Determination of Carbonyls Compound, Ketones and Aldehydes Emissions from CI Diesel Engines Fueled with Pure Diesel/Diesel Methanol Blends
Abstract
:1. Introduction
2. Experimental
2.1. IC Engine
2.2. Fuel
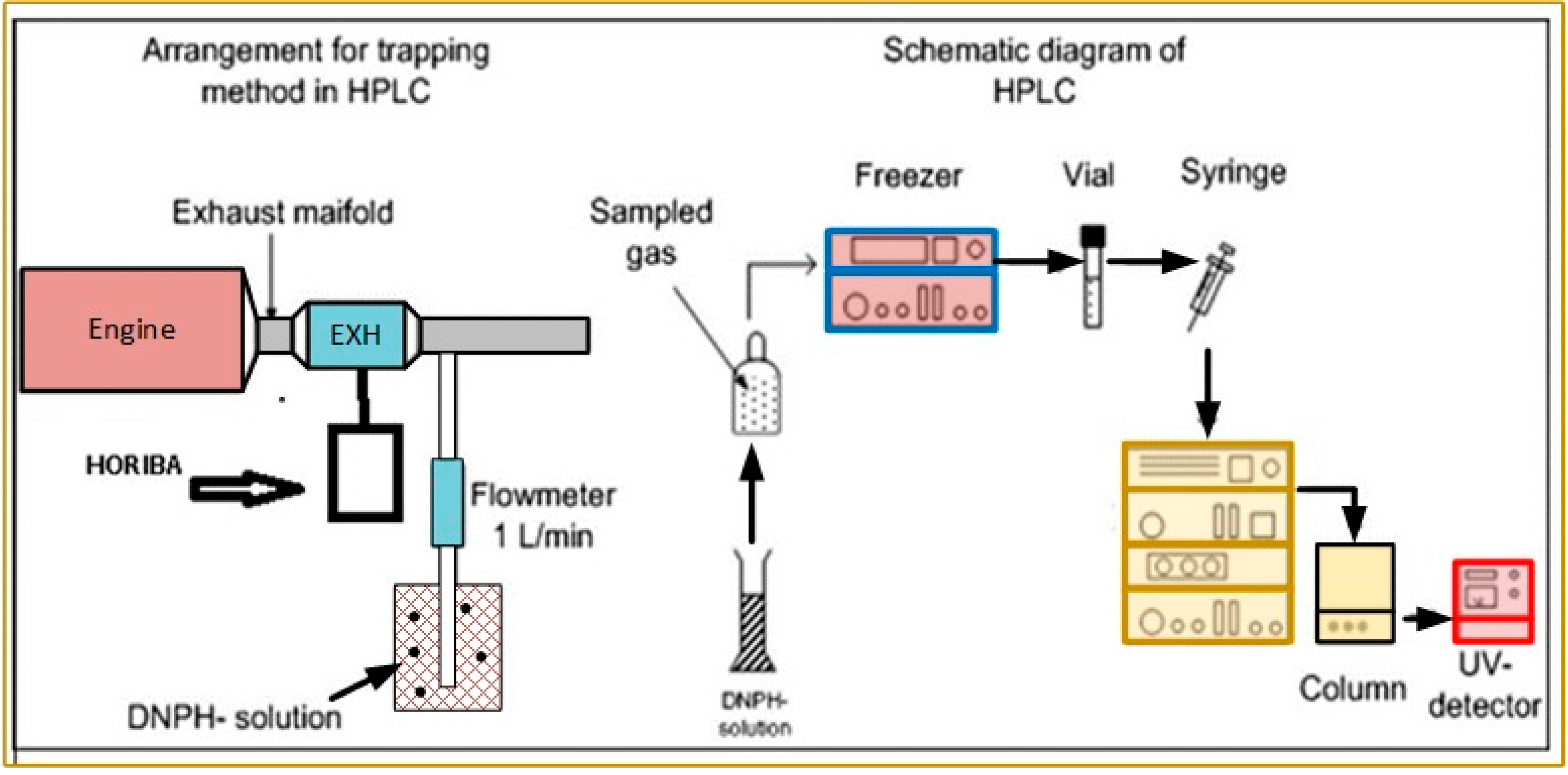
2.3. Emissions Analysis

3. Results and Desiccation
3.1. Exhaust Emissions
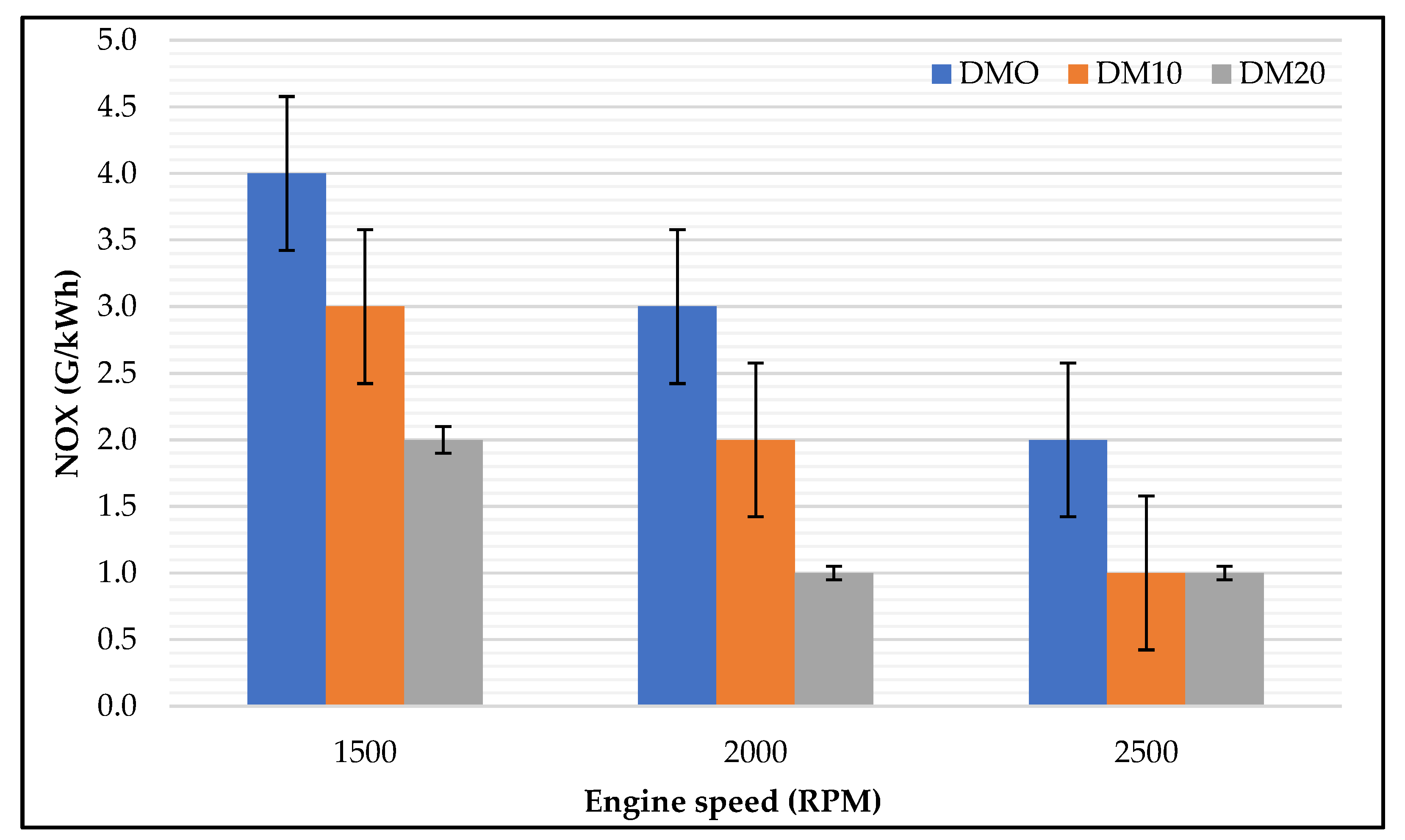
