 1.8 | 5.0 | 5.2 |
| 7 | Ru/C-KOH-2 | 2-propanol | 220 | 64.9 | 13.0 | 2.2 | 17.7 | 1.3 | 0.7 | 57.0 | 3.3 | 3.2 | 1.6 |
| 8 | Ru/C-KOH-2 | Tert-butanol | 220 | 47.1 | 0.8 | 6.9 | 10.2 | 0.7 | - | 14.2 | 13.3 | 14.4 | 39.5 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, X.-T.; Tran, V.A.; Tran, L.-T.T.; Nguyen, T.N.P.; Le, T.H.; Hoang, H.; Nguyen, T.-H.; Vu, K.B.; Phung, T.K. Hierarchical Porous Activated Carbon-Supported Ruthenium Catalysts for Catalytic Cleavage of Lignin Model Compounds. Energies 2022, 15, 8611. https://doi.org/10.3390/en15228611
Pham X-T, Tran VA, Tran L-TT, Nguyen TNP, Le TH, Hoang H, Nguyen T-H, Vu KB, Phung TK. Hierarchical Porous Activated Carbon-Supported Ruthenium Catalysts for Catalytic Cleavage of Lignin Model Compounds. Energies. 2022; 15(22):8611. https://doi.org/10.3390/en15228611
Chicago/Turabian StylePham, Xuan-Tien, Vy Anh Tran, Lan-Trinh Thi Tran, Tram Ngoc P. Nguyen, Thong Hoang Le, Huy Hoang, Thi-Hiep Nguyen, Khanh B. Vu, and Thanh Khoa Phung. 2022. "Hierarchical Porous Activated Carbon-Supported Ruthenium Catalysts for Catalytic Cleavage of Lignin Model Compounds" Energies 15, no. 22: 8611. https://doi.org/10.3390/en15228611
APA StylePham, X.-T., Tran, V. A., Tran, L.-T. T., Nguyen, T. N. P., Le, T. H., Hoang, H., Nguyen, T.-H., Vu, K. B., & Phung, T. K. (2022). Hierarchical Porous Activated Carbon-Supported Ruthenium Catalysts for Catalytic Cleavage of Lignin Model Compounds. Energies, 15(22), 8611. https://doi.org/10.3390/en15228611







