Physical-Chemical Properties Modification of Hermetia Illucens Larvae Oil and Diesel Fuel for the Internal Combustion Engines Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
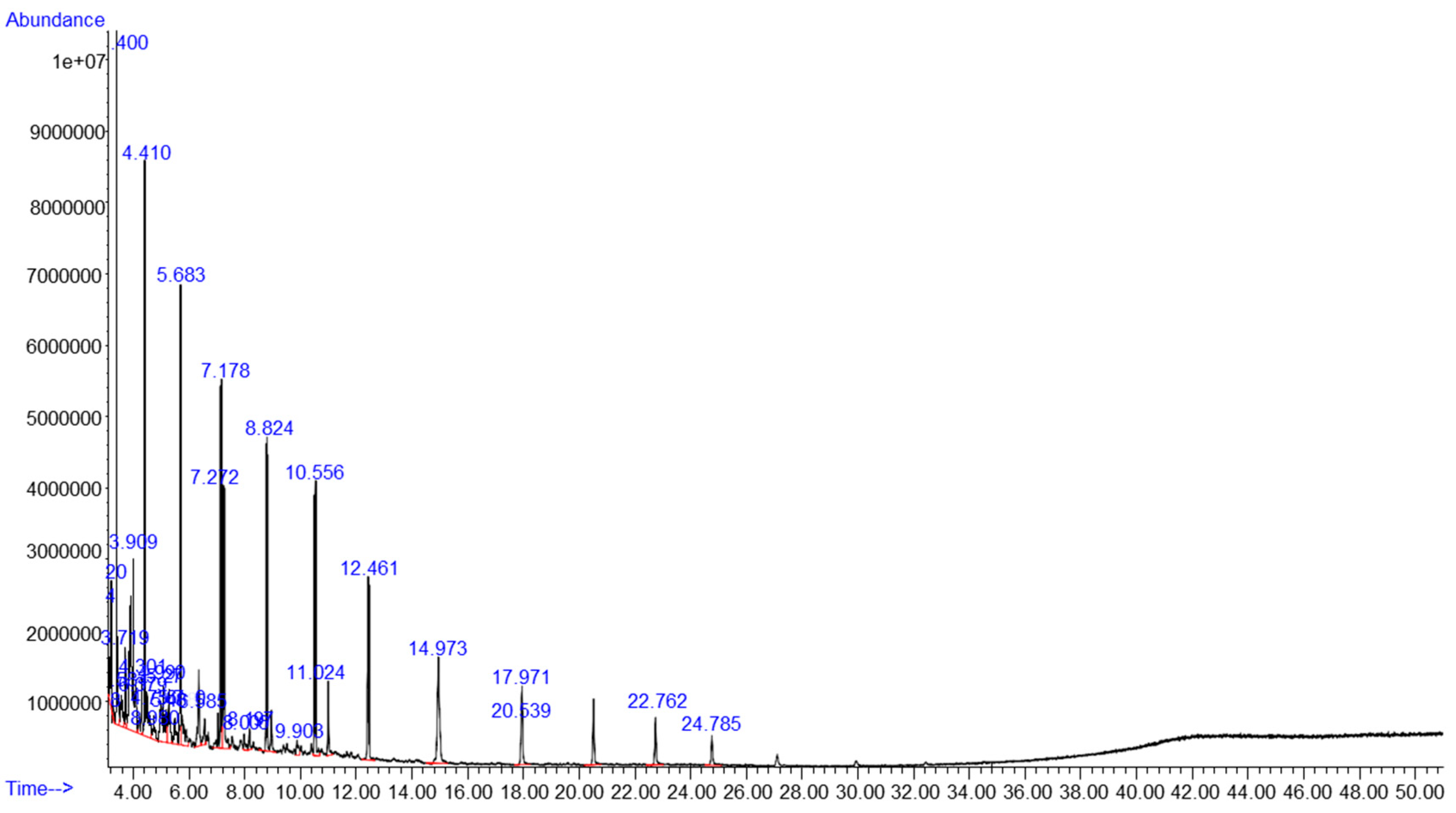
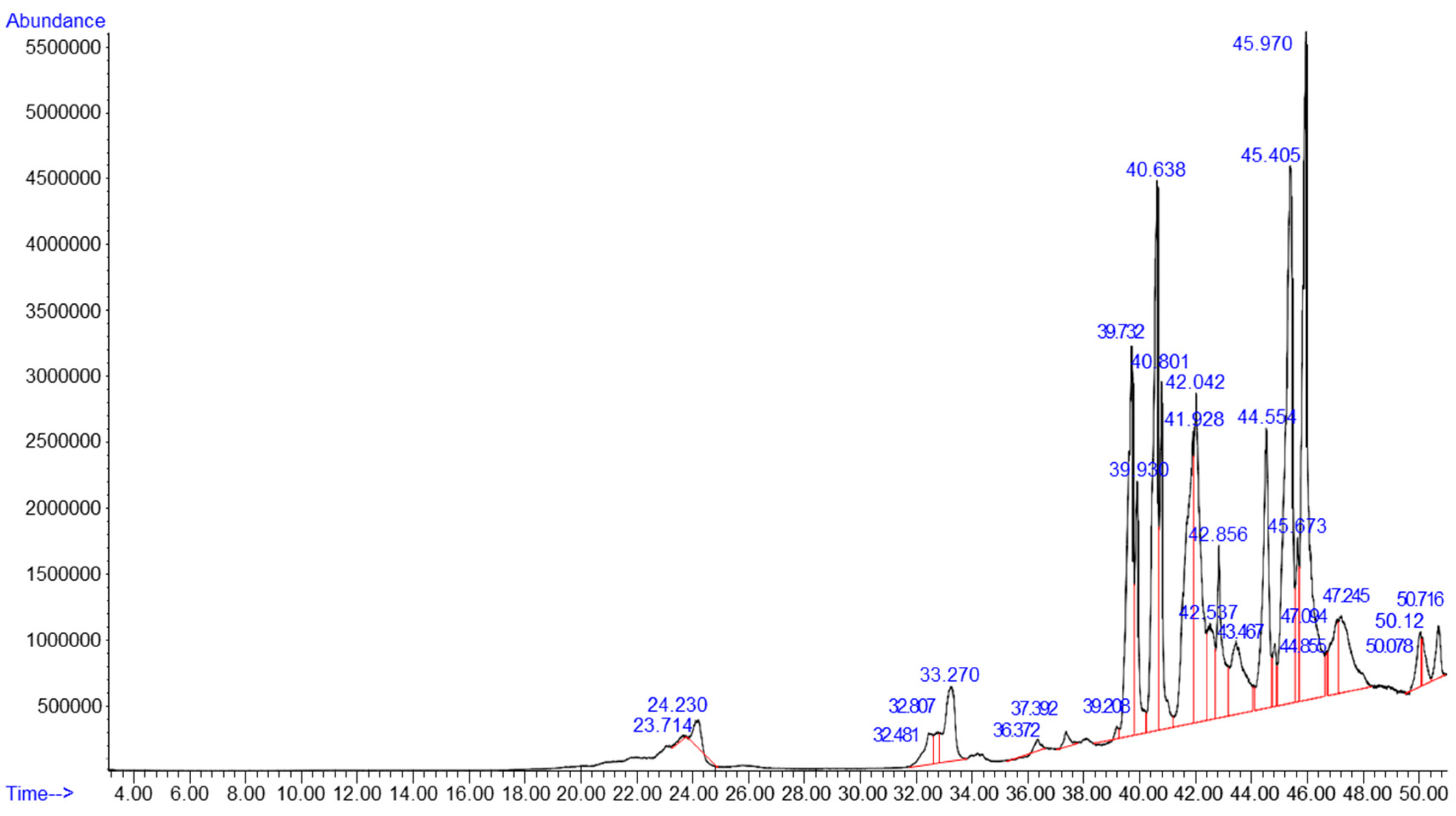
2.2. Chemical Composition
2.3. CHNOS Elements
2.4. Density
2.5. Viscosity
2.6. Heating Value
2.7. Cetane Number
2.8. Flash Point
3. Results and Discussion
3.1. Chemical Composition of HILO and Diesel Fuel
3.2. Influence of HILO on Density
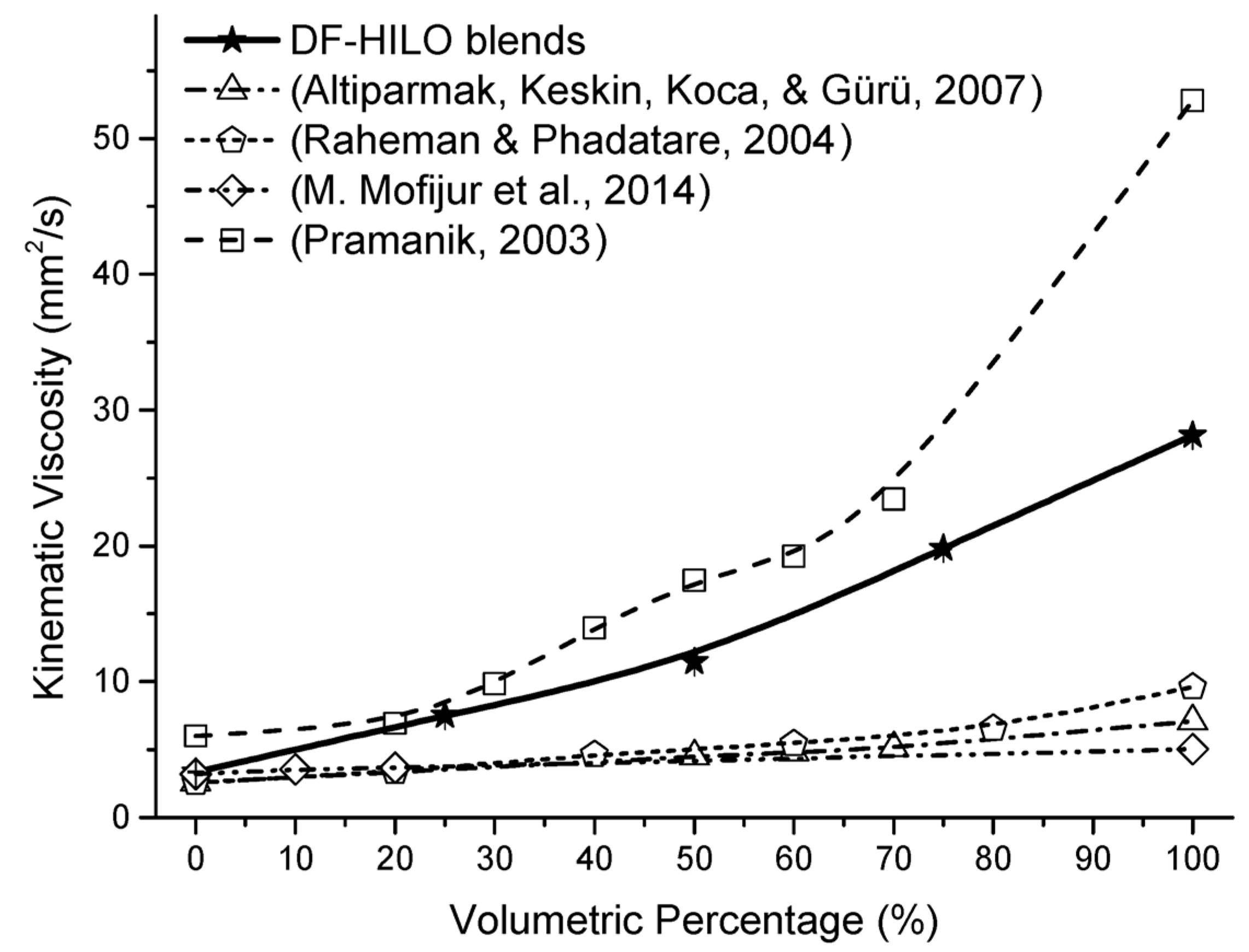
3.3. Influence of HILO on Viscosity
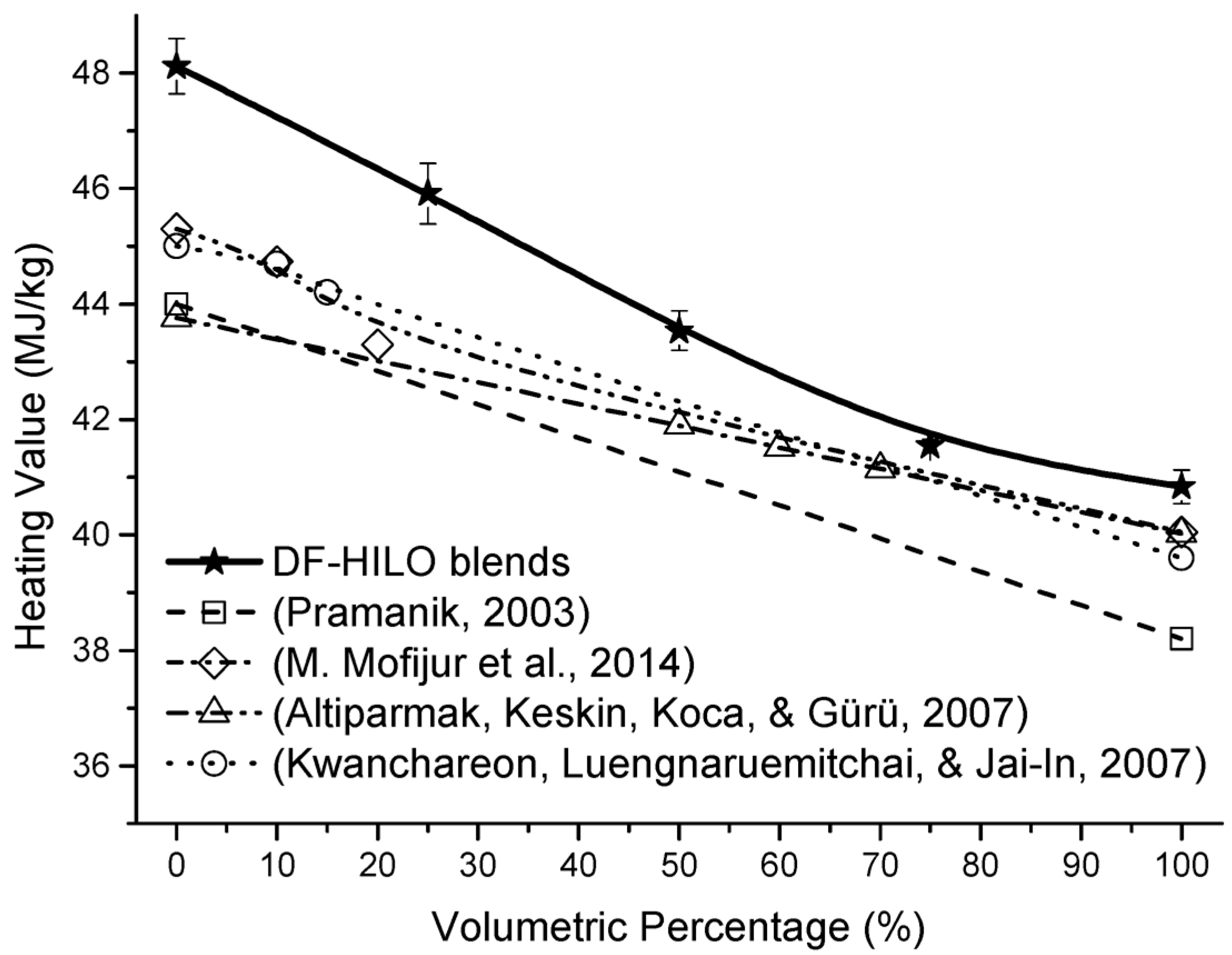
3.4. Influence of HILO on Heating Value
3.5. Influence of HILO on Cetane Number
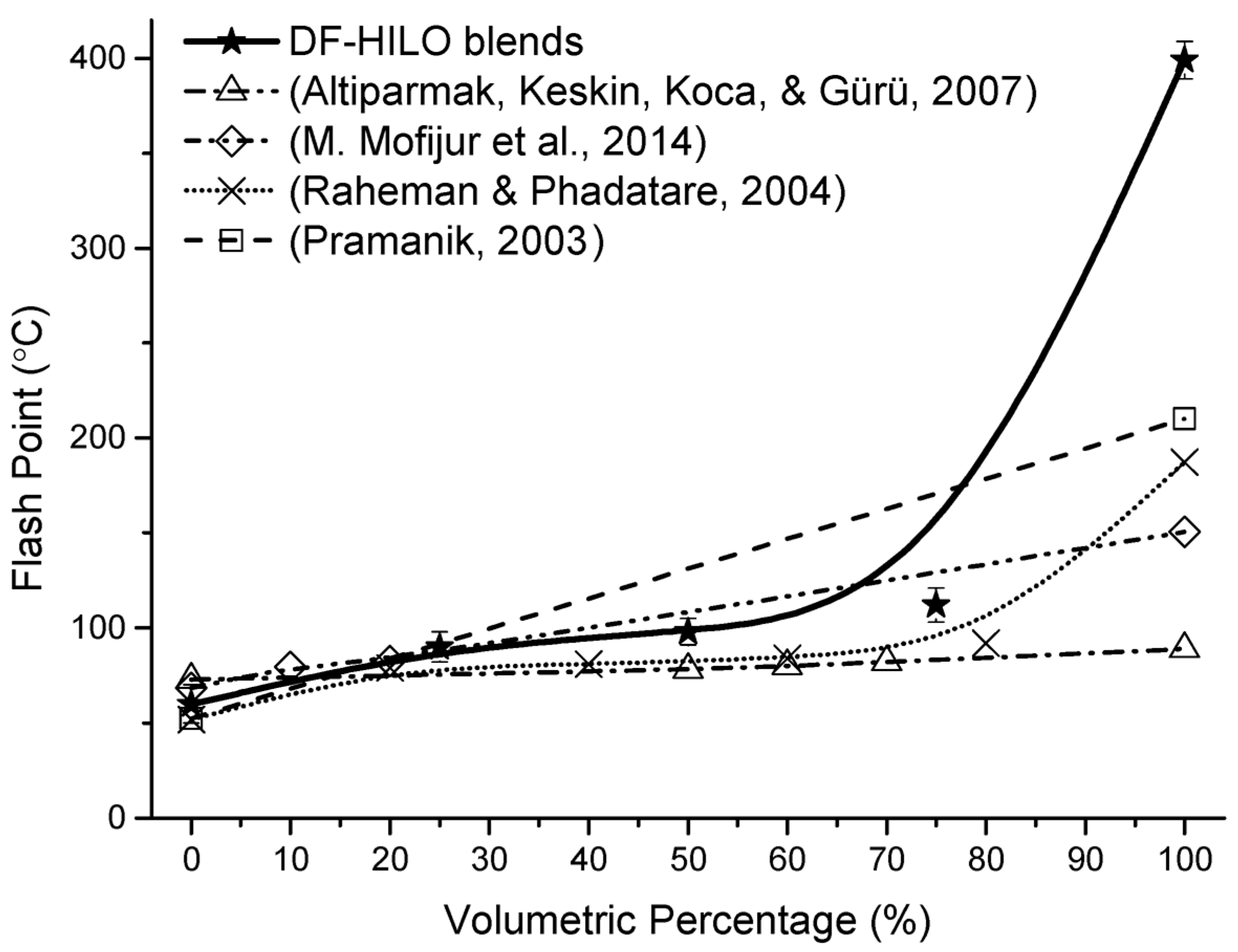
3.6. Influence of HILO on Flash Point
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | Compound Name | Structure/Shorthand | Formula | Molar Mass (g/mol) | Hydrocarbon Family | Saturated/Unsaturated | Stability | DF (wt.%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Naphthalene, 1,6-dimethyl- | C12:5 | C12H12 | 156.22 | Aromatics | Highly unsaturated | Most unstable | 10.0334 |
| 2 | Pentadecane | C15:0 | C15H32 | 212.421 | Paraffin | Saturated | Stable | 9.4548 |
| 3 | Tetradecane | C14:0 | C14H30 | 198.39 | Paraffin | Saturated | Stable | 8.7604 |
| 4 | Heptadecane | C17:0 | C17H36 | 240.5 | Paraffin | Saturated | Stable | 7.7215 |
| 5 | Hexadecane | C16:0 | C16H34 | 226.44 | Paraffin | Saturated | Stable | 7.3136 |
| 6 | Octadecane | C18:0 | C18H38 | 254.5 | Paraffin | Saturated | Stable | 6.2162 |
| 7 | Pentadecane, 2,6,10,14-tetramethyl- | C19:0 | C19H40 | 268.5 | Paraffin | Saturated | Stable | 6.194 |
| 8 | Nonadecane | C19:0 | C19H40 | 268.5 | Paraffin | Saturated | Stable | 5.7505 |
| 9 | Eicosane | C20:0 | C20H42 | 282.5 | Paraffin | Saturated | Stable | 4.8809 |
| 10 | Heneicosane | C21:0 | C21H44 | 296.6 | Paraffin | Saturated | Stable | 4.8401 |
| 11 | Naphthalene, 1,6,7-trimethyl- | C13:5 | C13H14 | 170.25 | Aromatics | Highly unsaturated | Most unstable | 3.8814 |
| 12 | Eicosane | C20:0 | C20H42 | 282.5 | Paraffin | Saturated | Stable | 2.4984 |
| 13 | Pentadecane, 2,6,10-trimethyl- | C18:0 | C18H38 | 254.5 | Paraffin | Saturated | Stable | 2.1935 |
| 14 | Docosane, 11-decyl- | C32:0 | C32H66 | 450.9 | Paraffin | Saturated | Stable | 1.897 |
| 15 | Dodecane, 2,6,11-trimethyl- | C15:0 | C15H32 | 212.41 | Paraffin | Saturated | Stable | 1.7759 |
| 16 | Anthracene, 1,2,3,4-tetrahydro- | C14:5 | C14H14 | 182.266 | Aromatics | Highly unsaturated | Most unstable | 1.737 |
| 17 | 17-Pentatriacontene | C35:1 | C35H70 | 490.9 | Olefins | Unsaturated | Unstable | 1.6309 |