3.1.1. NOx Emissions
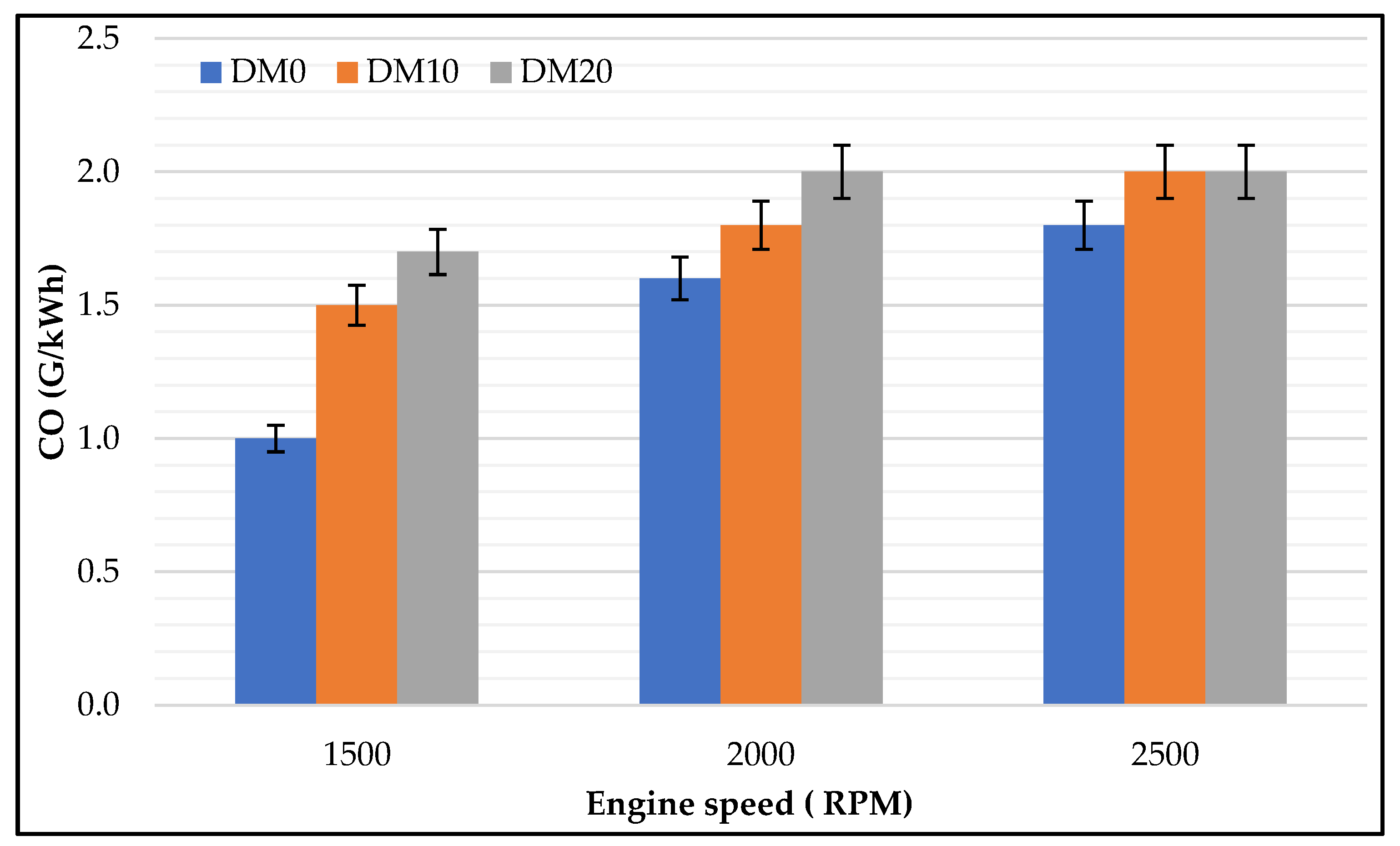
3.1.2. HC, CO Emissions
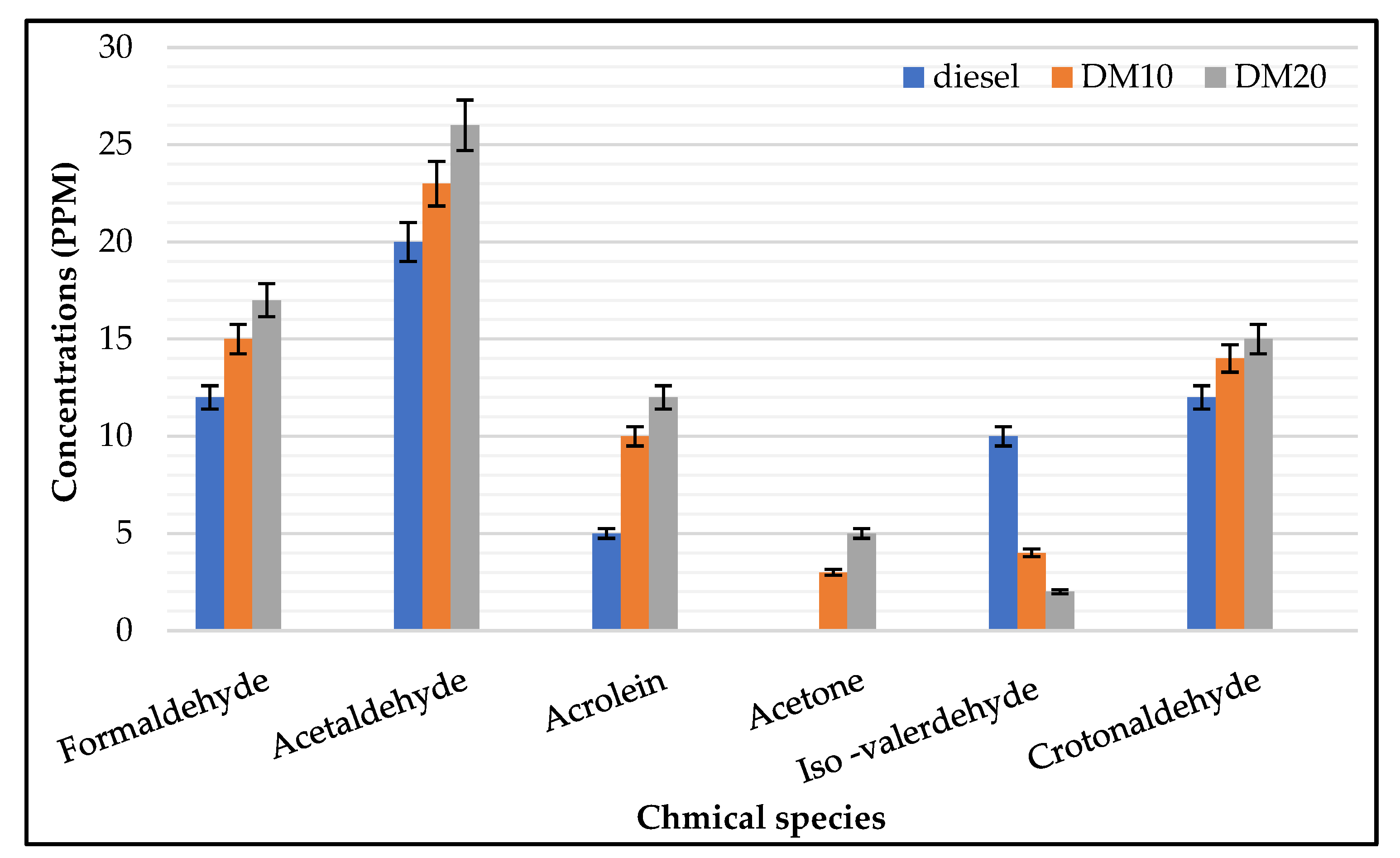
3.1.3. UN Regulated HC Emissions
3.2. Engine Characteristics
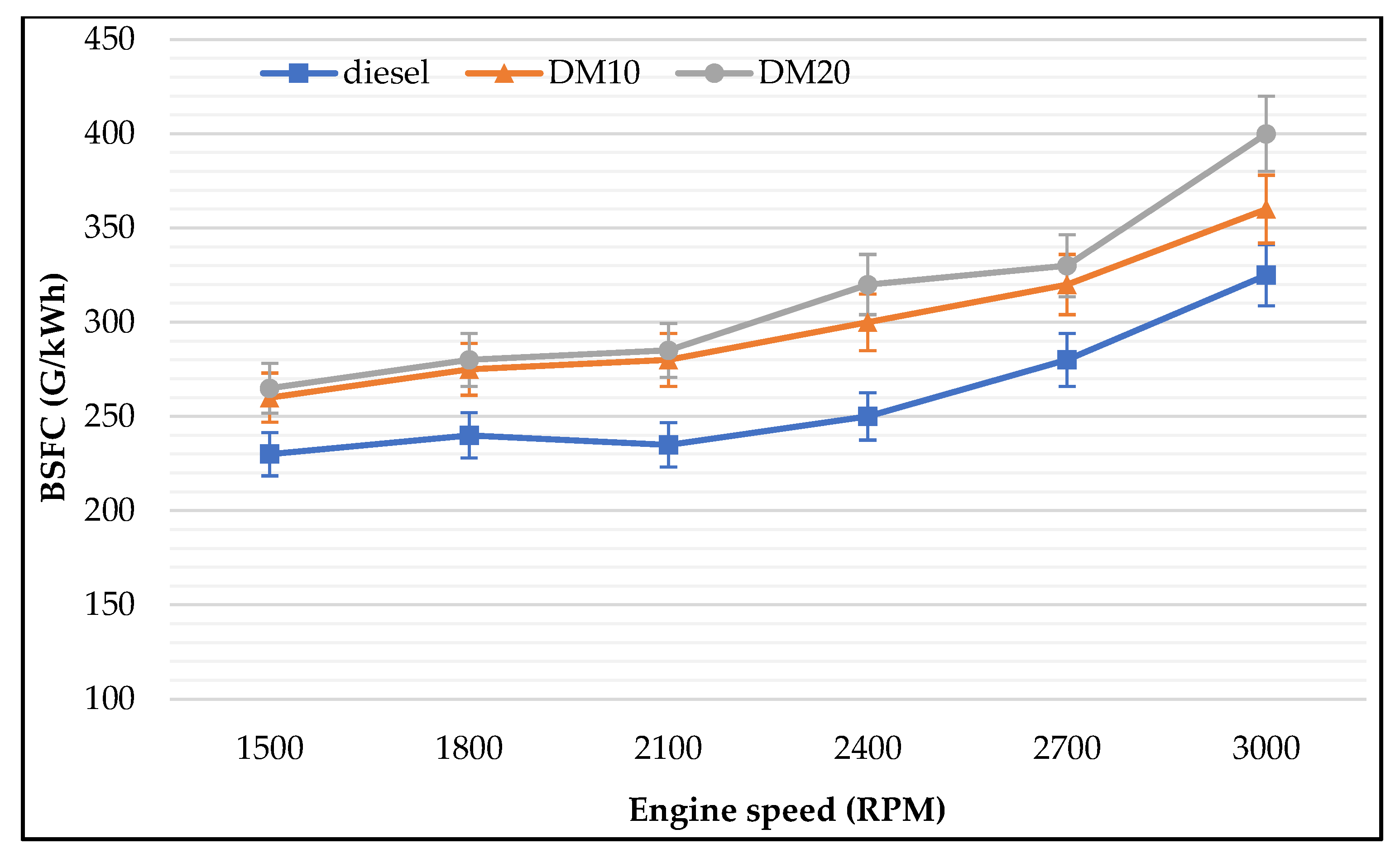
3.2.1. Brake Specific Fuel Consumption
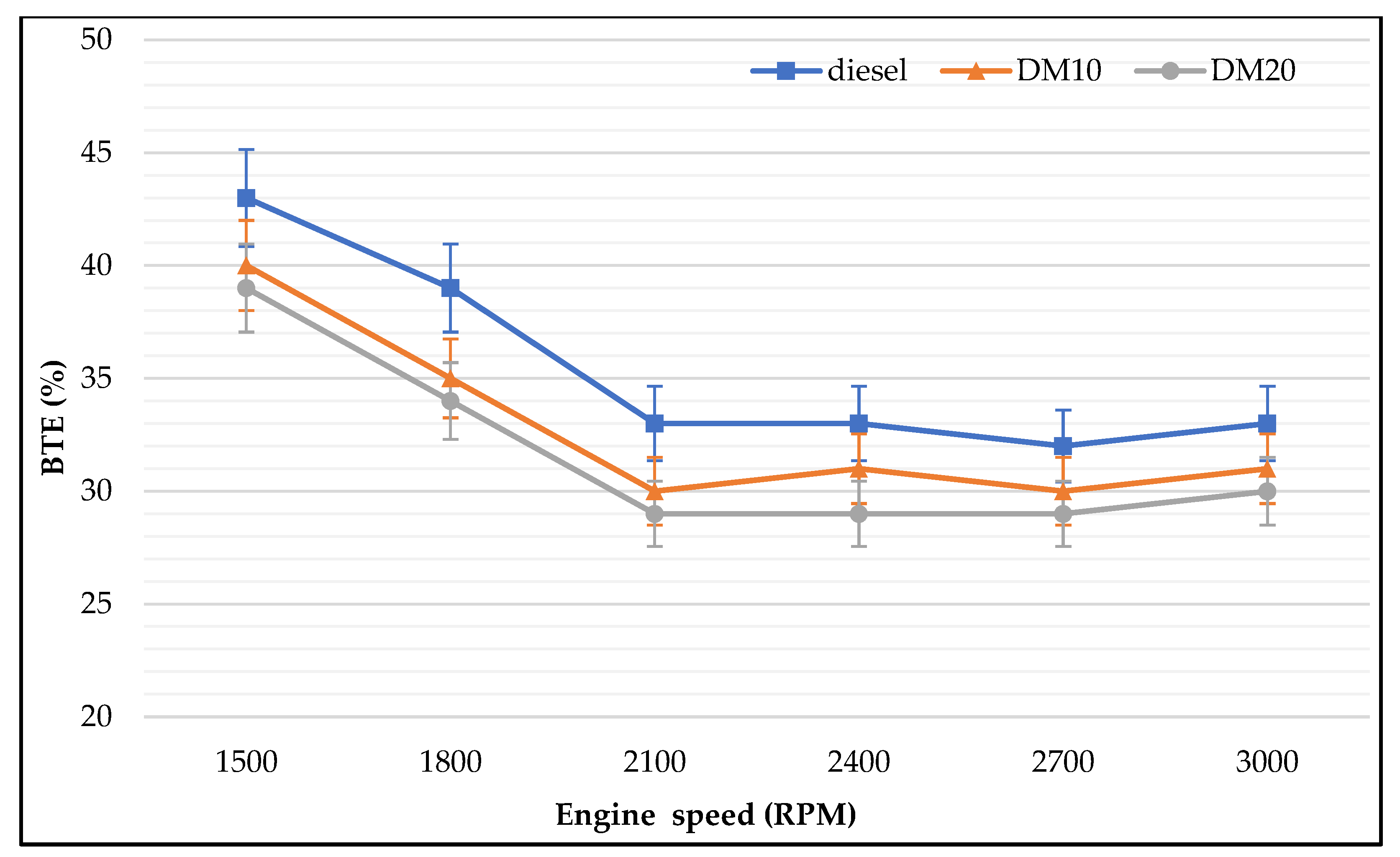
3.2.2. Brake Thermal Efficiency
