Impact of Temperature and Nanoparticle Concentration on Turbulent Forced Convective Heat Transfer of Nanofluids
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Nanofluids
2.2. Correlations for Forced Convection Heat Transfer inside Horizontal Tubes
2.3. Correlations for Thermophysical Properties of Nanofluids
3. Results and Discussion
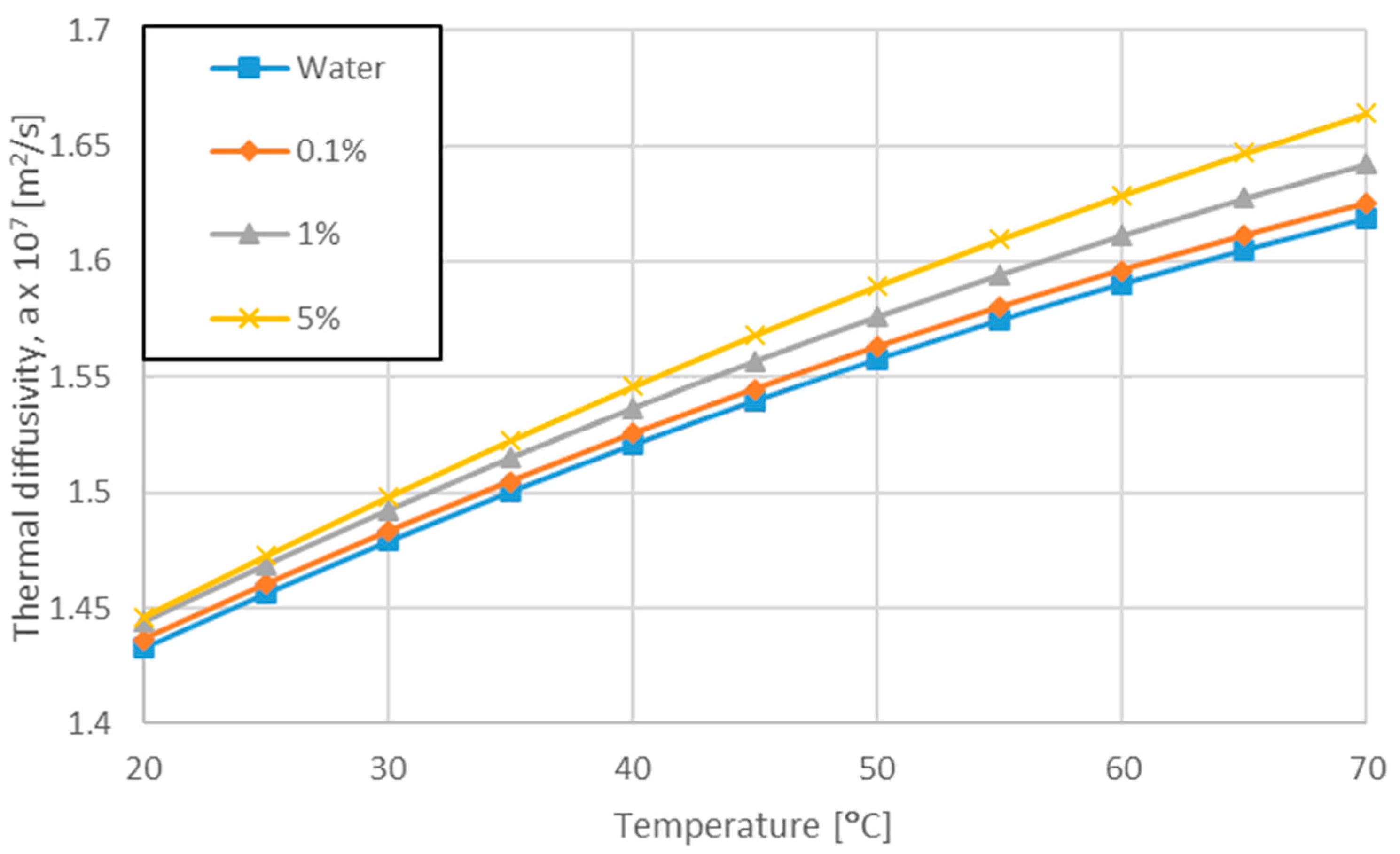
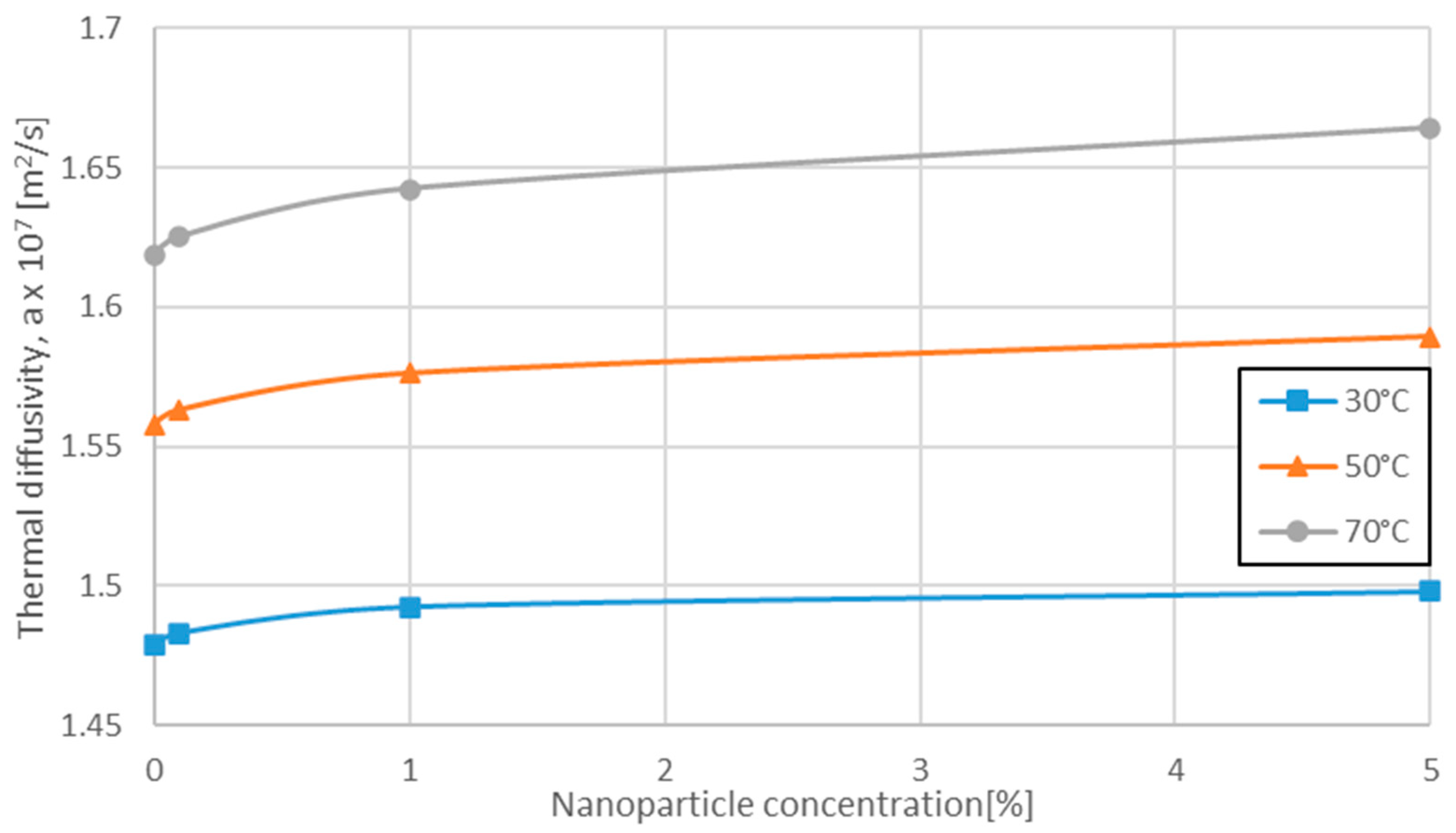
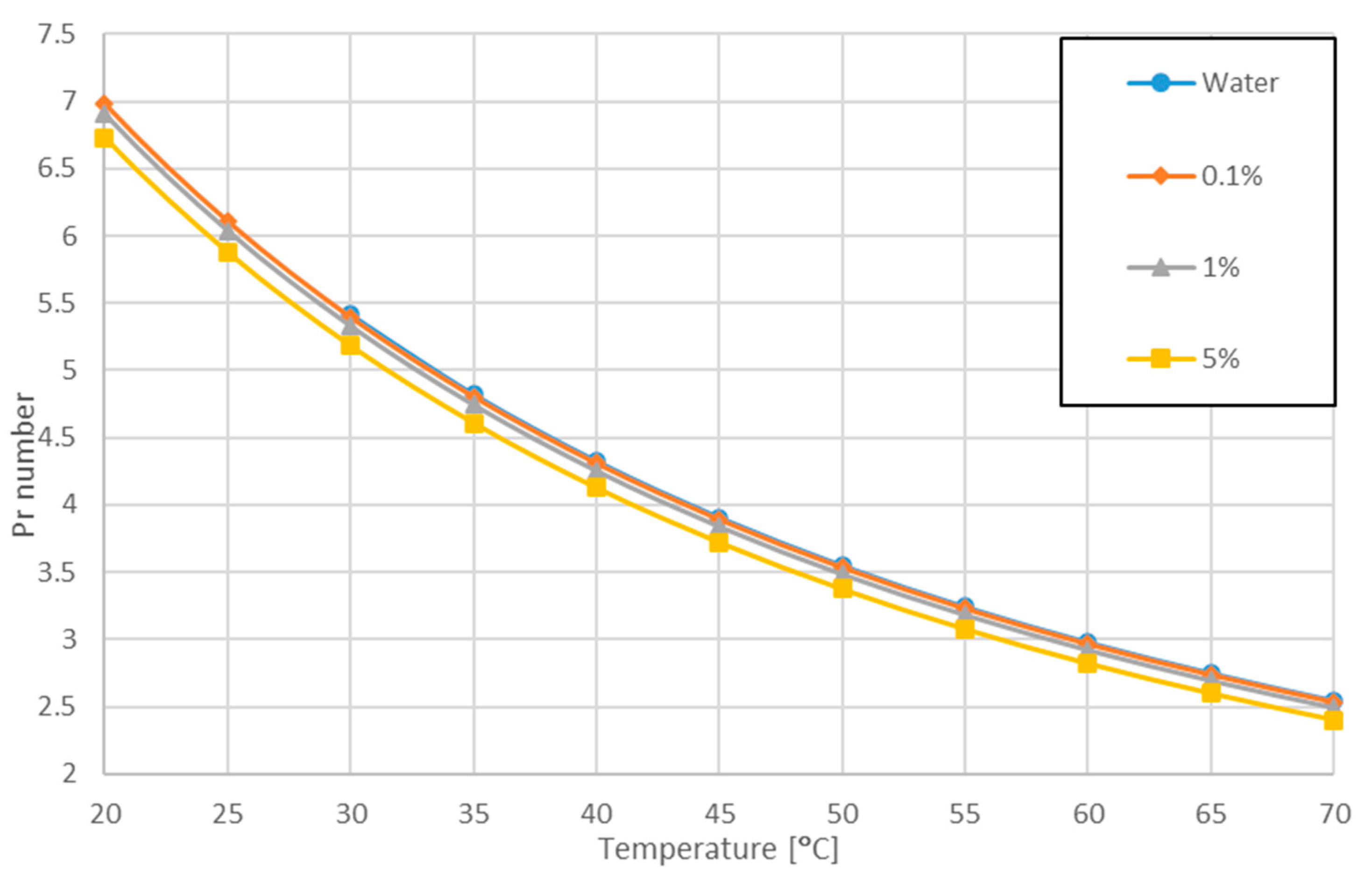
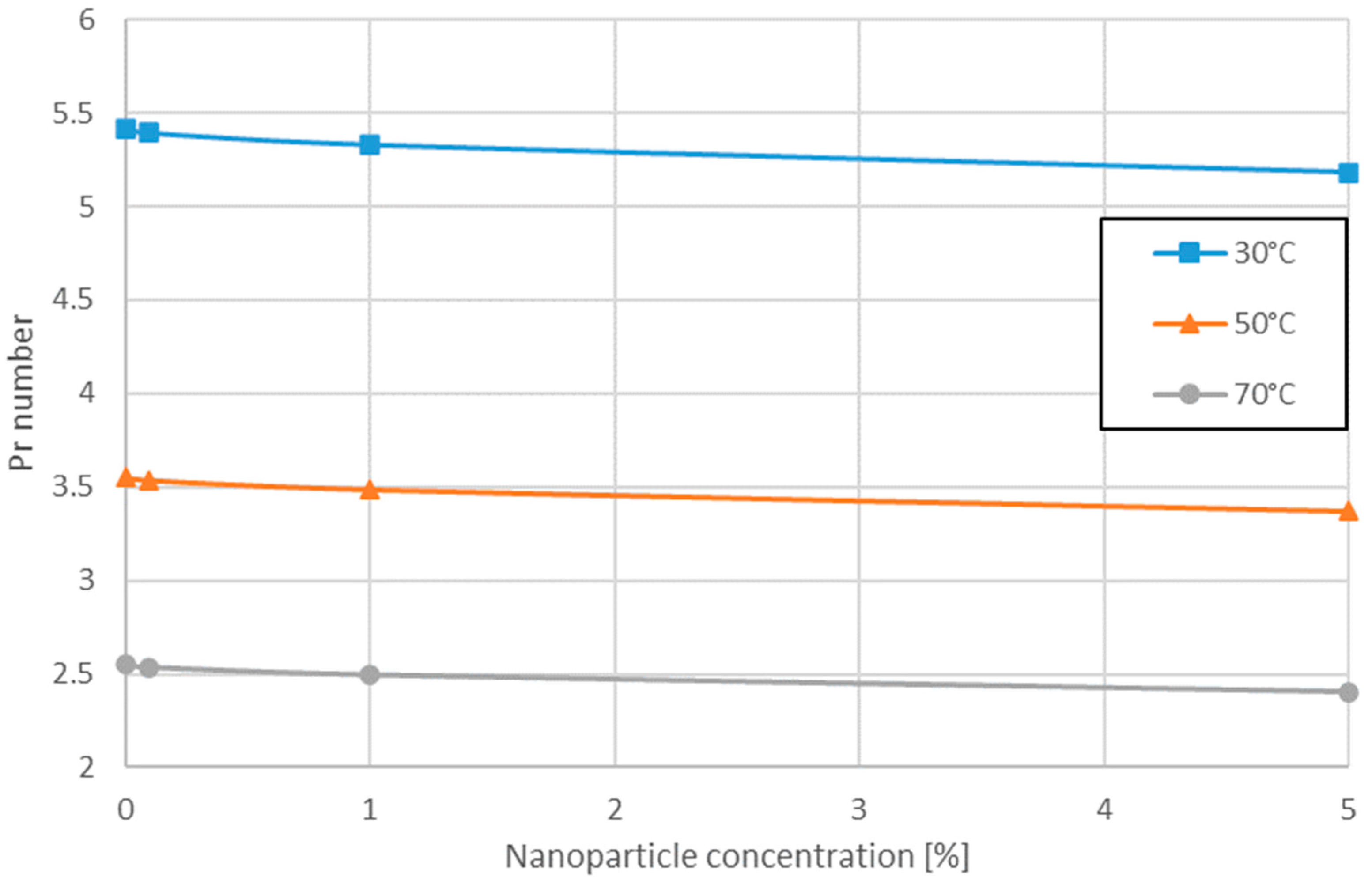
3.1. Variation of Pr Number
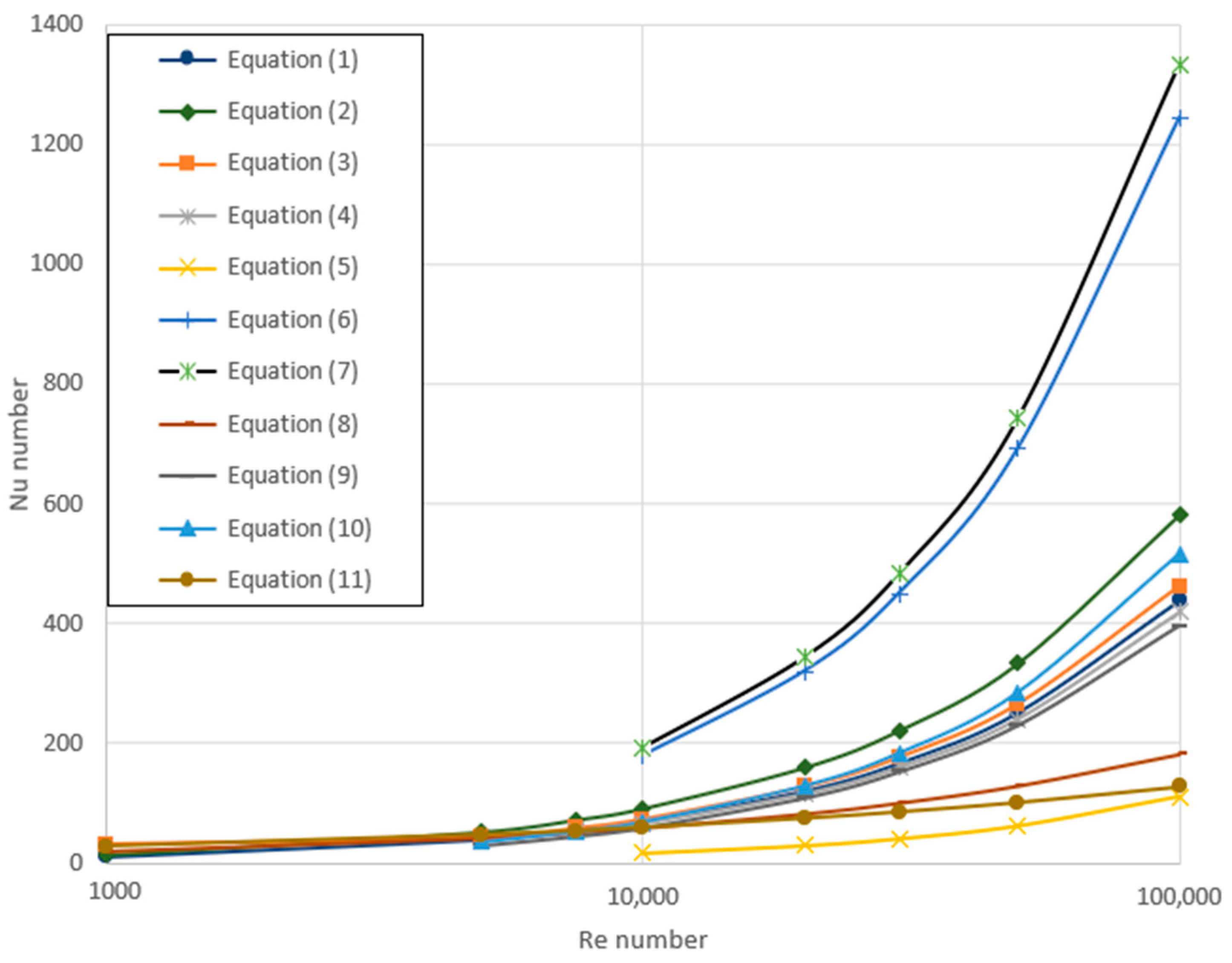
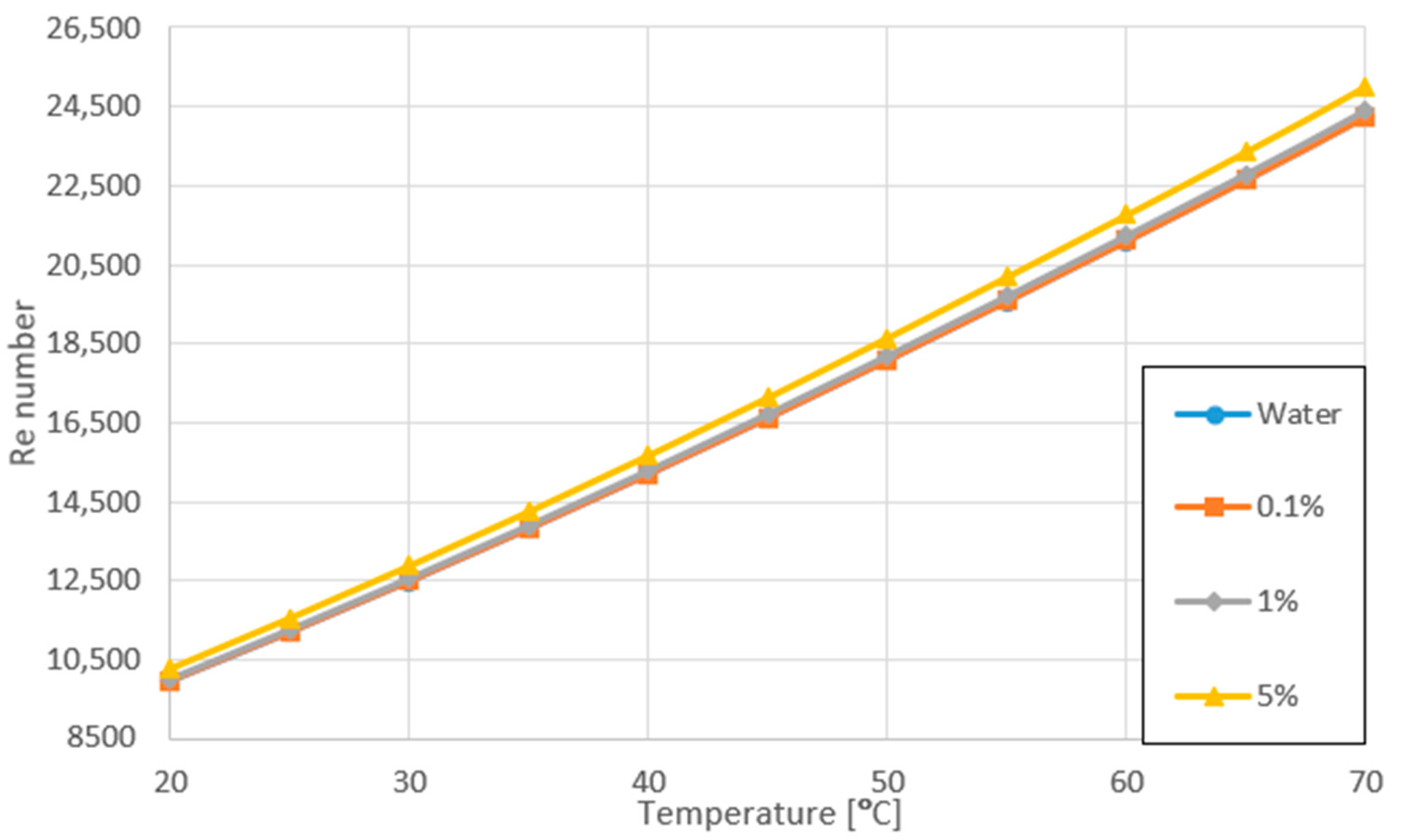
3.2. Variation of Re Number
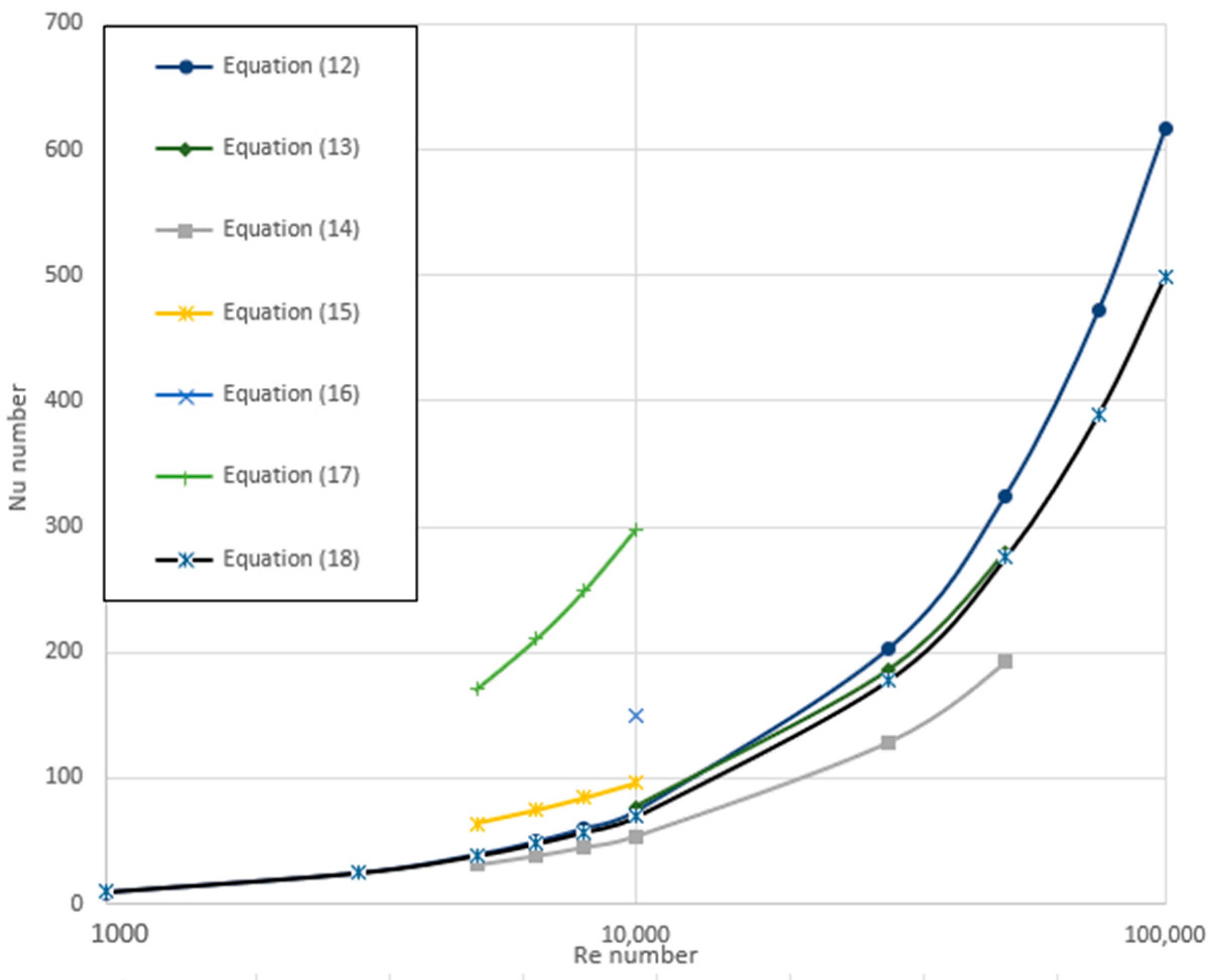
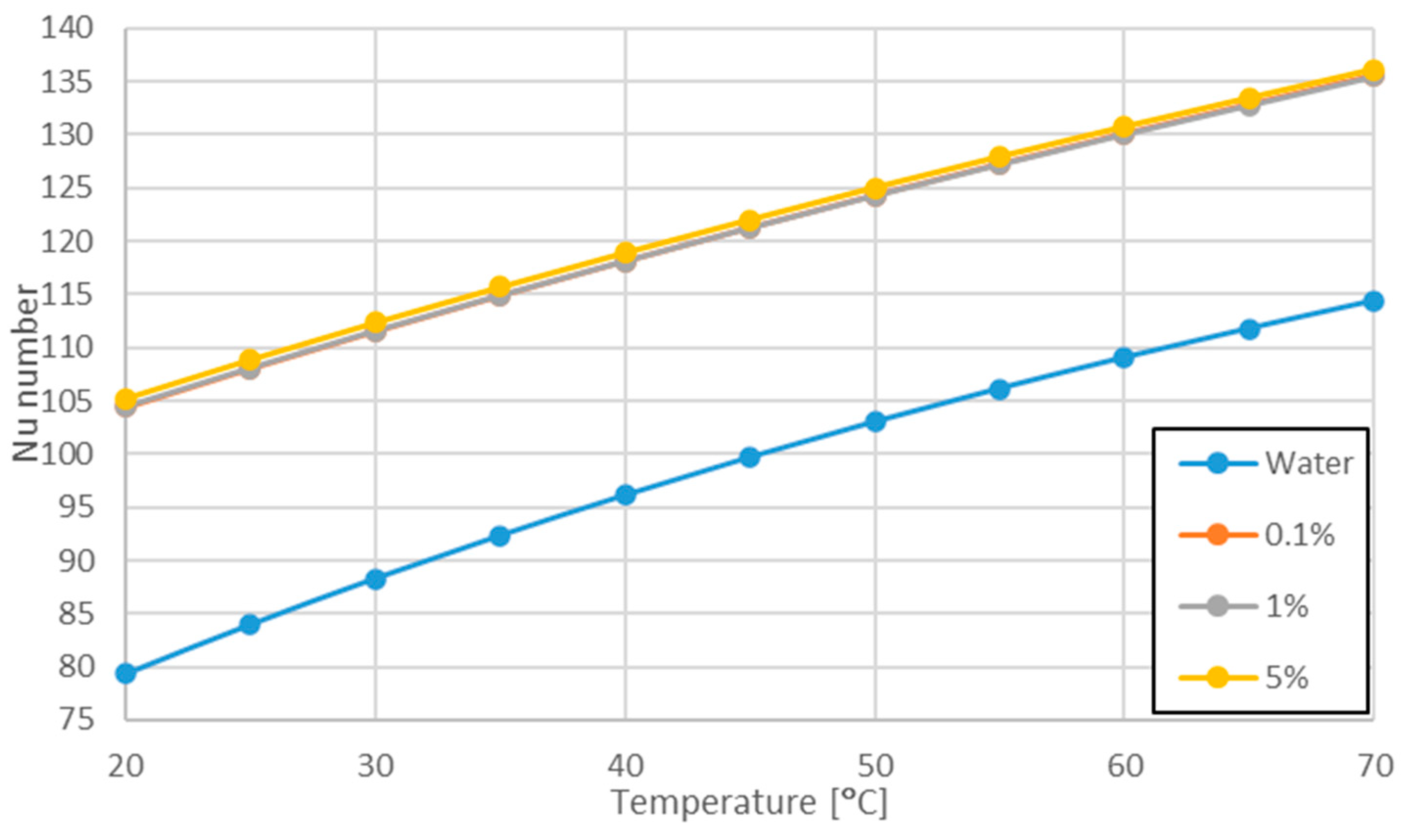
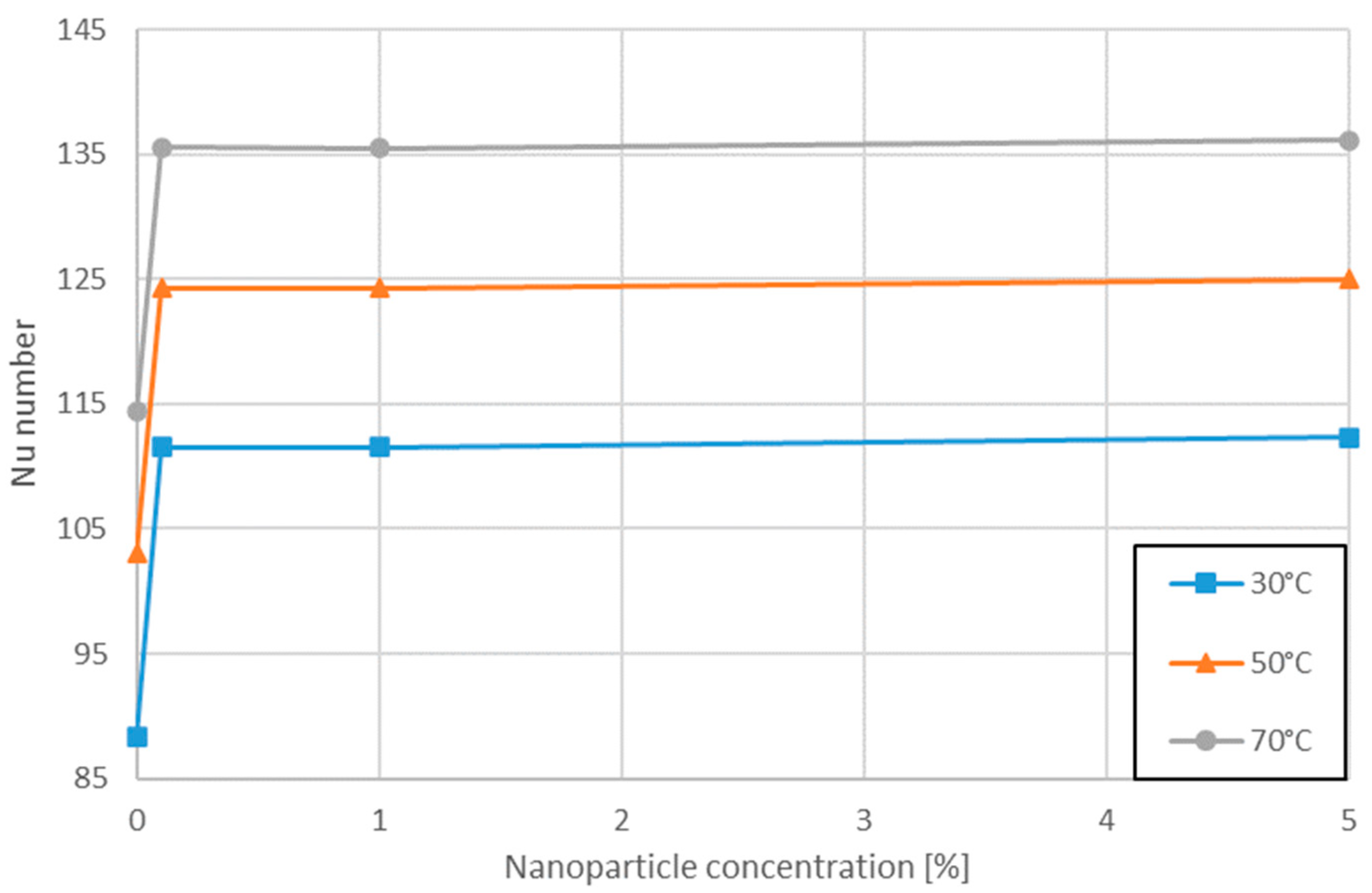
3.3. Variation of Nu Number
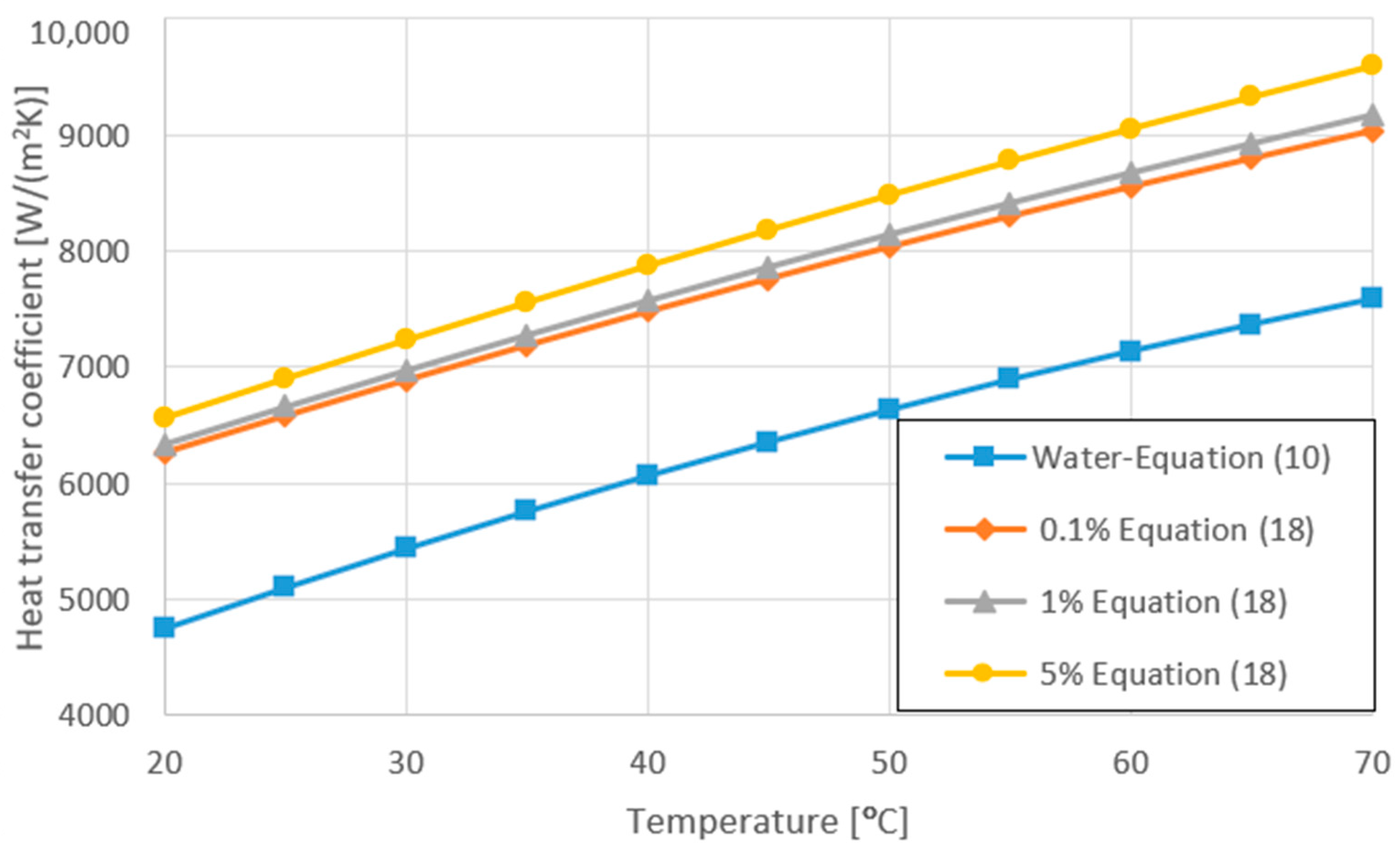
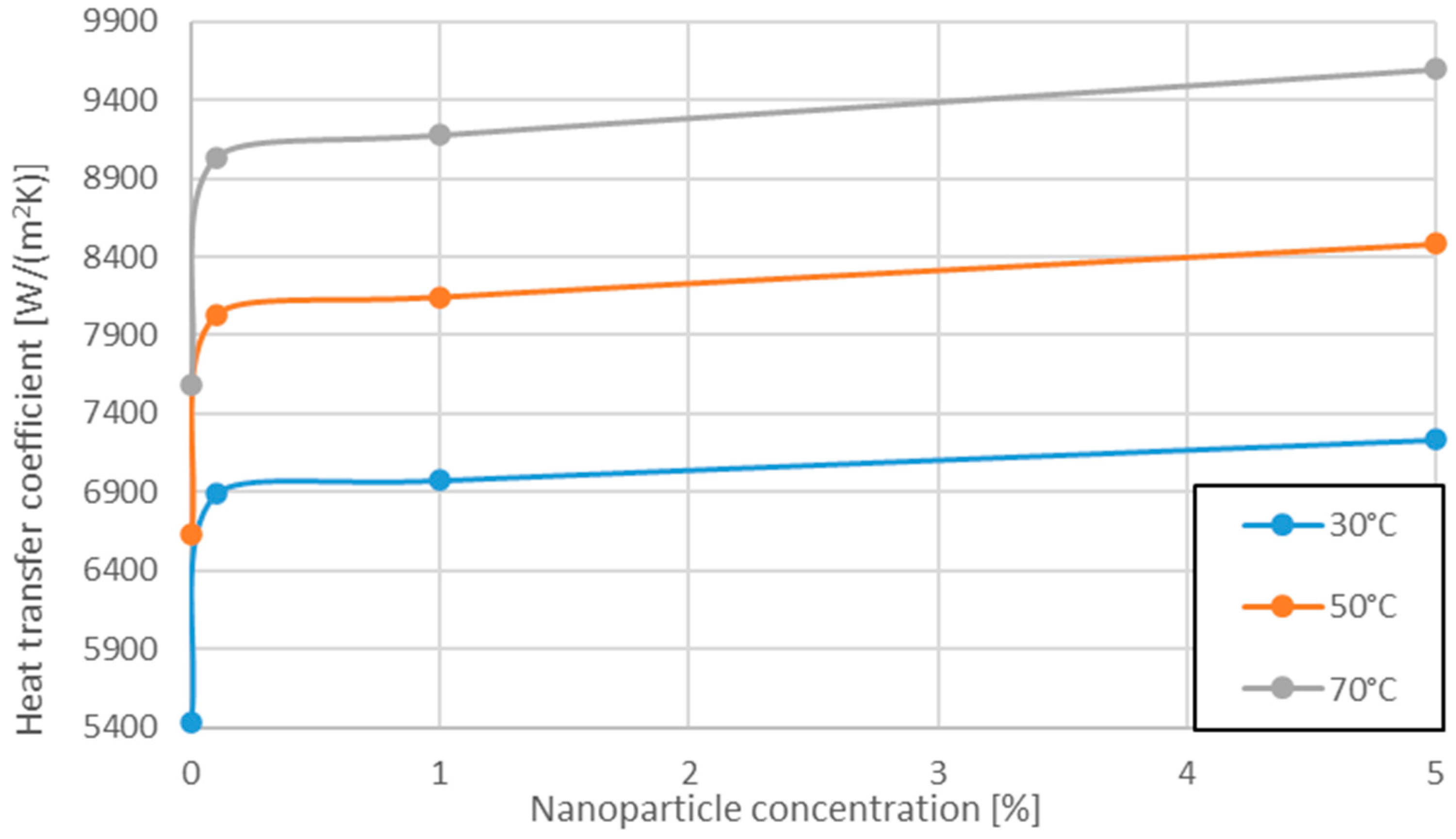
3.4. Variation of Heat Transfer Coefficient
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| a | Thermal diffusivity | (m2/s) |
| cp | Specific heat | (J/(kgK)) |
| Base fluid molecule diameter | (m) | |
| Particle diameter | (m) | |
| D | Inside diameter of tube | (m) |
| f | Friction factor | (-) |
| h | Local heat transfer coefficient | (W/(m2 K)) |
| Average heat transfer coefficient | (W/(m2 K)) | |
| k | Thermal conductivity | (W/(m K)) |
| L | Length | (m) |
| Local Nusselt number | (-) | |
| Average Nusselt number | (-) | |
| Peclet number | (-) | |
| Peclet number related to dp | (-) | |
| Prandtl number | (-) | |
| Heat flux | (W/m2) | |
| Reynolds number | (-) | |
| Temperature | [°C] | |
| Temperature | [K] | |
| w | Velocity | (m/s) |
| x | Axial coordinate | (m) |
| Greek symbols | ||
| µ | Dynamic viscosity | (Pas) |
| Kinematic viscosity | (m2/s) | |
| ρ | Density | (kg/m3) |
| Nanoparticle concentration | (-) | |
| Subscripts | ||
| bf | Base fluid | |
| f | Fluid | |
| m | Mass | |
| nf | Nanofluid | |
| nl | Nanolayer | |
| p | Particle | |
| v | Volume | |
| w | Wall | |
| Abbreviations | ||
| HTC | Heat transfer coefficient | |
| NPC | Nanoparticle concentration | |
References
- Webb, R.L. Principles of Enhanced Heat Transfer; John Wiley & Sons, Inc.: New York, NY, USA, 1994. [Google Scholar]
- Xie, S.; Beni, M.S.; Cai, J.; Zhao, J. Review of critical-heat-flux enhancement methods. Int. J. Heat Mass Transf. 2018, 122, 275–289. [Google Scholar] [CrossRef]
- Mousa, M.H.; Miljkovic, N.; Nawaz, K. Review of heat transfer enhancement techniques for single phase flows. Renew. Sustain. Energy Rev. 2021, 137, 110566. [Google Scholar] [CrossRef]
- Choi, S. Enhancing thermal conductivity of fluids with nanoparticles. In Developments and Applications of Non-Newtonian Flows; ASME: New York, NY, USA, 1995; Volume 231/MD, pp. 99–105. [Google Scholar]
- Liang, G.; Mudawar, I. Review of single-phase and two-phase nanofluid heat transfer in macro-channels and micro-channels. Int. J. Heat Mass Transf. 2019, 136, 324–354. [Google Scholar] [CrossRef]
- Lodhi, M.S.; Sheorey, T.; Dutta, G. Single-phase fluid flow and heat transfer characteristics of nanofluid in a circular microchannel: Development of flow and heat transfer correlations. Proc. Inst. Mech. Eng. C J. Mech. Eng. Sci. 2020, 234, 3689–3708. [Google Scholar] [CrossRef]
- Cheng, L.; Filho, E.P.B.; Thome, J.R. Nanofluid Two-Phase Flow and Thermal Physics: A New Research Frontier of Nanotechnology and Its Challenges. J. Nanosci. Nanotechnol. 2008, 8, 3315–3332. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Sahu, D.S. Nanofluid in the multiphase flow field and heat transfer: A review. Heat Transf. 2021, 50, 3722–3775. [Google Scholar] [CrossRef]
