Thermal Energy Storage in Concentrating Solar Power Plants: A Review of European and North American R&D Projects
Abstract
1. Introduction
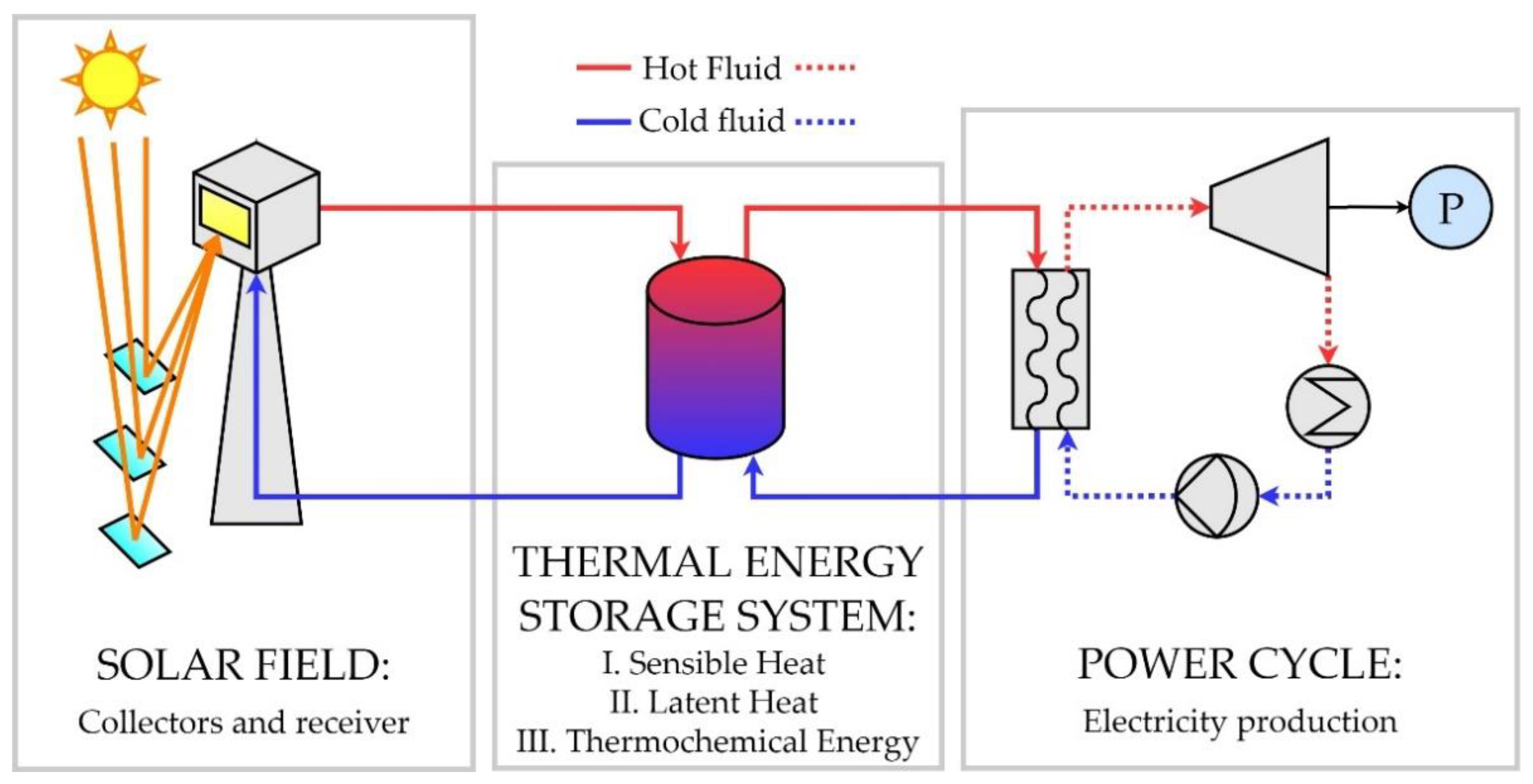
2. Potential Integration of TES in CSP Plants
2.1. CSP Technologies
2.2. TES Technologies/Systems
2.2.1. Sensible Heat Storage (SHS)
2.2.2. Latent Heat Storage (LHS)
2.2.3. Thermochemical Energy Storage (TCES)
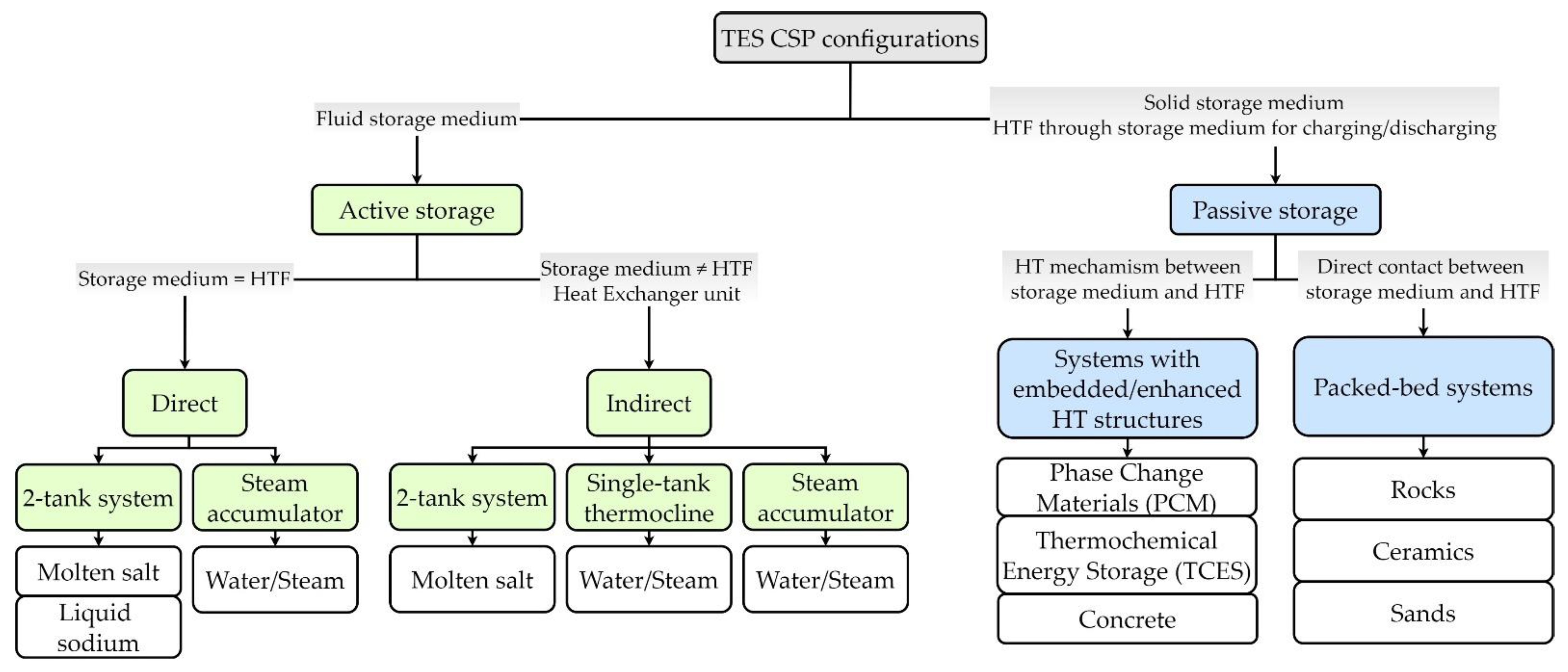
2.3. TES CSP Integrated Configurations
- The heat storage capacity, which defines the thermal energy which can be stored in the system for a given process, medium and size of the storage system. The larger energy density of the storage medium (MJ/m3), the smaller storage volume required.
- The storage/discharge rates related to the speed and time elapsed in each charge or discharge process.
- The period of time during which energy can be stored. It will depend on the storage medium, from hours to months.
- The chemical compatibility of the storage medium with the CSP plant. The storage medium must be mechanically and chemically stable, minimizing its degradation after each charge/discharge cycle.
- The energy storage efficiency which relates the energy retrieved from the storage medium and the energy required in the storage process, accounting for the energy losses between each charge/discharge cycle. Thus, excellent heat transfer must occur between the HTF and the storage medium to improve the energy efficiency above 95%. Besides, there must be high chemical compatibility between HTF, heat exchanger and storage medium, with minimum thermal losses.
- The compatibility with the power block associated to the CSP plant. The higher operating temperature of the storage medium, the greater overall efficiency of the CSP plant. Up until now, the Rankine power cycles have been the most widespread in CSP plants using molten salts as the storage medium. However, novel storage materials currently under development withstand operating temperatures above 700 °C and can improve the efficiency of the CSP system by coupling to Brayton cycles.
- The cost of the storage medium including capital and operation and maintenance costs. The longer lifetime and the lower cost of a storage medium, the better the economic and commercial feasibility.
