Abstract
Wastewater treatment plants are considered to be not only as treatment facilities, but also essential elements of the circular economy. Wastewater treatment plants can be essential chains of the circular economy cycle. Despite this, sewage sludge management and utilization are mostly limited to biodegradation and further agricultural uses or incineration. The recovery of valuable products is mainly limited to nitrogen and phosphorus compounds. Fewer analyses focus on generating, recovering, and removing various polymers from sewage sludge, such as cellulose or extracellular polymeric substances (EPS). On the other hand, sewage sludge also contains polymeric pollutants, such as microplastics. The recovery and use of biopolymers is significant considering the problems connected with the presence and effects of artificial polymers (microplastics) in the environment. Despite the technical possibilities, not many technical scale installations are operated. Law regulations should make some incentives to develop the technologies and sell the recovered polymers in the market not as waste material, but as a valuable product. This paper presents state-of-the-art technologies for selected polymers’ recovery from sludge, including technical parameters of the processes and possible applications of recovered products, but it also considers the possibility of microplastics’ removal from this waste material.
1. Introduction
At present, the management and utilization of sewage sludge is mostly limited to biodegradation under anaerobic (methane digestion) or aerobic conditions (aerobic digestion), and further agricultural uses or incineration [1]. Anaerobic digestion is the most frequently used method of sludge processing in wastewater treatment plants because of the possibility of energy recovery via methane generation and utilization [1]. To increase methane production, co-fermentation units which utilize high-energy efficient wastes, such as fats, have been built. Many experiments on other energy-generating processes are frequently undertaken, including the generation of hydrogen or ethanol from sludge to produce biofuels or other products [1,2].
More and more frequently, wastewater treatment plants have also been considered essential objects in a circular economy, which means the installations for recovering valuable substrates from wastewater and sludge. Some wastewater treatment plants recover phosphorus and ammonium nitrogen as MAP (magnesium ammonium phosphate) [1]. Fewer analyses focus on generating, recovering, and removing various polymers from sewage sludge, such as cellulose or EPS [3]. On the other hand, a significant problem (but as a pollutant) is microplastics’ removal from sewage sludge. This problem has not been solved yet, but it is an important technological and environmental issue [4].
The paper aims to present state-of-the-art recovery technologies for cellulose and extracellular polymeric substances, including the technological parameters and applications of the recovered products.
2. Cellulose Recovery from Sewage Sludge
2.1. Cellulose Sources and Content in the Sludge
Cellulose is an organic polymer (polysaccharide) composed of D-glucose units linked by β-1,4 glucose bonds. Cellulose chains usually contain more than 3000 glucose units but no more than 14,000 [5]. It has about 85% crystalline areas and about 15% amorphic ones. Crystalline areas are strongly resistant to degradation, whereas amorphic ones are more susceptible to destruction [6]. Chemically clean cellulose fibers are odorless, white powdery fibers. The fibers are simultaneously strong and flexible. This polymer is insoluble in water and organic solvents, but it dissolves in sulfuric acid and concentrated solutions of zinc chloride, as well as in aqueous solutions of cupric ammonium chloride [7]. It is one of the most common organic polymers on Earth. It is the fundamental structural component of plants’ cell walls and is concomitant with the hemicellulose and lignin in them [8]. Hemicellulose does not have a significant commercial value, contrary to other polysaccharides, such as cellulose, starch, or pectins, but it can affect the extraction of cellulose and contribute to the quality of the fibers [7]. It is bonded to cellulose mainly via hydrogen bonds. Its susceptibility to degradation depends on the side groups of hemicellulose chains, including the number of methylene and acetate groups [9]. Lignin is a natural phenolic polymer, with a high molecular weight, and is an essential component of a plant cell wall. It also plays a vital role as the barrier that protects plants against pests and pathogens [10].
The source of cellulose in municipal wastewater is mainly toilet paper [11]. Other sources can be some food additives (E460, E461-469) [12]. Among them, E460 is crystalline cellulose (cellulose powder), E461 is methylcellulose, E462 is ethylcellulose, E463 is hydroxypropyl cellulose, E464 is hydrohypropylmethyl cellulose, E465 is methyl ethyl cellulose, E466 is sodium carboxymethyl cellulose, E467 is ethulose, E468 is sodium carboxymethyl cellulose, and E469 is enzymatically hydrolyzed carboxymethyl cellulose [13]. As sources of cellulose, fiber (the average discharge of which is estimated in Poland to be 4 kg per person per year) and vegetable residues are also categorized [12,14].