| 18 | Pentadecanoic acid, 14-methyl-, methyl ester | C17:0 | C17H34O2 | 270.5 | Carboxylic acids | Saturated | Very stable | 1.6144 |
| 19 | Naphthalene, 1,4,6-trimethyl- | C13:5 | C13H14 | 170.25 | Aromatics | Highly unsaturated | Most unstable | 1.3927 |
| 20 | 11,12-Dibromo-tetradecan-1-ol acetate | C16:0 | C16H30Br2O2 | 414.2 | Carboxylic acids | Saturated | Very stable | 1.3603 |
| 21 | Tricosane | C23:0 | C23H48 | 324.6 | Paraffin | Saturated | Stable | 1.3463 |
| 22 | Decahydro-4,4,8,9,10-pentamethylnaphthalene | C15:0 | C15H28 | 208.38 | Aromatics | Highly unsaturated | Most unstable | 1.3274 |
| 23 | Naphthalene, 1,5-dimethyl- | C12:5 | C12H12 | 156.22 | Aromatics | Highly unsaturated | Most unstable | 1.2802 |
| 24 | Nonacosane | C29:0 | C29H60 | 408.8 | Paraffin | Saturated | Stable | 1.0636 |
| 25 | Naphthalene, 1,4,5-trimethyl- | C13:5 | C13H14 | 170.25 | Aromatics | Highly unsaturated | Most unstable | 1.0588 |
| 26 | Tetracosane | C24:0 | C24H50 | 338.7 | Paraffin | Saturated | Stable | 0.8935 |
| 27 | Naphthalene, 1,6,7-trimethyl- | C13:5 | C13H14 | 170.25 | Aromatics | Highly unsaturated | Most unstable | 0.8599 |
| 28 | Benzene, (4,5,5-trimethyl-1,3-cyclopentadien-1-yl)- | C14:5 | C14H16 | 184.28 | Aromatics | Highly unsaturated | Most unstable | 0.5142 |
| 29 | Octadecane, 1-(ethenyloxy)- | C20:0 | C20H40O | 296.5 | Paraffin | Saturated | Stable | 0.5093 |
| 100.0001 |
Appendix B
| No. | Compound Name | Structure/Shorthand | Formula | Molar Mass (g/mol) | Hydrocarbon Family | Saturated/Unsaturated | Stability | HILO (wt.%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Dodecanoic acid, 1,2,3-propanetriyl ester | C39:0 | C39H74O6 | 639.00 | Carboxylic acids | Saturated | Very stable | 74.051 |
| 2 | 3-[7-Bromo-5-(2-chloro-phenyl)-2-oxo-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-ylamino]-benzoic acid | - | C19H14BrClN2O5 | 465.7 | Carboxylic acids | Saturated | Very stable | 13.858 |
| 3 | Indole-3-carboxylic acid, 1-benzyl-5-methoxy-2-methyl-, ethyl ester | - | - | - | Carboxylic acids | - | - | 3.6025 |
| 4 | Aprobarbital, bis(trimethylsilyl)- | - | - | - | - | - | - | 3.4296 |
| 5 | Carbamic acid, N-(3-chloro-4-methoxyphenyl)-, glycidyl ester | - | C10H12ClNO2 | 213.66 | Carboxylic acids | Saturated | Very stable | 2.6763 |
| 6 | Lauric anhydride | C24:0 | C24H46O3 | 382.6 | Carboxylic acids | Saturated | Very stable | 0.9252 |
| 7 | N-Trifluoroacetyldemecolcine | - | C23H24F3NO6 | 467.4 | - | - | - | 0.6915 |
| 8 | Iron, cyclopentadienyl-ethyl-1,2-diisopropylphosphinoethane | - | C21H44FeP2+6 | 414.4 | - | - | - | 0.6359 |
| 9 | Trans-4′-Dimethylamino-4-(methylthio)chalcone | - | C17H17NO | 251.32 | Ketones | - | - | 0.0771 |
