1. Introduction
Rechargeable lithium-ion batteries are the go-to technology to power mobile machines as well as battery-electric vehicles. The demand for lithium-ion batteries has been rising for years and further growth is expected for years to come, as vehicles powered by combustion engines have to be phased out in order to reduce greenhouse gas emissions in the mobility sector and ways to store energy produced by fluctuating renewable sources are urgently needed [
1,
2]. At the same time, higher energy density, better fast-charging capability, reliable safety concepts and declining acquisition costs are expected [
3]. In this regard, huge efforts are made to develop new or improve existing production methods.
Within pouch cell assembly, the alternate stacking of anode, separator and cathode is a time-consuming task as it affords many sequential pick-and-place operations. Thus, it represents a bottleneck in pouch cell production and drives up costs [
4]. One way to speed up the stacking process is to reduce the number of components that need to be handled in the production chain. This can be achieved by tightly connecting the separators to the electrodes by applying heat and pressure, forming so called electrode-separator laminates (ESLs) for further processing. These ESLs can be sub-units of a cell stack, usually consisting of up to five components (e.g. two cathodes, two separators and one anode in bi-cell arrangement). Alternative sub-unit compounds to create a continuous stack are possible as well. Asides from reducing the number of necessary handling steps in cell stacking, the components are less flexible and sensitive to mechanical stress and thus enable a faster process speed, less susceptibility to damage and better placement accuracy [
3]. Further savings can also be expected regarding storage time and cost, as it has been reported that lamination improves the wetting behavior of electrode-separator assemblies [
5]. In addition to the production benefits, recent studies on laminated cells have shown improved electrochemical performance in terms of fast-charging capability as well as high capacity retention, since the adhesion between electrode and separator obtained by lamination reduces impedance build up during cycling [
6,
7,
8].
At the moment, however, lamination is not possible with most types of commercially available separators, like polyolefin membranes (mostly PE or PP) or polymer separators with ceramic coatings [
9]. To overcome this limitation, functional coating layers can be added to the separators using various techniques. A very promising coating method to achieve this refinement is to use electrospinning, which enables the application of the necessary thermoplastic binder polymers to the separator as a micro- or nanofiber non-woven surface layer. This technology is already capable of managing high throughputs in industrial scale up to 14.4 kg of polymer fibers per day [
10]. Electrospinning allows high control over fiber morphology, thickness and mass loading of the applied coating layer. It allows for low polymer loadings, saving material and leading to a fairly low increase in separator thickness below 1 µm. By only partially covering the surface, the majority of the separator’s pores remain open and impairment of ion diffusion is minimized. On that account, electrospinning could enable more flexible production. By adding an electrospinning module to the separator feed, intrinsically non-laminable separators become available for assembly in existing lamination-based high-speed production lines. Besides, commercially available separators could be refined inhouse by suppliers to meet cell manufacturers’ demands for laminable separators without the need to develop a completely new product.
The feasibility of this method has already been demonstrated by Lee et al., who created an adhesive interface by hot-pressing a polyolefin separator with an electrospun layer consisting of a PVdF copolymer onto a LFP battery cathode [
11]. Successful lamination has been demonstrated using a ceramic separator with electrospun beaded PVdF-nanofibers and a NMC cathode [
12]. Electrochemical studies on separators with electrospun fiber coatings are currently not known to the authors. To successfully implement electrospinning at developing laminable separators, a number of dependencies have to be considered. For good battery performance, the material properties of separator, electrodes and coating polymers must be precisely matched. This applies in particular to their melting behavior and thermal stability. In order to form adhesion between the cell components, the binder should be softened at the process conditions used, while the pore-structure of the separator and its coating should be retained so that ion transport between the electrodes is not impaired and materials are not irreversibly decomposed. Leithoff et. al recently addressed some of the process-product interdependencies for lamination and consider in-depth understanding crucial on the path towards quality-assured cell production [
13]. They investigated the influence of the lamination parameters feed rate, contact pressure and process temperature to the properties of intermediate products and battery cells. When laminating a PVdF/Al
O
coated polyolefin separator to the cathode, it was observed, that inappropriately high temperature and contact pressure significantly deteriorate the cell capacity in ageing tests.