The use of toilet paper varies between countries. It is estimated that the highest usage of paper is noticed in the United States, and it is estimated to be about 18 ± 8 kg per person per year [15,16]. In Europe, toilet paper usage is estimated to be 11 ± 7 kg per person per year. In Asia, use is up to 2 kg per person per year, and in Africa it is up to 0.4 kg per person per year [15]. Based on these estimates, loads of toilet paper that inflow into wastewater treatment plants, depending on its size, could be 1800 tons per year for every 100,000 inhabitants in the United States, on average. In Europe, it could be 1100 tons per year for every 100,000 inhabitants. In Asia and Africa, the amounts of cellulose in municipal wastewater are significantly lower; for the large wastewater treatment plants in Asia, the amount of potential cellulose incoming to the installation is about 30 tons per year for every 100,000 inhabitants.
Cellulose is estimated to be up to 30% of the total COD (chemical oxygen demand) in wastewater treatment plants’ influents in such countries as the Netherlands [17].
The data on cellulose content in wastewater and sewage sludge are listed in Table 1.

Table 1.
The cellulose content in wastewater and sewage sludge according to various sources.
Cellulose fibers are resistant to degradation [18], and cellulose concentrations in digested sewage sludge are similar to those before fermentation [12]. The first stage of degradation under aerobic and anaerobic conditions is enzymatic hydrolysis by cellulases. The microorganisms which can digest cellulose are, among others, mainly Actinomycetales and Clostridiales. Bacteria which are capable for cellulose hydrolysis are both aerobic ones (including such genes as Acidothermus, Bacillus, Caldibacillus, Cellulomonas, Cellvibrio, Cytophaga, Erwinia, Micromonospora, Pseudomonas, Sporocythophaga, Rhodothermus, Streptomyces, Thermobifida), and anaerobic ones (including genes such as Acetivibrio, Anaerocellum, Butyryvibrio, Caldicellulsiruptor, Clostridium, Eubacterium, Fervidobacterium, Fibrobacter, Halocella, Ruminococcus, Spirochaete, Thermotoga). These microorganisms are commonly present in wastewater and sewage sludge [21].
It has been stated that anaerobic bacteria attack cellulose mainly via complex cellulase systems, exemplified by the well-characterized polycellulosome organelles. Their cellulolytic enzymes are distributed both in a liquid phase and on the surface of the cells. They release quite measurable amounts of extracellular enzymes to the external environment. In the case of aerobic bacteria, the enzymes are mainly complexed directly on the cell’s surface [21]. The form of the enzymes affects, among others, the possibility of their recovery from the reaction environment and their use for the technological purposes [5]. What is important is that facultative anaerobes or aerobes, often present in wastewater treatment systems, are not involved in cellulose hydrolysis [21].
In the case of the fungi capability of cellulose, hydrolysis is widely distributed. Capable of this are protist-like, primitive Chytridomycetes and advanced Basidomycetes [21]. It should be emphasized that the degradation of the cellulose biomass is more complex than that of pure cellulose because of the fact that biomass also contains other polymers, such as hemicellulose or lignin. In the case of biomass, the structure of the cellulose particles affects the process and the problem with diffusion and transport of the enzymes into the site of the “attack” [21]. The complete decomposition of cellulose lasts for several weeks under anaerobic methane digestion. In the case of aerobic stabilization of sludge, it was evaluated that a 40-day solids retention time yielded 83% degradation of toilet paper in activated sludge systems [22]. It is estimated that, in conventional wastewater treatment plants, less than 60% of entering cellulose can be degraded at an SRT (solids retention time) of 4–5 weeks [23]. Lignin is more resistant to decomposition than cellulose [24].
If one has decided to degrade cellulose in sewage sludge, some strategies for the enhancement of this process, including physical (e.g., thermal conditioning at a temperature of 40 to 250 °C, sonication), physicochemical (e.g., use of water vapor), and chemical ones (conditioning with acids or bases) can be used [25]. In the case of cellulose recovery, the methods mentioned above are, however, not applicable because the technique aims to separate cellulose fibers from the wastewater or sludge, and further decomposition depends on the other application of the separated biomass.
2.2. Cellulose Recovery Technologies
The first step of cellulose recovery is usually to separate the primary sludge with a high cellulose content on the sieves. Therefore, the recovery of the cellulose from the sewage requires replacing conventional primary sedimentation in primary sedimentation tanks by sieves or other more effective separation systems. Under technical conditions, it is estimated that the application of sieves is more expensive than the usage of the primary settlement tanks [16].
