Influence of Several Phosphate-Containing Additives on the Stability and Electrochemical Behavior of Positive Electrolytes for Vanadium Redox Flow Battery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the V(V) Electrolyte Solution
2.3. Thermal Stability Test of V(V)
2.4. Electrochemical Tests
3. Results and Discussion
3.1. Effect of Additives on the Stability of the V(V) Electrolyte
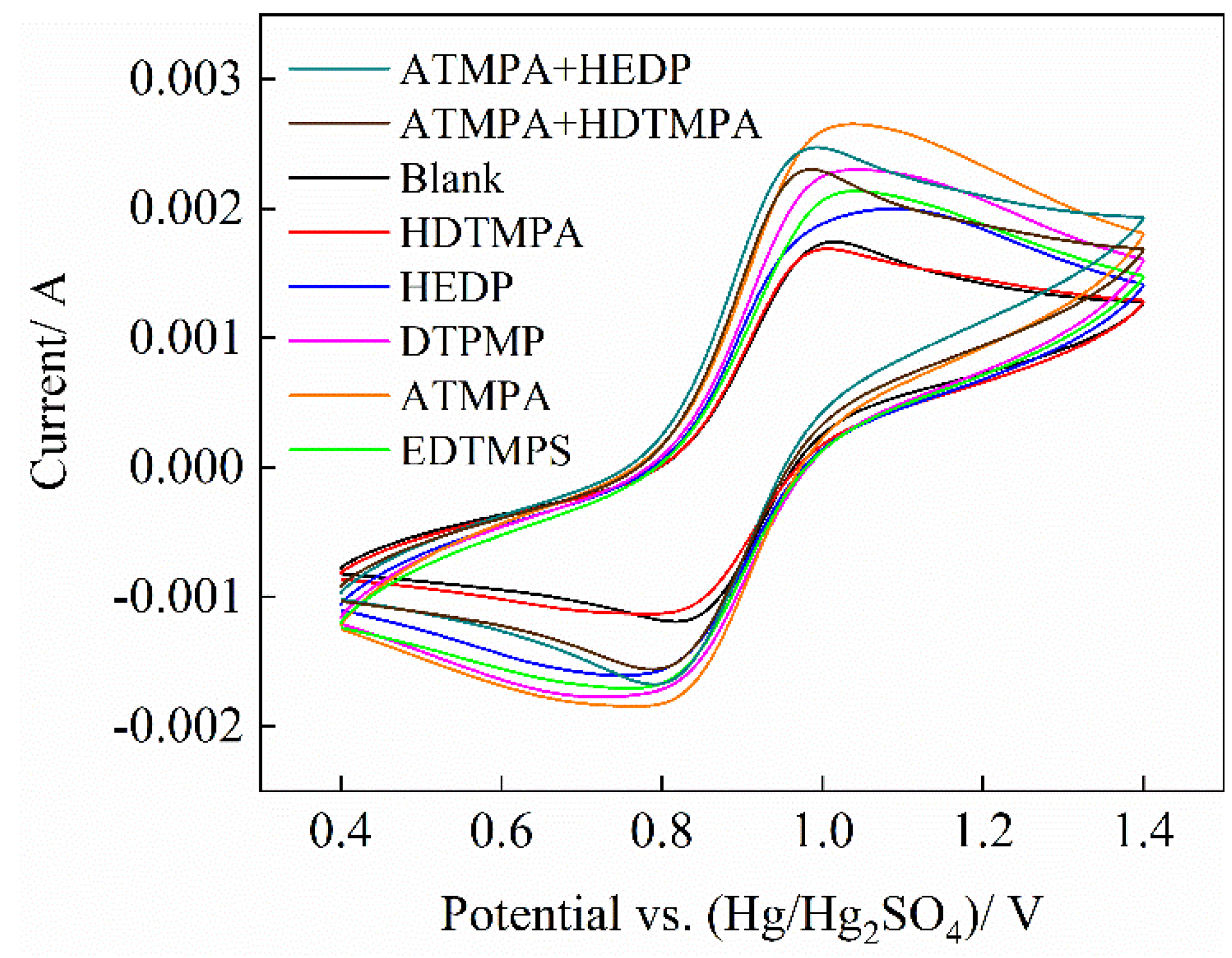
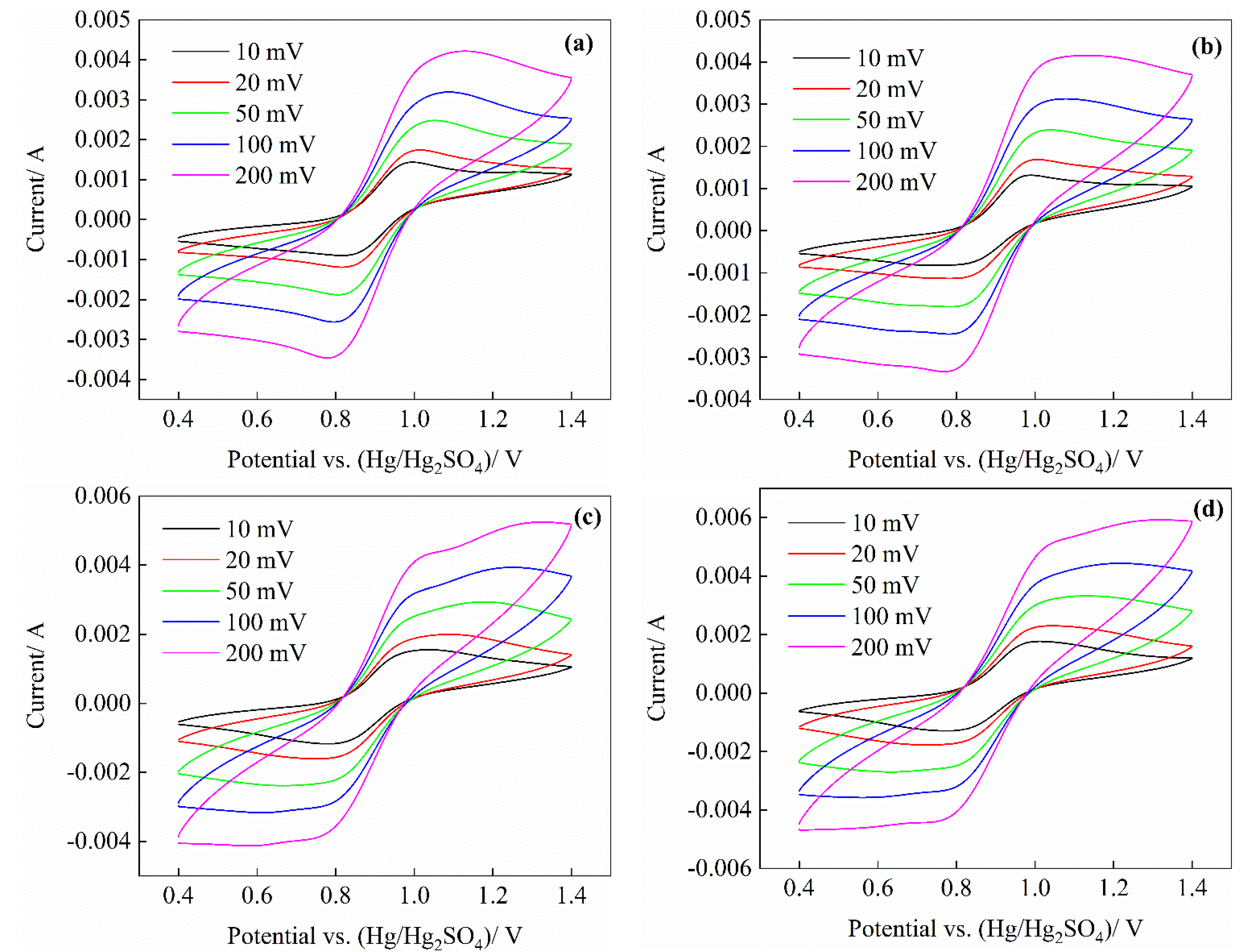
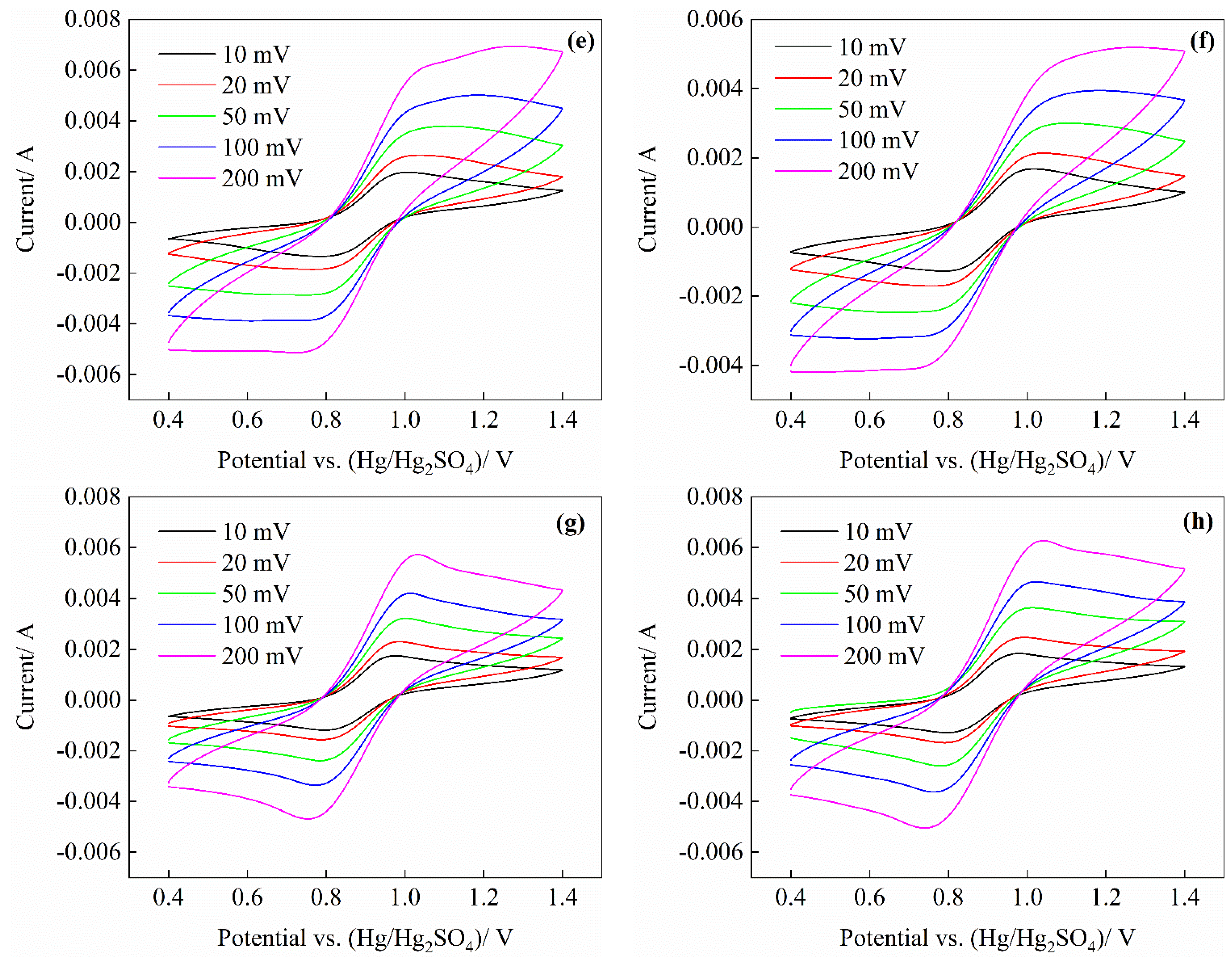
3.2. CV Test
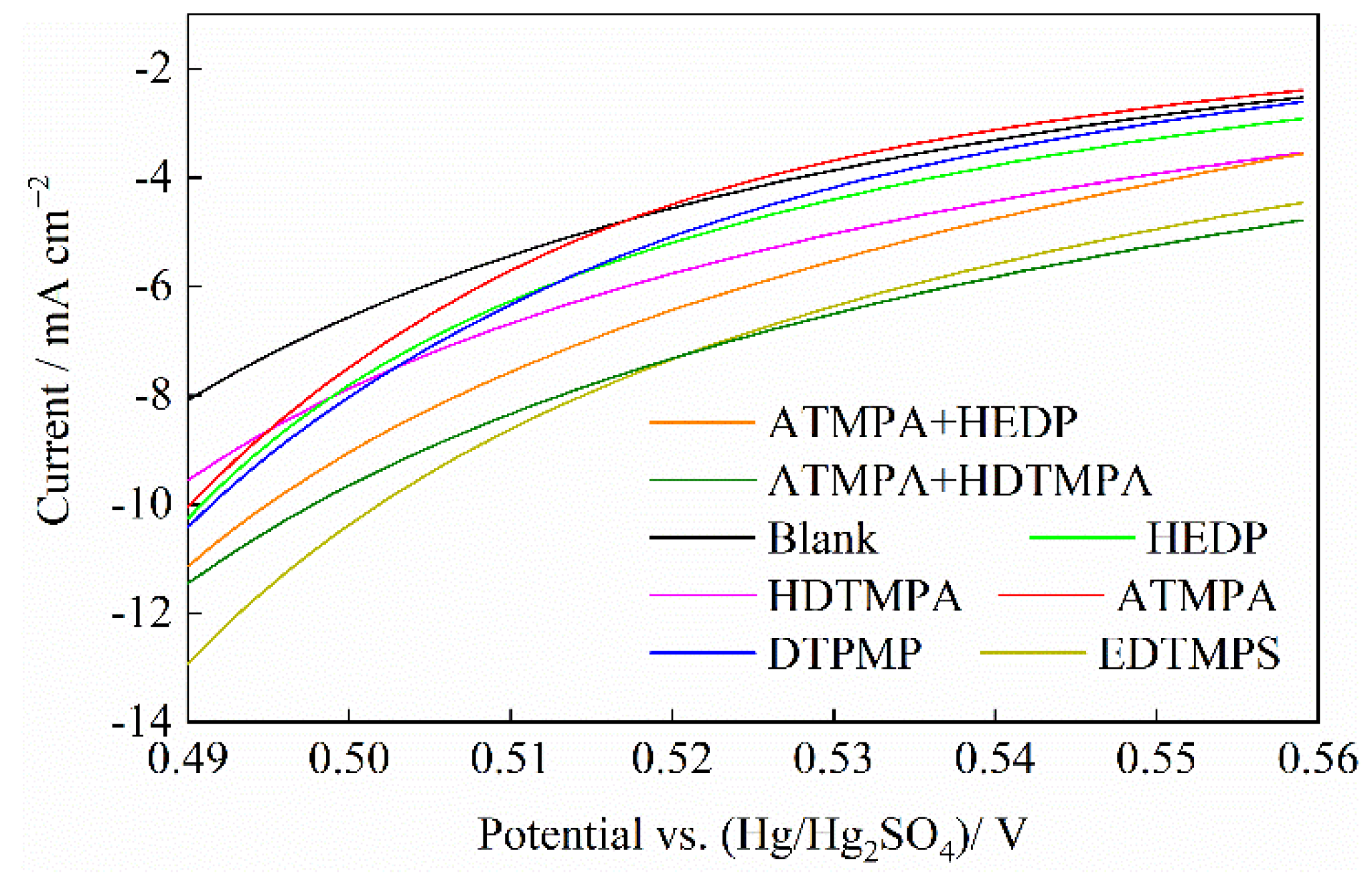