To the best of our knowledge we are the first to give an overview on the challenges in separator modification by electrospinning for subsequent lamination and present a systematic approach to surmount certain obstacles. Our work includes characterization of separators, polymer selection, analysis of electrospun-nonwovens, mechanical tests of the laminates as well as comprehensive electrochemical testing of lithium-ion pouch cells, assembled using the modified separators. In order to determine the process-condition window for lamination, the separator must at first be characterized in terms of its robustness against thermo-mechanical stress. Air permeability measurements on the sole separator are used to detect structural changes in the porous membrane. The determined maximum processing window sets the frame conditions for the selection of the coating polymer. To determine the applicability of a binder in terms of bonding between cell components, measurements of the adhesion strength between laminated components are conducted. To validate the procedure and to investigate electrochemical effects of the separator modification, pouch-type NMC622/graphite battery cells are assembled and tested.
2. Materials and Methods
2.1. Electrodes
All electrodes were obtained from UniverCell Holding GmbH, Flintbek, Germany. The cathode is composed of NMC622 (94.5 wt.%) active material, PVdF (3%) binder and Carbon Black (2.5%) conductive additive, coated on aluminium foil. It has an areal capacity of 3.0 mAh/cm2. The anode is composed of graphite (94%), CMC/SBR (4.5%) and Carbon Black (1.5%), coated on copper foil and has an areal capacity of 2.7 mAh/cm2.
2.2. Separators & Coating
Two different separators were used for the following experiments. The first separator is a ceramic impregnated polyester nonwoven (FS3011, Freudenberg Performance Materials SE & Co. KG, Weinheim, Germany) with a thickness of 22 µm. It is referred to as ceramic separator in the following. The second separator used is a single layer polyethylene (PE) separator (SK Innovation, Seoul, South Korea) with a thickness of 16 µm and referred to as PE separator.
Polymer A used for coating of the separators is a PVdF-co-HFP (KYNAR Powerflex LBG PWD, ARKEMA, Colombes Cedex, France; melting point: 154 °C). For electrospinning, 12.5 wt.% of the polymer was fully dissolved in a 70/30 vol.% mixture of N,N-dimethylformamide (Carl Roth, Karlsruhe, Germany) and acetone (VWR, Darmstadt, Germany) on a magnetic stirrer for ≥12 h at room temperature. Polymer B is a lower-melting fluoroelastomer (vinylidene fluoride, hexafluoropropylene, and tetrafluoroethylene; 3M DYNEON FPO 3850, 3M Company, St. Paul, USA; melting range 50–140 °C). For electrospinning a 10 wt.% solution in acetone was prepared analogously.
2.3. Electrospinning
All electrospinning experiments were performed on a commercially available single nozzle electrospinning machine (NE300-XP, Inovenso Inc., Woburn, MA, USA), equipped with an rotating drum collector on which sheets of the separators were mounted. During spinning, a polymer solution was fed to the needle emitter with a controlled flow rate of 4.0 mL/h. For homogeneous coating, the rotating collector was continuously moving bidirectionally along the rotation axis with an amplitude of 120 mm. After the specified duration, the separator was turned over and the other side was coated under the same conditions.
Polymer solution A was spun at a distance of 225 mm at a voltage range between 25 and 26 kV. Polymer B was applied at 120 mm and 18–20 kV.
To determine the area mass loading of the polymer coating on the separator, non-coated and modified separator samples with a total area of 40 cm were weighed on a precision balance. The separators were coated at different spinning times with the above mentioned parameters. As a linear dependency between spinning time and area mass loading was observed, polymer loads were estimated by interpolation.
According to this method, 467 mg/m2 of Polymer A was applied to each side of the ceramic separators used in electrochemical measurements. Analogously 245 mg/m2 of Polymer B was applied to the PE separator if not mentioned otherwise.
2.4. Lamination & Pouch Cell Assembly
Anode, separator and cathode were punched to rectangular shape and stacked concentrically. The dimensions of the respective components were 54 × 84 mm2, 58 × 88 mm and 50 × 80 mm2. The resulting cell stack was laminated using a roller laminator (BLE 282 D, Arcotronics Italia, Bologna; now: MANZ Italia) with a roller speed of 21 mm/s. Lamination temperature and line force were varied across different experiments and are mentioned at the respective positions. Generally, a temperature ramp was applied, rising along machine feed direction from T − 10 K up to the stated lamination temperature T.