| 10 | Aluminum, bis(2-methylpropyl)(2,4-pentanedionato-O,O’)-, (T-4)- | - | - | - | - | - | - | 0.0527 |
| 99.9998 |
References
- Kalu-Uka, G.M.; Kumar, S.; Kalu-Uka, A.C.; Vikram, S.; Okorafor, O.O.; Kigozi, M.; Ihekweme, G.O.; Onwualu, A.P. Prospects for biodiesel production from Macrotermes nigeriensis: Process optimization and characterization of biodiesel properties. Biomass Bioenergy 2021, 146, 105980. [Google Scholar] [CrossRef]
- Kadirgama, G.; Kamarulzaman, M.K.; Ramasamy, D.; Kadirgama, K.; Hisham, S. Classification of Lubricants Base Oils for Nanolubricants Applications—A Review. In Technological Advancement in Mechanical and Automotive Engineering; Springer: Singapore, 2023; pp. 205–213. [Google Scholar]
- De Oliveira, A.; Valente, O.S.; Sodré, J.R. Effects of ethanol addition to biodiesel-diesel oil blends (B7 and B20) on engine emissions and fuel consumption. MRS Adv. 2017, 2, 4005–4015. [Google Scholar] [CrossRef]
- Ali, M.H.; Adam, A.; Yasin, M.H.M.; Kamarulzaman, M.K.; Othman, M.F. Mitigation of NOx emission by monophenolic antioxidants blended in POME biodiesel blends. Greenh. Gases Sci. Technol. 2020, 10, 829–839. [Google Scholar] [CrossRef]
- Mihankhah, T.; Delnavaz, M.; Khaligh, N.G. Application of TiO2 nanoparticles for eco-friendly biodiesel production from waste olive oil. Int. J. Green Energy 2018, 15, 69–75. [Google Scholar] [CrossRef]
- Bakar, R.; Kadirgama, K.; Ramasamy, D.; Yusaf, T.; Kamarulzaman, M.; Aslfattahi, N.; Samylingam, L.; Alwayzy, S.H. Experimental analysis on the performance, combustion/emission characteristics of a DI diesel engine using hydrogen in dual fuel mode. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Inda, C.S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A comprehensive review of physicochemical properties, production process, performance and emissions characteristics of 2nd generation biodiesel feedstock: Jatropha curcas. Fuel 2021, 285, 119110. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Aransiola, E.; Ehinmitola, E.; Adebimpe, A.; Shittu, T.; Solomon, B. Prospects of Biodiesel Feedstock as an Effective Ecofuel Source and Their Challenges. In Advances in Eco-Fuels for a Sustainable Environment; Woodhead Publishing: Sawston, UK, 2019; pp. 53–87. [Google Scholar]
- Das, P.; Gundimeda, H. Is biofuel expansion in developing countries reasonable? A review of empirical evidence of food and land use impacts. J. Clean. Prod. 2022, 372, 133501. [Google Scholar] [CrossRef]
- Knápek, J.; Králík, T.; Vávrová, K.; Valentová, M.; Horák, M.; Outrata, D. Policy implications of competition between conventional and energy crops. Renew. Sustain. Energy Rev. 2021, 151, 111618. [Google Scholar] [CrossRef]