As Li et al. [16] calculated, fine screens with mesh openings of 3 mm yielded a recovery of 45.7% of the toilet paper in sewage. In contrast, sieves with meshes lower than 1 mm allowed for the separation of up to 94.5% of this material. According to Ruiken et al. [17], sieves of fine mesh sizes (of <0.35 mm) yield an effective recovery of cellulose from raw sewage. The problem connected with cellulose recovery on very fine screens is the clogging of the openings [16]. Additionally, rotating belt filters (RBFs) with mesh sizes of 50–500 µm, with the most frequently applied 350 µm, are considered effective in cellulose recovery from wastewater. These methods have been commercially applied. Commercially used RBFs unit with mesh sizes of 350 µm had a submerged area of 0.1 to 2.2 m2 per unit [23]. Using them means > 50% of total suspended solids can be removed from raw wastewater [23]. The advantage of RBFs compared to conventional clarifiers is less space, and compared to sieves, the possibility to apply small meshes without the problem of clogging [23].
The original separation method was presented by Glińska et al. [26], who used tetrakis (hydroxymethyl) phosphonium chloride ionic liquid to separate hydrocarbons, including cellulose, from sewage sludge. This last method, however, has only been used at a laboratory scale, and its effectiveness (at average 25%) was low compared to the previously mentioned separation methods [26].
Some authors also indicate that cellulose in sewage is present in two forms: cellulose fibers and nanocellulose. Until now, technologies have been focused on the recovery of cellulose fibers, and the fibers’ recovery is mainly considered from the inflow stream by sieving. Nanocellulose can also be recovered from excess sludge [27].
Despite its resistance to degradation, the problem connected with cellulose recovery is that sieving the sewage with very fine sieves decreases energy production during the digestion of sewage sludge [17]. On the other hand, the removal of cellulose fibers during primary treatment yields a reduction in energy use in an aeration tank. According to the data given by Marcelis and Wessels [28], by the removal of cellulose fibers, a decrease in energy consumption in aeration tanks by 10–15% can be achieved.
The simple technology of cellulose recovery from the primary sewage sludge was given by Honda et al. [18]. They observed that, when fibrous cellulose is suspended in 0.3% sulfuric acid and autoclaved at 130 °C for 60 min, about 85–88% of initial solids remained not dissolved. Under the same conditions, the sludge without cellulose fibers was hydrolyzed, and about 7% of solids remained.
An interesting recovery method was examined by Macfarlane et al. [29]. The authors applied simultaneous precipitation and dissolved air flotation for the recovery of organosolv lignin. Dissolved air flotation is a method of low energy and maintenance requirements, in comparison to centrifugation and filtration. The most important parameters of the process were identified to be temperature, mixing regime, and air saturation. As the temperature was above 30 °C, the yield of lignin recovery decreased, but faster settling was observed. Clarification was also affected by air saturation. A higher air saturation resulted in faster clarification of the solution. At temperatures over 40 °C, a very fine and not-settleable precipitate was formed.
On a technical scale, Cellvation technology was applied in the Netherlands to separate cellulose from wastewater. The technology uses RBFs of 350 µm mentioned above. Before RBF filtration, grit is removed from the wastewater in gravity grit-removal chambers. This step aims to avoid clogging RBFs. Afterwards, the grit-removal chambers’ cellulose washer is applied to remove, among other items, hair and plastics. The next step is the filtration of rotating belt filters of 350 µm mesh sizes. The wastewater is fed to the filter from the top and forms a precoat on the filter cloth, creating an even smaller mesh size. The sievings collected during filtration are partially dewatered on the filter cloth, which yields a reduction in solid material obtained. After sieving, the solids are, however, additionally dewatered (up to 20–30%) and hygenized. The obtained product is dried and broken up into smaller pieces. Recovered cellulose can then be turned into fluffy fibers formed into pellets (called Recell), depending on the requirements of the customer [23,28,30]. Until now, technologies of cellulose recovery, by separating the cellulose on RBFs, have been applied at a technical or pilot scale, among others, at Breivika WWTPs in Tromsø (Norway), WWTPs in California, and WWTPs in Geestmerambacht [23,28]. The recovery rates of cellulose during the process were 38-> 86%. In Geestmerambacht WWTPs, 400 kg of cellulose was recovered daily.
Despite the technological aspects of cellulose recovery from sewage, the vital problem is the further management of cellulose fibers. In the Netherlands, bike roads are constructed from materials that contain recovered cellulose fibers. Other solutions are also considered, e.g., the production of ethanol based on recovered lignocellulose. This type of ethanol can be a viable alternative to petroleum-based fuels [31]. Energy recovery from produced ethanol is a promising alternative, especially under the current political situation. Other energy production methods, including the burning of the cellulose, seems to be not economically effective because the heat of combustion of sewage sludge is equal to 18–21.5 MJ/kg, whereas for cellulose, it is 17–18 MJ/kg [25]. It is not economically effective to recover cellulose from sludge and then burn it, whereas the drying of sewage sludge is much simpler and more commonly used under technical-scale technology.