- The storage medium must be safe and environmentally-friendly, considering its lifetime.
3. European and North American TES CSP R&D Projects Review
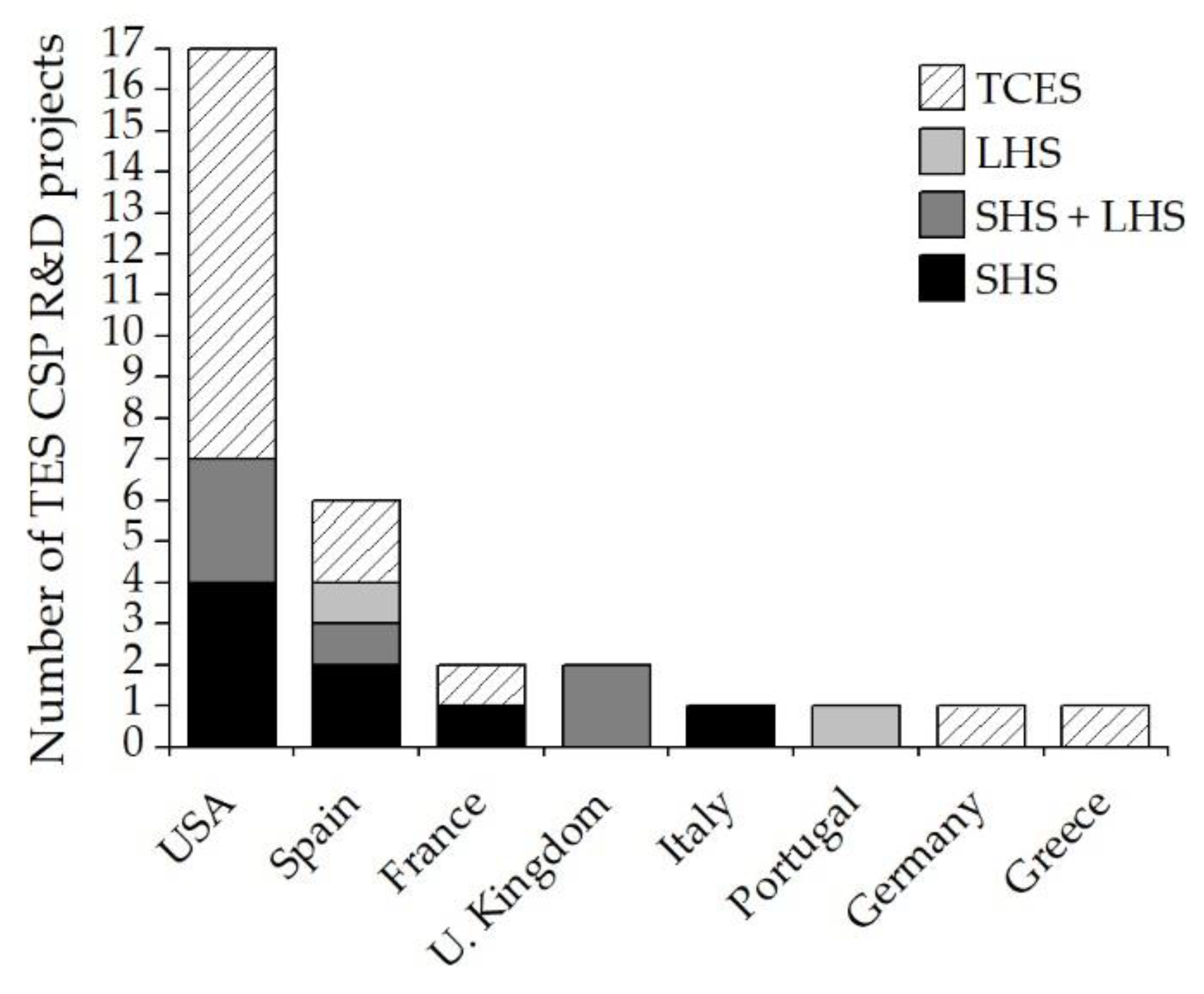
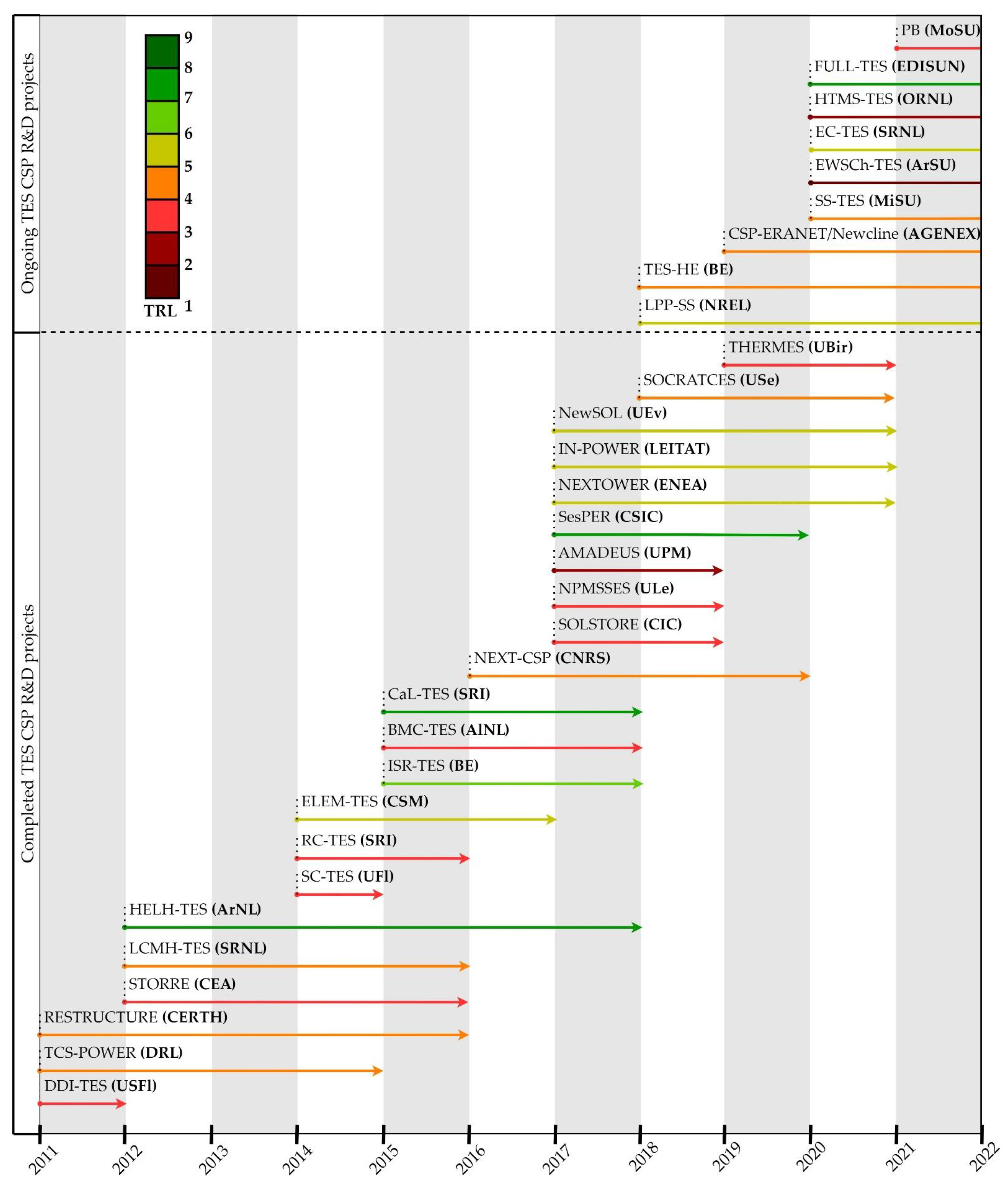
3.1. Summary of R&D Projects (2011–2022): Timeline and TRL
3.2. TES CSP R&D Projects Completed between 2011–2022
3.2.1. TCS-Power
3.2.2. RESTRUCTURE
3.2.3. STORRE
3.2.4. CaL-TES
3.2.5. NEXT-CSP
3.2.6. NEXTOWER
3.2.7. IN-POWER
3.2.8. NewSOL
3.2.9. SOCRATCES
3.2.10. Other TES CSP R&D Projects (Materials, Concepts, Technology)
3.3. Ongoing TES CSP R&D Projects Lauched between 2011–2022
4. Discussion and Conclusions
5. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbols | |
| a | fraction reacted, - |
| c | specific heat, J/kg∙K |
| f | melt fraction, - |
| m | mass of storage medium, kg |
| Q | heat, J |
| T | temperature, °C |
| ∆H | heat of reaction, J/kg |
| ∆q | latent heat of fusion, J/kg |
| Subscripts and superscripts | |
| f | final |
| I | initial |
| m | melting |
| max | maximum |
| out | outlet |
| p | constant pressure |
| pl | liquid phase |
| ps | solid phase |
| r | reaction |
| s | storage/stored |
| Acronyms and abbreviations | |
| CC | Combined Cycle |
| CORDIS | Community Research and Development Information Service |
| CSP | Concentrating Solar Power |
| GHG | Greenhouse Gases |
| HT | Heat Transfer |
| HTF | Heat Transfer Fluid |
| IPCC | Intergovernmental Panel on Climate Change |
| LCOE | Levelized Cost Of Electricity |
| LFR | Linear Fresnel Reflectors |
| LHS | Latent Heat Storage |
| PDC | Parabolic Dish Collectors |
| PMCs | Phase Change Materials |
| PTC | Parabolic Trough Collectors |
| RES | Renewable Energy Sources |
| R&D | Research and Development |
| sCO2 | Supercritical CO2 |
| SETO | Solar Energy Technologies Office |
| SHS | Sensible Heat Storage |
| SPT | Solar Power Towers |
| TCES | Thermochemical Energy Storage |
| TES | Thermal Energy Storage |
| TRL | Technology Readiness Level |
| USA | United States of America |
Appendix A. List of R&D Projects, Co-Ordinators and Abbreviations
| Abbreviation | Project Name |
|---|---|
| AMADEUS | Next GenerAtion MateriAls and Solid State DevicEs for Ultra High Temperature Energy Storage and Conversion |
| BMC-TES | Binary Metal Chalcogenides for High Temperature Thermal Storage |
| CaL-TES | Demonstration of High-Temperature Calcium-Based Thermochemical Energy Storage System for Use with Concentrating Solar Power Facilities |
| CSP-ERANET (Newcline) | Advanced thermocline concepts for thermal energy storage for CSP |
| DDI-TES | Development and Demonstration of an Innovative Thermal Energy Storage System for Baseload Power Generation |
| EC-TES | Eutectic Carbonates for Low Cost-Efficient Thermochemical Heat Storage System |
| ELEM-TES | Efficiently Leveraging Equilibrium Mechanisms for Engineering New Thermochemical Storage |
| EWSCh-TES | Economic Weekly and Seasonal Thermochemical and Chemical Energy Storage for Advanced Power Cycles |
| FULL-TES | Development, Build and Operation of a Full-Scale, Nominally 5MWe, Supercritical Carbon Dioxide Power Cycle Coupled with Solid Media Energy Storage |
| HELH-TES | High Efficiency Latent Heat Based Thermal Energy Storage System Compatible with Supercritical Carbon Dioxide Power Cycle |
| HTMS-TES | Simplified High-Temperature Molten Salt Concentrating Solar Power Plant Preconceptual Design |
| IN-POWER | Advanced Materials technologies to QUADRUPLE the Concentrated Solar Thermal current POWER GENERATION |
| ISR-TES | Integrated Solar Receiver with Thermal Storage for an sCO2 Power Cycle |
| LMMH-TES | Low-Cost Metal Hydride Thermal Energy Storage System for Concentrating Solar-Thermal Power Systems |
| LPP-SS | Liquid-Phase Pathway to SunShot |
| NewSOL | New StOrage Latent and sensible concept for high efficient CSP Plants |
| NEXT-CSP | High Temparature concentrated solar thermal power plan with particle receiver and direct thermal storage |
| NEXTOWER | Advanced materials solutions for next generation high efficiency concentrated solar power (CSP) tower systems |
| NPMSSES | Nanoparticle Enhanced Molten Salts for Solar Energy Storage |