- Nkurikiyimfura, I.; Wang, Y.; Pan, Z. Heat transfer enhancement by magnetic nanofluids—A review. Renew. Sustain. Energy Rev. 2013, 21, 548–561. [Google Scholar] [CrossRef]
- Shaw, S.; Mabood, F.; Muhammad, T.; Nayak, M.K.; Alghamdi, M. Numerical simulation for entropy optimized nonlinear radiative flow of GO-Al2O3 magneto nanomaterials with auto catalysis chemical reaction. Numer. Methods Partial Differ. Equ. 2022, 38, 329–358. [Google Scholar]
- Sivaraj, R.; Banerjee, S. Transport properties of non-Newtonian nanofluids and applications. Eur. Phys. J. Special Top. 2021, 230, 1167–1171. [Google Scholar] [CrossRef]
- Shamshuddin, M.D.; Ghaffari, A.; Usman. Radiative heat energy exploration on Casson-type nanoliquid induced by a convectively heated porous plate in conjunction with thermophoresis and Brownian movements. Int. J. Ambient Energy 2022. [Google Scholar] [CrossRef]
- Hayat, T.; Aziz, A.; Muhammad, T.; Alsaedi, A. Model and Comparative Study for Flow of Viscoelastic Nanofluids with Cattaneo-Christov Double Diffusion. PLoS ONE 2017, 12, e0168824. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Al-Khaled, K.; Gouadria, S.; El-Zahar, E.R.; Usman; Khan, S.U.; Khan, M.I.; Malik, M.Y. Numerical simulations for three-dimensional rotating porous disk flow of viscoelastic nanomaterial with activation energy, heat generation and Nield boundary conditions. Waves Random Complex Media 2022. [Google Scholar] [CrossRef]
- Mahato, R.; Das, M.; Sen, S.S.S.; Shaw, S. Entropy generation on unsteady stagnation-point Casson nanofluid flow past a stretching sheet in a porous medium under the influence of an inclined magnetic field with homogeneous and heterogeneous reactions. Heat Transf. 2022, 51, 5723–5747. [Google Scholar] [CrossRef]
- Khan, S.U.; Usman; Al-Khaled, K.; Hussain, S.M.; Ghaffari, A.; Khan, M.I.; Ahmed, M.W. Implication of Arrhenius Activation Energy and Temperature-Dependent Viscosity on Non-Newtonian Nanomaterial Bio-Convective Flow with Partial Slip. Arab. J. Sci. Eng. 2022, 47, 7559–7570. [Google Scholar] [CrossRef]
- Zapata, K.; Rodríguez, Y.; Lopera, S.H.; Cortes, F.B.; Franco, C.A. Development of Bio-Nanofluids Based on the Effect of Nanoparticles’ Chemical Nature and Novel Solanum torvum Extract for Chemical Enhanced Oil Recovery (CEOR) Processes. Nanomatererials 2022, 12, 3214. [Google Scholar] [CrossRef]
- Mandal, S.; Shit, G.C.; Shaw, S.; Makinde, O.D. Entropy analysis of thermo-solutal stratification of nanofluid flow containing gyrotactic microorganisms over an inclined radiative stretching cylinder. Therm. Sci. Eng. Prog. 2022, 34, 101379. [Google Scholar] [CrossRef]
- Mehta, R.N.; Chakraborty, M.; Parikh, P.A. Nanofuels: Combustion, engine performance and emissions. Fuel 2014, 120, 91–97. [Google Scholar] [CrossRef]
- Cieśliński, J.T.; Krzyżak, J.; Kropiwnicki, J.; Kneba, Z. Experiments on compression ignition engine powered by nano-fuels. Combust. Engines 2022, 188, 55–59. [Google Scholar] [CrossRef]
- Taylor, R.; Coulombe, S.; Otanicar, T.; Phelan, P.; Gunawan, A.; Lv, W.; Rosengarten, G.; Prasher, R.; Tyagi, H. Small particles, big impacts: A review of the diverse applications of nanofluids. J. Appl. Phys. 2013, 113, 011301. [Google Scholar] [CrossRef]
- Huminic, G.; Huminic, A. Application of nanofluids in heat exchangers: A review. Renew. Sustain. Energ. Rev. 2012, 16, 5625–5638. [Google Scholar] [CrossRef]
- Sajid, M.U.; Ali, H.M. Recent advances in application of nanofluids in heat transfer devices: A critical review. Renew. Sustain. Energy Rev. 2019, 103, 556–592. [Google Scholar] [CrossRef]
