Transition Metal Carbides Filler-Reinforced Composite Polymer Electrolyte for Solid-State Lithium-Sulfur Batteries at Room Temperature: Breakthrough
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the NiWC/SBA-15-IL
2.3. Preparation of the Solid Electrolyte (PEO-LiTFSI-NiWC/SBA-15-IL-LCO)
2.4. Preparation of the Cathode (S-CoS@CNT-PEO-LiTFSI-NiWC/SBA-15-IL-LCO)
2.5. Materials Characterization
2.6. The Assembly of the Cells
2.7. Electrochemical Measurements
3. Results
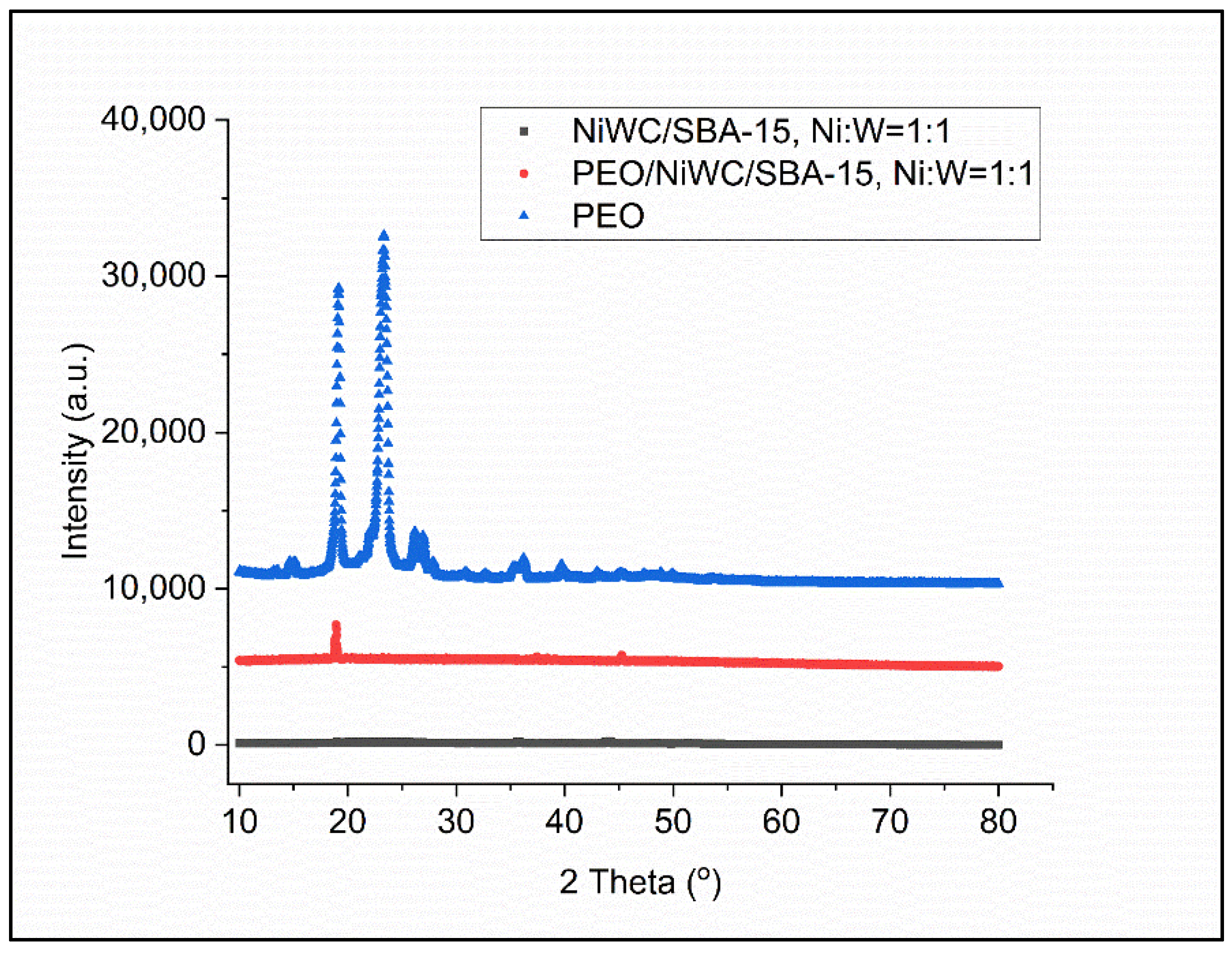
3.1. Materials Crystallography
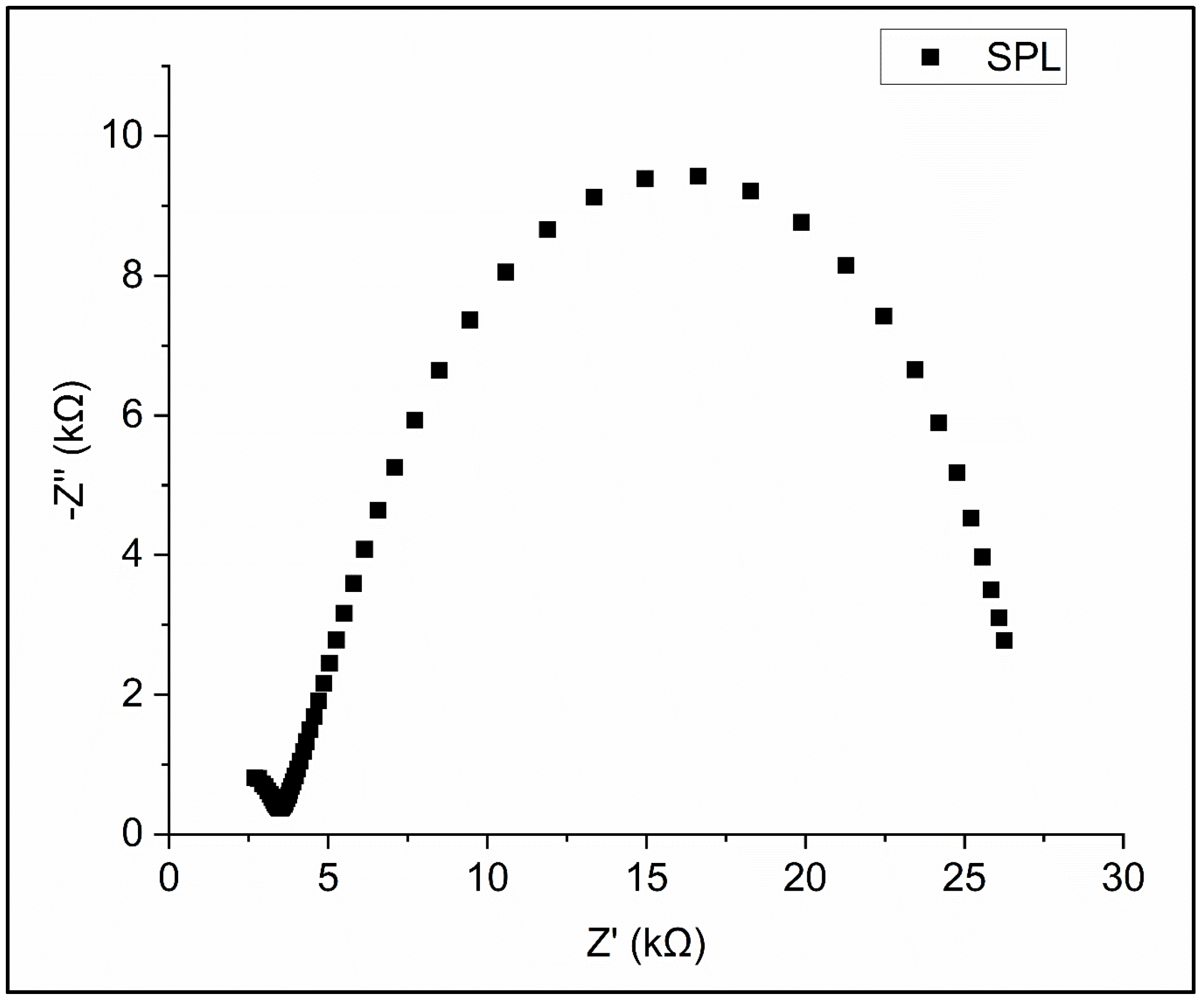
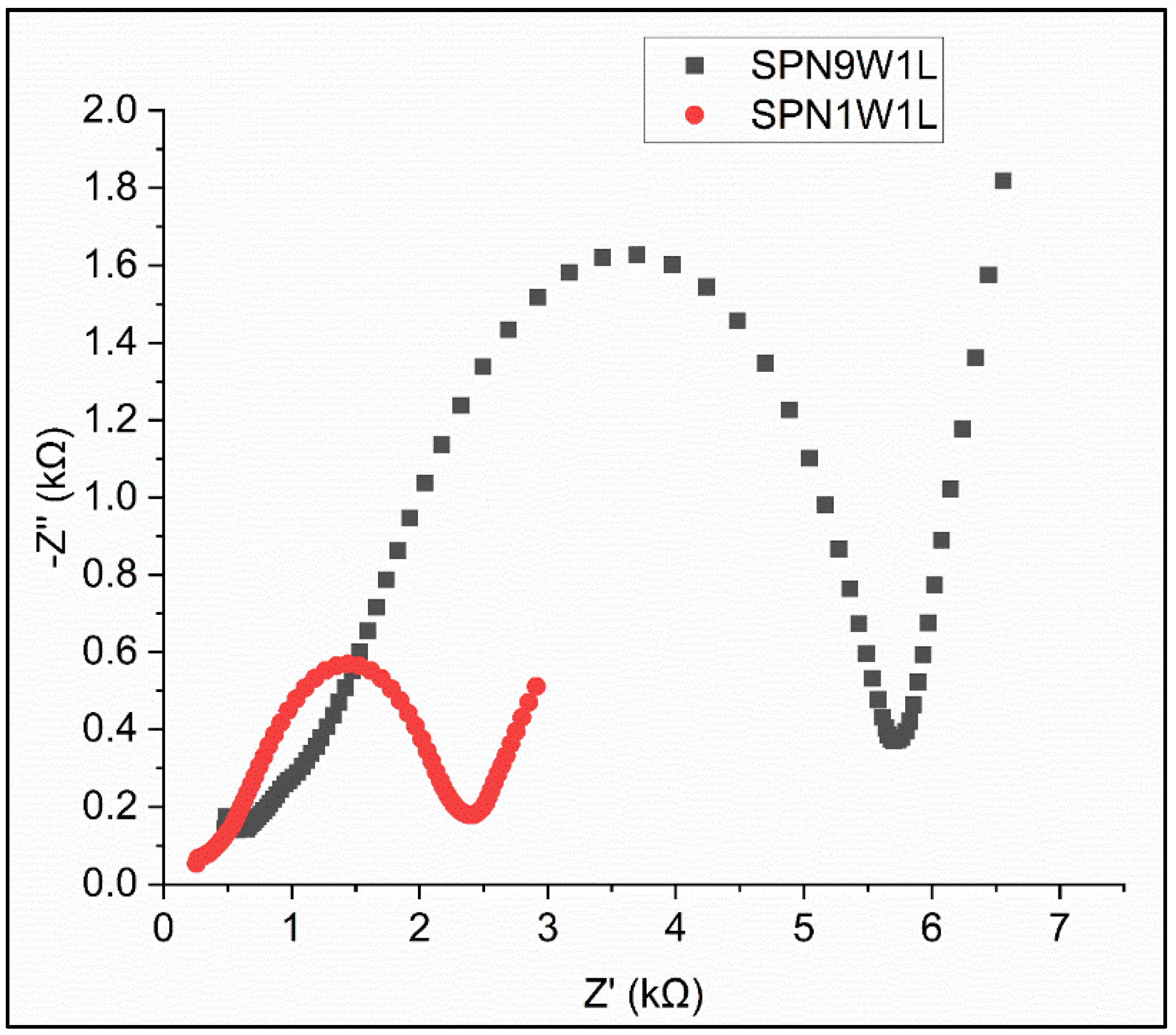
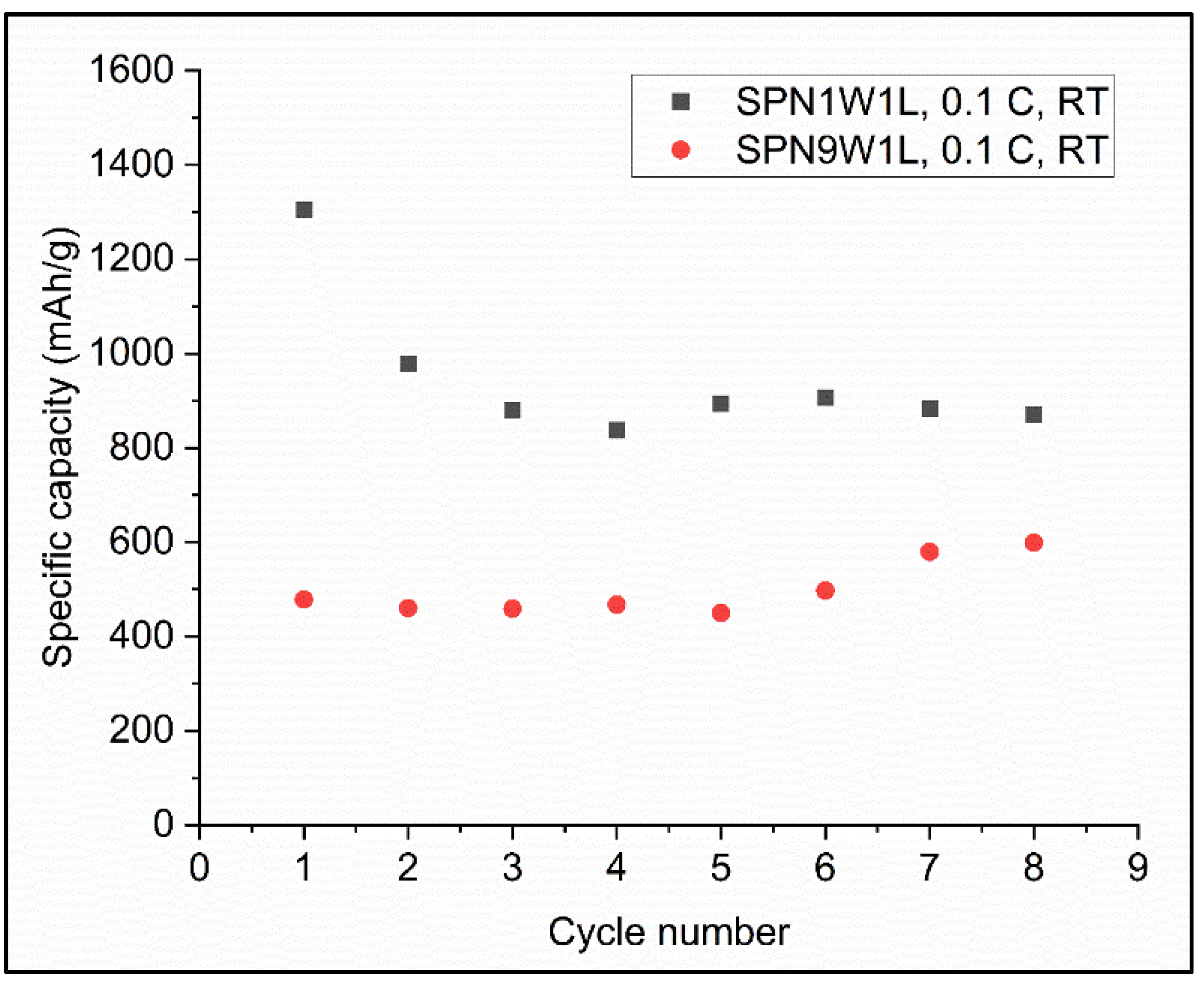
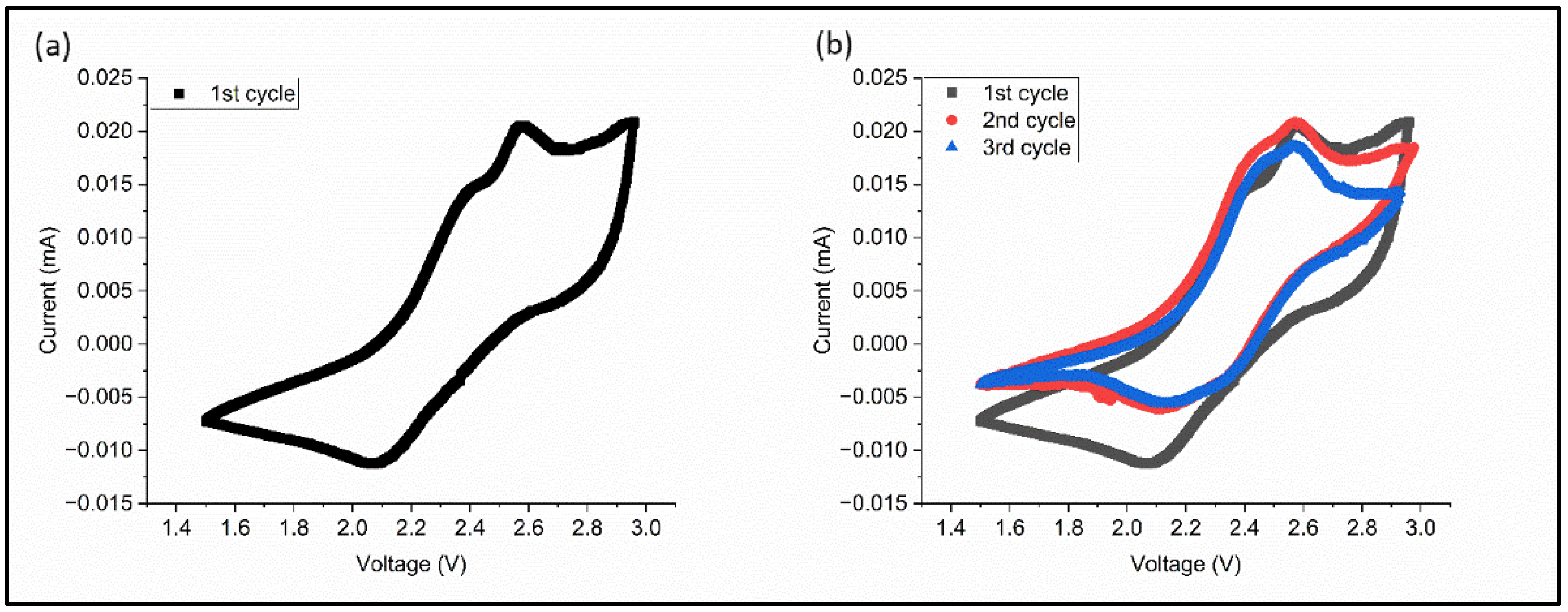
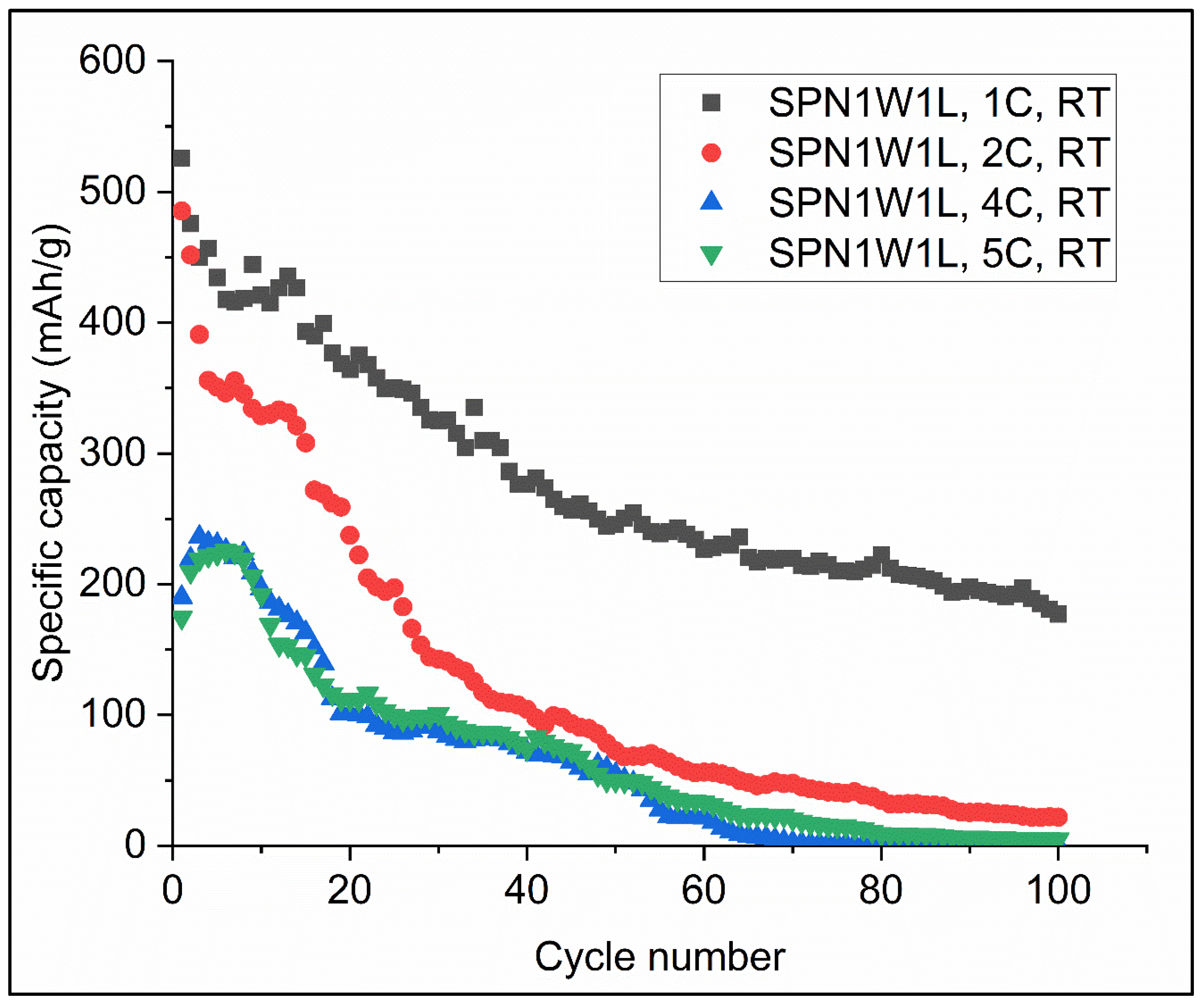
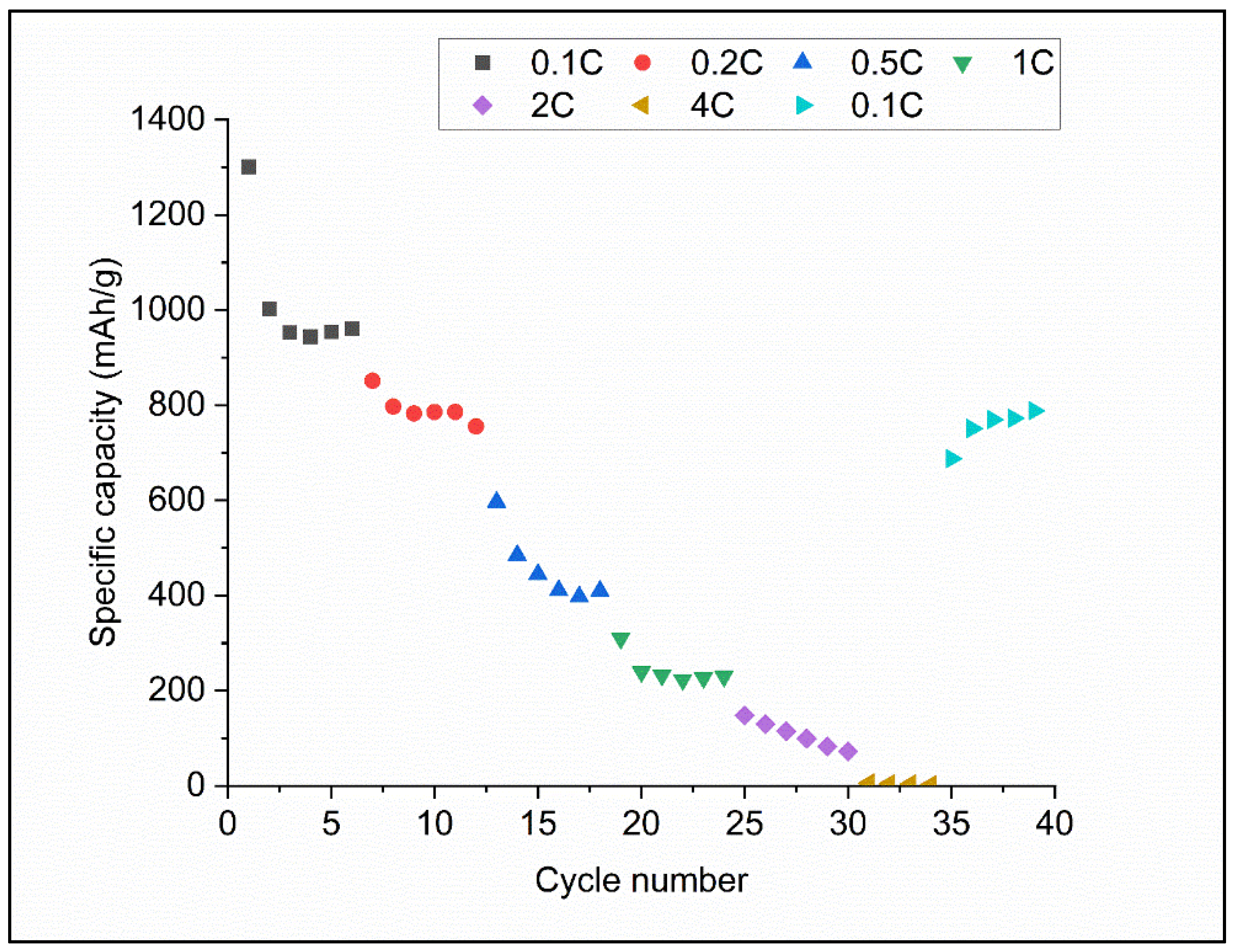
3.2. The Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Liu, B.; Li, Y.H.; Shen, S.H.; Deng, S.J.; Wang, X.L.; Xia, X.H.; Tu, J.P. Electrode Design for Lithium–Sulfur Batteries: Problems and Solutions. Adv. Funct. Mater. 2020, 30, 1910375. [Google Scholar] [CrossRef]
- Chen, X.; Hou, T.; Persson, K.A.; Zhang, Q. Combining theory and experiment in lithium–sulfur batteries: Current progress and future perspectives. Mater. Today 2019, 22, 142–158. [Google Scholar] [CrossRef]