Other usages of recovered cellulose include the production of building bioblocs, bioplastics, and flocculants [23]. Cellulose recovered from sewage has since been used as a stabilizer in asphalt binders or additives (project VAZENA in the Netherlands) as an environmentally friendly reinforcing material component in binder-based materials, in the production of cellulose composites, and as insulation material. It can also be used in hydraulic lime mortar [23]. Another potential application of recovered cellulose is the production of activated carbons via hydrothermal carbonization (temperature 180–250 °C, pressure of 10–40 bar, 6–10 h) [32].
3. Extracellular Polymeric Substances (EPS) Recovery from Sewage Sludge
Extracellular polymeric substances (EPSs) are the main components of bacterial biofilms. It is estimated that about 50–90% of organic carbon in the biofilm is composed of these biopolymers. EPSs are composed mainly of polysaccharides of various physical and chemical properties (the abundant polysaccharides are hexose, hexosamine, and ketose). It also contains other molecules, such as proteins, lipids, and extracellular DNA, which are secreted by the bacteria and released from the dead cells [33]. In biofilms, significant amounts of humic substances can also be present in wastewater treatment systems [34]. They contribute to the electrically neutral or electrically negative properties of the biofilms. It was confirmed that various bacteria produce various types and quantities of EPS; moreover, the biofilm’s age affects EPS production. EPSs are generally hydrophobic, but significant amounts of water are bound in it via hydrogen bonds, simultaneously [35]. PHA (polyhydroxyalkanoates) components of EPSs play an important role in the cell adhesion and cohesion of sludge and affects its functional integrity, strength, dewaterability, and biodegradability. They can form various fractions, including tightly bounded EPSs (TB-EPSs), soluble EPSs (S-EPSs), and loosely bound EPSs (LB-EPSs). TB-EPSs accumulate both on the outside of cells and in the interior of the microbial aggregates. S-EPSs are well distributed in the aqueous phase, whereas LB-EPSs form a highly porous slime layer. By creating a biofilm, EPSs act as a protective layer against stressful external disturbances and carbon reserves during biological processes [36]. The distribution of EPS in the sludge is highly heterogeneous. EPSs are polymers capable of forming hydrogels and interacting with other EPSs and pollutants via hydrogen bonds, electrostatic forces, attractive ionic forces, or biochemical reactions [37].
From a technological point of view, EPS formation in wastewater treatment plants is affected by parameters, such as aeration, temperature, the salinity of sewage, pH, presence of metal ions, and dilution rate. As in other biological systems, EPS production is also affected by the accessibility of carbon sources, nitrogen, phosphates, and sulfates. Particularly, a C/N ratio is essential for EPS generation. However, phototrophic biofilms can be formed by cyanobacteria [38].
When we consider extraction methods of EPSs from the sludge, it is crucial to note the difference between TB-EPSs and LB-EPSs. LB-EPSs are easier to separate than TB-EPSs. Suitable extraction methods should avoid the cell death and disruption of the EPS structure [34]. Methods of EPS extractions can be divided into:
- physical (sonication, centrifugation, heating),
- chemical (application of the alkali and acids, extraction by ethylene diamine tetra acetic acid (EDTA), cation exchange, ethanol, NaCl, sulfides),
- biological (by enzymes).
Chemical methods seem to be more effective in EPS recovery; however, they have many limitations connected with the properties of chemical agents, such as damage of the microbial cells [35].
EPSs in WWTPs can be recovered from excess sludge, both conventional and granular; however, granular-activated sludge is considered a promising material for the technological recovery of some EPSs [37,39]. In Table 2, the technological parameters of various extraction methods are presented. The methods mentioned above are helpful in separating EPSs from the biomass. Further treatment is, however, necessary to obtain polymers in a form that will be valuable in the market.

Table 2.
Technological parameters of selected EPS extraction from the sludge.
Most methods examined for EPS removal from activated sludge are at a laboratory scale. It is visible that EPSs can be directly extracted from sludge by both physical and chemical methods. The advantage of physical methods is that sonication lasts for a short time, i.e., a few minutes. In addition, centrifugation is a process that is relatively short (several minutes). Direct centrifugation is the process that is effective both for aerobic and anaerobic sewage sludge. Heating is considered an effective method for EPS extraction from sludge, which yields an extraction from sludge even if the EPS is firmly bound to flocks [42].
Under laboratory conditions, various chemicals have been used, including sodium carbonate, formaldehyde, NaCl, ethanol, and EDTA (ethylenediamine tetraacetic acid). Among the various extrahents, NaCl is considered to be the most widely accepted because the resin used for the selective extraction of EPS can be easily removed and recycled [40].