- Mahian, O.; Kolsi, L.; Amani, M.; Estellé, P.; Ahmadi, G.; Kleinstreuer, C.; Marshall, J.S.; Taylor, R.A.; Abu-Nada, E.; Rashidi, S.; et al. Recent advances in modeling and simulation of nanofluid flows-Part II: Applications. Phys. Rep. 2019, 791, 1–59. [Google Scholar] [CrossRef]
- Cieśliński, J.T. Application of Nanofluids in Thermal Technologies; Contemporary Issues of Heat and Mass Transfer; Publishing House of the Koszalin University of Technology: Koszalin, Poland, 2019; Tom 1, No. 360; pp. 33–48. [Google Scholar]
- Patil, M.; Mehta, D.S.; Guvva, S. Future impact of nanotechnology on medicine and dentistry. J. Indian Soc. Periodontol. 2008, 12, 34–40. [Google Scholar]
- Shaw, S.; Shit, G.C.; Tripathi, D. Impact of drug carrier shape, size, porosity and blood rheology on magnetic nanoparticle-based drug delivery in a microvessel. Colloids Surf. A Physicochem. Eng. Asp. 2022, 639, 128370. [Google Scholar] [CrossRef]
- Kalbande, V.P.; Walke, P.V.; Kriplani, C.V.M. Advancements in Thermal Energy Storage System by Applications of Nanofluid Based Solar Collector: A Review. Environ. Clim. Technol. 2020, 24, 310–340. [Google Scholar] [CrossRef]
- Li, Z.; Cui, L.; Li, B.; Du, X. Mechanism exploration of the enhancement of thermal energy storage in molten salt nanofluid. Phys. Chem. Chem. Phys. 2021, 23, 13181. [Google Scholar] [CrossRef] [PubMed]
- Sundararaj, A.J.; Pillai, B.C.; Asirvatham, L.G. Convective heat transfer analysis of refined kerosene with alumina particles for rocketry application. J. Mech. Sci. Technol. 2018, 32, 1685–1691. [Google Scholar] [CrossRef]
- Kakaç, S.; Pramuanjaroenki, J.A. Single-phase and two-phase treatments of convective heat transfer enhancement with nanofluids—A state-of-the-art review. Int. J. Therm. Sci. 2016, 100, 75–97. [Google Scholar] [CrossRef]
- Mahian, O.; Kolsi, L.; Amani, M.; Estellé, P.; Ahmadi, G.; Kleinstreuer, C.; Marshall, J.S.; Siavashi, M.; Taylor, R.A.; Niazmad, H.; et al. Recent advances in modeling and simulation of nanofluid flows—Part I: Fundamentals and theory. Phys. Rep. 2019, 790, 1–48. [Google Scholar] [CrossRef]
- Solangi, K.H.; Sharif, S.; Sadiq, I.O.; Hisam, M.J. Experimental and numerical investigations on heat transfer and friction loss of functionalized GNP nanofluids. Int. J. Mech. Eng. Technol. 2019, 10, 61–77. [Google Scholar]
- Singh, P.; Oberoi, A.S.; Nijhawan, P. Experimental heat transfer analysis of copper oxide nanofluids through a straight tube. IJATCSE 2019, 8, 495–500. [Google Scholar] [CrossRef]
- Karabulut, K.; Buyruk, E.; Kilinc, F. Experimental and numerical investigation of convection heat transfer in a circular copper tube using graphene oxide nanofluid. J. Braz. Soc. Mech. Sci. 2020, 42, 230. [Google Scholar] [CrossRef]
- Kong, M.; Lee, S. Performance evaluation of Al2O3 nanofluid as an enhanced heat transfer fluid. Adv. Mech. Eng. 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Boertz, H.; Baars, A.J.; Cieśliński, J.T.; Smolen, S. Numerical Study of Turbulent Flow and Heat Transfer of Nanofluids in Pipes. Heat Transf. Eng. 2018, 39, 241–251. [Google Scholar] [CrossRef]
- Minea, A.A.; Buonomo, B.; Burggraf, J.; Ercole, D.; Karpaiya, K.R.; Pasqua, A.D.; Sekrani, G.; Steffens, J.; Tibaut, J.; Wichmann, N.; et al. NanoRound: A benchmark study on the numerical approach in nanofluids’ simulation. Int. Commun. Heat Mass Transf. 2019, 108, 104292. [Google Scholar] [CrossRef]
- Onyiriuka, E.J.; Ikponmwoba, E.A. A numerical investigation of mango leaves-water nanofluid under laminar flow regime. Niger. J. Technol. 2019, 38, 348–354. [Google Scholar] [CrossRef]