The laminated cell stacks were then partially sealed into pouch foil (Gelon-LIB) and dried under vacuum at 70 °C for at least 16 h. In an argon-filled glovebox (MB20, MBraun, Garching, Germany; O-content ≤0.1 ppm, HO-content ≤2 ppm), all cells were filled with 1500 µL of electrolyte (LP57 containing 2% vinylene carbonate, each Gotion Inc., Independence, OH, USA) before the pouch bag was completely sealed at an evacuation pressure of 80 mbar. For complete wetting with electrolyte, the cells rested at room temperature for 12 h before formation. To remove formation gases, the cells were cut open, evacuated and resealed again after formation. Pouch cell assembly was performed on a small scale professional battery manufacturing line (Solith, Sovema Group, Villafranca di Verona, Italy).
For each state, at least 3 cells were investigated. An overview of all cells can be found in
Appendix A.
2.5. Cell Testing
The charge and discharge cycles were performed using a battery test system (CTS/ CTS-LAB, BaSyTec, Asselfingen, Germany). In all test procedures, cells were charged using the CC-CV method (constant current until 4.2 V terminal voltage; then the voltage was held constant until the current dropped below 0.05 C) and discharged using the CC method (constant discharge current until 3.0 V). For formation, 3 cycles at a low current rate of 0.1 C were performed. The coulombic efficiency was above 99% for all cells in the last formation cycle. The mean discharge capacity in the 3rd cycle of all cells in a series of experiments was set as the cell nominal capacity for further cycling. Following the formation, decoupled discharge and charge rate tests were performed. First the discharge rate, then the charge rate was increased step-wise up to 5 C, with the other partial cycle being performed at 0.2 C in each case. For charge test evaluation, only the CC part of the half cycle was considered. To evaluate the longevity of the cells, they were then cyclically charged and discharged at 1 C. Electrochemical Impedance Spectroscopy (EIS) measurements were performed using an optimized potentiostat (Vertex.C.EIS, EKTechnologies, Wesel, Germany) channel, shielded and tempered at 25 °C in a refrigeration incubator (INCU-Line® IL 56 PR, VWR, Darmstadt, Germany). At each cell, a series of six EIS measurements was conducted in potentiostatic mode (100 kHz—10 mHz, amplitude 10 mVpeak) at different cut-off voltages (3.0 V, 3.6 V, 3.8 V, 4.0 V, 4.2 V) of the same charging step. Previous to each EIS measurement, cells passed a 2 h OCV period after last charge/discharge step. EIS cut-off voltages were applied by charging at 0.2 C in CC mode (initial discharging to 3.0 V in CC mode, final charging to 4.2 V in CCCV mode). EIS data were processed in a MATLAB environment (MATLAB R2017b, MathWorks, Natick, USA), including the open source Z-fit protocol. Equivalent circuit fit (see
Supplementary Material Figure S2) was conducted in identical manner like we discussed in a previous study [
14], using the same fit model due to analogous conditions of the measurement environment. Fundamentals on the chosen fit model and its scientific background were provided in detail in this study [
14]. EIS datasets and fit parameters were normalized to the geometric area of active cathode coating in our single cells (40 cm
). EIS equivalent circuit fits had an averaged
of 0.07.
2.6. Membrane Permeability
Air permeability measurements were performed on a gurley densometer (model 4110, Gurley Precision Instruments, Troy, NY, USA) with a test volume of 100 cm3 and a one square inch orifice. Each sample was measured 5 times.
2.7. SEM Analysis
Microscope images were taken on a field emission scanning electron microscope (FE-SEM, Merlin Compact, Carl Zeiss Microscopy GmbH, Germany) with a secondary electron detector. Gold sputtering for sample preparation was done using a sputter coater (SCD 050, Bal-Tec Balzers, Liechtenstein).
2.8. Adhesion Measurements
The modified separators were cut into strips of 25 mm width and laminated to a graphite anode as described above. The interface between separator and anode is prioritized for investigation, since adhesion there is generally lower compared with the separator-cathode interface due to the use of non-thermoplastic binders in the anode. Moreover, recent studies have shown that the improved cell performance due to lamination is predominantly attributed to changes on the anode side [
6]. The copper foil of the graphite anode was attached to a guided carriage of an uniaxial material testing machine (Z005, ZwickRoell GmbH & Co. KG, Ulm, Germany) using double-sided adhesive tape (Tesa 4970). The separator was then pulled off at a constant pull-off angle of 90° at a speed of 1 mm/s, while the required force was continuously recorded by a 50 N load cell. The tensile bond strength P is calculated as the quotient of the mean measured force F and the specimen width w.