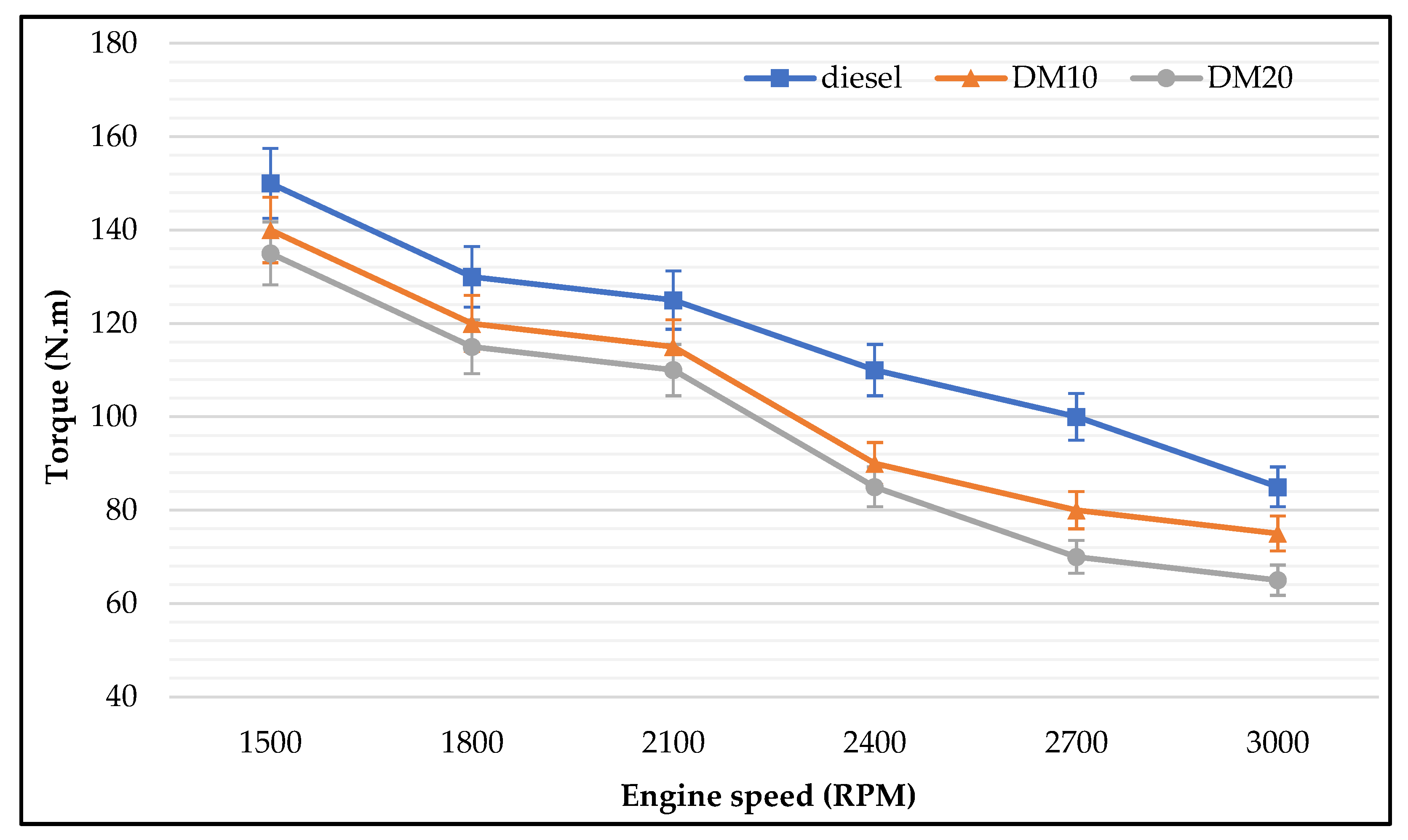
3.2.3. Engine Torque
3.2.4. Engine Brake Power
4. Conclusions
- Engine–exhaust emissions: Hydrocarbon, carbon monoxide, and nitrogen oxide emissions were strongly influenced by engine speeds and diesel methanol blends. HC and CO emissions had a similar trend at different engine speeds and were slightly higher at lower engine speeds, while with pure diesel, NOx concentrations presented with higher levels at all engine speeds; with diesel blended methanol at lower speeds NOx showed lower concentrations. Using diesel blended methanol fuel had a remarkable result regarding exhaust emissions; HC, CO, and NOx were heavily reduced.