A comparison of the total EPS extracted from various cultures by various extraction methods is presented in Table 3. As can be seen based on collected data, EPS which can be extracted from the sludge varies a lot, not only depending on the extraction method but also on the kind of the substrate used for EPS extraction: it can be from several mg/g d.w. to about 800 mg/g d.w. In each case, laboratory tests should be made to identify optimal parameters and extracting agents.

Table 3.
Comparison of the total EPS extracted from various cultures by various extraction methods (based on the data collected by Nouha et al. [42]).
Based on the data collected in Table 3, it can be suggested that such methods as NaOH, EDTA, formaldehyde, and NaOH are some of the most effective ones for EPS extraction from sludge. This last method is considered to be especially effective by Lui et al. [42,43], who stated that this method yields EPSs not polluted by extracellular substances.
The limitations and advantages of the particular extraction methods used for EPS recovery from the sludge are listed in Table 4.

Table 4.
Limitations and advantages of selected EPS extraction methods (“−” poor, “+” good, “++” very good, “+/−” average).
The ideal extraction method for EPS should be effective, cause minimal cell lysis, and not disrupt the EPS structure.
However, some industrial installations are also operated, e.g., in the Netherlands, a commercial company produces EPS in the form of Kaumera Gum. A full-scale installation was opened in the Netherlands in 2019 in the WWTPs, according to Zupthen [44]. The technology applied in the installation is presented in Figure 1. Collected sewage sludge of 3–7% TSS is stored in a special tank. The process of EPS isolation from the sludge begins by heating alkalized to pH 9–11 sludge to 80 °C for 0.5 to 2.0 h. To separate the centrifugate from the sludge, centrifugation is applied. A supernatant, which contains EPSs, is then acidified to precipitate the product EPS Kaumera. The remaining dewatered sludge is managed separately. EPS Kaumera is separated from the liquid phase via centrifugation. The ready product, acidic Kaumera, is then stored and can be treated in various ways. Further treatment can provide dialysis against ultrapure water for 24 h and the extract EPS in acidic form by adding 1 M HCl, followed by centrifugation. The product is a gel-like pellet. Additionally, a sodium or potassium form can be obtained by adding NaOH or KOH [37].
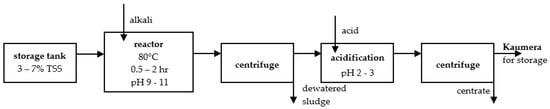
Figure 1.
Kaumera Gum recovery process [44].
Kaumera recovery yield is equal to 25–35% of organic matter in the sludge, depending on pH and extraction time. The recovery efficiency increases as the pH and reaction time of recovery in the reactor increases.
The amount of EPSs that can be recovered from conventional activated sludge are in the range of 90–190 mg/g of VSSs (volatile suspended solids). The obtained polymers had a similarity of about 60% to commercial alginate, and they consisted of poly (guluronic acid) blocks (20–30%) and poly (guluronic acid-mannuronic acid) blocks (8–28%). They had a relatively high gel-forming capacity [38].
Recovered EPSs can be used as bioflocullants and for the removal of toxic substances, such as heavy metals or dyes [34].
4. Microplastics Removal from Sewage Sludge
Because of the common pollution of wastewater by microplastics, not only natural biopolymers are present in the sludge, but also nondegradable, artificial polymers. Microplastics have been found in almost all sludge generated in WWTPs worldwide. A wide analysis of microplastic contamination of sludge conducted by Rolsky et al. [45] has shown that average particle counts in sludge for microplastics is 12.8 ± 5.2 MP/g, but it can even reach 168,000 MP/g [41,45].
The main problem connected with sewage sludge pollution by microplastics is the discharge of these micropollutants into the soil during applications of the sludge, e.g., in agriculture. The most frequently conducted studies on microplastics in the sludge focus on the concentration of these pollutants in the waste material. Few studies have been undertaken on the possibilities of microplastic removal from sludge.
Such studies suggest that the processes used for sludge stabilization (in Europe, it is mainly anaerobic digestion) are inefficient in removing microplastics from sludge [46,47]. Some other ones, however, suggest that these processes affect the characteristics of the sludge. The effects of various treatment methods on the characteristics of microplastics are presented in Table 5.

Table 5.
The effect of the sludge treatment method on the characteristics of microplastics in the sludge.