3.3. Steady-State Polarization Test
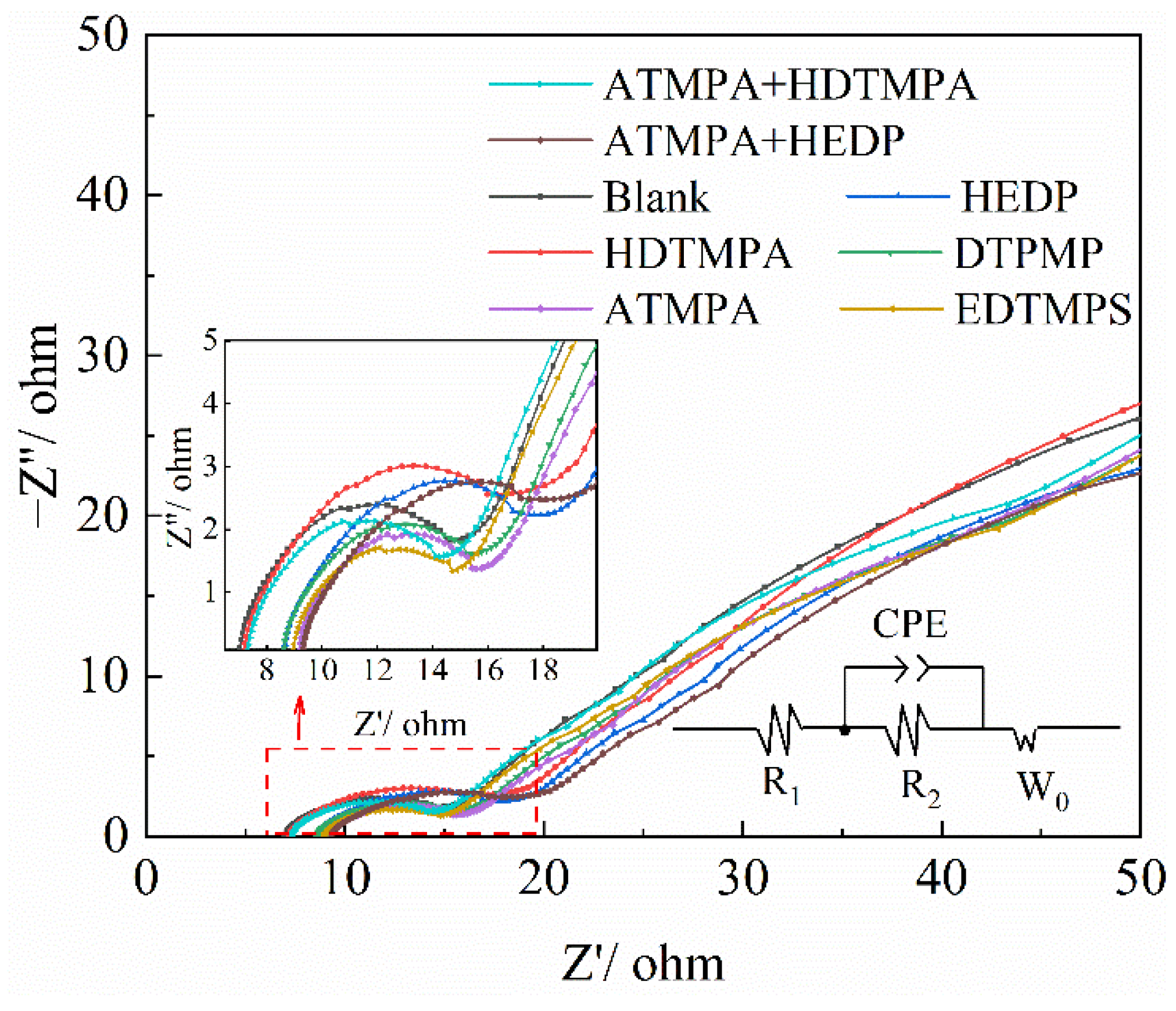
3.4. Electrochemical Impedance Spectroscopy Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hsieh, C.; Tsai, P.; Hsu, N.; Chen, Y. Effect of compression ratio of graphite felts on the performance of an all-vanadium redox flow battery. Energies 2019, 12, 313. [Google Scholar] [CrossRef]
- Chen, L.; Liu, T.; Zhang, Y.; Liu, H.; Ding, M.; Pan, D. Mitigating Capacity Decay by Adding Carbohydrate in the Negative Electrolyte of Vanadium Redox Flow Battery. Energies 2022, 15, 2454. [Google Scholar] [CrossRef]