| PB | Efficient Thermal Energy Storage with Radial Flow in Packed Beds |
| RC-TES | Regenerative Carbonate-Based Thermochemical Energy Storage System for Concentrating Solar Power |
| RESTRUCTURE | Redox Materials-based Structured Reactors/Heat Exchangers for Thermo-Chemical Heat Storage Systems in Concentrated Solar Power Plants |
| SC-TES | Carbon Dioxide Shuttling Thermochemical Storage Using Strontium Carbonate |
| SesPER | Solar Energy Storage PERovskites |
| SOCRATCES | SOlar Calcium-looping integRAtion for Thermo-Chemical Energy Storage |
| SOLSTORE | Solid-state reactions for thermal energy storage |
| SS-TES | Solid State Solar Thermochemical Fuel for Long-Duration Storage |
| STORRE | High temperature thermal energy Storage by Reversible thermochemical Reaction |
| TCS-Power | Thermochemical Energy Storage for Concentrated Solar Power Plants |
| TES-HE | Integrated Thermal Energy Storage Heat Exchanger for Concentrating Solar Power Applications |
| THERMES | A new generation high temperature phase change microemulsion for latent thermal energy storage in dual loop solar field |
| Abbreviation | Project Coordinator |
|---|---|
| AGENEX | Agencia Extremeña de la Energía |
| AlNL | Los Alamos National Laboratory |
| ArNL | Argonne National Laboratory |
| ArSU | Arizona State University |
| BE | Brayton Energy |
| CEA | Commissariat a l’Energie Atomique et aux Energies Alternatives |
| CERTH | Ethniko Kentro Erevnas kai Technologikis Anaptyxis |
| CIC | Centro de Investigación Cooperativa de Energías Alternativas |
| CNRS | Centre National de la Recherche Scientifique |
| CSIC | Agencia Estatal Consejo Superior de Investigaciones Científicas |
| CSM | Colorado School of Mines |
| DRL | Deutsches Zentrum für Luft-und Raumfahrt e.V. |
| EDISUN | Edisun |
| ENEA | Italian National Agency for New Technologies, Energy and Sustainable Economic Development |
| LEITAT | Acondicionamiento Tarrasense Asociación |
| MiSU | Michigan State University |
| MoSU | Montana State University |
| NREL | National Renewable Energy Laboratory |
| ORNL | Oak Ridge National Laboratory |
| SRI | Southern Research Institute |
| SRNL | Savannah River National Laboratory |
| UBir | University of Birmingham |
| UEv | Universidade de Évora |
| UFl | University of Florida |
| ULe | Univertisty of Leeds |
| UPM | Universidad Politécnica de Madrid |
| USe | Universidad de Sevilla |
| USFl | University of South Florida |
References
- IPCC. Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Shukla, P.R., Skea, J., Slade, R., Al Khourdajie, A., van Diemen, R., McCollum, D., Pathak, M., Some, S., Vyas, P., Fradera, R., et al., Eds.; Cambridge University Press: Cambridge UK; New York, NY, USA, 2022. [Google Scholar]
- Renewables 2022. Global Status Report; Zervos, A., Adib, R., Eds.; REN21 Secretariat: Paris, France, 2022; ISBN 978-3-948393-04-5. [Google Scholar]
- Morante, J.R. El Almacenamiento de Electricidad—Fundación Gas Natural Fenosa; Fundación Gas Natural Fenosa, Ed.; Fundación Gas Natural Fenosa: Barcelona, Spain, 2014; ISBN 9788469598979. [Google Scholar]
- Peón Menendez, R. Optimización del Control del Sistema de Almacenamiento Térmico en Centrales Solares Termoeléctricas; Universidad de Oviedo: Oviedo, Spain, 2012. [Google Scholar]
- Kunwer, R.; Pandey, S.; Pandey, G. Technical Challenges and Their Solutions for Integration of Sensible Thermal Energy Storage with Concentrated Solar Power Applications—A Review. Process Integr. Optim. Sustain. 2022, 6, 559–585. [Google Scholar] [CrossRef]
- International Renewable Energy Agency (IRENA). Innovation Outlook: Thermal Energy Storage; International Renewable Energy Agency (IRENA): Abu Dhabi, United Arab Emirates, 2020; ISBN 978-92-9260-279-6. [Google Scholar]
- Goyal, N.; Aggarwal, A.; Kumar, A. Concentrated solar power plants: A critical review of regional dynamics and operational parameters. Energy Res. Soc. Sci. 2022, 83, 102331. [Google Scholar] [CrossRef]
- Kraemer, S. CSP Doesn’t Compete with PV—It Competes with Gas. Available online: Solarpaces.org/csp-competes-with-natural-gas-not-pv/ (accessed on 1 July 2022).