- Mairizal, A.Q.; Awad, S.; Priadi, C.R.; Hartono, D.M.; Moersidik, S.S.; Tazerout, M.; Andres, Y. Experimental study on the effects of feedstock on the properties of biodiesel using multiple linear regressions. Renew. Energy 2020, 145, 375–381. [Google Scholar] [CrossRef]
- Mukhtar, A.; Saqib, S.; Lin, H.; Shah, M.U.H.; Ullah, S.; Younas, M.; Rezakazemi, M.; Ibrahim, M.; Mahmood, A.; Asif, S. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew. Sustain. Energy Rev. 2022, 157, 112012. [Google Scholar] [CrossRef]
- Kamarulzaman, M.K.; Abdullah, A.; Mamat, R. Combustion, performances, and emissions characteristics of Hermetia illucens larvae oil in a direct injection compression ignition engine. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1483–1496. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Prathima, A.; Periyasamy, M.; Mahendran, G. Assessment of the use of Codium Decorticafum [Green seaweed] biodiesel and pyrolytic waste tires oil blends in CI engine. Mater. Today Proc. 2020, 33, 4224–4227. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; Mata, T.; Martins, A.; Freitas, M.; Caetano, N. Economic analysis of microalgae biodiesel production in a small-scale facility. Energy Rep. 2020, 6, 325–332. [Google Scholar] [CrossRef]
- Tran, N.N.; Tišma, M.; Budžaki, S.; McMurchie, E.J.; Ngothai, Y.; Morales Gonzalez, O.M.; Hessel, V. Production of Biodiesel from Recycled Grease Trap Waste: A Review. Ind. Eng. Chem. Res. 2021, 60, 16547–16560. [Google Scholar] [CrossRef]
- Konur, O. Waste Oil-Based Biodiesel Fuels: A Scientometric Review of the Research. In Biodiesel Fuels Based on Edible and Nonedible Feedstocks, Wastes, and Algae; CRC Press: Boca Raton, FL, USA, 2021; pp. 599–622. [Google Scholar]
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The challenge of feeding the world. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef]
- Kolet, M.; Zerbib, D.; Nakonechny, F.; Nisnevitch, M. Production of Biodiesel from Brown Grease. Catalysts 2020, 10, 1189. [Google Scholar] [CrossRef]
- Mizik, T.; Gyarmati, G. Economic and sustainability of biodiesel production—A systematic literature review. Clean Technol. 2021, 3, 19–36. [Google Scholar] [CrossRef]
- Kamarulzaman, M.K.; Abdullah, A. Multi-objective optimization of diesel engine performances and exhaust emissions characteristics of Hermetia illucens larvae oil-diesel fuel blends using response surface methodology. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–14. [Google Scholar] [CrossRef]