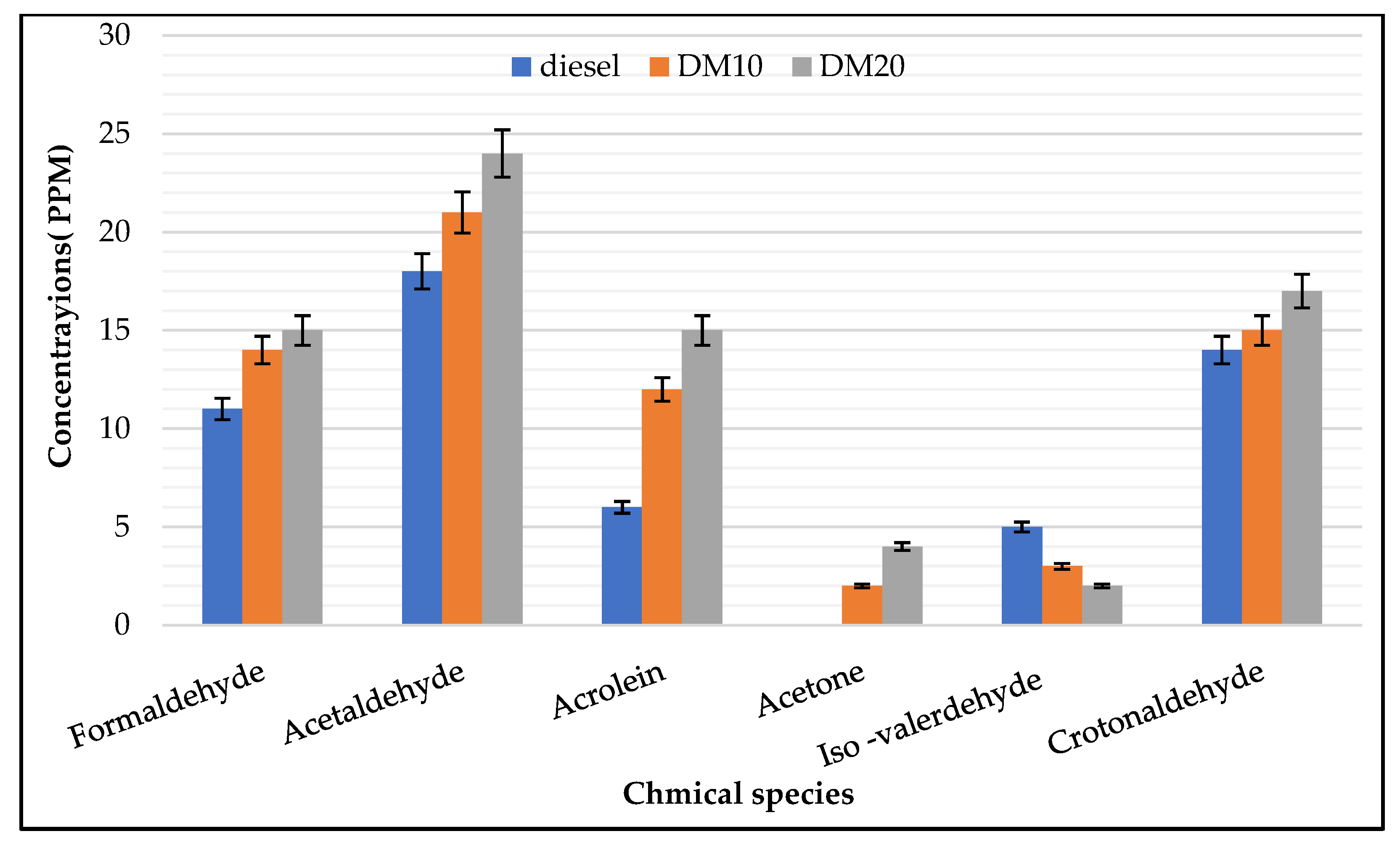
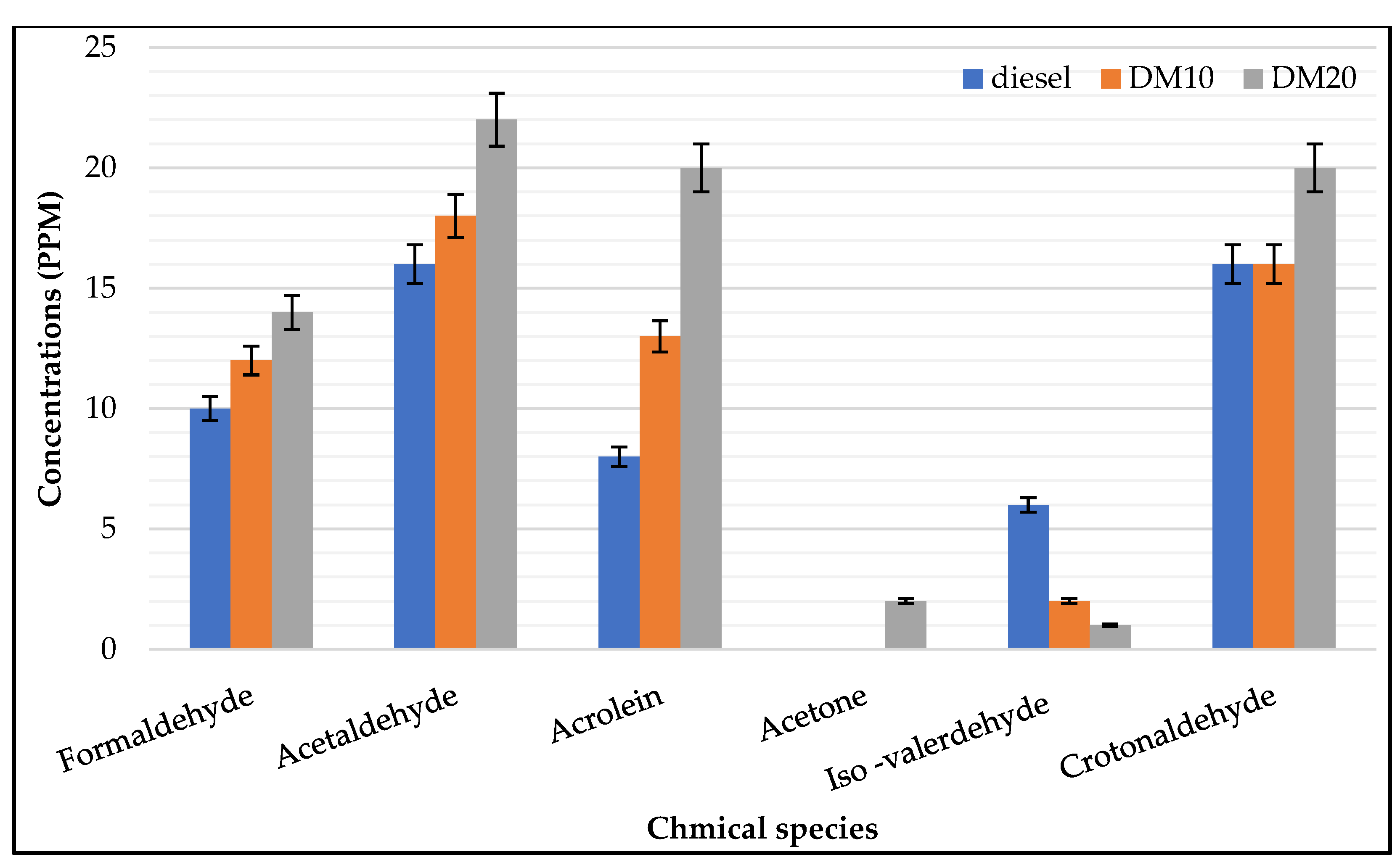
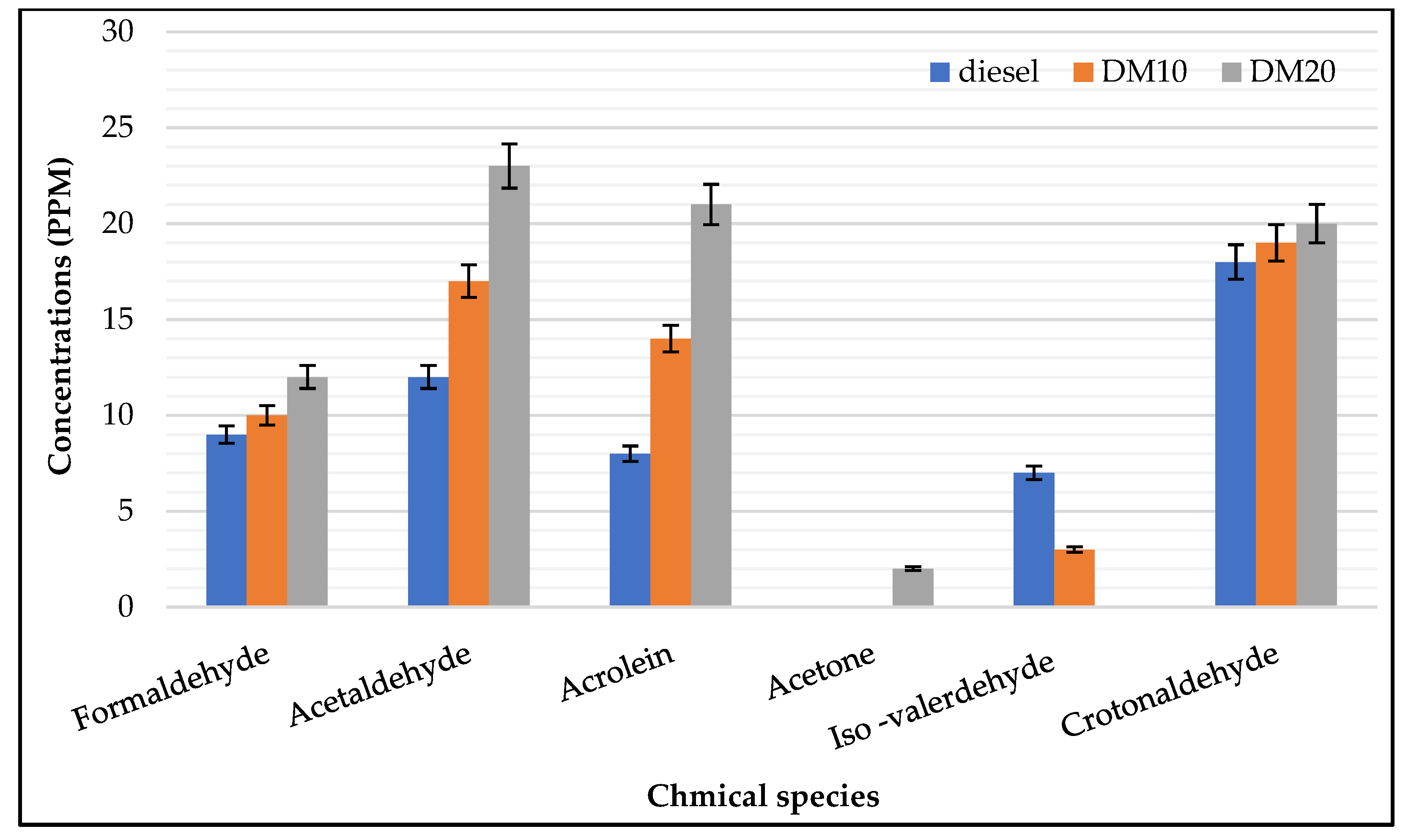
- Engine–UN regulated HC: Engine loads has a strong influence on saturated aldehyde (e.g., formaldehyde, acetaldehyde); a considerable amount of exhaust gas emissions was presented at lower loads, which agrees with previous work when it was found that the highest hydrocarbon engine-out emissions were produced for HCCI engine at low engine load operations [43] while unsaturated aldehydes were less sensitive to higher loads. In combustion fuel modes and engine operating parameters, carbonyl chemical species were strongly influenced (i.e., pure diesel or diesel methanol blends); saturated species such as acetaldehyde, formaldehyde, and croton aldehyde were mainly found with higher concentrations in the exhaust of both engine fuel modes. Carbonyl’s highest concentrations were found in higher loads, and acrolein concentration was higher in higher load and exceeded formaldehyde concentrations. During engine operations at a low engine load, the highest amount of hydrocarbon emissions was produced.
- Engine performance: Diesel methanol blends (30% and 40% methanol) showed less improvement in engine brake power than lower portions of methanol blended. Results showed that BTE was dominated by engine speed. There was no difference in BTE for different proportions of methanol, which increased when engine speeds increased. Moreover, BTE increments were different among tested fuels. Methanol blends improved engine tailpipe emissions rather than improving engine performance and reducing engine emissions, according to the study results. At low engine speeds, pure diesel produced the highest BTE followed by 10% and 20% methanol blends. As noticed in the presented results, BSFC was dominated by the methanol concentration with different engine speeds.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| BP | brake power |
| BSFC | brake specific fuel consumption |
| BTE | brake thermal efficiency |
| BTEX | benzene, toluene, ethyl benzene, xylene |
| CI | compression ignition |
| CO | carbon monoxide |
| DM10 | diesel blended with 10% methanol |
| DM20 | diesel blended with 20% methanol |
| DNPH | 2,4-dinitrophenyl hydrazine |
| EGR | exhaust gas recirculation |
| EPY | environmental protection agency |
| HPLC | high performance liquid chromatography |
| IARC | international agency for research on cancer |
| NOx | nitrogen oxide |
| OH | hydroxyl radical |
| O2 | oxygen |
| PM | particulate matter |
| RPM | revolution per minute |
| SOA | secondary organic aerosol |
| STP | standard temperature pressure |
| THC | total hydrocarbon |
| UV | ultraviolet |
| UV/VIS | ultraviolet/visible spectroscopy |
| UN HC | unburned hydro carbon |
| VOC | volatile organic compound |
References
- Hasan, A.O.; Osman, A.I.; Al-Muhtaseb, A.H.; Al-Rawashdeh, H.; Abu-jrai, A.; Ahmad, R.; Gomaa, M.R.; Deka, T.J.; Rooney, D.W. An Experimental Study of Engine Characteristics and Tailpipe Emissions from Modern DI Diesel Engine Fuelled with Methanol/Diesel Blends. Fuel Process. Technol. 2021, 220, 106901. [Google Scholar] [CrossRef]