- Cabeza, L.F.; de Gracia, A.; Zsembinszki, G.; Borri, E. Perspectives on thermal energy storage research. Energy 2021, 231, 120943. [Google Scholar] [CrossRef]
- Islam, T.; Huda, N.; Abdullah, A.B.; Saidur, R. A comprehensive review of state-of-the-art concentrating solar power (CSP) technologies: Current status and research trends. Renew. Sustain. Energy Rev. 2018, 91, 987–1018. [Google Scholar] [CrossRef]
- Achkari, O.; El Fadar, A. Latest developments on TES and CSP technologies—Energy and environmental issues, applications and research trends. Appl. Therm. Eng. 2020, 167, 114806. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Alim, M.A.; Alam, T.; Mofijur, M.; Ahmed, S.F.; Perkins, G. A critical review on the development and challenges of concentrated solar power technologies. Sustain. Energy Technol. Assess. 2021, 47, 101434. [Google Scholar] [CrossRef]
- Calderón, A.; Barreneche, C.; Prieto, C.; Segarra, M.; Fernández, A.I. Concentrating Solar Power Technologies: A Bibliometric Study of Past, Present and Future Trends in Concentrating Solar Power Research. Front. Mech. Eng. 2021, 7, 682592. [Google Scholar] [CrossRef]
- Palacios, A.; Barreneche, C.; Navarro, M.E.; Ding, Y. Thermal energy storage technologies for concentrated solar power—A review from a materials perspective. Renew. Energy 2020, 156, 1244–1265. [Google Scholar] [CrossRef]
- Alnaimat, F.; Rashid, Y. Thermal energy storage in solar power plants: A review of the materials, associated limitations, and proposed solutions. Energies 2019, 12, 4164. [Google Scholar] [CrossRef]
- Calderón, A.; Palacios, A.; Barreneche, C.; Segarra, M.; Prieto, C.; Rodriguez-Sanchez, A.; Fernández, A.I. High temperature systems using solid particles as TES and HTF material: A review. Appl. Energy 2018, 213, 100–111. [Google Scholar] [CrossRef]
- El Alami, K.; Asbik, M.; Agalit, H. Identification of natural rocks as storage materials in thermal energy storage (TES) system of concentrated solar power (CSP) plants—A review. Sol. Energy Mater. Sol. Cells 2020, 217, 110599. [Google Scholar] [CrossRef]
- Opolot, M.; Zhao, C.; Liu, M.; Mancin, S.; Bruno, F. A review of high temperature (≥ 500 °C) latent heat thermal energy storage. Renew. Sustain. Energy Rev. 2022, 160, 112293. [Google Scholar] [CrossRef]
- Liu, M.; Omaraa, E.S.; Qi, J.; Haseli, P.; Ibrahim, J.; Sergeev, D.; Müller, M.; Bruno, F.; Majewski, P. Review and characterisation of high-temperature phase change material candidates between 500 C and 700 °C. Renew. Sustain. Energy Rev. 2021, 150, 111528. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Recent Advances in Thermochemical Energy Storage via Solid–Gas Reversible Reactions at High Temperature. Energies 2020, 13, 5859. [Google Scholar] [CrossRef]
- Carrillo, A.J.; González-Aguilar, J.; Romero, M.; Coronado, J.M. Solar Energy on Demand: A Review on High Temperature Thermochemical Heat Storage Systems and Materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef] [PubMed]
- Zsembinszki, G.; Solé, A.; Barreneche, C.; Prieto, C.; Fernández, A.; Cabeza, L. Review of Reactors with Potential Use in Thermochemical Energy Storage in Concentrated Solar Power Plants. Energies 2018, 11, 2358. [Google Scholar] [CrossRef]
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Pelay, U.; Luo, L.; Fan, Y.; Stitou, D.; Rood, M. Thermal energy storage systems for concentrated solar power plants. Renew. Sustain. Energy Rev. 2017, 79, 82–100. [Google Scholar] [CrossRef]
- Concentrating Solar Power Projects. National Renewable Energy Laboratory. Available online: https://solarpaces.nrel.gov/projects (accessed on 1 July 2022).
- CORDIS. EU Research Results. European Commission. Available online: https://cordis.europa.eu/projects/en (accessed on 15 July 2022).
- Solar Energy Research Database. Solar Energy Technologies Office. U.S. Department of Energy. Available online: https://www.energy.gov/eere/solar/solar-energy-research-database (accessed on 15 July 2022).
- Teske, S.; Leung, J. Solar Thermal Electricity—Global Outlook 2016; Rochon, E., Ed.; Greenpeace International: Amsterdam, The Netherlands; European Solar Thermal Electricity Association (ESTELA): Bruxelles, Belgium; SolarPACES Secretariate: Almeria, Spain, 2016. [Google Scholar]
- Pavlovic, T.; Radonjic, I. A review of concentrating solar power plants in the world and their potential use in Serbia. Renew. Sustain. Energy Rev. 2012, 16, 12. [Google Scholar] [CrossRef]
- Chacartegui, R.; Alovisio, A.; Ortiz, C.; Valverde, J.M.; Verda, V.; Becerra, J.A. Thermochemical energy storage of concentrated solar power by integration of the calcium looping process and a CO2 power cycle. Appl. Energy 2016, 173, 589–605. [Google Scholar] [CrossRef]
- Ortiz, C.; Chacartegui, R.; Valverde, J.M.; Alovisio, A.; Becerra, J.A. Power cycles integration in concentrated solar power plants with energy storage based on calcium looping. Energy Convers. Manag. 2017, 149, 815–829. [Google Scholar] [CrossRef]
- Tesio, U.; Guelpa, E.; Verda, V. Integration of thermochemical energy storage in concentrated solar power. Part 2: Comprehensive optimization of supercritical CO2 power block. Energy Convers. Manag. 2020, 6, 100038. [Google Scholar] [CrossRef]
- Arias, I.; Cardemil, J.; Zarza, E.; Valenzuela, L.; Escobar, R. Latest developments, assessments and research trends for next generation of concentrated solar power plants using liquid heat transfer fluids. Renew. Sustain. Energy Rev. 2022, 168, 112844. [Google Scholar] [CrossRef]
- Barlev, D.; Vidu, R.; Stroeve, P. Innovation in concentrated solar power. Sol. Energy Mater. Sol. Cells 2011, 95, 2703–2725. [Google Scholar] [CrossRef]
- Wagner, S.J.; Rubin, E.S. Economic implications of thermal energy storage for concentrated solar thermal power. Renew. Energy 2014, 61, 81–95. [Google Scholar] [CrossRef]
- Kuravi, S.; Trahan, J.; Goswami, D.Y.; Rahman, M.M.; Stefanakos, E.K. Thermal energy storage technologies and systems for concentrating solar power plants. Prog. Energy Combust. Sci. 2013, 39, 285–319. [Google Scholar] [CrossRef]
- Dizaji, H.B.; Hosseini, H. A review of material screening in pure and mixed-metal oxide thermochemical energy storage (TCES) systems for concentrated solar power (CSP) applications. Renew. Sustain. Energy Rev. 2018, 98, 9–26. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Bolivar, F.J.; Isaza-ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Liu, C.; Rao, Z. Challenges in various thermal energy storage technologies. Sci. Bull. 2017, 62, 231–233. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Qi, C.; Ling, X.; Peng, H. State of the art on the high-temperature thermochemical energy storage systems. Energy Convers. Manag. 2018, 177, 792–815. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, S.K. A Review on Thermal Energy Storage Unit for Solar Thermal Power Plant Application. Energy Procedia 2015, 74, 462–469. [Google Scholar] [CrossRef]
- Khare, S.; Dell’Amico, M.; Knight, C.; McGarry, S. Selection of materials for high temperature sensible energy storage. Sol. Energy Mater. Sol. Cells 2013, 115, 114–122. [Google Scholar] [CrossRef]
- Tiddens, A.; Röger, M.; Stadler, H.; Hoffschmidt, B. Air return ratio measurements at the solar tower Jülich using a tracer gas method. Sol. Energy 2017, 146, 351–358. [Google Scholar] [CrossRef]
- Gil, A.; Medrano, M.; Martorell, I.; Lázaro, A.; Dolado, P.; Zalba, B.; Cabeza, L.F. State of the art on high temperature thermal energy storage for power generation. Part 1-Concepts, materials and modellization. Renew. Sustain. Energy Rev. 2010, 14, 31–55. [Google Scholar] [CrossRef]
- Aggarwal, A.; Goyal, N.; Kumar, A. Thermal characteristics of sensible heat storage materials applicable for concentrated solar power systems. Mater. Today Proc. 2021, 47, 5812–5817. [Google Scholar] [CrossRef]
- El Alami, K.; Asbik, M.; Boualou, R.; Ouchani, F.-Z.; Agalit, H.; Bennouna, E.G.; Rachidi, S. A critical overview of the suitability of natural Moroccan rocks for high temperature thermal energy storage applications: Towards an effective dispatching of concentrated solar power plants. J. Energy Storage 2022, 50, 104295. [Google Scholar] [CrossRef]