3. Results
3.1. Separator Characterization & Polymer Selection
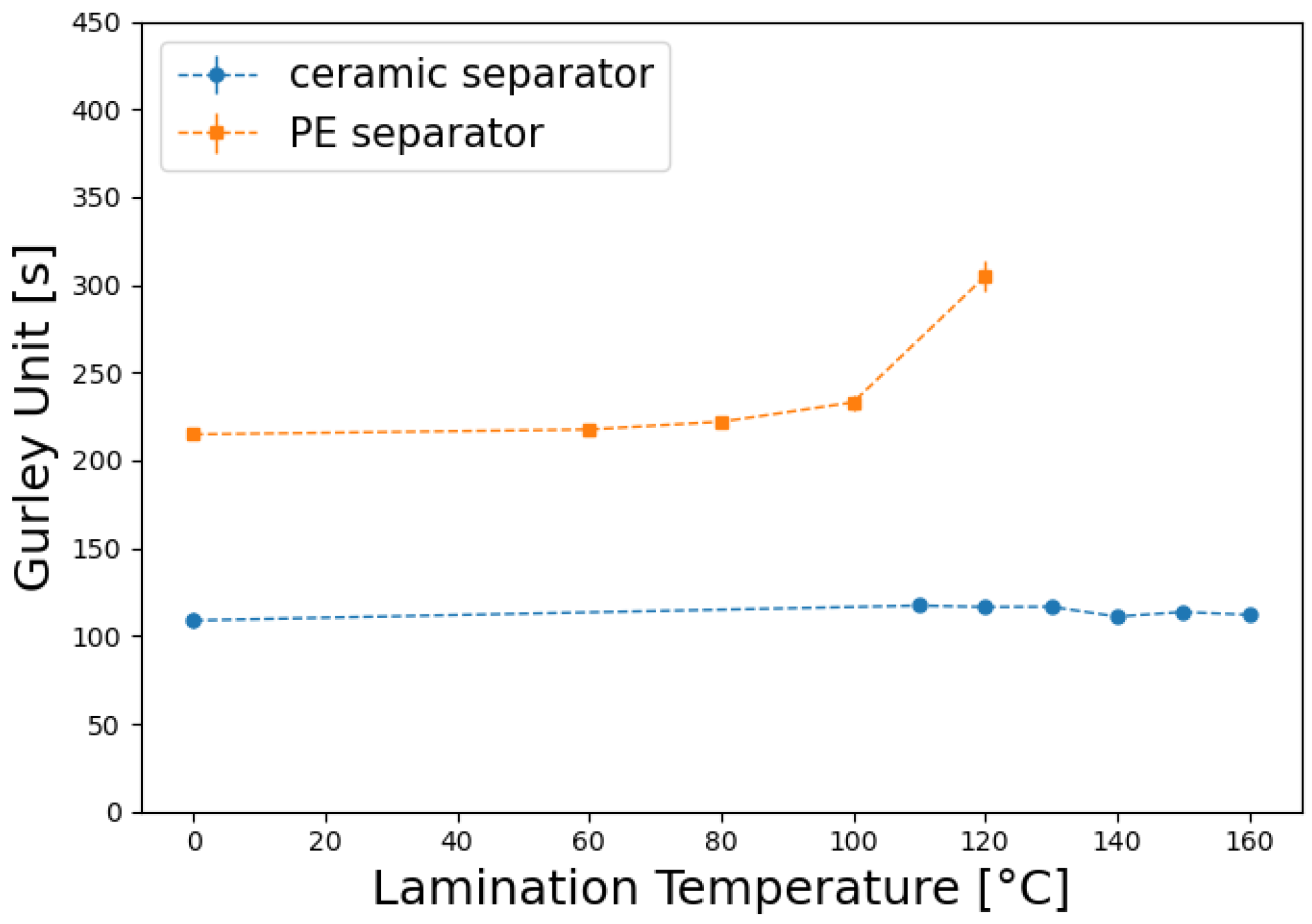
In order to explore the limits of reasonable lamination conditions for a given separator, the gurley value was used to indicate at which thermo-mechanical stress changes in the pore structure of the separator occur.
Figure 1 shows, that there is no significant change in the permeability of the ceramic separator even when the lamination temperature is elevated up to 160 °C at a line force of 8 N/mm. The wide processable temperature range sets rather low demands on the melting behavior of its functional coating layer. Therefore Polymer A (PVdF-co-HFP)—a well established polymer for separator coatings—with a melting point of 154 °C was used in combination with this separator.