- Mustafa, R.J.; Al-Rawashdeh, H.A.; Hasan, A.O.; Gomaa, M.R. Enhancement of a Hydrogen Engine Cavitation Utilizing Mixed Fuel: A Review and Experimental Case Study. Int. Rev. Mech. Eng. 2020, 14, 33–42. [Google Scholar] [CrossRef]
- Alahmer, A.; Rezk, H.; Aladayleh, W.; Mostafa, A.O.; Abu-Zaid, M.; Alahmer, H.; Gomaa, M.R.; Alhussan, A.A.; Ghoniem, R.M. Modeling and Optimization of a Compression Ignition Engine Fueled with Biodiesel Blends for Performance Improvement. Mathematics 2022, 10, 420. [Google Scholar] [CrossRef]
- Hasan, A.O.; Al-Rawashdeh, H.; Abu-jrai, A.; Gomaa, M.R.; Jamil, F. Impact of variable compression ratios on engine performance and unregulated HC emitted from a research single cylinder engine fueled with commercial gasoline. Int. J. Hydrogen Energy 2022, in press. [Google Scholar] [CrossRef]
- Mohamed, R.G.; Talib, K.M.; Abu-Jrai, A.; Hegazy Rezk, M.; Altarawneh, A.; Marashli, A. Experimental Investigation on Waste Heat Recovery from a Cement Factory to Enhance Thermoelectric Generation. Sustainability 2022, 14, 10146. [Google Scholar] [CrossRef]
- Hasan, A.O.; Essa, K.; Gomaa, M.R. Synthesis, structure characterization and study of a new kind of catalyst: A monolith of nickel made by additive manufacturing coated with platinum. Energies 2022, 15, 7575. [Google Scholar] [CrossRef]
- Karavalakis, G.; Poulopoulos, S.; Zervas, E. Impact of Diesel Fuels on the Emissions of Non-Regulated Pollutants. Fuel 2012, 102, 85–91. [Google Scholar] [CrossRef]
- Rantanen, L.; Mikkonen, S.; Nylund, L.; Kociba, P.; Lappi, M.; Nylund, N.O. Effect of Fuel on the Regulated, Unregulated and Mutagenic Emissions of Di Diesel Engines. In Proceedings of the 1993 SAE International Fuels and Lubricants Meeting and Exposition, Pennsylvania, PA, USA, 18–21 October 1993; SAE International: Warrendale, PA, USA, 1993. [Google Scholar]
- Ge, J.C.; Kim, H.Y.; Yoon, S.K.; Choi, N.J. Reducing Volatile Organic Compound Emissions from Diesel Engines Using Canola Oil Biodiesel Fuel and Blends. Fuel 2018, 218, 266–274. [Google Scholar] [CrossRef]
- Hu, N.; Tan, J.; Wang, X.; Zhang, X.; Yu, P. Volatile Organic Compound Emissions from an Engine Fueled with an Ethanol-Biodiesel-Diesel Blend. J. Energy Inst. 2017, 90, 101–109. [Google Scholar] [CrossRef]
- Tian, J.; Tan, J.; Hu, N.; Liu, T.; Wang, Y.; Zhong, H.; Cheng, J.; Zhang, X. Characteristics Analysis for Total Volatile Organic Compounds Emissions of Methanol-Diesel Fuel. J. Energy Inst. 2018, 91, 527–533. [Google Scholar] [CrossRef]
- Harrenstien, M.S.; Rhee, K.T.; Adt, R.R. Determination of Individual Aldehyde Concentrations in the Exhaust of a Spark Ignited Engine Fueled by Alcohol/Gasoline Blends; SAE Technical Papers; SAE International: Warrendale, PA, USA, 1979. [Google Scholar]
- IARC. WHO IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 88 Formaldehyde, 2-Butoxyethanol and 1-Tert-Butoxypropan-2-Ol; IARC: Lyon, France, 2006; p. 88. [Google Scholar]
- Harrenstien, M.S.; Rhee, K.T.; Adt, R.R., Jr. Carcinogenicity of Formaldehyde in Rats and Mice after Long-Term Inhalation Exposure1 | Cancer Research | American Association for Cancer Research. Available online: https://aacrjournals.org/cancerres/article/43/9/4382/488333/Carcinogenicity-of-Formaldehyde-in-Rats-and-Mice (accessed on 14 May 2022).