Based on the available data, it can be stated that during sludge treatment processes, mainly mechanical crushing occurs. Chemicals used for the treatment of sludge, such as lime, also affect the surface of microplastics particles by damaging its surface. No degradation of microplastics occurs. At present, any uses, despite incineration, do not protect the environment against microplastics in sludge [49,50]. In addition, other strategies for decreasing MP particle concentration in sludge have been developed, e.g., improving MPs removal during the grease separation step in WWTPs, and treating the grease separately from sewage sludge. This strategy prevents a large number of microplastics in sewage sludge [50]. Additionally, pyrolysis processes are considered, including thermal pyrolysis, catalytic pyrolysis, and microwave-assisted pyrolysis [51]. Another valuable alternative in the case of solid matrices is a separation of MPs based on density differences between MPs and the solids by using saturated salt solutions [6,50].
Some technologies involving enzymes are also considered to remove microplastics from sewage sludge, but all of them are currently tested only on a laboratory scale, e.g., within the ENZYCYCLE project, the scientist considered using enzymes to remove microplastics from sewage sludge. According to their idea, 87% of tiny microplastic particles can be separated by using this method. Bacterial strains that are capable of degrading microplastics will be included in a biofilter for biodegradation. The project is in progress [52].
5. Conclusions
Based on the literature review given above, the following conclusions can be formulated:
- Wastewater treatment plants can be essential chains of the circular economy cycle.
- Recovery of other products than P- and N-salts is technically possible.
- Recovery and use of biopolymers is significant considering the problems connected with the presence and effects of artificial polymers (microplastics) in the environment.
- Despite technical possibilities, not many technical scale installations are operated. Law regulations should make some incentives to develop technologies and sell the recovered polymers in the market not as waste material, but as a valuable product.
- No effective method of microplastic removal from sewage sludge has been developed.
- Further research works in this area should focus on economically effective and environmentally friendly methods of EPS and microplastic recovery from sludge.
Funding
The research was funded by Czestochowa University of Technology under statutory funds.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rosiek, K. Directions and challenges in the management on municipal sewage sludge in Poland in the context of the circular economy. Sustainability 2020, 12, 3686. [Google Scholar] [CrossRef]
- Gottardo, M.; Micolucci, F.; Mattioli, A.; Faggian, S.; Cavinato, C.; Pavan, P. Hydrogen and methane production from biowaste and sewage sludge by two phases anaerobic digestion. Chem. Eng. Trans. 2015, 43, 379–384. [Google Scholar]
- Huang, L.; Jin, Y.; Zhou, D.; Liu, L.; Huang, S.; Zhao, Y.; Chen, Y. A review of the role of extracellular polymeric substances (EPS) in wastewater treatment systems. Int. J. Environ. Res. Public Health 2022, 19, 12191. [Google Scholar] [CrossRef] [PubMed]
- Wiśniowska, E.; Moraczewska-Majkut, K.; Nocoń, W. Efficiency of microplastics removal in selected wastewater treatment plants—preliminary studies. Desalination Water Treat. 2018, 134, 36–323. [Google Scholar] [CrossRef]
- Poszytek, K. Microbial utilisation of cellulose. Postępy Mikrobiol. 2016, 55, 132–146. (In Polish) [Google Scholar]
- Cellulose. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/CELLULOSE (accessed on 7 September 2022).
- Gregory, A.; Bolwell, G.P. Carobohydrates and their derivarives including tannins, cellulose, and related lignins. In Comprehensive Natural Products Chemistry; Fraser-Reid, B.O., Tatsuta, K., Thiem, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Shang, Y. Celluloze Mineralization in Two-Stage Anaerobic Digestion Systems. Ph.D. Thesis, IOWA State University, Ames, IA, USA, 2000. [Google Scholar]
- Kancelista, A. Biodegradation of Lignocellulose Watses by Filamentus Fungi. Ph.D. Thesis, Wrocław University of Environmental and Life Sciences, Wrocław, Poland, 2012. (In Polish). [Google Scholar]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Gupta, M.; Ho, D.; Santoro, D.; Torfs, E.; Doucet, J.; Vanrolleghem, P.A.; Nakhla, G. Experimental assessment and validation of quantification methods for cellulose content in municipal wastewater and sludge. Environ. Sci. Pollut. Res. Int. 2018, 25, 16743–16753. [Google Scholar] [CrossRef] [PubMed]
- Cellulose in Sewage Sludge. Available online: https://seidel-przywecki.eu/2020/11/16/celuloza-w-osadzie-sciekowym/ (accessed on 7 September 2022).
- E469—Enzymatically Hydrolyzed Carboxymethylcellulase. Available online: https://world.openfoodfacts.org/additive/en:e469-enzymatically-hydrolysed-carboxymethylcellulose (accessed on 7 September 2022).