In contrast, when using the same line force, structural changes in the PE separator seem to start occurring at temperatures of 80 °C upwards, as the Gurley value slightly increases. The separator becomes impermeable when exposed to temperatures above 130 °C. To establish adhesion within this limited temperature range, this separator was coated with Polymer B. Polymer B shares the typical chemical stability of fluoropolymers, has a low glass transition temperature of −6 °C and shows swelling in the commonly used battery electrolyte LP572, without dissolution. This ensures adhesion after electrolyte filling.
3.2. Separator Modification & Interface Stability
The electrospinning of the high-melting Polymer A leads to a homogeneous surface coverage with fiber diameters between approximately 100 nm and 1.5 µm. The fiber morphology is smooth in general with a small amount of beaded fibers (see
Figure 2C,D). After 2 min of spinning, the area mass loading of the coating is estimated to 467 mg/m
2, which means a relative increase in the separators area weight of about 1.4 % (initially: 33 g/m
2).
The spinning of Polymer B produces a very homogeneous structure of fibers in the diameter range of 100 nm to 2 µm without any beads or other noticeable irregularities (see
Figure 2A,B). After 1 min of electrospinning, the polymer fibers cover about 10% of the separator surface. According to the previously listed estimation (see
Section 2.3), the coating load is about 123 mg/m
2 at this stage. After 4 min, which corresponds to 191 mg/m
2, the degree of coverage is above 50%.
To quantify the interface stability that the coating layer forms between electrode and separator, 90° peeling tests on separators laminated to the graphite anode were carried out.
During peeling tests with the PE separator, breakage mainly occurred as adhesive failure between separator and anode. For measured forces above 15 N/m, an increasing amount of graphite particles was ripped out of the anode. This corresponds to the beginning of cohesive breakage inside the anode. The dependence between peeling strength and electrospinning duration follows an almost linear trend with just a small saturation effect (see
Figure 3). This indicates, that adhesion between electrode and separator mainly depends on the area mass loading of polymer applied to the separator. The adhesion also highly depends on lamination conditions. With lamination temperature increasing from 60 °C to 100 °C as well as line force increasing from 4 N/mm to 12 N/mm, the adhesive force between separator and anode more than quintuples.
The interlayer formed by Polymer A only forms good adhesion when laminated at temperatures above 150 °C (see
Figure 4). Already at adhesive forces from about 6 N/m, cohesive failure of the ceramic separator occurs to a noticeable extent. Measured values above this mark can no longer be compared meaningfully.
3.3. Electrochemical Testing—Ceramic Separator
For the examination of C-rate capability and aging behavior, a series of pouch cells was investigated, consisting of a modified separator laminated to both anode and cathode at a high temperature of 160 °C and a line force of 10 N/mm. For reference, cells were built using the same, but unmodified separator and without lamination.
Figure 5 shows the mean discharge capacities of the cells when discharged and charged at increasing C-rates. When discharged at 1 C and 2 C, the capacities of laminated cells start to spread noticeably, but the deviation of mean capacity always stays within ±2 mAh (<2% of initial capacity). This could be due to irregularities on the separator coating layer. Besides, no significant differences between laminated and non-laminated cells were observed in discharge, or charge rate stability. Fit parameters of EIS measurements (see
Supplementary Material, Figure S1) provide significant deviation of non laminated/laminated state in case of surface resistance fit parameter (see
Figure 6, more details in
Supplementary Material, Figures S3–S5). Any trends to Warburg extension (CPE-element) fit parameters are considered to arise from solid state diffusion characteristics (closed Warburg regime) and are therefore excluded from analysis and discussion due to the focus on electrode surface phenomena.
Any cell specific fit parameter datasets reveal negligible dependency of surface resistance with state of charge (SOC). While surface resistance fit parameters are well aligned for both non laminated cells, the average surface resistance fit parameter of the laminated cells show clear deviation (more details in
Table 1).
In the cycling test, on the other hand, the laminated cells showed higher capacity retention then the reference cells (see
Figure 7). While non-laminated cells in cycle 600 only reached 68.9% of their initial capacity, the laminated cells still reached 82.6%. This represents a decrease in capacity fading of 44%.