- Zhao, H.; Deng, N.; Yan, J.; Kang, W.; Ju, J.; Ruan, Y.; Wang, X.; Zhuang, X.; Li, Q.; Cheng, B. A review on anode for lithium-sulfur batteries: Progress and prospects. Chem. Eng. J. 2018, 347, 343–365. [Google Scholar] [CrossRef]
- Fotouhi, A.; Auger, D.; O’Neill, L.; Cleaver, T.; Walus, S. Lithium-Sulfur Battery Technology Readiness and Applications—A Review. Energies 2017, 10, 1937. [Google Scholar] [CrossRef]
- Rong, G.; Zhang, X.; Qiu, Y.; Liu, M.; Ye, F.; Xu, Y.; Chen, J.; Hou, Y.; Li, W.; Zhang, Y. Liquid-Phase Electrochemical Scanning Electron Microscopy for In Situ Investigation of Lithium Dendrite Growth and Dissolution. Adv. Mater. 2017, 29, 1606187. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbonsulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Shen, J.; Moganty, S.S.; Corona, A.; Archer, L.A. Porous hollow carbon@sulfur composites for high-power lithium-sulfur batteries. Angew Chem. Int. Ed. 2011, 50, 5904–5908. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Xu, Y.; Wang, C. Sulfur-impregnated disordered carbon nanotubes cathode for lithium-sulfur batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, C.; Lou, J.; Xia, Y.; Liang, C.; Huang, H.; Gan, Y.; Tao, X.; Zhang, W. Poly(ethylene