- Aramendia, I.; Fernandez-Gamiz, U.; Martinez-San-Vicente, A.; Zulueta, E.; Lopez-Guede, J.M. Vanadium redox flow batteries: A review oriented to fluid-dynamic optimization. Energies 2020, 14, 176. [Google Scholar] [CrossRef]
- Leung, P.; Mohamed, M.; Shah, A.; Xu, Q.; Conde-Duran, M. A mixed acid based vanadium–cerium redox flow battery with a zero-gap serpentine architecture. J. Power Sources 2015, 274, 651–658. [Google Scholar] [CrossRef]
- Behi, B.; Baniasadi, A.; Arefi, A.; Gorjy, A.; Jennings, P.; Pivrikas, A. Cost–benefit analysis of a virtual power plant including solar PV, flow battery, heat pump, and demand management: A western australian case study. Energies 2020, 13, 2614. [Google Scholar] [CrossRef]
- Rahman, F.; Skyllas-Kazacos, M. Vanadium redox battery: Positive half-cell electrolyte studies. J. Power Sources 2009, 189, 1212–1219. [Google Scholar] [CrossRef]
- Cao, L.; Skyllas-Kazacos, M.; Menictas, C.; Noack, J. A review of electrolyte additives and impurities in vanadium redox flow batteries. J. Energy Chem. 2018, 27, 1269–1291. [Google Scholar] [CrossRef]
- Kazacos, M.; Skyllas-Kazacos, M. High Energy Density Vanadium Electrolyte Solutions, Methods of Preparation Thereof and All-Vanadium Redox Cells and Batteries Containing High Energy Vanadium Electrolyte Solutions. W.O. Patent 9,635,239 A1, 7 November 1996. [Google Scholar]
- Skyllas-Kazacos, M.; Kazacos, M. High Energy Density Vanadium Electrolyte Solutions, Methods of Preparation Thereof and All-Vanadium Redox Cells and Batteries Containing High Energy Vanadium Electrolyte Solutions. U.S. Patent 7,078,123 B2, 18 July 2006. Available online: https://patentimages.storage.googleapis.com/eb/d1/4a/83586dc481f159/US7078123.pdf (accessed on 15 September 2022).