- Liu, R.; Li, Y.; Zhang, M.; Hao, X.; Liu, J. Review on the fate of cellulose in wastewater treatment. Resour. Conserv. Recycl. 2022, 184, 10635. [Google Scholar] [CrossRef]
- Palmieri, S.; Cipoletta, G.; Pastore, C.; Giosue, C.; Akyol, C.; Eusebi, A.L.; Frison, N.; Tittarelli, F.; Fatone, F. Pilot scale cellulose recovery from sewage sludge and reuse in building and concstruction material. Waste Manag. 2019, 100, 208–218. [Google Scholar]
- Li, S.; Wu, Z.; Wu, Z.; Liu, G. Enhancing fiber recovery from wastewater may require toilet paper redesign. J. Clean. Prod. 2020, 261, 121138. [Google Scholar] [CrossRef]
- Ruiken, C.J.; Breuer, G.; Klaversma, E.; Santiago, T.; van Loosdrecht, M.C.M. Sieving wastewater—Cellulose recovery, economic and energy evaluation. Water Res. 2013, 47, 3–48. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Miyata, N.; Iwahori, K. Recovery of biomass cellulose from waste sewage sludge. J. Mater. Cycles Waste Manag. 2002, 4, 46–50. [Google Scholar]
- Ahmed, A.S.; Bahreini, G.; Ho, D.; Sridhar, G.; Gupta, M.; Wessels, C.; Marcelis, P.; Elbeshbishy, E.; Rosso, D.; Nakhla, G. Fate of cellulose in primary and secondary treatment at municipal water resource facilities. Water Environ. Res. 2019, 91, 1479–1489. [Google Scholar] [CrossRef]
- Champagne, P.; Caijian, L. Enzimatic hydrolysis of cellulosic municipal wastewater treatment proces residuals as feedstocs for the recovery of simple sugars. Bioresour. Technol. 2009, 100, 5700–5706. [Google Scholar] [CrossRef]
- Lynd, L.R.; Weimer, P.J.; van Zyl, W.H.; Pretorius, I.S. Microbial Cellulose Utilization: Fundamentals and Biotechnology. Microbiol. Mol. Biol. Rev. 2002, 66, 506–577. [Google Scholar] [CrossRef]
- Li, S.; Wu, Z.; Liu, G. Degradation konetics of toilet paper fiber during wastewater treatment: Effects of solids retention time and microbial community. Chemosphere 2019, 225, 915–926. [Google Scholar] [CrossRef]
- Akyol, C.; Eusebi, A.L.; Cipoletta, G.; Bruni, C.; Foglia, A.; Giosue, C.; Frison, N.; Tittarelli, F.; Canestrari, F.; Fatone, F. Cellulosic materials recovery from municipal wastewater: From treatment plants to the market. In Clean Energy and Resource Recovery: Wastewater Treatment Plants as Biorefineries; Tyagi, K.V., An, K.J., Cetecioglu, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Saker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A review of the role of critical parameters in the design and operation of biogas production plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Wiśniowska, E. Integrated Systems of Waste Utilisation in Wastewater Treatment Plants; Publishing House of Czestochowa University of Technology: Czestochowa, Poland, 2016. (In Polish) [Google Scholar]
- Glińska, K.; Stuber, F.; Fabregat, E.; Giralt, J.; Font, J.; Mateo-Sanz, J.M.; Torrens, M.; Bengoa, C. Moving WWTP towards circular economy: Cellulose recovery from primary sludge with ionic liquid. Resour. Conserv. Recycl. 2020, 154, 104626. [Google Scholar] [CrossRef]
- Pereira-Espindola, S.; Pronk, M.; Zlopasa, J.; Picken, S.; van Loosdrecht, C.M. Nanoclellulose recovery from domestic wastewater. J. Clean. Prod. 2021, 280, 124507. [Google Scholar] [CrossRef]
- Cellvation® Process. Available online: https://www.cell-vation.com/cellvation-process (accessed on 7 September 2022).
- Macfarlane, A.L.; Prestidge, R.; Farid, M.M.; Chen, J.J.J. Dissolved air flotation: A novel approach to recovery of organosolv lignin. Chem. Eng. J. 2009, 148, 15–19. [Google Scholar] [CrossRef]
- Smart-Plant Pilot Project: Recovering Cellulose from Salsnes Filter Sludge. Available online: https://www.salsnes-filter.com/2017/07/06/smart-plant-pilot-project-recycling-salsnes-filter-sludge/ (accessed on 7 September 2022).
- Yuan, J.S.; Tiller, K.H.; Al-Ahmad, H.; Stewart, N.R.; Neal Stewart, C., Jr. Plants to power, bioenergy to fuel the future. Trends Plant Sci. 2008, 13, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk-Juśko, A.; Szymańska, M. Fermentative Supernatant ad a Nutrient for Agriculture. Available online: http://ksow.pl/files/Bazy/Biblioteka/files/Poferment_nawozem_dla_rolnictwa_01.pdf (accessed on 7 September 2022). (In Polish).