- Jamali, M.; Toghraie, D. Investigation of heat transfer characteristics in the developing and the developed flow of nanofluid inside a tube with different entrances in the transition regime. J. Therm. Anal. Calorim. 2020, 139, 685–699. [Google Scholar] [CrossRef]
- Fadodun, O.G.; Amosun, A.A.; Salau, A.O.; Olaloye, D.O.; Ogundeji, J.A.; Ibitoye, F.I.; Balogun, F.A. Numerical investigation and sensitivity analysis of turbulent heat transfer and pressure drop of Al2O3/H2O nanofluid in straight pipe using response surface methodology. Arch. Thermodyn. 2020, 41, 3–30. [Google Scholar]
- Uribe, S.; Zouli, N.; Cordero, M.E.; Al-Dahhan, M. Development and validation of a mathematical model to predict the thermal behaviour of nanofluids. Heat Mass Transf. 2021, 57, 93–110. [Google Scholar] [CrossRef]
- Angayarkanni, S.A.; Philip, J. Review on thermal properties of nanofluids: Recent developments. Adv. Colloid Interface Sci. 2015, 225, 146–176. [Google Scholar] [CrossRef]
- Ilyas, S.U.; Pendyala, R.; Marneni, N. Stability of Nanofluids. Topics in Mining, Metallurgy and Materials Engineering. In Engineering Applications of Nanotechnology. From Energy to Drug Delivery; Korada, V.S., Hamid, N.H.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Akbari, M.; Galanis, N.; Behzadmehr, A. Comparative assessment of single and two-phase models for numerical studies of nanofluid turbulent forced convection. Int. J. Heat Fluid Flow 2012, 37, 136–146. [Google Scholar] [CrossRef]
- Moraveji, M.K.; Esmaeili, E. Comparison between Single-Phase and Two-Phases CFD Modeling of Laminar Forced Convection Flow of Nanofluids in Circular Tube under Constant Heat Flux. Int. Commun. Heat Mass Transf. 2012, 39, 1297–1302. [Google Scholar] [CrossRef]
- Duan, F. Thermal Property Measurement of Al2O3-Water Nanofluids. Smart Nanoparticles Technology; Abbass, H., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0500-8. [Google Scholar]
- Buongiorno, J.; Venerus, D.C.; Prabhat, N.; McKrell, T.; Townsend, J.; Chrisianson, R.; Tolmachev, Y.V.; Keblinski, P.; Hu, L.-w.; Alvarado, J.L.; et al. A benchmark study on the thermal conductivity of nanofluids. J. Appl. Phys. 2009, 106, 094312. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. Specific heat measurement of three nanofluids and development of new correlations. ASME J. Heat Transf. 2009, 131, 071601–071607. [Google Scholar] [CrossRef]
- Dittus, F.W.; Boelter, L.M.K. Heat transfer in automobile radiators of the tubular type. Univ. Calif. Publ. Eng. 1930, 2, 443–461. [Google Scholar] [CrossRef]
- Kraußold, H. Die Wärmeübertragung an Flüssigkeiten in Rohren bei turbulenter Strömung. Forschung auf dem Gebiet des Ingenieurwesens; Springer: Berlin/Heidelberg, Germany, 1933; Band 1; pp. 39–44. [Google Scholar]
- Sieder, E.N.; Tate, G.E. Heat transfer and pressure drop of liquids in tubes. Ind. Eng. Chem. Res. 1936, 28, 1429–1435. [Google Scholar] [CrossRef]
- Mikhejev, M.A.; Mikhejeva, I.M. Teplootdacza pri Turbulentom Dwiżenji w Trubach; Osnovy teploperedczi, Energija: Moscow, Russia, 1973. (In Russian) [Google Scholar]
- Petukhov, B.S. Heat Transfer and Friction in Turbulent Pipe Flow with Variable Physical Properties. Adv. Heat Transf. 1970, 503–564. [Google Scholar]
- Notter, R.H.; Sleicher, C.A. A solution to the turbulent Graetz problem—III fully developed and entry region heat transfer rates. Chem. Eng. Sci. 1972, 27, 2073–2093. [Google Scholar] [CrossRef]
- Churchill, S.W.; Ozoe, H. Correlations for laminar forced convection in flow over an flat plate and in developing and fully developed flow in a tube. J. Heat Trans-T ASME 1973, 95, 78–84. [Google Scholar] [CrossRef]