- Gutierrez, A.; Miró, L.; Gil, A.; Rodríguez-Aseguinolaza, J.; Barreneche, C.; Calvet, N.; Py, X.; Inés Fernández, A.; Grágeda, M.; Ushak, S.; et al. Advances in the valorization of waste and by-product materials as thermal energy storage (TES) materials. Renew. Sustain. Energy Rev. 2016, 59, 763–783. [Google Scholar] [CrossRef]
- Ortega-Fernández, I.; Calvet, N.; Gil, A.; Rodríguez-Aseguinolaza, J.; Faik, A.; D’Aguanno, B. Thermophysical characterization of a by-product from the steel industry to be used as a sustainable and low-cost thermal energy storage material. Energy 2015, 89, 601–609. [Google Scholar] [CrossRef]
- Al-Azawii, M.M.S.; Alhamdi, S.F.H.; Braun, S.; Hoffmann, J.F.; Calvet, N.; Anderson, R. Experimental study on packed-bed thermal energy storage using recycled ceramic as filler materials. J. Energy Storage 2021, 44, 103375. [Google Scholar] [CrossRef]
- Bradshaw, R.W.; Siegel, N.P. Molten nitrate salt development for thermal energy storage in parabolic trough solar power systems. In Proceedings of the ASME 2008 2nd International Conference on Energy Sustainability, Jacksonville, FL, USA, 10–14 August 2008; Volume 2, pp. 631–637. [Google Scholar]
- Peng, Q.; Wei, X.; Ding, J.; Yang, J.; Yang, X. High-temperature thermal stability of molten salt materials. Int. J. Energy Res. 2008, 32, 1164–1174. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Wu, Z.G. Thermal property characterization of a low melting-temperature ternary nitrate salt mixture for thermal energy storage systems. Sol. Energy Mater. Sol. Cells 2011, 95, 3341–3346. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Arvay, A.; Hsu, K.; Kannan, A.M. Heat transfer fluids for concentrating solar power systems—A review. Appl. Energy 2015, 146, 383–396. [Google Scholar] [CrossRef]
- Caraballo, A.; Galán-Casado, S.; Caballero, Á.; Serena, S. Molten salts for sensible thermal energy storage: A review and an energy performance analysis. Energies 2021, 14, 1197. [Google Scholar] [CrossRef]
- Ding, W.; Bauer, T. Progress in Research and Development of Molten Chloride Salt Technology for Next Generation Concentrated Solar Power Plants. Engineering 2021, 7, 334–347. [Google Scholar] [CrossRef]
- Aljaerani, H.A.; Samykano, M.; Saidur, R.; Pandey, A.K.; Kadirgama, K. Nanoparticles as molten salts thermophysical properties enhancer for concentrated solar power: A critical review. J. Energy Storage 2021, 44, 103280. [Google Scholar] [CrossRef]
- Pistocchini, L.; Size, P.M.; Plant, P.; Motta, M.; Lambruschini, V. Feasibility Study of an Innovative Dry-Cooling System With Phase-Change Material Storage for Concentrated Solar. Sol. Energy Eng. 2011, 133, 031010. [Google Scholar] [CrossRef]
- González-Roubaud, E.; Pérez-Osorio, D.; Prieto, C. Review of commercial thermal energy storage in concentrated solar power plants: Steam vs. molten salts. Renew. Sustain. Energy Rev. 2017, 80, 133–148. [Google Scholar] [CrossRef]
- CMI Solar. Khi Solar One South Africa 50 MW CMI ’s First Thermo Solar Receiver; CMI-Abengoa Solar, Ed.; CMI Solar: Seraing, Belgium, 2016. [Google Scholar]
- Serge, E.; Edem, K.; Tsoukpoe, N.; Ouédraogo, I.W.K.; Coulibaly, Y.; Py, X.; Marie, F.; Ouédraogo, A.W. Energy for Sustainable Development Jatropha curcas crude oil as heat transfer fl uid or thermal energy storage material for concentrating solar power plants. Energy Sustain. Dev. 2017, 40, 59–67. [Google Scholar] [CrossRef]
- Molina, S.; Haillot, D.; Deydier, A.; Bedecarrats, J. Material screening and compatibility for thermocline storage systems using thermal oil. Appl. Therm. Eng. 2018, 146, 252–259. [Google Scholar] [CrossRef]
- Bruch, A.; Molina, S.; Esence, T.; Couturier, R.; Molina, S.; Esence, T.; Couturier, R. Experimental investigation of cycling behaviour of pilot-scale thermal oil packed-bed thermal storage system. Renew. Energy 2016, 103, 277–285. [Google Scholar] [CrossRef]
- EASE/EERA. European Energy Storage Technology Development Roadmap towards 2030. 2017. Available online: https://eera-es.eu/wp-content/uploads/2016/03/EASE-EERA-Storage-Technology-Development-Roadmap-2017-HR.pdf (accessed on 11 September 2022).
- Jodeiri, A.M.; Orozco, C. Thermal Energy Storage in CSP Technologies: From Commercialized to Innovative Solutions; Technical Report; French National Centre for Scientific Research: Paris, France, 2018. [Google Scholar] [CrossRef]
- Tofani, K.; Tiari, S. Nano-enhanced phase change materials in latent heat thermal energy storage systems: A review. Energies 2021, 14, 3821. [Google Scholar] [CrossRef]
- Kasaeian, A.; Pourfayaz, F.; Khodabandeh, E. Experimental studies on the applications of PCMs and nano-PCMs in buildings: A critical review. Energy Build. 2017, 154, 96–112. [Google Scholar] [CrossRef]
- Xu, B.; Li, P.; Chan, C. Application of phase change materials for thermal energy storage in concentrated solar thermal power plants: A review to recent developments. Appl. Energy 2015, 160, 286–307. [Google Scholar] [CrossRef]
- Konuklu, Y.; Şahan, N.; Paksoy, H. 2.14 Latent Heat Storage Systems. In Comprehensive Energy Systems; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 396–434. ISBN 9780128095973. [Google Scholar]
- Reddy, K.S.; Mudgal, V.; Mallick, T.K. Review of latent heat thermal energy storage for improved material stability and effective load management. J. Energy Storage 2018, 15, 205–227. [Google Scholar] [CrossRef]
- Crespo, A.; Barreneche, C.; Ibarra, M.; Platzer, W. Latent thermal energy storage for solar process heat applications at medium- high temperatures—A review. Sol. Energy 2018, 192, 3–34. [Google Scholar] [CrossRef]
- Sharma, S.D. Latent heat storage materials and systems: A review. Int. J. Green Energy 2005, 2, 1–56. [Google Scholar] [CrossRef]
- Saha, S.; Ruslan, A.R.M.; Monjur Morshed, A.K.M.; Hasanuzzaman, M. Global prospects and challenges of latent heat thermal energy storage: A review. Clean Technol. Environ. Policy 2021, 23, 531–559. [Google Scholar] [CrossRef]
- Mumtaz, M.; Khan, A.; Saidur, R.; Al-sulaiman, F.A. A review for phase change materials (PCMs) in solar absorption refrigeration systems. Renew. Sustain. Energy Rev. 2017, 76, 105–137. [Google Scholar] [CrossRef]
- Kenisarin, M.M. High-temperature phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2010, 14, 955–970. [Google Scholar] [CrossRef]
- Ge, H.; Li, H.; Mei, S.; Liu, J. Low melting point liquid metal as a new class of phase change material: An emerging frontier in energy area. Renew. Sustain. Energy Rev. 2013, 21, 331–346. [Google Scholar] [CrossRef]
- Luo, L.; Le Pierrès, N. Chapter 3—Innovative Systems for Storage of Thermal Solar Energy in Buildings. In Solar Energy Storage; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015; pp. 27–62. ISBN 9780124095403. [Google Scholar]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Su, W.; Darkwa, J.; Kokogiannakis, G. Review of solid—Liquid phase change materials and their encapsulation technologies. Renew. Sustain. Energy Rev. 2015, 48, 373–391. [Google Scholar] [CrossRef]
- Nomura, T.; Akiyama, T. High-temperature latent heat storage technology to utilize exergy of solar heat and industrial exhaust heat. Int. J. Energy Res. 2016, 41, 240–251. [Google Scholar] [CrossRef]
- Michels, H.; Pitz-paal, R. Cascaded latent heat storage for parabolic trough solar power plants. Sol. Energy 2007, 81, 829–837. [Google Scholar] [CrossRef]
- Bhale, P.V.; Rathod, M.K.; Sahoo, L. Thermal analysis of a solar concentrating system integrated with sensible and latent heat storage. Energy Procedia 2015, 75, 2157–2162. [Google Scholar] [CrossRef]
- Bayón, R.; Rojas, E.; Valenzuela, L.; Zarza, E.; León, J. Analysis of the experimental behaviour of a 100 kW th latent heat storage system for direct steam generation in solar thermal power plants. Appl. Therm. Eng. 2010, 30, 2643–2651. [Google Scholar] [CrossRef]
- Garcia, P.; Olcese, M.; Rougé, S. Experimental and numerical investigation of a pilot scale latent heat thermal energy storage for CSP power plant. Energy Procedia 2015, 69, 842–849. [Google Scholar] [CrossRef]