oxide) reinforced Li6PS5Cl composite solid electrolyte for allsolid-state lithium battery: Enhanced electrochemical performance, mechanical property and interfacial stability. J. Power Sources 2019, 412, 78–85. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Y.; Liang, Y.; Robinson, J.T.; Li, Y.; Jackson, A.; Cui, Y.; Dai, H. Graphene-wrapped sulfur particles as a rechargeable lithium-sulfur battery cathode material with high capacity and cycling stability. Nano Lett. 2011, 11, 2644–2647. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bai, Y.; Zhao, Y.; Yao, H.; Pang, H. MoS2/graphene composites: Fabrication and electrochemical energy storage. Energy Storage Mater. 2020, 33, 470–502. [Google Scholar] [CrossRef]
- Judez, X.; Zhang, H.; Li, C.; Eshetu, G.G.; González-Marcos, J.A.; Armand, M.; Rodriguez-Martinez, L.M. Review—Solid Electrolytes for Safe and High Energy Density Lithium-Sulfur Batteries: Promises and Challenges. J. Electrochem. Soc. 2018, 165, A6008–A6016. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Lin, Z.; Liang, C. Lithium–sulfur batteries: From liquid to solid cells. J. Mater. Chem. A 2015, 3, 936. [Google Scholar] [CrossRef]