- Ali, S.S.; Al-Tohamy, R.; Mahmoud, Y.A.-G.; Kornaros, M.; Sun, S.; Sun, J. Recent advances in the life cycle assessment of biodiesel production linked to azo dye degradation using yeast symbionts of termite guts: A critical review. Energy Rep. 2022, 8, 7557–7581. [Google Scholar] [CrossRef]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. Fat body—Multifunctional insect tissue. Insects 2021, 12, 547. [Google Scholar] [CrossRef]
- Manzano-Agugliaro, F.; Sanchez-Muros, M.; Barroso, F.; Martínez-Sánchez, A.; Rojo, S.; Pérez-Bañón, C. Insects for biodiesel production. Renew. Sustain. Energy Rev. 2012, 16, 3744–3753. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Cai, H.; Garza, E.; Yu, Z.; Zhou, S. From organic waste to biodiesel: Black soldier fly, Hermetia illucens, makes it feasible. Fuel 2011, 90, 1545–1548. [Google Scholar] [CrossRef]
- Surendra, K.; Olivier, R.; Tomberlin, J.K.; Jha, R.; Khanal, S.K. Bioconversion of organic wastes into biodiesel and animal feed via insect farming. Renew. Energy 2016, 98, 197–202. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Zheng, L.; Wang, Y.; Zhang, J.; Yu, Z.; Zhang, Y. Potential biodiesel and biogas production from corncob by anaerobic fermentation and black soldier fly. Bioresour. Technol. 2015, 194, 276–282. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, L.; Qiu, N.; Cai, H.; Tomberlin, J.K.; Yu, Z. Bioconversion of dairy manure by black soldier fly (Diptera: Stratiomyidae) for biodiesel and sugar production. Waste Manag. 2011, 31, 1316–1320. [Google Scholar] [CrossRef]
- Furman, D.P.; Young, R.D.; Catts, P.E. Hermetia illucens (Linnaeus) as a factor in the natural control of Musca domestica Linnaeus. J. Econ. Entomol. 1959, 52, 917–921. [Google Scholar] [CrossRef]
- Wang, C.; Qian, L.; Wang, W.; Wang, T.; Deng, Z.; Yang, F.; Xiong, J.; Feng, W. Exploring the potential of lipids from black soldier fly: New paradigm for biodiesel production (I). Renew. Energy 2017, 111, 749–756. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Sheppard, D.C. Factors influencing mating and oviposition of black soldier flies (Diptera: Stratiomyidae) in a colony. J. Entomol. Sci. 2002, 37, 345–352. [Google Scholar] [CrossRef]
- Myers, H.M.; Tomberlin, J.K.; Lambert, B.D.; Kattes, D. Development of black soldier fly (Diptera: Stratiomyidae) larvae fed dairy manure. Environ. Entomol. 2014, 37, 11–15. [Google Scholar] [CrossRef]
- Parra Paz, A.S.; Carrejo, N.S.; Gómez Rodríguez, C.H. Effects of larval density and feeding rates on the bioconversion of vegetable waste using black soldier fly larvae Hermetia illucens (L.), (Diptera: Stratiomyidae). Waste Biomass Valorization 2015, 6, 1059–1065. [Google Scholar] [CrossRef]
- Sealey, W.M.; Gaylord, T.G.; Barrows, F.T.; Tomberlin, J.K.; McGuire, M.A.; Ross, C.; St-Hilaire, S. Sensory analysis of rainbow trout, Oncorhynchus mykiss, fed enriched black soldier fly prepupae, Hermetia illucens. J. World Aquac. Soc. 2011, 42, 34–45. [Google Scholar] [CrossRef]