- Alhuyi Nazari, M.; Maleki, A.; Assad, M.E.H.; Rosen, M.A.; Haghighi, A.; Sharabaty, H.; Chen, L. A review of nanomaterial incorporated phase change materials for solar thermal energy storage. Sol. Energy 2021, 228, 725–743. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Liu, D. Progress in thermochemical energy storage for concentrated solar power: A review. Int. J. Energy Res. 2018, 42, 4546–4561. [Google Scholar] [CrossRef]
- Estado de las Tecnologías de Almacenamiento. Resumen Ejecutivo; Grupo Interplataformas de Almacenamiento (GIA), Ed.; International Telecommunication Union: Geneva, Switzerland, 2016. [Google Scholar]
- Bellan, S.; Kodama, T.; Gokon, N.; Matsubara, K. A review on high-temperature thermochemical heat storage: Particle reactors and materials based on solid–gas reactions. WIREs Energy Environ. 2022, 11, e440. [Google Scholar] [CrossRef]
- Alvarez Barcia, L. Optimizacion del Control del Sistema de Aceite Termico en Centrales Solares Termoeletricas; Universidad de Oviedo: Oviedo, Spain, 2015. [Google Scholar]
- Nie, F.; Bai, F.; Wang, Z.; Li, X.; Yang, R. Solid particle solar receivers in the next-generation concentrated solar power plant. EcoMat 2022, 4, e12207. [Google Scholar] [CrossRef]
- Ortega-Fernández, I.; Hernández, A.B.; Wang, Y.; Bielsa, D. Performance assessment of an oil-based packed bed thermal energy storage unit in a demonstration concentrated solar power plant. Energy 2021, 217, 119378. [Google Scholar] [CrossRef]
- Basildo Garcia, J.A. Balance de explotación de la Planta Termosolar Puerto Errado 2. Conferencia y ponencias invitadas. In Proceedings of the III Encuentro de Ingeniería de la Energía del Campus Mare Nostrum, Murcia, Spain, 27 September 2016. [Google Scholar]
- Maccari, A.; Bissi, D.; Casubolo, G.; Guerrini, F.; Lucatello, L.; Luna, G.; Rivaben, A.; Savoldi, E.; Tamano, S.; Zuanella, M. Archimede Solar Energy molten salt parabolic trough demo plant: A step ahead towards the new frontiers of CSP. Energy Procedia 2015, 69, 1643–1651. [Google Scholar] [CrossRef]
- Kong, L.; Chen, X.; Gong, J.; Fan, D.; Wang, B.; Li, S. Optimization of the hybrid solar power plants comprising photovoltaic and concentrating solar power using the butterfly algorithm. Energy Convers. Manag. 2022, 257, 115310. [Google Scholar] [CrossRef]
- Romero, M.; González-Aguilar, J. 7—Next generation of liquid metal and other high-performance receiver designs for concentrating solar thermal (CST) central tower systems. In Advances in Concentrating Solar Thermal Research and Technology; Blanco, M.J., Santigosa, L.R., Eds.; Woodhead Publishing Series in Energy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 129–154. ISBN 9780081005163. [Google Scholar]
- Zhu, Z.; Zhang, D.; Mischke, P.; Zhang, X. Electricity generation costs of concentrated solar power technologies in China based on operational plants. Energy 2015, 89, 65–74. [Google Scholar] [CrossRef]
- Platzer, W.J.; Mills, D.; Gardner, W. Chapter 6—Linear Fresnel Collector (LFC) solar thermal technology. In Concentrating Solar Power Technology, 2nd ed.; Lovegrove, K., Stein, W., Eds.; Woodhead Publishing Series in Energy; Elsevier: Amsterdam, The Netherlands, 2021; pp. 165–217. ISBN 9780128199701. [Google Scholar]
- Xu, E.; Yu, Q.; Wang, Z.; Yang, C. Modeling and simulation of 1 MW DAHAN solar thermal power tower plant. Renew. Energy 2011, 36, 848–857. [Google Scholar] [CrossRef]
- Grogan, D.C.P. Development of Molten-Salt Heat Transfer Fluid Technology for Parabolic Trough Solar Power Plants—Public Final Technical Report; Abengoa Solar, LLC: Lakewood, CO, USA, 2013; pp. 303–323. [Google Scholar] [CrossRef]
- Tilley, D.; Kelly, B.; Burkholder, F. Baseload Nitrate Salt Central Receiver Power Plant Design; Final Report; Abengoa Solar LLC: Lakewood, CO, USA, 2014. [Google Scholar] [CrossRef]
- Wang, T.; Mantha, D.; Reddy, R.G. High Thermal Energy Storage Density LiNO3-NaNO3-KNO3-KNO2 Quaternary Molten Salts for Parabolic Trough Solar Power Generation. In Energy Technology 2012; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 73–84. [Google Scholar]
- Shin, D.; Banerjee, D. Experimental Investigation of Molten Salt Nanofluid for Solar Thermal Energy Application. In Proceedings of the ASME/JSME 2011 8th Thermal Engineering Joint Conference, Honolulu, HI, USA, 13–17 March 2011. [Google Scholar]
- Li, P.W.; Lew, J.V.; Karaki, W.; Chan, C.L.; Stephens, J.; O’Brien, J.E. Transient Heat Transfer and Energy Transport in Packed Bed Thermal Storage Systems. In Developments in Heat Transfer; InTech: Bolton, UK, 2011. [Google Scholar]
- John, E.E.; Hale, W.M.; Selvam, R.P. Development of a High-Performance Concrete to Store Thermal Energy for Concentrating Solar Power Plants. In Proceedings of the ASME 2011 5th International Conference on Energy Sustainability, Parts A, B, and C, Washington, DC, USA, 7–10 August 2011; pp. 523–529. [Google Scholar]
- Villarroel, E.; Fernandez-Pello, C.; Lenartz, J.; Parysek, K. High Efficiency Thermal Storage System for Solar Plants (HELSOLAR); Final Report; SENER Engineering and Systems, Inc.: San Francisco, CA, USA, 2013. [Google Scholar] [CrossRef]
- Newmarker, M.; Campbell, M. Indirect, Dual-Media, Phase Changing Material Modular Thermal Energy Storage System; Final Technical Report; ACCIONA SOLAR POWER, INC.: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Mathur, A. Heat Transfer and Latent Heat Storage in Inorganic Molten Salts for Concentrating Solar Power Plants; Final Report; Terrafore Inc.: Riverside, CA, USA, 2013. [Google Scholar] [CrossRef]
- Robak, C.W.; Bergman, T.L.; Faghri, A. Enhancement of latent heat energy storage using embedded heat pipes. Int. J. Heat Mass Transf. 2011, 54, 3476–3484. [Google Scholar] [CrossRef]
- Goswami, D.Y. Development and Demonstration of an Innovative Thermal Energy Storage System for Baseload Power Generation; Final Report; University of South Florida: Tampa, FL, USA, 2012. [Google Scholar] [CrossRef]
- Linder, M. Thermochemical Energy Storage for Concentrated Solar Power Plants (TCSPower). Final Report. Available online: https://cordis.europa.eu/project/id/282889/reporting/es (accessed on 22 July 2022).
- Karagiannakis, G. Redox Materials-based Structured Reactors/Heat Exchangers for Thermo-Chemical Heat Storage Systems in Concentrated Solar Power Plants (RESTRUCTURE). Final Report. Available online: https://cordis.europa.eu/project/id/283015/reporting (accessed on 22 July 2022).
- STORRE High Temperature Thermal Energy Storage by Reversible Thermochemical Reaction (STORRE). Final Report. Available online: https://cordis.europa.eu/project/id/282677/reporting (accessed on 22 July 2022).
- Project Profile: Low-Cost Metal Hydride Thermal Energy Storage System. United States. Available online: https://www.energy.gov/eere/solar/project-profile-low-cost-metal-hydride-thermal-energy-storage-system (accessed on 22 July 2022).
- Singh, D.; Yu, W.; France, D.M. High Efficiency Latent Heat Based Thermal Energy Storage System Compatible with Supercritical CO2 Power Cycle; Final Report; Argonne National Lab. (ANL): Argonne, IL, USA, 2019. [Google Scholar] [CrossRef]
- Mei, R. Carbon Dioxide Shuttling Thermochemical Storage Using Strontium Carbonate; Final Report; Argonne National Lab. (ANL): Argonne, IL, USA, 2015. [Google Scholar] [CrossRef]
- Gangwal, S.; Muto, A. Regenerative Carbonate-Based Thermochemical Energy Storage System for Concentrating Solar Power; Final Report; Southern Research Inst.: Durham, NC, USA, 2017. [Google Scholar] [CrossRef]
- Project Profile: High-Temperature Thermochemical Storage with Redox-Stable Perovskites for Concentrating Solar Power. United States. Available online: https://www.energy.gov/eere/solar/project-profile-high-temperature-thermochemical-storage-redox-stable-perovskites (accessed on 22 July 2022).
- Project Profile: Integrated Solar Receiver with Thermal Storage for an sCO2 Power Cycle. United States. Available online: https://www.energy.gov/eere/solar/project-profile-brayton-energy (accessed on 22 July 2022).
- Project Profile: Binary Metal Chalcogenides for High Temperature Thermal Storage. United States. Available online: https://www.energy.gov/eere/solar/project-profile-binary-metal-chalcogenides-high-temperature-thermal-storage-sunlamp (accessed on 22 July 2022).