- Umeshbabu, E.; Zheng, B.; Yang, Y. Recent Progress in All-Solid-State Lithium−Sulfur Batteries Using High Li-Ion Conductive Solid Electrolytes. Electrochem. Energ. Rev. 2019, 2, 199. [Google Scholar] [CrossRef]
- Wang, Y.; Sahadeo, E.; Rubloff, G.; Lin, C.F.; Lee, S.B. High-capacity lithium sulfur battery and beyond: A review of metal anode protection layers and perspective of solid-state electrolytes. J. Mater. Sci. 2019, 54, 3671. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Huang, J.Q.; Zhao, C.Z.; Zhang, Q. A review of solid electrolytes for safe lithium-sulfur batteries. Sci. China Chem. 2017, 60, 1508–1526. [Google Scholar] [CrossRef]
- Bachman, J.C.; Muy, S.; Grimaud, A.; Chang, H.H.; Pour, N.; Lux, S.F.; Paschos, O.; Maglia, F.; Lupart, S.; Lamp, P.; et al. Inorganic Solid-State Electrolytes for Lithium Batteries: Mechanisms and Properties Governing Ion Conduction. Chem. Rev. 2016, 116, 140–162. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, K.; Ding, F.; Liu, X. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res. 2017, 10, 4139–4174. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Yang, K.; Wang, H.; Yu, C.; Xu, D.; Xu, B.; Wang, L.M. Superior Blends Solid Polymer Electrolyte with Integrated Hierarchical Architectures for All-Solid-State Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 36886–36896. [Google Scholar] [CrossRef]