- Kamarulzaman, M.K.; Hafiz, M.; Abdullah, A.; Chen, A.F.; Awad, O.I. Combustion, performances and emissions characteristics of black soldier fly larvae oil and diesel blends in compression ignition engine. Renew. Energy 2019, 142, 569–580. [Google Scholar] [CrossRef]
- Zheng, L.; Li, Q.; Zhang, J.; Yu, Z. Double the biodiesel yield: Rearing black soldier fly larvae, Hermetia illucens, on solid residual fraction of restaurant waste after grease extraction for biodiesel production. Renew. Energy 2012, 41, 75–79. [Google Scholar] [CrossRef]
- John, C.B.; Raja, S.A.; Deepanraj, B.; Ong, H. Palm stearin biodiesel: Preparation, characterization using spectrometric techniques and the assessment of fuel properties. Biomass Convers. Biorefinery 2022, 12, 1679–1693. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E.; Ledakowicz, S. Stoichiometry of the aerobic biodegradation of the organic fraction of municipal solid waste (MSW). Biodegradation 2003, 14, 51–56. [Google Scholar] [CrossRef]
- Dogan, B.; Cakmak, A.; Yesilyurt, M.K.; Erol, D. Investigation on 1-heptanol as an oxygenated additive with diesel fuel for compression-ignition engine applications: An approach in terms of energy, exergy, exergoeconomic, enviroeconomic, and sustainability analyses. Fuel 2020, 275, 117973. [Google Scholar] [CrossRef]
- Misra, N.N.; Rai, D.K.; Hossain, M. Chapter 10—Analytical Techniques for Bioactives from Seaweed. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 271–287. [Google Scholar]
- Bacha, J.; Freel, J.; Gibbs, A.; Gibbs, L.; Hemighaus, G. Diesel Fuels Technical Review; Chevron Production Company: San Ramon, CA, USA, 2007. [Google Scholar]
- Pulkrabek, W.W. Engineering Fundamentals of the Internal Combustion Engine; Pearson Prentice Hall: Hoboken, NJ, USA, 2004. [Google Scholar]
- Ganesan, V. Internal Combustion Engines; McGraw-Hill: New York, NY, USA, 1994. [Google Scholar]
- Ganesan, V. Internal Combustion Engines, 3rd ed.; McGraw-Hill Education (India) Pvt Limited: Karnataka, India, 2008. [Google Scholar]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.; Masjuki, H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Altıparmak, D.; Keskin, A.; Koca, A.; Gürü, M. Alternative fuel properties of tall oil fatty acid methyl ester–diesel fuel blends. Bioresour. Technol. 2007, 98, 241–246. [Google Scholar] [CrossRef]
- Pramanik, K. Properties and use of jatropha curcas oil and diesel fuel blends in compression ignition engine. Renew. Energy 2003, 28, 239–248. [Google Scholar] [CrossRef]