3.4. Electrochemical Testing—Polyethylene Separator
Since the PE separator is—in contrast to the ceramic separator—sensitive to thermal stress, the edge cases of strong adhesion accompanied by high thermo-mechanical stress as well as moderate thermal stress leading to weak adhesion between cell components are examined.
At first, the high stress, strong adhesion limit was tested. For this purpose, ESLs were laminated at 100 °C and 12 N/mm. According to the gurley tests, structural changes on the separator cannot be excluded here. The influence of coating thickness was also taken into consideration. Pouch cells were built and tested for each of four different surface loadings. As a reference, cells with the unmodified PE separator were built without laminating its components.
At low currents, the cells with modified separators showed no distinctive behavior, but when the charge and discharge currents were increased to more than 1 C, the capacity of the laminated cells with coated separators was lower compared to the reference. While the reference cells with PE separator could still deliver almost 80% of their nominal capacity when discharged at 2 C, the laminated cells did not even deliver 60% (see
Figure 8). No clear correlation between the amount of polymer applied and the cell performance could be observed. In the cycling test, the laminated cells showed a slightly increased aging rate compared with the non-laminated reference cells (see
Figure 9). After 500 cycles at 1 C, the cells with the thickest fiber layer reached 67% of their original capacity while the non-laminated cells remained at 73%.
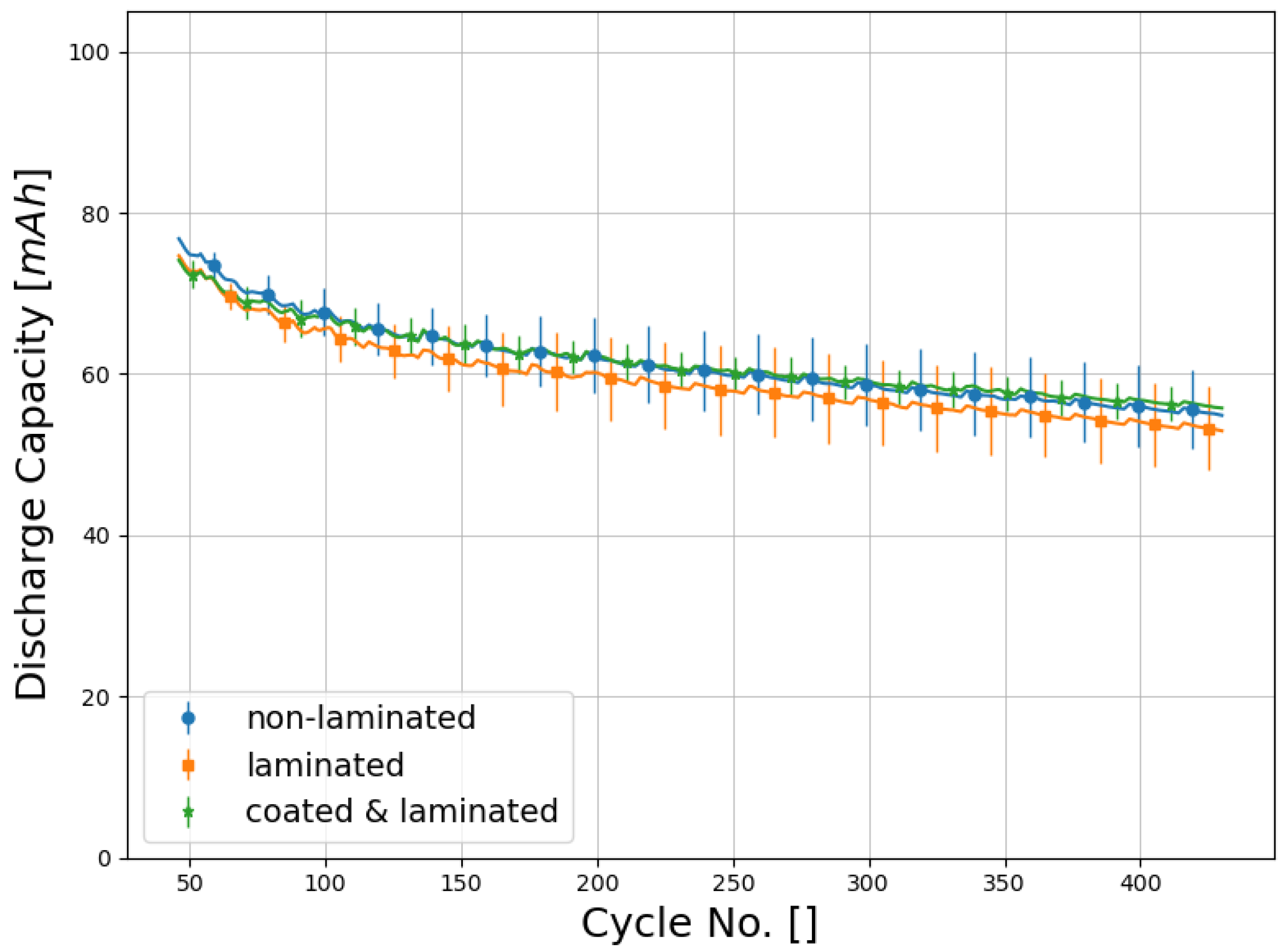
Secondly the low stress, low adhesion case was investigated. To reduce the thermal stress to which the separator is subjected, further cells were assembled using mild lamination conditions of 60 °C and 5 N/mm, at the expense of interface stability. The interfaces of ESLs assembled under these conditions do not break due to the load of their own weight, but they won’t withstand much higher forces. All non-reference cells employed separators coated with 245 mg/cm of polymer. ESLs with non-coated separators also passed the laminator at operating conditions. This way, no adhesion at all between cell components is achieved, but effects caused by the additional coating layer and effects caused by lamination can be distinguished from each other.