- Muto, A.; Hansen, T.A. Demonstration of High-Temperature Calcium-Based Thermochemical Energy Storage System for Use with Concentrating Solar Power Facilities; Final Technical Report; Southern Research Inst.: Birmingham, AL, USA, 2019. [Google Scholar] [CrossRef]
- Baeyens, J. High Temperature Concentrated Solar Thermal Power Plant with Particle Receiver and Direct Thermal Storage (NEXT-CSP); Centre National De La Recherche Scientifique CNRS: Paris, France, 2017. [Google Scholar] [CrossRef]
- Baeyens, J.; Siros, F.; Valentin, B.; Brau, J.-F. Report on Particle Handling Solutions for Large-Scale Facilities. Next-CSP Project. Available online: http://next-csp.eu/ (accessed on 22 July 2022).
- Concentrated Solar Power in Particles European Project (CSP2). Final Project Report. Available online: http://www.csp2-project.eu/ (accessed on 22 July 2022).
- Behar, O.; Grange, B.; Flamant, G. Design and performance of a modular combined cycle solar power plant using the fluidized particle solar receiver technology. Energy Convers. Manag. 2020, 220, 113108. [Google Scholar] [CrossRef]
- Reyes-Belmonte, M.A.; Sebastián, A.; Spelling, J.; Romero, M.; González-Aguilar, J. Annual performance of subcritical Rankine cycle coupled to an innovative particle receiver solar power plant. Renew. Energy 2019, 130, 786–795. [Google Scholar] [CrossRef]
- Rovense, F.; Reyes-Belmonte, M.A.; González-Aguilar, J.; Amelio, M.; Bova, S.; Romero, M. Flexible electricity dispatch for CSP plant using un-fired closed air Brayton cycle with particles based thermal energy storage system. Energy 2019, 173, 971–984. [Google Scholar] [CrossRef]
- Periodic Reporting for Period 1—SOLSTORE (Solid-State Reactions for Thermal Energy Storage). Available online: https://cordis.europa.eu/project/id/752520/reporting (accessed on 22 July 2022).
- Doppiu, S.; Dauvergne, J.-L.; Palomo del Barrio, E. Solid-State Reactions for the Storage of Thermal Energy. Nanomaterials 2019, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Periodic Reporting for Period 1—NPMSSES (Nanoparticle Enhanced Molten Salts for Solar Energy Storage). Available online: https://cordis.europa.eu/project/id/706788/reporting (accessed on 22 July 2022).
- Periodic Reporting for Period 2—AMADEUS (Next GenerAtion MateriAls and Solid State DevicEs for Ultra High Temperature Energy Storage and Conversion). Available online: https://www.amadeus-project.eu/news (accessed on 22 July 2022).
- Periodic Reporting for Period 2—SESPer (Solar Energy Storage PERovskites). Available online: https://cordis.europa.eu/project/id/746167/reporting (accessed on 22 July 2022).
- Advanced Materials Solutions for Next Generation High Efficiency Concentrated Solar Power (CSP) Tower Systems (NEXTOWER). Final Results. Available online: https://www.h2020-nextower.eu/ (accessed on 22 July 2022).
- Garcia, P.; Pouvreau, J. High temperature combined sensible-latent thermal energy storage. In Proceedings of the AIP Conference Proceedings 2126, Casablanca, Morocco, 2–5 October 2019; p. 200020. [Google Scholar]
- Gálvez, A.; Cubillo, J.J.; Guerreiro, L.; Azevedo, P.; Diamantino, T.; Alonso, M.C.; Bonk, A.; Bauer, T.; Franke, W.; Haselbacher, A.; et al. NEW StOrage Latent and Sensible Concept for High Efficient CSP Plants Preliminary Selection of Materials Compositions and TES System Predesign (NewSOL). Available online: http://www.newsol.uevora.pt/ (accessed on 22 July 2022).
- SOlar Calcium-looping IntegRAtion for ThermoChemical Energy Storage. Final Innovation. Evaluation Report. Available online: https://cordis.europa.eu/project/id/727348/results (accessed on 22 July 2022).
- Periodic Reporting for Period 1—SOCRATCES (SOlar Calcium-looping IntegRAtion for Thermo-Chemical Energy Storage). Available online: https://socratces.eu/ (accessed on 22 July 2022).
- A New Generation High Temperature Phase Change Microemulsion for Latent Thermal Energy Storage in Dual Loop Solar Field (THERMES). Fact Sheet. Available online: https://cordis.europa.eu/project/id/831756 (accessed on 22 July 2022).
- Turchi, C.; Gage, S.; Martinek, J.; Jape, S.; Armijo, K.; Coventry, J.; Pye, J.; Asselineau, C.-A.; Venn, F.; Logie, W.; et al. CSP Gen3: Liquid-Phase Pathway to SunShot; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2021. [Google Scholar] [CrossRef]
- Project Profile: Brayton Energy 2 (Gen3 CSP). United States. Available online: https://www.energy.gov/eere/solar/project-profile-brayton-energy-2-gen3-csp (accessed on 22 July 2022).
- NEWCLINE Project CSP ERANET: 1st Cofund Joint Call. Available online: http://www.newcline.eu/ (accessed on 22 July 2022).
- Robb, K. Simplified High-Temperature Molten Salt CSP Plant Preconceptual Design; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2022. [Google Scholar] [CrossRef]

| Excluded Phrase | Main Search Phrase | Complementary Search Phrase | Total Papers | Papers in the Last 5 Years (2018–2022) |
|---|---|---|---|---|
| Photovoltaic | Concentrated solar power | Review | 381 | 191 |
| Review + Technologies | 212 | 112 | ||
| Review + Thermal energy storage | 137 | 78 | ||
| Review + Sensible heat storage | 28 | 17 | ||
| Review + Latent heat storage | 31 | 18 | ||
| Review + Thermochemical energy storage | 34 | 19 | ||
| Thermal storage configuration | 207 | 104 | ||
| Thermal energy storage + Active system | 22 | 12 | ||
| Thermal energy storage + Passive system | 15 | 6 |
| Research Tool | Search by | Total TES CSP Facilities | Total TES CSP R&D Projects |
|---|---|---|---|
| SolarPACES | Operational status | 119 | - |
| Operational status + Thermal energy storage | 61 | - | |
| CORDIS | Concentrated solar power + Energy storage | - | 46 |
| SETO | Concentrated solar power + Thermal energy storage + Inactive | - | 38 |
| Concentrated solar power + Thermal energy storage + Active | - | 31 |
| CSP Technology | PTC | LFR | SPT | PDC |
|---|---|---|---|---|
| Solar concentration ratio | 70–80 | 60–100 | 1000–1500 | 1300–3000 |
| Operating temperature (°C) | <400 | <300 | <1000 | <1500 |
| Nominal capacity (MW) | 10–280 | 9–125 | 10–377 | <1.5 |
| Average specific cost (€/kW) | 7399 | 5054 | 6052 | - |
| Average LCOE (€/kWh) | 0.24 | 0.16 | 0.15 | - |
| Thermodynamic efficiency | ↓↓ | ↓ | ↑ | ↑↑ |
| Advantages | ● Commercial scale. ● Modularity. Good land-use factor | ● Readily available. Low manufacturing cost. | ● High conversion. High temperature storage. ● Optimal for dry cooling. | ● Good land-use factor. ● With/out heat transfer fluid. |
| Disadvantages | ● Fluid working temperatures up to 400 °C. | ● Small plants. Recent entrance in market. | ● Low land-use factor. ● Larger-scale operation required. | ● Further experimental feedback required. |
| TES Technology | SHS | LHS | TCES |
|---|---|---|---|
| TRL | 8–9 | 6–9 | 4–7 |
| Energy density | Low | Medium | High |
| Heat transfer | Good | Slow | Slow |
| Materials costs | Low, except liquid metals and thermal oils | Low | Low, except design and installation of reactors |
| Required area | High | Medium | Low |
| Timescale | Hours–Seasonal | Days–Months | Hours–Years |
| Lifetime | Long | Limited | Depends on reactant |
| Storage temperature | High | High | Low |
| Flexibility | Fast switch charge/discharge | Fast switch charge/discharge | Slow switch charge/discharge |
| Advantages | ● Large experimental and industrial feedback. ● Easy implementation. | ● Short distance transport. ● Small volumes. ● Constant temperatures for charge/discharge. | ● Long distance transport. ● Small volumes. ● Long storage periods without losses. |