- Hausen, H. Erweiterte Gleichung für den Wärmeübergang in Rohren bei turbulenter Strömung. Wärme Stoffübertragung 1974, 7, 222–225. [Google Scholar] [CrossRef]
- Gnieliński, V. Neue Gleichungen für den Wärme—und den Stoffübergang in turbulent durchströmten Rohren und Kanälen. In Forschung in Ingenieurwesen; Springer: Berlin/Heidelberg, Germany, 1975; Band 41; pp. 8–16. [Google Scholar]
- Kutateladze, S.S. Osnovy teorii Teploobmena; Atomizdat: Moscow, Russia, 1979. (In Russian) [Google Scholar]
- Xuan, Y.; Li, Q. Investigation on Convective Heat Transfer and Flow Features of Nanofluids. J. Heat Trans-T ASME. 2003, 125, 151–155. [Google Scholar] [CrossRef]
- Vasu, V.; Krishna, K.R.; Kumar, A.C.S. Analytical prediction of forced convective heat transfer of fluids embedded with nanostructured materials (nanofluids). PRAMANA J. Phys. 2007, 69, 411–421. [Google Scholar] [CrossRef]
- Hussein, A.M.; Sharma, K.V.; Bakar, R.A.; Kadirgama, K. The Effect of Nanofluid Volume Concentration on Heat Transfer and Friction Factor inside a Horizontal Tube. J. Nanomater. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Sahin, B.; Gültekin, G.G.; Manay, E.; Karagoz, S. Experimental investigation of heat transfer and pressure drop characteristics of Al2O3-water nanofluid. Exp. Therm. Fluid Sci. 2013, 50, 21–28. [Google Scholar] [CrossRef]
- Chavan, D.; Pise, A.T. Experimental investigation of convective heat transfer augmentation using Al2O3/water nanofluid in circular pipe. Heat Mass Transf. 2015, 51, 1237–1246. [Google Scholar] [CrossRef]
- Durga Prasad, P.V.; Gupta, A. Experimental investigation on enhancement of heat transfer using Al2O3/water nanofluid in a u-tube with twisted tape inserts. Int. Commun. Heat Mass Transf. 2016, 75, 154–161. [Google Scholar] [CrossRef]
- Saha, G.; Paul, M.C. Heat transfer and entropy generation of turbulent forced convection flow of nanofluids in a heated pipe. Int. Commun. Heat Mass Transf. 2015, 61, 26–36. [Google Scholar] [CrossRef]
- Meyer, J.P.; Adio, S.A.; Sharifpur, M.; Nwosu, P.N. The Viscosity of Nanofluids: A Review of the Theoretical, Empirical, and Numerical Models. Heat Transf. Eng. 2016, 37, 387–421. [Google Scholar] [CrossRef]
- Bashirnezhad, K.; Bazri, S.; Safaei, M.R.; Goodarzi, M.; Dahari, M.; Mahian, O.; Dalkılıça, A.S.; Wongwises, S. Viscosity of nanofluids: A review of recent experimental studies. Int. Commun. Heat Mass Transf. 2016, 73, 114–123. [Google Scholar] [CrossRef]
- Krieger, I.M.; Dougherty, T.J. A mechanism for non-newtonian flow in suspensions of rigid spheres. Trans. Soc. Rheology. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- Palm, S.J.; Roy, G.; Nguyen, C.T. Heat transfer enhancement with the use of nanofluids in radial flow cooling systems considering temperature dependent properties. Appl. Therm. Eng. 2006, 26, 2209–2218. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Desgranges, F.; Roy, G.; Galanis, N.; Mare, T.; Boucher, S.; Mintsa, A. Temperature and particle-size dependent viscosity data for water-based nanofluids—Hysteresis phenomenon. Int. J. Heat Fluid Flow 2007, 28, 1492–1506. [Google Scholar] [CrossRef]
- Khanafer, K.; Vafai, K. A critical synthesis of thermophysical characteristics of nanofluids. Int. J. Heat Mass Transf. 2011, 54, 4410–4428. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.J.; Lugo, L.; Legido, J.L.; Piñeiro, M.M. Thermal conductivity and viscosity measurements of ethylene glycol-based Al2O3 nanofluids. Nanoscale Res. Lett. 2011, 6, 221. [Google Scholar] [CrossRef]
- Corcione, M. Empirical correlating equations for predicting the effective thermal conductivity and dynamic viscosity of nanofluids. Energy Convers. Manag. 2011, 52, 789–793. [Google Scholar]