In contrast to the previous experiment, no significant deterioration in either C-rate (
Figure 10), or cycling stability (
Figure 11) was observed. In cycle 430, non-laminated cells in average showed a capacity of 71.5% compared to the start of the cycling test. Laminated cells with unmodified separator behaved in a similar manner (70.9%), whereas degradation of laminated cells with coated separators was slightly slower (75.3%).
4. Discussion
In this study we demonstrated, that electrospinning is a well suited technique for applying functional polymer layers to commercial battery separators, enabling lamination in cell assembly. Stable interfaces between the cell components can be created, what enables higher process speeds in battery production.
The alternate production route shown, including electrospinning and lamination processes, is well applicable to thermally stable separators with low requirements to the melting properties of the applied polymer.
This was demonstrated on a ceramic impregnated polyester nonwoven separator. No significant changes in Gurley porosity up to temperatures of 160 °C and 8 N/mm line force were found, setting low limitations for the thermo-mechanical stress applied in the lamination step. In consequence we selected a well-established PVdF-HFP-copolymer with a melting point of 154 °C as coating material. Lamination of the coated separator and a graphite anode results in stable interfaces for temperatures above 160 °C—corresponding to the polymer’s melting point. Significant improvements in rate stability could be observed, while the current rate stability remains unchanged. This means that the porous coating doesn’t impair ion transport between the electrodes. EIS fit parameters reveal typical trends that are well known for NMC graphite single cells. While surface capacitance fit parameters (see
Supplementary Material, Figure S4) are found in a typical range of double layer capacitances [
15], there is a clear deviation by three orders of magnitude to the charge transfer (CT) capacitance fit parameters (see
Supplementary Material, Figure S4). This is a well known artifact arising from normalization to geometric electrode surface (despite BET electrode surface) and can therefore act as an indicator for correct assignment of the semicircle fit parameters [
6,
15]. Surface resistance fit parameters (see
Figure 6) in general are significantly higher than in any of our previous studies that included EIS on NMC—graphite single cells [
6,
7,
8,
14], or in comparable literature [
16,
17]. This might either suggest an extraordinary high contribution of the cathode current collector interface (non calendered cathode) or indicate an additional contribution of the separator itself, which was not reported in literature so far to our best knowledge. In our earlier studies, we found clear indications of lamination to both reduce cathode current collector (cathodic lamination) and SEI (anodic lamination) contributions to the surface resistance. Though in this case, due to the large spreading in case of laminated cells, a significant effect of the lamination to the surface resistance is statistically not evident. Nevertheless, the suggested tendency for surface resistance reduction (while increasing statistical spreading) would directly imply analogous trends to voltage overpotentials during charge/discharge cycles, with rising relevance at elevated C rates. This trend suggested by EIS correlates very well with our findings in lamination influences onto rate capability of cells assembled with ceramic separator (see
Figure 5).
In expanding the field of application of the method to more thermo-sensitive separators, additional dependencies have to be considered. By close examination of the separator’s load limits, the choice of a special coating polymer well adapted to it and the selection of appropriate process parameters, this obstacle can be overcome. In particular, good adhesion at the electrode-separator interface and low stress to the cell components are competing parameters, where the improvement of one generally leads to a deterioration of the other.