- Roznyatovskaya, N.V.; Roznyatovsky, V.A.; Höhne, C.-C.; Fühl, M.; Gerber, T.; Küttinger, M.; Noack, J.; Fischer, P.; Pinkwart, K.; Tübke, J. The role of phosphate additive in stabilization of sulphuric-acid-based vanadium (V) electrolyte for all-vanadium redox-flow batteries. J. Power Sources 2017, 363, 234–243. [Google Scholar] [CrossRef]
- Park, S.K.; Shim, J.; Yang, J.H.; Jin, C.S.; Lee, B.S.; Lee, Y.S.; Shin, K.H.; Jeon, J.D. Effect of inorganic additive sodium pyrophosphate tetrabasic on positive electrolytes for a vanadium redox flow battery. Electrochim. Acta 2014, 121, 321–327. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Nie, Z.; Chen, B.; Vijayakumar, M.; Kim, S.; Wang, W.; Schwenzer, B.; Liu, J.; Yang, Z. Effects of additives on the stability of electrolytes for all-vanadium redox flow batteries. J. Appl. Electrochem. 2011, 41, 1215–1221. [Google Scholar] [CrossRef]
- Li, S.; Huang, K.; Liu, S.; Fang, D.; Wu, X.; Lu, D.; Wu, T. Effect of organic additives on positive electrolyte for vanadium redox battery. Electrochim. Acta 2011, 56, 5483–5487. [Google Scholar] [CrossRef]
- Zhang, Y.; Xi, J.; Liu, L.; Wu, Z. Boosting the thermal stability of electrolytes in vanadium redox flow batteries via 1-hydroxyethane-1, 1-diphosphonic acid. J. Appl. Electrochem. 2020, 50, 255–264. [Google Scholar] [CrossRef]
- Roe, S.; Menictas, C.; Skyllas-Kazacos, M. A high energy density vanadium redox flow battery with 3 M vanadium electrolyte. J. Electrochem. Soc. 2015, 163, A5023. [Google Scholar] [CrossRef]
- Rahman, F.; Skyllas-Kazacos, M. Evaluation of additive formulations to inhibit precipitation of positive electrolyte in vanadium battery. J. Power Sources 2017, 340, 139–149. [Google Scholar] [CrossRef]
- Kausar, N.; Mousa, A.; Skyllas-Kazacos, M. The effect of additives on the high-temperature stability of the vanadium redox flow battery positive electrolytes. ChemElectroChem 2016, 3, 276–282. [Google Scholar] [CrossRef]
- Xuewen, W.; Suqin, L.; Kelong, H. Characteristics of CTAB as electrolyte additive for vanadium redox flow battery. J. Inorg. Mater. 2010, 25, 641–646. [Google Scholar]
- Wu, X.; Liu, S.; Wang, N.; Peng, S.; He, Z. Influence of organic additives on electrochemical properties of the positive electrolyte for all-vanadium redox flow battery. Electrochim. Acta 2012, 78, 475–482. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Skyllas-Kazacos, M. Stabilized Vanadium Electrolyte Solutions for All-Vanadium Redox Cells and Batteries. U.S. Patent 6,562,514, 13 May 2003. [Google Scholar]
- Sun, H.; Yang, B.; Zhu, Z.; Yin, W.; Sheng, Q.; Hou, Y.; Yao, J. New insights into selective-depression mechanism of novel depressant EDTMPS on magnesite and quartz surfaces: Adsorption mechanism, DFT calculations, and adsorption model. Miner. Eng. 2021, 160, 106660. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Y.; Xie, M.; Huang, M.; Chen, G. Characterization of scalants and strategies for scaling mitigation in membrane distillation of alkaline concentrated circulating cooling water. Desalination 2022, 527, 115534. [Google Scholar] [CrossRef]