- Tsuneda, S.; Aikawa, H.; Hayashi, H.; Yuasa, A.; Hirata, A. Extracellular polymeric substances responsible for bacterial adhesion onto solid surface. FEMS Microbiol. Lett. 2003, 223, 287–292. [Google Scholar] [CrossRef]
- Mohanty, A.; Meena, S.S.; Pathak, P.R.; Rout, P.R. Chapter 23. Cellulose and extracellular polymers recovery from sludge, in: Clean Energy and Resource recovery. Wastewater Plants Biorefineries 2022, 2, 395–405. [Google Scholar]
- Lee, K.S.; Kwon, T.H.; Park, T.; Jeong, M.S. Chapter 2. Microbiology and Microbial products for Enhanced Oil Recovery. In Theory and Practice in Microbial Enhanced Oil Recovery; Gulf Professional Publishing: Houston, TX, USA; Elsevier: Houston, TX, USA, 2020. [Google Scholar]
- Guangyin, Z.; Youcai, Z. Harvest of Bioenergy from Sewage Sludge by Anaerobic Digestion. In Pollution Control and Resource Recovery for Sewage Sludge; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Felz, S.; Al-Zuhairy, S.; Aarstad, O.A.; van Loosdrecht, M.C.M.; Lin, Y.M. Extraction of Structural Extracellular Polymeric Substances from Aerobic Granular Sludge. J. Vis. Exp. 2016, 115, 54534. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Bharti, A.; Prasanna, R. Chapter 14: Algal biofilma and their biotechnological signifficane. In Algal green Chemistry, Recent Progress in Biotechnology; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 285–303. [Google Scholar]
- Li, J.; Hao, X.; Gan, W.; van Loosdrecht, M.C.M.; Wu, Y. Recovery of extracellular polymers from conventional activated sludge: Potential, characteristisc and limitation. Water Res. 2021, 205, 117706. [Google Scholar] [CrossRef]
- D’Abzack, P.; Bordas, F.; Van Hullebush, E.; Lens, P.N.L.; Guibaud, G. Extraction of extracellular polymeric substances (EPS) from anaerobic granular sludges: Comparison of chemical and physical extraction protocols. Appl. Microbiol. Biotechnol. 2010, 85, 1589–1599. [Google Scholar] [CrossRef]
- Aguilera, A.; Souza-Egipsy, V.; San Martin-Uriz, P.; Amils, R. Extraction of extracellular polymeric substances from extreme acidic microbial film. Appl. Microbiol. Biotechnol. 2008, 78, 1079–1088. [Google Scholar] [CrossRef]
- Nouha, K.; Kumar, R.S.; Balasubramanian, S.; Tyagi, R.D. Critical review of EPS production, synthesis and composition for sludge flocculation. J. Environ. Sci. 2018, 66, 225–245. [Google Scholar] [CrossRef]
- Hong, L.; Fang, H.P. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar]
- Kaumera Nereda Gum. Report No. 14. Available online: https://edepot.wur.nl/501893 (accessed on 7 September 2022).
- Rolsky, C.; Kelkar, V.; Driver, E.; Halden, R.U. Municipal sewage sludge as a source of microplastics in the environment. Curr. Opin. Environ. Sci. Health 2020, 14, 14–22. [Google Scholar] [CrossRef]
- Harley-Nyang, D.; Memon, F.A.; Jones, N.; Galloway, T. Investigation and analysis of microplastics in sewage sludge and biosolids; a case study from one wastewater treatment works in the UK. Sci. Total Environ. 2022, 823, 153735. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, H.; Wang, L.; Guo, H.; Zhang, J.; Gao, D. Effects of typical sludge treatments on microplastics in China—Characteristics, abundance and micro-morphological evidence. Sci. Total Environ. 2022, 826, 154206. [Google Scholar] [CrossRef]
- Zubris, K.; Richards, B. Synthetic fibers as an indicator of land application of sludge. Environ. Pollut. 2005, 138, 201–211. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics in wastewater treatment plants and their environmental dispersion with effluent and sludge. Environ. Pollut. 2019, 259, 113837. [Google Scholar] [CrossRef] [PubMed]
- Vahvaselka, M.; Winquist, E. Existing and Emerging Technologies for Microplastics Removal: Review Report of the FanpLESStic-Sea Project; Natural Resources and Bioeconomy Studies 81/2021; Natural Resources Institute: Helsinki, Finland, 2021. [Google Scholar]
- Doñate Hernández, S. Applying Enzymes for Microplastic Degradation in Sewage Sludge. Available online: https://www.enzycle.eu/enzymes-for-microplastic-degradation/ (accessed on 7 September 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).