These interrelations were illustrated on a monolayer PE separator. In contrast to the ceramic, that PE separator already shows a slight increase in Gurley value when laminated at ≥80 °C with 8 N/mm and its pores completely close at >130 °C. So, for its coating, a novel fluoroelastomer, with adjusted thermal properties was introduced for battery applications. The adhesion force measured between separator and anode was found to increase linearly with the area mass loading of the interlayer. Increasing lamination temperatures and line force also strongly correlate with the adhesive strength on the interface. The impact of these variables on full cell performance was demonstrated at two fringe cases: (a) strong adhesion as result of high thermo-mechanical stress with moderate damage to the separator and (b) minimal adhesion needed for further processing in order to preserve the separator. In the first case, deterioration both in the C-rate test and during cycling was observed. One reason for the reduced rate stability of these cells could be, that the binder closes the pores of the separator during lamination, inhibiting ion transport. However, the structure of the separator itself could also have been altered by the effects of pressure and temperature, thereby increasing the cell’s internal resistance.
As no clear correlation between the amount of polymer applied and the cell performance could be observed, the second hypothesis of damages to the separator structure itself during lamination seems more likely.
Our data also indicates, that Gurley measurements are indeed a simple and capable method to detect a separator’s upper limits for lamination conditions. Determination of ionic conductivity and tortuosity could provide more detailed insights into these relationships [
18].
In contrast, lamination at mild conditions has no negative effect on electro-chemical performance. This shows, that the method can even be used to take advantage of the benefits of lamination even when using common polyolefin separators. In this case, the lower limit for adhesion depends on the mechanical stress which ESLs are subjected to in the following process steps. It can be assumed, that optimized adhesion, without detrimental—or even positive—effects to battery performance can be achieved for lamination parameter sets in between the two investigated fringe cases.
Table 2 presents an overview of observed effects on electrochemical performance for all investigated cell series.
The discussed effects of lamination on battery performance are in line with the observations of R. Leithoff et al. [
13], who also reported significantly reduced discharge capacities for cells with polyolefin-based separators laminated at elevated temperature and pressure during cycling tests.
Our findings in identical (
Figure 5 and
Figure 10) or slightly diminished (
Figure 8) C-rate capability of laminated cells compared to non-laminated cells is in contrast to our earlier findings on lamination of specialized laminable separators (PVdF-Al
O
), where we found indications that lamination reduces internal resistances upon cathode-separator lamination [
6], as well as to clearly improve C-rate capability upon full (anode-separator-cathode) lamination [
7]. These findings were attributed to additional cathode contraction, similar to known layered-oxide-cathode calendering effects [
16,
17], of improving the cathode-current collector interface. As we didn’t find such changes in C-rate capability here, we consider our pristine reference cathode yet at a fully optimized compression/adhesion state.
Also, in our earlier studies we presented first hints for a mechanistic model of lamination to change aging phenomena: Fundamental homogenization of pore sizes at the electrode-separator interface [
7] is expected to reduce local spots of increased resistance [
6,
7]. The resulting homogenized current distribution/reduced polarization tendency is expected to reduce the risk of lithium-plating and unfavorable SEI growth at high charge currents [
6,
7,
8].
In our current study, a significant pore size reduction at the electrode-separator interface can almost be neglected as the amount of thermoplastic (laminable) polymer is minimal due to our electrospinning approach. So changes of lithium plating trend or SEI growth are not expected to change in a significant extent after electrospinning & lamination.
Nevertheless, we found clear improvements in capacity retention of laminated cells even at a moderate 1 C cycling test (see
Figure 7). As the effects are not expected to arise from lithium plating trends or SEI growth phenomena, it is clear that our earlier model on electrochemical effects of electrode-separator lamination cannot explain all apparent effects. It might be necessary to also consider gassing phenomena (and their interaction with electrode-separator lamination) as a partial driving force of capacity retention during cycling test of non-compressed pouch cells more than typically considered today. So far, gassing phenomena and fundamental influences to internal cell pressure are typically in focus during formation cycles [
19]. Possibly, our reported effects of electrospinning & lamination during 1 C cycling test might indicate similarities to known effects of permanent cell compression [
20,
21], though only occurring selectively in special cases (compare
Figure 7,
Figure 9 and
Figure 11).