- Peng, S.; Wang, N.-F.; Wu, X.-J.; Liu, S.-Q.; Fang, D.; Liu, Y.-N.; Huang, K.-L. Vanadium species in CH3SO3H and H2SO4 mixed acid as the supporting electrolyte for vanadium redox flow battery. Int. J. Electrochem. Sci. 2012, 7, 643–649. [Google Scholar]
- Wang, G.; Chen, J.; Xu, Y.; Yan, B.; Wang, X.; Zhu, X.; Zhang, Y.; Liu, X.; Wang, R. Several ionic organic compounds as positive electrolyte additives for a vanadium redox flow battery. RSC Adv. 2014, 4, 63025. [Google Scholar] [CrossRef]
- Wu, T.; Huang, K.; Liu, S.; Zhuang, S.; Fang, D.; Li, S.; Lu, D.; Su, A. Hydrothermal ammoniated treatment of PAN-graphite felt for vanadium redox flow battery. J. Solid State Electrochem. 2012, 16, 579–585. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Menictas, C.; Kazacos, M. Thermal stability of concentrated V (V) electrolytes in the vanadium redox cell. J. Electrochem. Soc. 1996, 143, L86. [Google Scholar] [CrossRef]
- Huang, F.; Zhao, Q.; Luo, C.; Wang, G.; Yan, K.; Luo, D. Influence of Cr3+ concentration on the electrochemical behavior of the anolyte for vanadium redox flow batteries. Chin. Sci. Bull. 2012, 57, 4237–4243. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 2022. [Google Scholar]
- Xue, F.; Wang, Y.; Wang, W.; Wang, X. Investigation on the electrode process of the Mn (II)/Mn (III) couple in redox flow battery. Electrochim. Acta 2008, 53, 6636–6642. [Google Scholar] [CrossRef]
- Gao, C.; Wang, N.; Peng, S.; Liu, S.; Lei, Y.; Liang, X.; Zeng, S.; Zi, H. Influence of Fenton’s reagent treatment on electrochemical properties of graphite felt for all vanadium redox flow battery. Electrochim. Acta 2013, 88, 193–202. [Google Scholar] [CrossRef]

| Additive | Amount | V(V)/M | H2SO4/M | Temperature/°C | Effect of Thermal Stability | References |
|---|---|---|---|---|---|---|
| Sodium tripolyphosphate | 1 wt% | 2 | - | 44 | Improved | [8] |
| Sodium hexametaphosphate | 1 wt% | 2 | - | 44 | Improved | [9] |
| Sodium pyrophosphate | 0.05 M | 2 | 4 | 25 | Improved | [11] |
| glucose | 1 wt% | 1.8 | 4.8 | 20–60 | Improved | [13] |
| K3PO4 | 1 wt% | 3 | 5 | 30/50 | Improved | [15] |
| Polyacrylic acid | 0.5 wt/vol% | 4.7 | 6 | 50 | Slightly improved | [16] |
| (NH4)2SO4 | 2 wt% | 1.8 | 5 | 50 | Improved | [17] |
| H3PO4 | 1 wt% | 2 | 5 | 50 | Significantly improved | [17] |
| CH3SO3H | 2.1–3 wt% | 2 | 5 | 40 | Improved | [12] |
| Hexadecyl trimethyl ammonium bromide (CTAB) | 0.00106–0.0053 M | 1.5 | 4.5 | 45 | Improved | [18] |
| Phytic acid | N/A | 1.8 | 3 | 25–60 | Improved | [19] |
| Chemical Name (Short Form) | Molecular Structure |
|---|---|
| Hexamethylenediamine tetramethylene phosphonic acid (HDTMPA) |  |
| 1-hydroxyethylidene-1,1-diphosphonic acid (HEDP) |  |
| Amino trimethylene phosphonic acid (ATMPA) | 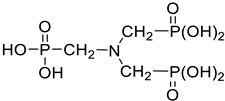 |
| Sodium ethylenediamine tetramethylene phosphonate (EDTMPS) |  |
| Diethyl triamine pentamethylene phosphonic acid (DTPMP) | 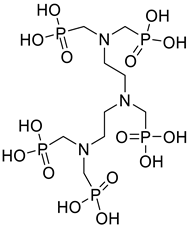 |
| Blank | HDTMPA | HEDP | ATMPA | EDTMPS | DTPMP | |
|---|---|---|---|---|---|---|