- Kwanchareon, P.; Luengnaruemitchai, A.; Jai-In, S. Solubility of a diesel–biodiesel–ethanol blend, its fuel properties, and its emission characteristics from diesel engine. Fuel 2007, 86, 1053–1061. [Google Scholar] [CrossRef]
- Mofijur, M.; Masjuki, H.; Kalam, M.; Atabani, A.; Arbab, M.; Cheng, S.; Gouk, S. Properties and use of Moringa oleifera biodiesel and diesel fuel blends in a multi-cylinder diesel engine. Energy Convers. Manag. 2014, 82, 169–176. [Google Scholar] [CrossRef]
- Torres-Jimenez, E.; Jerman, M.S.; Gregorc, A.; Lisec, I.; Dorado, M.P.; Kegl, B. Physical and chemical properties of ethanol–diesel fuel blends. Fuel 2011, 90, 795–802. [Google Scholar] [CrossRef]
- Kegl, B. Experimental investigation of optimal timing of the diesel engine injection pump using biodiesel fuel. Energy Fuels 2006, 20, 1460–1470. [Google Scholar] [CrossRef]
- Raheman, H.; Phadatare, A.G. Diesel engine emissions and performance from blends of karanja methyl ester and diesel. Biomass Bioenergy 2004, 27, 393–397. [Google Scholar] [CrossRef]
- Demirbas, A. Relationships derived from physical properties of vegetable oil and biodiesel fuels. Fuel 2008, 87, 1743–1748. [Google Scholar] [CrossRef]
- Atmanli, A. Effects of a cetane improver on fuel properties and engine characteristics of a diesel engine fueled with the blends of diesel, hazelnut oil and higher carbon alcohol. Fuel 2016, 172, 209–217. [Google Scholar] [CrossRef]
- Fayyazbakhsh, A.; Pirouzfar, V. Investigating the influence of additives-fuel on diesel engine performance and emissions: Analytical modeling and experimental validation. Fuel 2016, 171, 167–177. [Google Scholar] [CrossRef]
- Jose, T.K.; Anand, K. Effects of biodiesel composition on its long term storage stability. Fuel 2016, 177, 190–196. [Google Scholar] [CrossRef]
- Mosarof, M.; Kalam, M.; Masjuki, H.; Ashraful, A.; Rashed, M.; Imdadul, H.; Monirul, I. Implementation of palm biodiesel based on economic aspects, performance, emission, and wear characteristics. Energy Convers. Manag. 2015, 105, 617–629. [Google Scholar] [CrossRef]
- Bhuiya, M.; Rasul, M.; Khan, M.; Ashwath, N.; Azad, A.; Hazrat, M. Prospects of 2nd generation biodiesel as a sustainable fuel–Part 2: Properties, performance and emission characteristics. Renew. Sustain. Energy Rev. 2016, 55, 1129–1146. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Characterization of the key fuel properties of methyl ester–diesel fuel blends. Fuel 2009, 88, 75–80. [Google Scholar] [CrossRef]
- Kinast, J.A. Production of Biodiesels from Multiple Feedstocks and Properties of Biodiesels and Biodiesel/Diesel Blends: Final Report; Report 1 in a Series of 6; National Renewable Energy Lab.: Golden, CO, USA, 2003. [Google Scholar]


| Fuel | HILO (vol.%) | Diesel Fuel, DF (vol.%) |
|---|---|---|