- Puthirath, A.B.; Patra, S.; Pal, S.; Manoj, M.; Puthirath Balan, A.; Jayalekshmi, S.; Tharangattu, N.N. Transparent flexible lithium ion conducting solid polymer electrolyte. J. Mater. Chem. A 2017, 5, 11152–11162. [Google Scholar] [CrossRef]
- Zhang, J.; Zang, X.; Wen, H.; Dong, T.; Chai, J.; Li, Y.; Chen, B.; Zhao, J.; Dong, S.; Ma, J.; et al. High-voltage and free-standing poly(propylene carbonate)/Li6.75La3Zr1.75Ta0.25O12 composite solid electrolyte for wide temperature range and flexible solid lithium ion battery. J. Mater. Chem. A 2017, 5, 4940–4948. [Google Scholar] [CrossRef]
- Sun, L.; Park, S.S.; Sheberla, D.; Dinca, M. Measuring and Reporting Electrical Conductivity in Metal–Organic Frameworks: Cd2(TTFTB) as a Case Study. J. Am. Chem. Soc. 2016, 138, 14772–14782. [Google Scholar] [CrossRef]
- Park, S.S.; Tulchinsky, Y.; Dinca, M. Single-Ion Li+, Na+, and Mg2+ Solid Electrolytes Supported by a Mesoporous Anionic Cu–Azolate Metal–Organic Framework. J. Am. Chem. Soc. 2017, 139, 13260–13263. [Google Scholar] [CrossRef]
- Wu, J.; Guo, X. MOF-derived nanoporous multifunctional fillers enhancing the performances of polymer electrolytes for solid-state lithium batteries. J. Mater. Chem. A 2019, 7, 2653–2659. [Google Scholar] [CrossRef]