- Chon, C.H.; Kihm, K.D. Empirical correlation finding the role of temperature and particle size for nanofluid (Al2O3) thermal conductivity enhancement. Appl. Phys. Lett. 2005, 87, 153107. [Google Scholar] [CrossRef]
- Purohit, N.; Purohit, V.A.; Purohit, K. Assessment of nanofluids for laminar convective heat transfer: A numerical study. Eng. Sci. Technol. Int. J. 2016, 19, 574–586. [Google Scholar] [CrossRef]
- Saeed, F.R.; Al-Dulaimi, M.A. Numerical investigation for convective heat transfer of nanofluid laminar flow inside a circular pipe by applying various models. Arch. Thermodyn. 2021, 42, 71–95. [Google Scholar]
- Lemmon, E.W.; Huber, M.L.; McLinden, M.O. NIST Standard Reference Database 23, Reference Fluid Thermodynamic and Transport Properties (REFPROP), Version 9.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Kleinstreuer, C.; Feng, Y. Experimental and theoretical studies of nanofluid thermal conductivity enhancement: A review. Nanoscale Res. Lett. 2011, 6, 229. [Google Scholar] [CrossRef]
- Aybar, H.Ş.; Sharifpur, M.; Azizian, M.R.; Mehrabi, M.; Meyer, J.P. A Review of Thermal Conductivity Models for Nanofluids. Heat Transf. Eng. 2015, 36, 1085–1110. [Google Scholar] [CrossRef]
- Chen, G. Nonlocal and nonequilibrium heat conduction in the vicinity of nanoparticles. J. Heat Trans-T ASME 1996, 118, 539–545. [Google Scholar] [CrossRef]
- Hassani, S.; Saidur, R.; Mekhilef, S.; Hepbasli, A. A new correlation for predicting the thermal conductivity of nanofluids; using dimensional analysis. Int. J. Heat Mass Transfer. 2015, 90, 121–130. [Google Scholar] [CrossRef]
- Sawicka, D.; Cieśliński, J.T.; Smolen, S.A. Comparison of Empirical Correlations of Viscosity and Thermal Conductivity of Water-Ethylene Glycol-Al2O3 Nanofluids. Nanomaterials 2020, 10, 1487. [Google Scholar] [CrossRef] [PubMed]
- Pak, B.C.; Cho, Y.I. Hydrodynamic and heat transfer study of dispersed fluids with submicron metallic oxide particles. Exp. Heat Transf. 1998, 11, 151–170. [Google Scholar] [CrossRef]
- Sharifpur, M.; Yousefi, S.; Meyer, J.P. A new model for density of nanofluids including nanolayer. Int. Commun. Heat Mass Transf. 2016, 78, 168–174. [Google Scholar] [CrossRef]
- Saha, G.; Paul, M.C. Numerical analysis of the heat transfer behaviour of water based Al2O3 and TiO2 nanofluids in a circular pipe under the turbulent flow condition. Int. Commun. Heat Mass Transf. 2014, 56, 96–108. [Google Scholar] [CrossRef]
- Shahrul, I.M.; Mahbubul, I.M.; Khaleduzzaman, S.S.; Saidur, R.; Sabri, M.F.M. A comparative review on the specific heat of nanofluids for energy perspective. Renew. Sustain. Energy Rev. 2014, 38, 88–98. [Google Scholar] [CrossRef]
- Riazi, H.; Murphy, T.; Webber, G.B.; Atkin, R.; Tehrani, S.S.M.; Taylor, R.A. Specific heat control of nanofluids: A critical review. Int. J. Therm. Sci. 2016, 107, 25–38. [Google Scholar] [CrossRef]
- Hentschke, R. On the specific heat capacity enhancement in nanofluids. Nanoscale Res. Lett. 2016, 11, 88. [Google Scholar] [CrossRef]
- Williams, W.; Buongiorno, J.; Hu, L.W. Experimental investigation of turbulent convective heat transfer and pressure loss of alumina/water and zirconia/water nanoparticle colloids (nanofluids) in horizontal tubes. J. Heat Trans-T ASME 2008, 130, 042412. [Google Scholar] [CrossRef]
- Corcione, M.; Cianfrini, M.; Quintino, A. Heat transfer of nanofluids in turbulent pipe flow. Int. J. Therm. Sci. 2012, 56, 58–69. [Google Scholar] [CrossRef]