| N0 | 0 | 100 |
| N25 | 25 | 75 |
| N50 | 50 | 50 |
| N75 | 75 | 25 |
| N100 | 100 | 0 |
| Properties | ASTM D975 | Error | DF | DF-HILO Blends | HILO | |||
|---|---|---|---|---|---|---|---|---|
| Standard | Limits | N0 | N25 | N50 | N75 | N100 | ||
| Density at 15 °C (kg/m3) | D4052 | - | 0.50 | 829.00 | 869.48 | 885.84 | 902.11 | 922.53 |
| Kinematic viscosity (mm2/s) | D445 | 1.9–4.1 | 0.05 | 3.35 | 7.52 | 11.47 | 19.81 | 28.15 |
| Heating value (MJ/kg) | D240 | - | 0.35 | 48.115 | 45.913 | 43.537 | 41.542 | 40.836 |
| Cetane number | D613 | Min. 40 | 0.50 | 52 | 55.9 | 59.2 | 60.8 | 61 |
| Flash point (°C) | D93 | Min. 52 | 9.00 | 60 | 90 | 98 | 112 | 399 |
| Element C (wt.%) | - | - | 0.01 | 87.17 | 83.49 | 81.61 | 78.43 | 76.44 |
| Element H (wt.%) | - | - | 0.01 | 12.76 | 13.11 | 13.31 | 13.62 | 13.77 |
| Element N (wt.%) | - | - | 0.01 | 0.07 | 0.12 | 0.21 | 0.27 | 0.31 |
| Element O (wt.%) | - | - | 0.01 | 0 | 3.25 | 4.82 | 7.62 | 9.39 |
| Element S (wt.%) | - | - | 0.01 | 0 | 0.03 | 0.05 | 0.06 | 0.09 |
| Hydrocarbon Family | Diesel Fuel (wt.%) | HILO (wt.%) |
|---|---|---|
| Paraffin (alkane) | 73.3095 | - |
| Olefins (alkenes) | 1.6309 | - |
| Aromatics | 22.085 | - |
| Carboxylic acids (fatty acids) | 2.9747 | 95.113 |
| Ketones | - | 0.0771 |
| Others | - | 4.8097 |
| 100.0001 | 99.9998 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusaf, T.; Kamarulzaman, M.K.; Adam, A.; Hisham, S.; Ramasamy, D.; Kadirgama, K.; Samykano, M.; Subramaniam, S. Physical-Chemical Properties Modification of Hermetia Illucens Larvae Oil and Diesel Fuel for the Internal Combustion Engines Application. Energies 2022, 15, 8073. https://doi.org/10.3390/en15218073
Yusaf T, Kamarulzaman MK, Adam A, Hisham S, Ramasamy D, Kadirgama K, Samykano M, Subramaniam S. Physical-Chemical Properties Modification of Hermetia Illucens Larvae Oil and Diesel Fuel for the Internal Combustion Engines Application. Energies. 2022; 15(21):8073. https://doi.org/10.3390/en15218073
Chicago/Turabian StyleYusaf, Talal, Mohd Kamal Kamarulzaman, Abdullah Adam, Sakinah Hisham, Devarajan Ramasamy, Kumaran Kadirgama, Mahendran Samykano, and Sivaraos Subramaniam. 2022. "Physical-Chemical Properties Modification of Hermetia Illucens Larvae Oil and Diesel Fuel for the Internal Combustion Engines Application" Energies 15, no. 21: 8073. https://doi.org/10.3390/en15218073
APA StyleYusaf, T., Kamarulzaman, M. K., Adam, A., Hisham, S., Ramasamy, D., Kadirgama, K., Samykano, M., & Subramaniam, S. (2022). Physical-Chemical Properties Modification of Hermetia Illucens Larvae Oil and Diesel Fuel for the Internal Combustion Engines Application. Energies, 15(21), 8073. https://doi.org/10.3390/en15218073