- Han, X.; Wu, T.; Gu, L.; Tian, D. A Li-based MOF-derived multifunctional PEO polymer solid-state electrolyte for lithium energy storage. New J. Chem. 2022, 46, 3747–3753. [Google Scholar] [CrossRef]
- Elizalde-Segovia, R.; Irshad, A.; Zayat, B.; Narayanan, S.R. Solid-State Lithium-Sulfur Battery Based on Composite Electrode and Bi-layer Solid Electrolyte Operable at Room Temperature. J. Electrochem. Soc. 2020, 167, 140529. [Google Scholar] [CrossRef]
- Al Alwan, B.; Sari, E.; Salley, S.O.; Ng, K.Y.S. Effect of Metal Ratio and Preparation Method on Nickel−Tungsten Carbide Catalyst for Hydrocracking of Distillers Dried Grains with Solubles Corn Oil. Ind. Eng. Chem. Res. 2014, 53, 6923–6933. [Google Scholar] [CrossRef]
- Ara, M.; Meng, T.; Nazri, G.; Salley, S.O.; Ng, K.Y.S. Ternary Imidazolium-Pyrrolidinium-Based Ionic Liquid Electrolytes for Rechargeable Li-O2 Batteries. J. Electrochem. Soc. 2014, 161, A1–A7. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, W.; Ng, K.Y.S. Facile Synthesis of CoS Nanoparticles Anchored on the Surface of Functionalized Multiwalled Carbon Nanotubes as Cathode Materials for Advanced Li−S Batteries. Ind. Eng. Chem. Res. 2022, 61, 9322–9330. [Google Scholar] [CrossRef]