- Sekhar, Y.R.; Sharma, K.V. Study of viscosity and specific heat capacity characteristics of water-based Al2O3 nanofluids at low particle concentrations. J. Exp. Nanosci. 2012, 56, 58–69. [Google Scholar]
- Cieśliński, J.T.; Kozak, P. Experimental investigation of forced convection of water-Al2O3 nanofluids inside horizontal tubes. In Proceedings of the XVI International Symposium Heat Transfer and Renewable Sources of Energy, Szczecin-Międzyzdroje, Poland, 10–13 September 2016. [Google Scholar]
- Godson, L.; Raja, B.; Lal, D.M.; Wongwises, S. Enhancement of heat transfer using nanofluids—An overview. Renew. Sust. Energy Rev. 2010, 14, 629–641. [Google Scholar] [CrossRef]
- Buongiorno, J. Convective Transport in Nanofluids. J. Heat Transfer 2006, 128, 240–250. [Google Scholar] [CrossRef]



| Thermal Conductivity kp [W/(mK)] | Density ρp [kg/m3] | Specific Heat cp,p [J/(kg K)] |
|---|---|---|
| 35 * | 3600 ** | 765 ** |
| Authors | Correlation | Range | Remarks | Equation |
|---|---|---|---|---|
| Dittus and Boelter [50] | Re > 104 0.7 < Pr < 100 | n = 0.4—heating n = 0.3—cooling | Equation (1) | |
| Krauβold [51] | Re > 104 | n = 0.37—heating n = 0.3—cooling | Equation (2) | |
| Sieder and Tate [52] | Re > 104 0.7 < Pr < 16,700 | Tw = const. | Equation (3) | |
| Mikhejev [53] | 104 < Re < 5 × 106 0.6 < Pr < 2500 | εL = f(L/D, Re) | Equation (4) | |
| Petukhov [54] | 104 < Re < 5 × 106 0.5 < Pr < 2000 | Equation (5) | ||
| Notter and Sleicher [55] | 104 < Re < 106 0.004 < Pr < 0.1 | Tw = const. qw = const. | Equation (6) Equation (7) | |
| Churchill and Ozoe [56] | Re > 100 10−4 < Pr → ∞ | qw = const. | Equation (8) | |
| Hausen [57] | 2300 < Re < 2 × 106 1.5 < Pr < 500 d/L < 1 | Equation (9) | ||
| Gnieliński [58] | 3 × 103 < Re < 5 × 106 0.5 < Pr < 2000 | Equation (10) | ||
| Kutateladze [59] | Pe > 12 d/L < 12 | Equation (11) |
| Authors | Equation | Remarks | Equation |
|---|---|---|---|
| Xuan and Li [60] | Equation (12) | ||
| Vasu et al. [61] | 104 < Re < 8 × 104 | Equation (13) | |
| Hussein et al. [62] | 5000 < Re < 5 × 104 6.8 < Pr < 11.97 | Equation (14) | |
| Sahin et al. [63] | 4000 < Re < 20,000 5 < Pr < 7 0.5% < φv < 4% | Equation (15) | |
| Chavan and Pise [64] | 6000 < Re < 14,000 0.3% < φ < 1% | Equation (16) | |
| Durga and Gupta [65] | 3000 < Re < 30,000 5.12 < Pr < 6.54 0% < φv < 0.03% | Equation (17) | |
| Saha and Paul [66] | 104 < Re < 105 8.45 < Pr < 20.29 4% < φv < 6% 10 < dp [nm] < 40 | Equation (18) |
| Authors | Correlation | Remarks | Equation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Krieger and Dougherty [69] | volume fraction of aggregates —volume fraction of the well-dispersed individual particles, —diameter of aggregates, —fractal dimension of aggregates —volume fraction of densely packed spheres | Equation (19) | ||||||||
| Palm et al. [70] | Equation (20) Equation (21) | |||||||||
| Nguyen et al. [71] | dp = 47 nm | Equation (22) Equation (23) | ||||||||
| Khanafer and Vafai [72] | Equation (24) | |||||||||
| Pastoriza-Gallego et al. [73] | Equation (25) | |||||||||
| ϕv | ||||||||||
| 0.000 | 0.005 | 0.010 | 0.015 | 0.021 | 0.031 | 0.048 | ||||
| A | −3.694 | −3.632 | −2.381 | −1.702 | −3.450 | −3.302 | −1.379 | |||
| B [K] | 999.0 | 999.0 | 689.3 | 534.7 | 999.0 | 999.0 | 518.4 | |||
| To [K] | 145.7 | 145.5 | 169.8 | 185.5 | 146.2 | 145.3 | 189.9 | |||
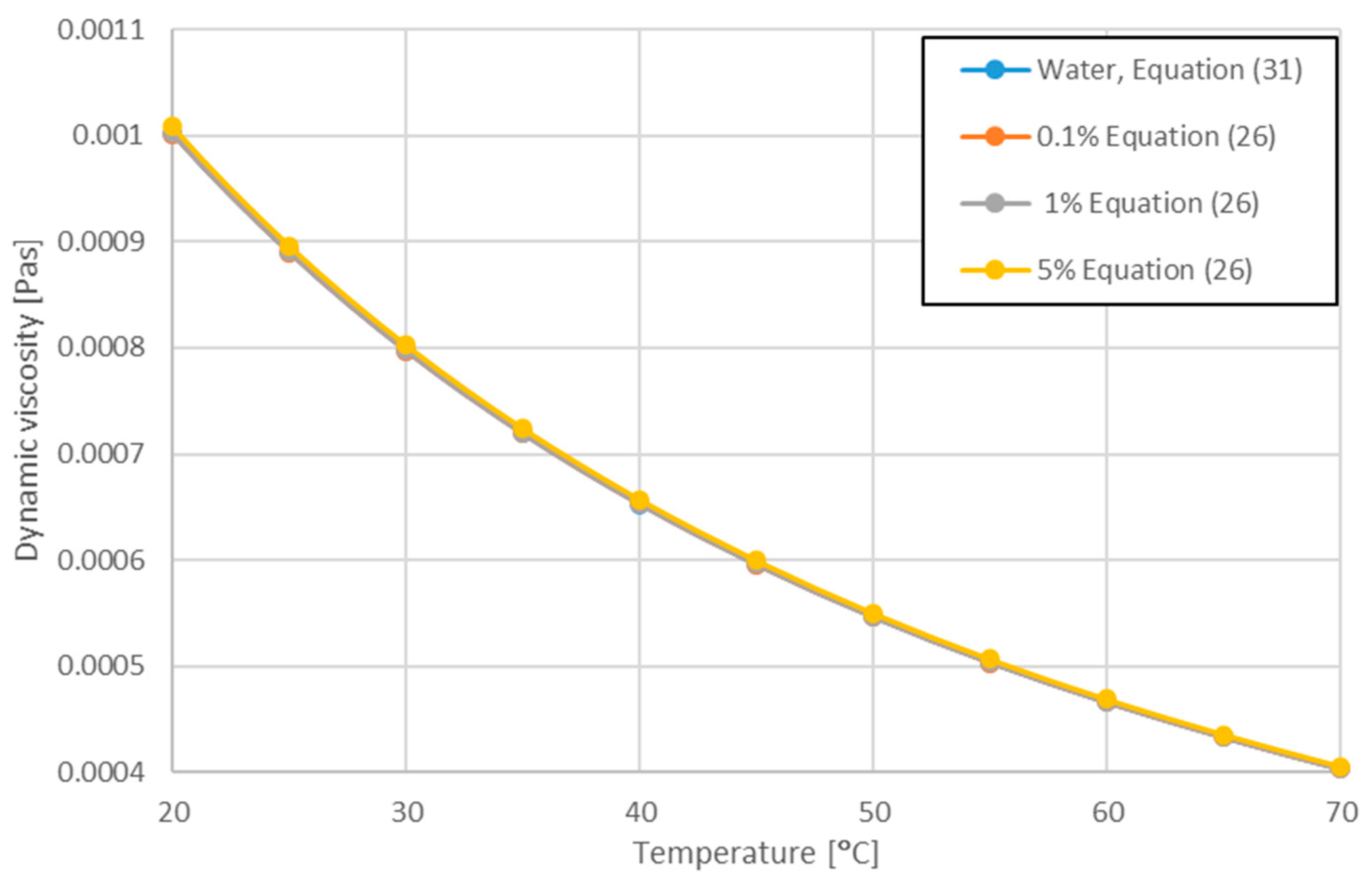
| Corcione [74] | df—equivalent diameter of liquid molecule M—molecular weight of water N—Avogadro number - density of water at To = 293 K | Equation (26) | ||||||||
| Authors | Correlation | Range | Equation |
|---|---|---|---|
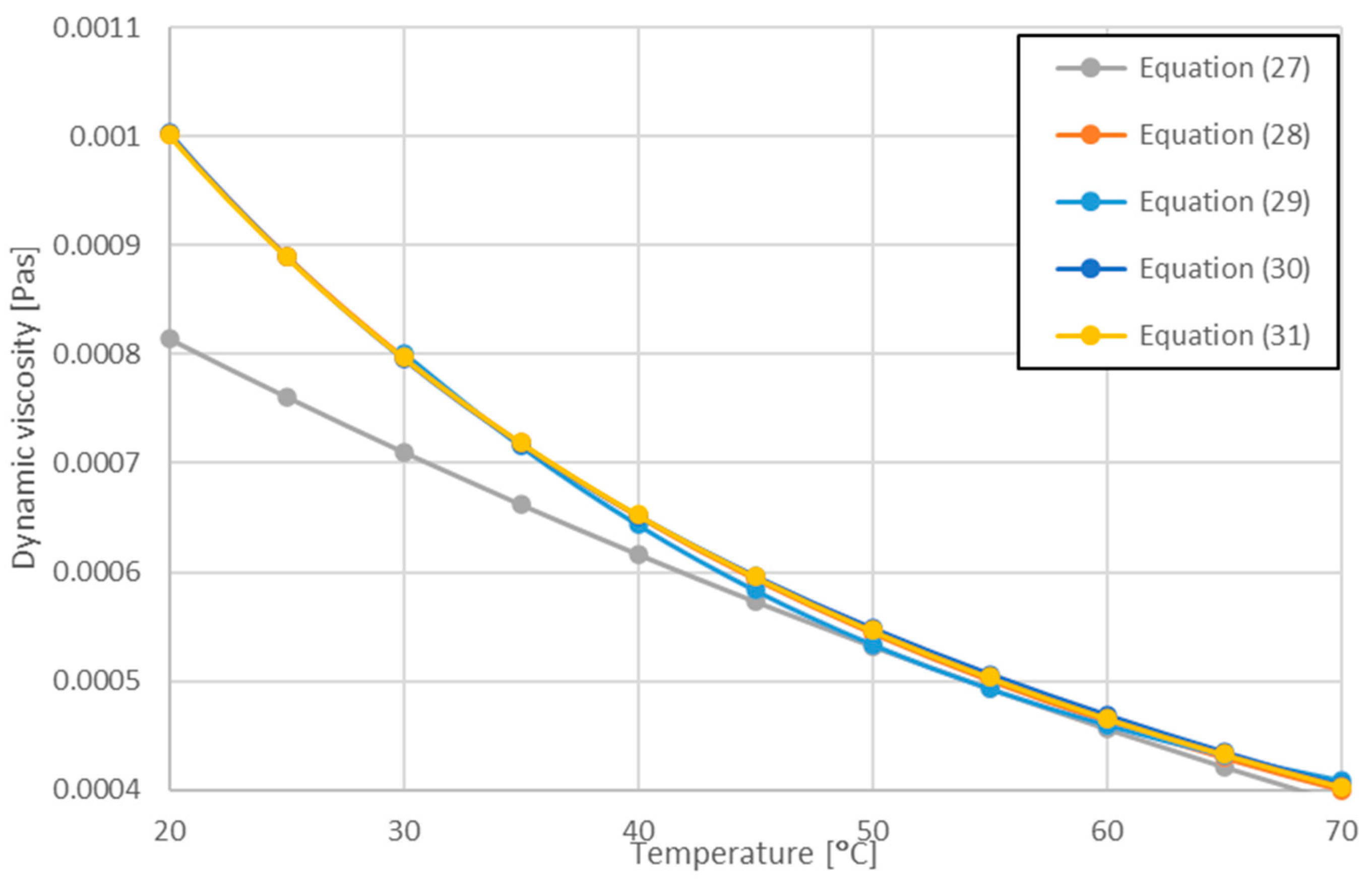
| Minea et al. Equation (31) in [36] | 294 < T < 344 | Equation (27) | |