| Disadvantages | ● High freezing point for liquid medium. ● Variable and unstable discharging temperature. ● Large volumes. | ● Corrosivity of materials. ● Large heat losses. ● Formation of solid deposits on the heat exchange area. | ● Complex technology. ● High capital costs. ● Technical issues: melting, incomplete reversibility, low reaction kinetics, sintering. ● Storage of gases. ● Required improvement of heat and mass transfer. ● Low charging rate. |
| TES CSP Integrated Configurations | Advantages | Limitations | |
|---|---|---|---|
| Active Direct Storage | 2-tank |
|
|
| Steam accumulator |
|
| |
| Active Indirect Storage | 2-tank |
|
|
| Single tank |
|
| |
| Steam accumulator |
|
| |
| Passive Storage | Embedded HT structures |
|
|
| Packed-bed systems |
|
| |
| TES CSP Configuration | Active Storage | Passive Storage | |||
|---|---|---|---|---|---|
| Direct | Indirect | Embedded HT Structures | |||
| 2-Tank | Steam Accumulator | 2-Tank | Single-Tank | ||
| CSP technology | SPT | SPT | PTC | LFR | LFR |
| TES medium | Molten salts | Water | Molten salts | Ruths tank | Concrete |
| HTF medium | Molten salts | Water | Thermal oil | Water | Water |
| Tout solar field (°C) | 565 | 250–530 | 393 | 270 | 450–550 |
| Expected production (GWh/year) | 110–500 | 23–180 | 158–944 | 49 | 75 |
| Nominal capacity (MWe) | 20–150 | 11 to 50 | 50–250 | 30 | 15 |
| Storage size (h) | 6 to 15 | 1 to 2 | 3 to 10 | 0.5 | 14 |
| Power block | Steam Rankine | Steam Rankine | Steam Rankine | Steam Rankine | Steam Rankine |
| Number of commercial TES CSP plants | 8 | 3 | 32 | 1 | 1 |
| TES | Project Name * | Location | Period | Coordinator * | Budget (M€) | Ref. |
|---|---|---|---|---|---|---|
| LHS | DDI-TES | Florida (USA) | 2011–2012 | USFl | 0.7 | [110] |
| TCES | TCS-Power | Germany | 2011–2015 | DRL | 4.4 | [111] |
| TCES | RESTRUCTURE | Greece | 2011–2016 | CERTH | 3 | [112] |
| TCES | STORRE | France | 2012–2016 | CEA | 2.9 | [113] |
| TCES | LCMH-TES | South Carolina (USA) | 2012–2016 | SRNL | 2.5 | [114] |
| LHS | HELH-TES | Illinois (USA) | 2012–2018 | ArNL | 1 | [115] |
| TCES | SC-TES | Florida (USA) | 2014–2015 | UFl | 0.4 | [116] |
| TCES | RC-TES | Alabama (USA) | 2014–2016 | SRI | 0.8 | [117] |
| TCES | ELEM-TES | Colorado (USA) | 2014–2017 | CSM | 1 | [118] |
| TCES | ISR-TES | New Hampshire (USA) | 2015–2018 | BE | 2.6 | [119] |
| TCES | BMC-TES | New Mexico (USA) | 2015–2018 | AlNL | 3.4 | [120] |
| TCES | CaL-TES | Alabama (USA) | 2015–2018 | SRI | 2.8 | [121] |
| SHS | NEXT-CSP | France | 2016–2020 | CNRS | 4.9 | [122,123,124,125,126,127] |
| Other | SOLSTORE | Spain | 2017–2019 | CIC | 0.1 | [128,129] |
| LHS | NPMSSES | United Kingdom | 2017–2019 | ULe | 0.2 | [130] |
| LHS | AMADEUS | Spain | 2017–2019 | UPM | 3.2 | [131] |
| TCES | SesPER | Spain | 2017–2020 | CSIC | 0.2 | [132] |
| SHS | NEXTOWER | Italy | 2017–2021 | ENEA | 6.2 | [133] |
| SHS + LHS | IN-POWER | Spain | 2017–2021 | LEITAT | 5.8 | [134] |
| SHS + LHS | NewSOL | Portugal | 2017–2021 | UEv | 5.6 | [135] |
| TCES | SOCRATCES | Spain | 2018–2021 | USe | 4.9 | [136,137] |
| LHS | THERMES | United Kingdom | 2019–2021 | UBir | 0.2 | [138] |
| SHS | LPP-SS | Colorado (USA) | 2018- | NREL | 8 | [139] |
| LHS | TES-HE | New Hampshire (USA) | 2018- | BE | 1.1 | [140] |
| SHS | CSP-ERANET (Newcline) | Spain | 2019–2024 | AGENEX | 13.8 | [141] |
| TCES | SS-TES | Michigan (USA) | 2020- | MiSU | 2 | [27] |
| TCES | EWSCh-TES | Arizona (USA) | 2020- | ArSU | 2.9 | [27] |
| TCES | EC-TES | South Carolina (USA) | 2020- | SRNL | 0.2 | [27] |
| SHS | HTMS-TES | Tennessee (USA) | 2020- | ORNL | 0.1 | [142] |
| SHS | FULL-TES | California (USA) | 2020- | EDISUN | 39 | [27] |
| SHS | PB | Montana (USA) | 2021- | MoSU | 0.1 | [27] |
| Project Name Abbreviation | TES CSP Configuration | TES Technology | Storage Size (h) | CSP Technology | HTF | Tmax ¥ (°C) | Power Block | TRL § | LCOE * (€/kWh) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| TCS-Power | Passive storage | Redox TCES | up to 12 | SPT | Air | 400–600 | Steam Rankine | 4–5 | 0.14 | [111] |
| Hydroxide TCES | PTC | Molten salts | 0.21 | |||||||
| RESTRUCTURE | Passive storage | Redox TCES | 6 to 13 | SPT | Air | up to 1000 | Air Brayton CC | 4–5 | <0.15 | [112] |
| STORRE | Active storage direct/indirect 2-tank | Hydroxide TCES | 6 to 13 | PTC, LFR | HTF = TES or HTF ≠ TES | 300–550 | Steam Rankine | 3–4 | - | [113] |
| CaL-TES | Passive storage | Carbonate TCES | - | SPT | sCO2 | 720 | Closed loop CO2 | 7–8 | 0.06 | [121] |
| NEXT-CSP | Active storage direct 2-tank | Solid particles SHS | up to 12 | SPT | HTF = TES | 650–750 | Gas turbine, Subcritical steam, Air Brayton | 5 | 0.1 | [122,123,124,125,126,127] |
| NEXTOWER | Active storage indirect single-tank | Liquid metal SHS | - | SPT | Air | 800 | Gas turbine | 6 | - | [133] |
| IN-POWER | Active storage single-tank & Passive storage | Molten salts SHS & PCM LHS | - | LFR, PTC | Molten salts | 600 | DSG | 6 | 0.1 | [134] |
| NewSOL | Passive storage | Concrete module | - | PTC | Ca-ternary molten salt mixture | up to 550 | Steam Rankine | 5–6 | 0.1–0.12 | [135] |
| Active storage single-tank & Passive storage | Molten salts SHS & PCM LHS | 8 | ||||||||
| SOCRATCES | Passive storage | Carbonate TCES | days/ months | SPT | HTF = TES | 600–1000 | Closed-loop CO2 Brayton | 5 | 0.07 | [137] |
| Project Name Abbreviation | TES CSP Configuration | TES Technology | CSP Technology | Tmax ¥ (°C) | Power Block | TRL § | LCOE * (€/kWh) | Ref. |
|---|---|---|---|---|---|---|---|---|
| LPP-SS | Active storage (2-tank indirect) | Molten chloride salts SHS | SPT | 740 | sCO2 | 6 | 0.06 | [139] |
| TES-HE | Passive storage | PCM LHS | SPT | >700 | sCO2 | 5 | - | [140] |
| CSP-ERANET (Newcline) | Active storage indirect single-tank & Passive storage | Ceramics/PCM LHS | PTC, SPT | - | - | 5 | - | [141] |
| SS-TES | Passive storage | Redox TCES | - | up to 1300 | - | 5 | - | [27] |
| EWSCh-TES | Passive storage | Multiple TCES | - | - | sCO2 | 1–2 | - | [27] |
| EC-TES | Passive storage | Carbonate TCES | - | - | - | 5–6 | - | [27] |
| HTMS-TES | Active storage indirect single-tank | Chloride salts SHS | - | - | More-efficient | 3 | - | [142] |
| FULL-TES | Passive storage packed-bed | Rocks SHS | SPT | 600 | sCO2 | 7–8 | <0.05 | [27] |
| PB | Passive storage packed-bed | SHS | - | - | - | 3–4 | - | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual, S.; Lisbona, P.; Romeo, L.M. Thermal Energy Storage in Concentrating Solar Power Plants: A Review of European and North American R&D Projects. Energies 2022, 15, 8570. https://doi.org/10.3390/en15228570
Pascual S, Lisbona P, Romeo LM. Thermal Energy Storage in Concentrating Solar Power Plants: A Review of European and North American R&D Projects. Energies. 2022; 15(22):8570. https://doi.org/10.3390/en15228570
Chicago/Turabian StylePascual, Sara, Pilar Lisbona, and Luis M. Romeo. 2022. "Thermal Energy Storage in Concentrating Solar Power Plants: A Review of European and North American R&D Projects" Energies 15, no. 22: 8570. https://doi.org/10.3390/en15228570
APA StylePascual, S., Lisbona, P., & Romeo, L. M. (2022). Thermal Energy Storage in Concentrating Solar Power Plants: A Review of European and North American R&D Projects. Energies, 15(22), 8570. https://doi.org/10.3390/en15228570