- Ge, Z.; Li, J.; Liu, J. Enhanced electrochemical performance of all-solid-state sodium-sulfur batteries by PEO-NaCF3SO3-MIL-53(Al) solid electrolyte. Ionics 2020, 26, 1787–1795. [Google Scholar] [CrossRef]
- Kumaresan, K.; Mikhaylik, Y.; White, R.E. A Mathematical Model for a Lithium–Sulfur Cell. J. Electrochem. Soc. 2008, 155, A576–A582. [Google Scholar] [CrossRef]
- Barchasz, C.; Molton, F.; Duboc, C. Lithium/sulfur cell discharge mechanism: An original approach for intermediate species identification. Anal. Chem. 2012, 84, 3973–3980. [Google Scholar] [CrossRef]
- Pletcher, D.; Greff, R.; Peat, R.; Peter, L.M.; Robinson, J. Instrumental Methods in Electrochemistry, 1st ed.; Woodhead Publishing: Philadelphia, PA, USA, 2001; p. 442. [Google Scholar]
- Mei, B.; Lau, J.; Lin, T.; Tolbert, S.H.; Dunn, B.S.; Pilon, L. Physical Interpretations of Electrochemical Impedance Spectroscopy of Redox Active Electrodes for Electrical Energy Storage. J. Phys. Chem. C 2018, 122, 24499–24511. [Google Scholar] [CrossRef]