| Time to precipitation | 5 days | 15 days | 30 days | 4 days | 10 days | 12 days |
| V(V) concentration after 30 days | 1.27 M | 1.46 M | 1.86 M | 1.20 M | 1.40 M | 1.44 M |
| Additives | Blank | HDTMPA | HEDP | DTPMP | ATMPA | EDTMPS | ATMPA + HEDP | ATMPA + HDTMPA |
|---|---|---|---|---|---|---|---|---|
| ΔVp/V | 0.241 | 0.231 | 0.340 | 0.320 | 0.275 | 0.285 | 0.208 | 0.200 |
| IpO/IpR | 1.462 | 1.496 | 1.242 | 1.299 | 1.432 | 1.246 | 1.470 | 1.474 |
| Additives | Diffusion Coefficient D1 and D2 of V(IV) Species (cm2 s−1) | ||
|---|---|---|---|
| D1 | D2 | Error (%) | |
| Blank | 2.20 × 10−7 | 1.88 × 10−7 | 1.9 |
| HDTMPA | 2.26 × 10−7 | 1.84 × 10−7 | 1.3 |
| HEDP | 3.89 × 10−7 | 3.15 × 10−7 | 1.5 |
| DTPMP | 4.86 × 10−7 | 3.94 × 10−7 | 1.3 |
| ATMPA | 6.80 × 10−7 | 5.52 × 10−7 | 1.3 |
| EDTMPS | 3.48 × 10−7 | 2.83 × 10−7 | 1.6 |
| ATMPA + HDTMPA | 4.34 × 10−7 | 3.53 × 10−7 | 1.8 |
| ATMPA + HEDP | 5.43 × 10−7 | 4.40 × 10−7 | 1.7 |
| Additives | Rct (Ω cm2) | i0 (mA cm−2) | k0 (10−5 cm s−1) |
|---|---|---|---|
| Blank | 12.40 | 2.07 | 1.07 |
| HDTMPA | 11.48 | 2.24 | 1.16 |
| HEDP | 9.40 | 2.73 | 1.42 |
| DTPMP | 8.84 | 2.91 | 1.51 |
| ATMPA | 9.01 | 2.85 | 1.48 |
| EDTMPS | 8.15 | 3.15 | 1.63 |
| ATMPA + HDTMPA | 10.35 | 2.48 | 1.29 |
| ATMPA + HEDP | 9.11 | 2.82 | 1.46 |
| Additives | R1/Ω cm2 | CPE/S sn cm−2 | R2/Ω cm2 | W0, Y0,2/S s−5 cm−2 | |
|---|---|---|---|---|---|
| Y0,1 | n | ||||
| Blank | 0.218 | 5.33 × 10−3 | 0.775 | 0.168 | 0.409 |
| HDTMPA | 0.223 | 9.11 × 10−3 | 0.727 | 0.215 | 0.411 |
| HEDP | 0.268 | 10.85 × 10−3 | 0.707 | 0.205 | 0.397 |
| DTPMP | 0.268 | 8.18 × 10−3 | 0.737 | 0.152 | 0.397 |
| ATMPA | 0.286 | 8.70 × 10−3 | 0.727 | 0.154 | 0.408 |
| EDTMPS | 0.279 | 5.61 × 10−3 | 0.769 | 0.116 | 0.390 |
| ATMPA + HDTMPA | 0.276 | 16.87 × 10−3 | 0.621 | 0.206 | 0.283 |
| ATMPA + HEDP | 0.282 | 18.63 × 10−3 | 0.552 | 0.186 | 0.288 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Meng, F.; Sun, L.; Zhu, Z.; Chen, D.; Wang, L. Influence of Several Phosphate-Containing Additives on the Stability and Electrochemical Behavior of Positive Electrolytes for Vanadium Redox Flow Battery. Energies 2022, 15, 7829. https://doi.org/10.3390/en15217829
Zhang X, Meng F, Sun L, Zhu Z, Chen D, Wang L. Influence of Several Phosphate-Containing Additives on the Stability and Electrochemical Behavior of Positive Electrolytes for Vanadium Redox Flow Battery. Energies. 2022; 15(21):7829. https://doi.org/10.3390/en15217829
Chicago/Turabian StyleZhang, Xukun, Fancheng Meng, Linquan Sun, Zhaowu Zhu, Desheng Chen, and Lina Wang. 2022. "Influence of Several Phosphate-Containing Additives on the Stability and Electrochemical Behavior of Positive Electrolytes for Vanadium Redox Flow Battery" Energies 15, no. 21: 7829. https://doi.org/10.3390/en15217829
APA StyleZhang, X., Meng, F., Sun, L., Zhu, Z., Chen, D., & Wang, L. (2022). Influence of Several Phosphate-Containing Additives on the Stability and Electrochemical Behavior of Positive Electrolytes for Vanadium Redox Flow Battery. Energies, 15(21), 7829. https://doi.org/10.3390/en15217829