| Chon and Khim [75] | 294 < T < 344 | Equation (28) | |
| Purohit et al. [76] | 300 < T < 350 | Equation (29) | |
| Saeed and Dulaimi [77] | Equation (30) | ||
| Present work [78] | 283 < T < 343 | Equation (31) |
| Authors | Correlation | Remarks | Equation |
|---|---|---|---|
| Khanafer and Vafai [72] | water-Al2O3 | Equation (32) | |
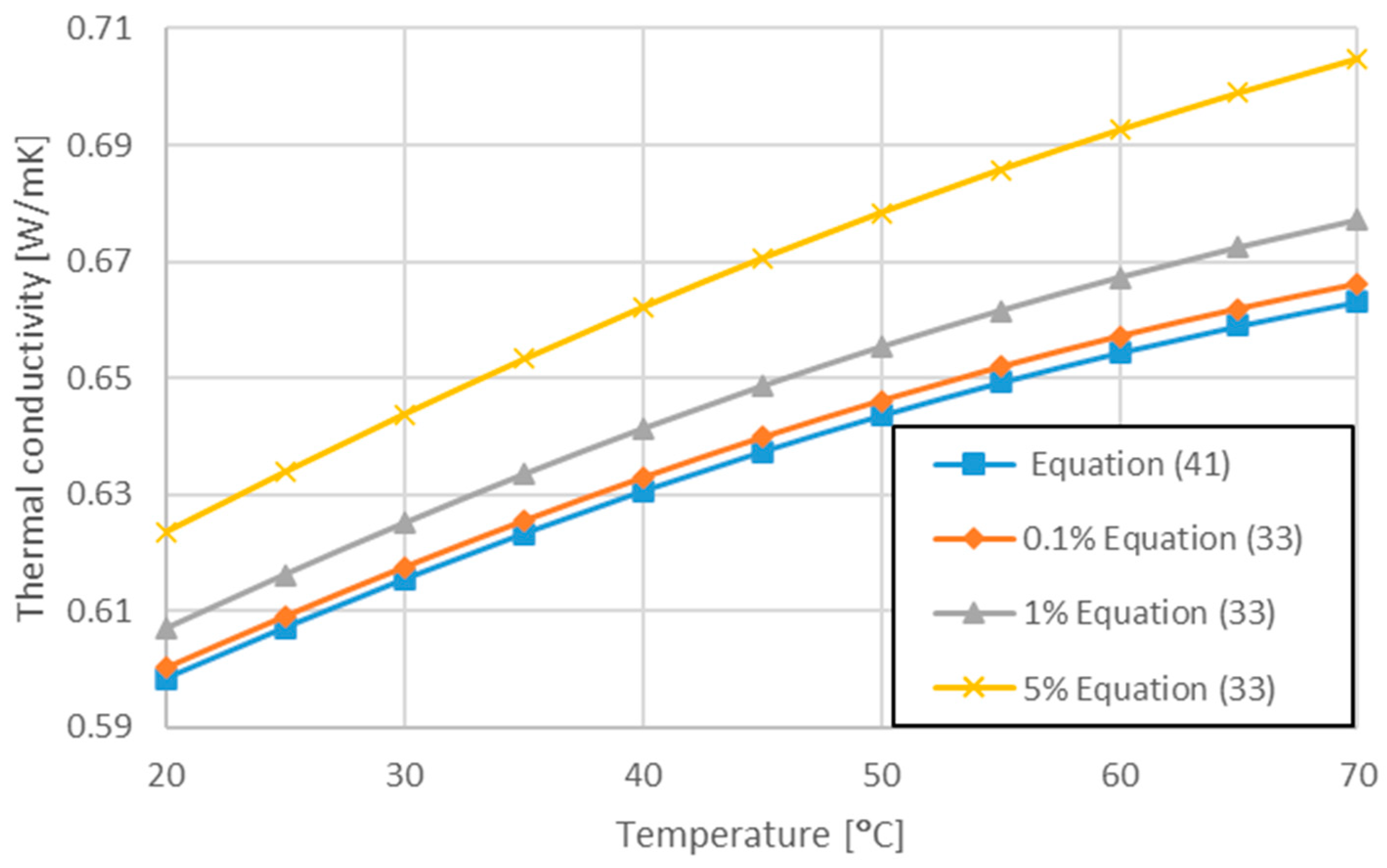
| Corcione [74] | —Re number based on nanoparticle diameter —Brownian velocity of the nanoparticle | Equation (33) | |
| Chen [81] | lp—mean free path of nanoparticle | Equation (34) | |
| Hassani et al. [82] | Various base fluids, metal and oxide nanoparticles | Equation (35) | |
| Sawicka et al. [83] | Equation (36) |
| Authors | Correlation | Range | Equation |
|---|---|---|---|
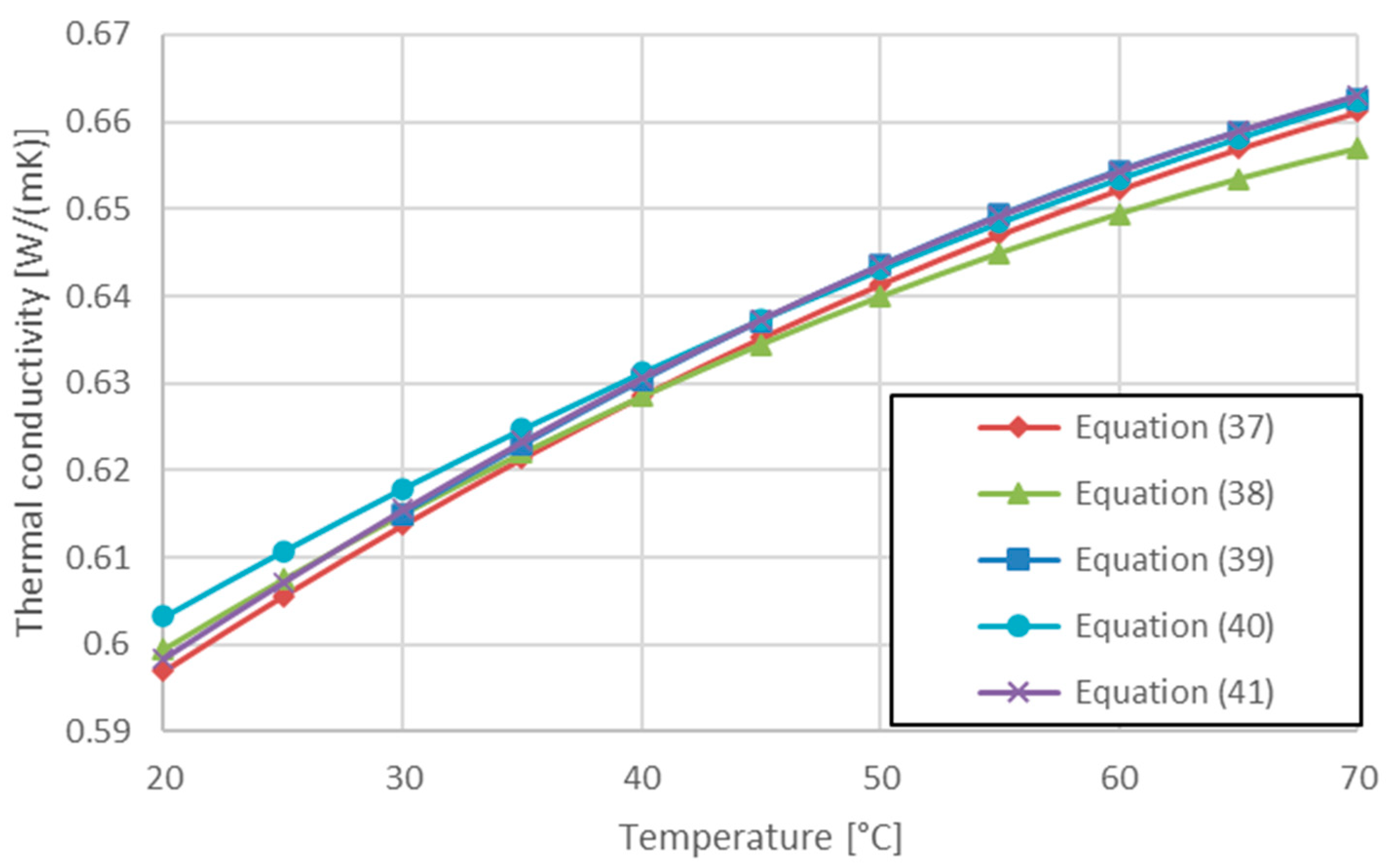
| Minea et al. Equation (22) in [36] | 294 < T < 344 | Equation (37) | |
| Minea et al. Equation (30) in [36] | 294 < T < 344 | Equation (38) | |
| Purohit [76] | 300 < T < 350 | Equation (39) | |
| Saeed and Dulaimi [77] | Equation (40) | ||
| Present work [78] | 283 < T < 343 | Equation (41) |
| Authors | Correlation | Remarks | Equation |
|---|---|---|---|
| Khanafer and Vafai [72] | water-Al2O3 | Equation (42) | |
| Pak and Cho [84] | Mixture model | Equation (43) | |
| Sharifpur et al. [85] | —radius of nanoparticle —nanolayer thickness | Equation (44) |
| Autors | Correlation | Range | Equation |
|---|---|---|---|
| Minea et al. Equation (20) in [36] | Equation (45) | ||
| Minea et al. Equation (28) in [36] | Equation (46) | ||
| Purohit et al. [76] | 300 < T < 350 | Equation (47) | |
| Saeed and Dulaimi [77] | Equation (48) | ||
| Present work [78] | Equation (49) | ||
| Saha and Paul [86] | 278 < T < 363 | Equation (50) |
| Authors | Correlation | Remarks | Equation |
|---|---|---|---|
| Pak and Cho [84] | Mixture model | Equation (51) | |
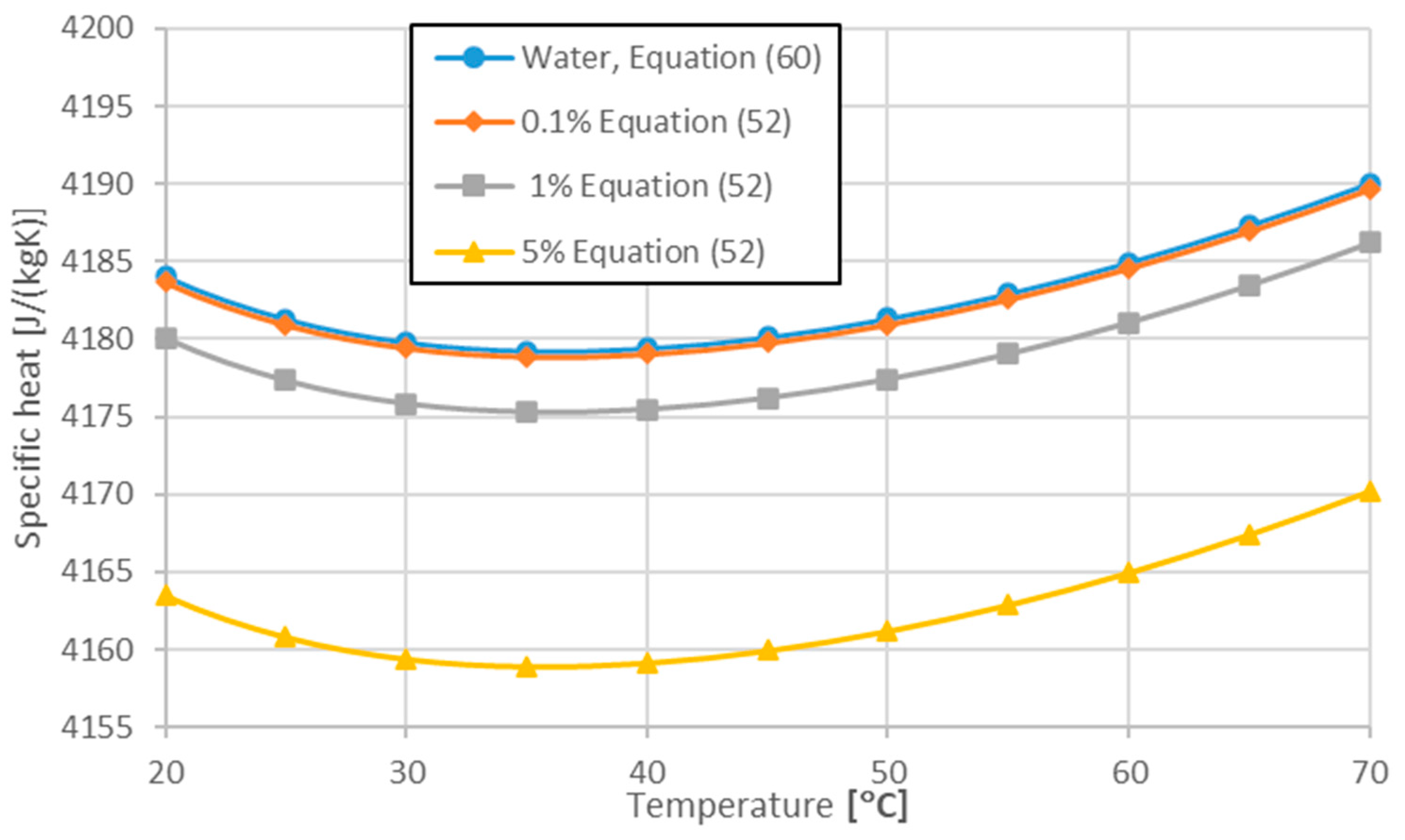
| Williams et al. [90] | water-Al2O3, water-ZrO2 | Equation (52) | |
| Corcione et al. [91] | water-Al2O3, water-CuO, water-TiO2 | Equation (53) | |
| Sekhar and Sharma [92] | water-Al2O3, water-CuO, water-TiO2, water-SiO2 , , | Equation (54) |
| Authors | Correlation | Range | Equation |
|---|---|---|---|
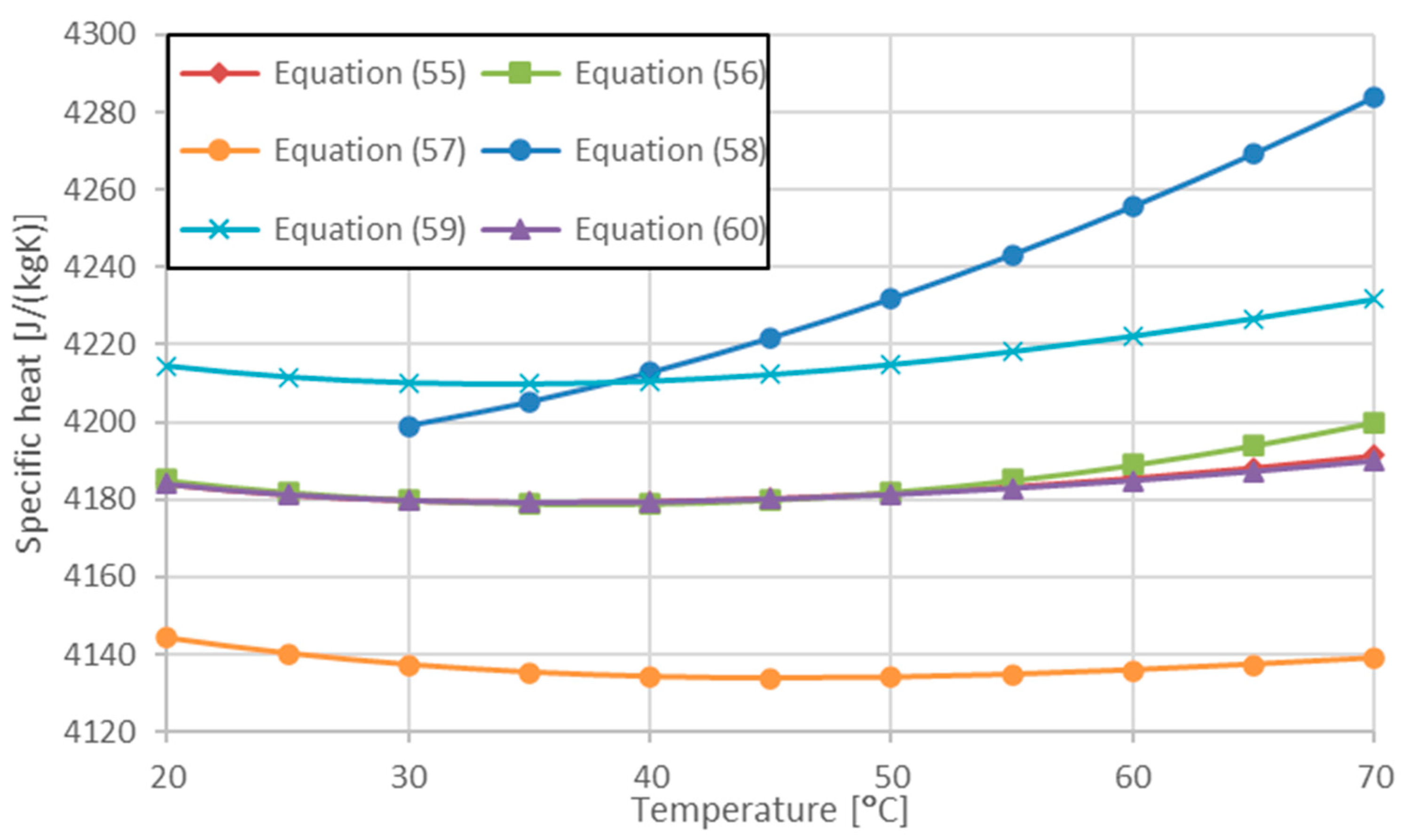
| Minea et al. Equation (21) in [36] | 293 < T < 313 | Equation (55) | |
| Minea et al. Equation (25) in [36] | 293 < T < 313 | Equation (56) | |
| Saha and Paul [66] | 278 < T < 363 | Equation (57) | |
| Purohit et al. [76] | 300 < T < 350 | Equation (58) | |
| Saeed and Dulaimi [77] | Equation (59) | ||
| Present work [78] | 283 < T < 343 | Equation (60) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cieśliński, J.T.; Lubocki, D.; Smolen, S. Impact of Temperature and Nanoparticle Concentration on Turbulent Forced Convective Heat Transfer of Nanofluids. Energies 2022, 15, 7742. https://doi.org/10.3390/en15207742
Cieśliński JT, Lubocki D, Smolen S. Impact of Temperature and Nanoparticle Concentration on Turbulent Forced Convective Heat Transfer of Nanofluids. Energies. 2022; 15(20):7742. https://doi.org/10.3390/en15207742
Chicago/Turabian StyleCieśliński, Janusz T., Dawid Lubocki, and Slawomir Smolen. 2022. "Impact of Temperature and Nanoparticle Concentration on Turbulent Forced Convective Heat Transfer of Nanofluids" Energies 15, no. 20: 7742. https://doi.org/10.3390/en15207742
APA StyleCieśliński, J. T., Lubocki, D., & Smolen, S. (2022). Impact of Temperature and Nanoparticle Concentration on Turbulent Forced Convective Heat Transfer of Nanofluids. Energies, 15(20), 7742. https://doi.org/10.3390/en15207742