- Das, S.; Ngene, P.; Norby, P.; Vegge, T.; Jongh, P.; Blanchard, D. All-Solid-State Lithium-Sulfur Battery Based on a Nanoconfined LiBH4 Electrolyte. J. Electrochem. Soc. 2016, 163, A2029–A2034. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Shi, F.; Zhan, J.; Tu, J.; Fan, H.J. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Alwan, B.; Wang, Z.; Fawaz, W.; Ng, K.Y.S. Transition Metal Carbides Filler-Reinforced Composite Polymer Electrolyte for Solid-State Lithium-Sulfur Batteries at Room Temperature: Breakthrough. Energies 2022, 15, 7827. https://doi.org/10.3390/en15217827
Al Alwan B, Wang Z, Fawaz W, Ng KYS. Transition Metal Carbides Filler-Reinforced Composite Polymer Electrolyte for Solid-State Lithium-Sulfur Batteries at Room Temperature: Breakthrough. Energies. 2022; 15(21):7827. https://doi.org/10.3390/en15217827
Chicago/Turabian StyleAl Alwan, Basem, Zhao Wang, Wissam Fawaz, and K. Y. Simon Ng. 2022. "Transition Metal Carbides Filler-Reinforced Composite Polymer Electrolyte for Solid-State Lithium-Sulfur Batteries at Room Temperature: Breakthrough" Energies 15, no. 21: 7827. https://doi.org/10.3390/en15217827
APA StyleAl Alwan, B., Wang, Z., Fawaz, W., & Ng, K. Y. S. (2022). Transition Metal Carbides Filler-Reinforced Composite Polymer Electrolyte for Solid-State Lithium-Sulfur Batteries at Room Temperature: Breakthrough. Energies, 15(21), 7827. https://doi.org/10.3390/en15217827