- Kataoka, H.; Kondo, T.; Sumida, A. Gas Chromatographic Determination of Aldehydes in Combustion Smoke Samples. Anal. Chim. Acta 1998, 358, 269–275. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Mubarak, A.T.; Marestani, Z.M.H.; Fawy, K.F. Highly Sensitive and Selective Catalytic Determination of Formaldehyde and Acetaldehyde. Talanta 2008, 74, 578–585. [Google Scholar] [CrossRef]
- Duan, J.; Tan, J.; Yang, L.; Wu, S.; Hao, J. Concentration, Sources and Ozone Formation Potential of Volatile Organic Compounds (VOCs) during Ozone Episode in Beijing. Atmos. Res. 2008, 88, 25–35. [Google Scholar] [CrossRef]
- Avigad, G. A Simple Spectrophotometric Determination of Formaldehyde and Other Aldehydes: Application to Periodate-Oxidized Glycol Systems. Anal. Biochem. 1983, 134, 499–504. [Google Scholar] [CrossRef]
- Ghadikolaei, M.A. Effect of Alcohol Blend and Fumigation on Regulated and Unregulated Emissions of IC Engines-A Review. Renew. Sustain. Energy Rev. 2016, 57, 1440–1495. [Google Scholar] [CrossRef]
- Anand, K.; Reitz, R.D. Exploring the Benefits of Multiple Injections in Low Temperature Combustion Using a Diesel Surrogate Model. Fuel 2016, 165, 341–350. [Google Scholar] [CrossRef]
- Aalam, C.S.; Saravanan, C.G.; Anand, B.P. Impact of High Fuel Injection Pressure on the Characteristics of CRDI Diesel Engine Powered by Mahua Methyl Ester Blend. Appl. Therm. Eng. 2016, 106, 702–711. [Google Scholar] [CrossRef]
- Sharma, P.; Sahoo, B.B. Precise prediction of performance and emission of a waste derived Biogas–Biodiesel powered Dual–Fuel engine using modern ensemble Boosted regression Tree: A critique to Artificial neural network. Fuel 2022, 321, 124131. [Google Scholar] [CrossRef]
- Sharma, P.; Sahoo, B.B. An ANFIS-RSM based modeling and multi-objective optimization of syngas powered dual-fuel engine. Int. J. Hydrogen Energy 2022, 47, 19298–19318. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. A Comprehensive Analysis of Biodiesel Impacts on Exhaust Emissions; Draft Technical Report EPA 420-P-02-001; US Environmental Protection Agency: Washington, DC, USA, 2002. [Google Scholar]
- Lowi, A.; Carter, W.P.L. A Method for Evaluating the Atmospheric Ozone Impact of Actual Vehicle Emissions. SAE Trans. 1990, 99, 303–311. [Google Scholar]
- Al-Rawashdeh, H.; Hasan, A.O.; Al-Shakhanbeh, H.A.; Al-Dhaifallah, M.; Gomaa, M.R.; Rezk, H. Investigation of the Effect of Solar Ventilation on the Cabin Temperature of Vehicles Parked under the Sun. Sustainability 2021, 13, 13963. [Google Scholar] [CrossRef]
- Gomaa, M.R.; Al-Dmour, N.; Shalby, M. Theoretical model of a fluidized bed solar reactor design with the aid of MCRT method and synthesis gas production. Renew. Energy 2020, 148, 91–102. [Google Scholar] [CrossRef]
- Gomaa, M.R.; Mustafa, R.J.; Al-Dmour, N. Solar Thermochemical Conversion of Carbonaceous Materials into Syngas by Co-Gasification. J. Clean. Prod. 2020, 248, 119185. [Google Scholar] [CrossRef]
- Imdadul, H.K.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Alabdulkarem, A.; Rashed, M.M.; Ashraful, A.M. Influences of Ignition Improver Additive on Ternary (Diesel-Biodiesel-Higher Alcohol) Blends Thermal Stability and Diesel Engine Performance. Energy Convers. Manag. 2016, 123, 252–264. [Google Scholar] [CrossRef]
- Imdadul, H.K.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.M.; Alabdulkarem, A.; Kamruzzaman, M.; Rashed, M.M. A Comparative Study of C4 and C5 Alcohol Treated Diesel-Biodiesel Blends in Terms of Diesel Engine Performance and Exhaust Emission. Fuel 2016, 179, 281–288. [Google Scholar] [CrossRef]
- Noorollahi, Y.; Azadbakht, M.; Ghobadian, B. The Effect of Different Diesterol (Diesel–Biodiesel–Ethanol) Blends on Small Air-Cooled Diesel Engine Performance and Its Exhaust Gases. Energy 2018, 142, 196–200. [Google Scholar] [CrossRef]
- Singh, M.; Sandhu, S.S. Performance, Emission and Combustion Characteristics of Multi-Cylinder CRDI Engine Fueled with Argemone Biodiesel/Diesel Blends. Fuel 2020, 265, 117024. [Google Scholar] [CrossRef]
- Hulwan, D.B.; Joshi, S.V. Performance, Emission and Combustion Characteristic of a Multicylinder DI Diesel Engine Running on Diesel-Ethanol-Biodiesel Blends of High Ethanol Content. Appl. Energy 2011, 88, 5042–5055. [Google Scholar] [CrossRef]
- He, B.Q.; Shuai, S.J.; Wang, J.X.; He, H. The Effect of Ethanol Blended Diesel Fuels on Emissions from a Diesel Engine. Atmos. Environ. 2003, 37, 4965–4971. [Google Scholar] [CrossRef]
- Brady, J.M.; Crisp, T.A.; Collier, S.; Kuwayama, T.; Forestieri, S.D.; Perraud, V.; Zhang, Q.; Kleeman, M.J.; Cappa, C.D.; Bertram, T.H. Real-Time Emission Factor Measurements of Isocyanic Acid from Light Duty Gasoline Vehicles. Environ. Sci. Technol. 2014, 48, 11405–11412. [Google Scholar] [CrossRef]
- Kar, K.; Cheng, W.K. Speciated Engine-out Organic Gas Emissions from a PFI-SI Engine Operating on Ethanol/Gasoline Mixtures. SAE Int. J. Fuels Lubr. 2010, 2, 91–101. [Google Scholar] [CrossRef]
- Donkerbroek, A.J.; van Vliet, A.P.; Somers, L.M.T.; Dam, N.J.; ter Meulen, J.J. Relation between Hydroxyl and Formaldehyde in a Direct-Injection Heavy-Duty Diesel Engine. Combust. Flame 2011, 158, 564–572. [Google Scholar] [CrossRef]
- Zervas, E.; Montagne, X.; Lahaye, J. Emission of Alcohols and Carbonyl Compounds from a Spark Ignition Engine. Influence of Fuel and Air/Fuel Equivalence Ratio. Environ. Sci. Technol. 2002, 36, 2414–2421. [Google Scholar] [CrossRef]
- Cheng, C.H.; Cheung, C.S.; Chan, T.L.; Lee, S.C.; Yao, C.D.; Tsang, K.S. Comparison of Emissions of a Direct Injection Diesel Engine Operating on Biodiesel with Emulsified and Fumigated Methanol. Fuel 2008, 87, 1870–1879. [Google Scholar] [CrossRef]
- Magnusson, R.; Nilsson, C.; Andersson, B. Emissions of Aldehydes and Ketones from a Two-Stroke Engine Using Ethanol and Ethanol-Blended Gasoline as Fuel. Environ. Sci. Technol. 2002, 36, 1656–1664. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C.S.; Park, S.; Gupta, J.G.; Maurya, R.K.; Agarwal, A.K. Spray Characteristics, Engine Performance and Emissions Analysis for Karanja Biodiesel and Its Blends. Energy 2017, 119, 138–151. [Google Scholar] [CrossRef]
- Hasan, A.O.; Leung, P.; Tsolakis, A.; Golunski, S.E.; Xu, H.M.; Wyszynski, M.L.; Richardson, S. Effect of Composite Aftertreatment Catalyst on Alkane, Alkene and Monocyclic Aromatic Emissions from an HCCI/SI Gasoline Engine. Fuel 2011, 90, 1457–1464. [Google Scholar] [CrossRef]
- Hasan, A.O.; Abu-jrai, A. Control of harmful hydrocarbon species in the exhaust of modern advanced GDI engines. Atmos. Environ. 2016, 129, 210–217. [Google Scholar] [CrossRef]


| Engine model | CI direct injection turbocharged diesel engine, four cylinders, four-stroke |
| Compression ratio | 18:1 |
| Torque | 350 Nm |
| Power | 80 kW at 2600 rpm |
| Displacement (Lit) | 3.8 |
| Engine speed | 1900 rpm |
| Fuel | Commercial diesel, diesel blends |
| Property | Method | Diesel |
|---|---|---|
| Pour point | D 98 | 0 °C |
| Flash point, closed cup | D 95 | 69 °C |
| Kinematical viscosity | D 555 | 3.03 mm2/s |
| Sulphated ash | D 899 | - |
| Total Sulphur | D 5663 | 0.05 wt% |
| Copper strip corrosion | D 125 | 1 a |
| Cloud point | D 2600 | 1.5 °C |
| ASTM | 1-D (S15) | - |
| S.N | Parameter Name | Instrument Uncertainty |
|---|---|---|
| 1 | NMEP (bar) | ±0.01 |
| 2 | Temperature | ±1 °C |
| 3 | Speed (rpm) | ±10 |
| 4 | CO (ppm)) | ±0.01 |
| 5 | HC (ppm) | ±0.01 |
| 6 | NOx (ppm) | ±0.01 |
| 7 | Aldehydes | ±0.01 |
| 8 | Brake Power (kW) | ±0.1 |
| 9 | Torque (N·m) | ±0.1 |
| 10 | BSFC (g/kWh) | ±0.1 |
| Model | Acclaim 120 by Dionex |
|---|---|
| Instrument Column | This is a C18 film with a diameter of 250 mm by 4.6 mm ID; a film length of 5 lumens |
| Detector | The UVD is 170 s and the wavelength is 365 nanometers |
| Pump | Low pressure quaternary P 580 |
| Flow rate | The eluent flow rate is one ml per minute |
| Peak No. | Compound | Retention Time (min) |
|---|---|---|
| 1 | Formaldehyde HCHO | 32.65 |
| 2 | Acetaldehyde CH3CHO | 42.88 |
| 3 | Acrolein CH3CH@CHCHO | 53.42 |
| 4 | Acetone CH3COCH3 | 54.63 |
| 5 | Propionaldehyde CH3CH2CHO | 58.34 |
| 6 | Crotonaldehyde CH3CH@CHCHO | 69.14 |
| 7 | Butyraldehyde CH3(CH2)2CHO | 70.05 |
| 8 | Benzaldehyde C6H5CHO | 80.38 |
| 9 | Isovaleraldehyde (CH3)2CH(CH92)CHO | 83.80 |
| 10 | PentanalCH3(CH2)3CHO | 85.57 |
| 11 | o-Tolualdehyde CH3(CH6H4)CHO | 88.7 |
| 12 | m-Tolualdehyde CH3(C6H4)CHO | 89.58 |
| 13 | p-Tolualdehyde CH3(C6H4)CHO | 90.13 |
| 14 | Hexanal CH3(CH2)4CHO | 94.98 |
| 15 | 2,5-Dimethylben (CH3)2(C6H4)CHO | 96.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rawashdeh, H.; Hasan, A.O.; Gomaa, M.R.; Abu-jrai, A.; Shalby, M. Determination of Carbonyls Compound, Ketones and Aldehydes Emissions from CI Diesel Engines Fueled with Pure Diesel/Diesel Methanol Blends. Energies 2022, 15, 7933. https://doi.org/10.3390/en15217933
Al-Rawashdeh H, Hasan AO, Gomaa MR, Abu-jrai A, Shalby M. Determination of Carbonyls Compound, Ketones and Aldehydes Emissions from CI Diesel Engines Fueled with Pure Diesel/Diesel Methanol Blends. Energies. 2022; 15(21):7933. https://doi.org/10.3390/en15217933
Chicago/Turabian StyleAl-Rawashdeh, Hani, Ahmad O. Hasan, Mohamed R. Gomaa, Ahmad Abu-jrai, and Mohammad Shalby. 2022. "Determination of Carbonyls Compound, Ketones and Aldehydes Emissions from CI Diesel Engines Fueled with Pure Diesel/Diesel Methanol Blends" Energies 15, no. 21: 7933. https://doi.org/10.3390/en15217933
APA StyleAl-Rawashdeh, H., Hasan, A. O., Gomaa, M. R., Abu-jrai, A., & Shalby, M. (2022). Determination of Carbonyls Compound, Ketones and Aldehydes Emissions from CI Diesel Engines Fueled with Pure Diesel/Diesel Methanol Blends. Energies, 15(21), 7933. https://doi.org/10.3390/en15217933








