Abstract
Oil well cement microcracks cause formation fluid channeling, compromising oil and gas extraction safety. Superabsorbent polymer (SAP) can absorb water and swell to prevent fluid channeling. In this study, an alkali-resistant and pH-sensitive SAP was prepared based on the properties of oil well cement slurry. The preparation of the SAP was optimized, including monomer ratio, cross-linking agent dosage, and monomer concentration. The pH sensitivity and alkali resistance of the SAP were evaluated. The results revealed that the SAP exhibited good pH sensitivity, with the absorption rate in water being 2.18 times that of cement slurry filtrate (CSF) at 95 °C. Furthermore, the FTIR spectrum showed that the SAP had a stable molecular structure. The secondary absorption rate in water of the SAP after soaking in CSF was not different from the original absorption rate. Styrene–butadiene latex (SBL) can be used to adjust the SAP’s absorption rate. The SAP’s absorption rate had a good exponential functional relationship with SBL dosage. The SBL dosage can be determined by the functional relationships to prepare a SAP with the required properties.
1. Introduction
The sealing integrity of the oil well cement sheath is crucial for the safe long-term production of oil and gas wells [1,2]. However, oil well cement stone, as a brittle material, is easily subjected to external stress during perforation and fracture, destroying the isolation and resulting in fluid channeling. The major approach for dealing with fluid channeling is to use cement squeezing techniques, which are not only expensive but also fail frequently. Self-healing cement (SHC) [3,4,5,6,7,8] in oil and gas wells is a novel technology that has been developed in the last 20 years to avoid fluid channeling.
SHC has been attempted in several studies depending on formation properties, fluid types, crack development, and self-healing materials. Self-healing microcapsules [9] have been prepared in many studies. The effective component for realizing the self-healing of microcracks in cement sheath is the core, which typically includes epoxy resin [10,11], sodium silicate [12], unhydrated cement particles [13,14], sodium potassium tartrate [15], superabsorpbent polymer [16,17], dimethyl thio-toluene diamine [18], magne sium oxide [19], etc. The main function of the shell is to carry the self-healing core while preventing interaction between the core and the cement slurry. Crystalline admixtures [20,21,22] minimize cement stone permeability while providing self-healing characteristics. ACaCO3 whisker has been discovered to induce deposition at high concentrations, and to increase cement stone’s self-healing capacity [23]. Bacteria-based SHC [24,25,26] is a newer technique that promotes self-healing, mostly through mineral precipitation produced by bacteria.
Cross-linked polymers with the ability to absorb formation fluids and expand can quickly fill and heal microcracks in cement stone. While cementing hydrocarbon-rich formations, oil swellable polymer [6,27,28,29,30,31] are added to oil well cement, which can heal microcracks in cement stone when absorbing oil or gas. For adjusting well cementing, which has high formation water content due to long-term water flooding, superabsorbent polymer (SAP) can absorb water and swell to prevent fluid channeling. The primary barrier to the use of SAPs in cement slurry is their ability to absorb the mixing water, making the cement slurry difficult to pump. The addition of extra water to the cement slurry is not permitted, since it reduces the density of the cement slurry, which is also a vital signal for cementing operations. Therefore, it is vital to regulate SAP’s ability to absorb water in the cement slurry.
Microcapsules composed of SAP cores can solve this problem [17]. However, the amount of encapsuled SAPs required to successfully plug micrcracks in cement stone is too high, exceeding 28% by weight of cement. It will undoubtedly raise the cementing cost significantly. SAPs should be added directly in the cement slurry to reduce the dosage and cost. Ions in the pore solution or pH of cement paste affect the water absorption of SAP. Calcium alginate hydrogel, as a kind of ion-responsive SAP, has a low swelling potential in cement slurry due to a high concentration [8]. It avoids the negative effect of SAPs on the cement paste and achieves the self-healing ability of cement paste in sodium silicate solution. However, the self-healing is accomplished by the use of sodium silicate solution, which is difficult to realize downhole. A pH-sensitive SAP, which is synthesized using 2-(Dimethylamino)ethyl methacrylate and acryloyloxyethyltrimethyl ammonium chloride, has a low absorption rate in cement slurry filtrate due to its high pH value [7]. It has a higher absorption rate in neutral and weak alkaline solutions, and achieves self-healing. However, the hydrolysis of ester groups of DMAEMA/DAC and the cross-linking of carboxyl groups ions increases the cross-linking density of SAP, thereby reducing the self-healing ability of the SAP at high temperature.
In this paper, two alkali-resistant monomers are used to prepare pH-sensitive SAP, N,N-Dimethylacrylamide (DMAA), and Diallyldimethylammonium chloride (DMDAAC). Using pH sensitivity as the assessment criterion, p(DMAA-co-DMDAAC) SAP’s molecular structure is adjusted, including the ratio of monomers and the amount of cross-linking agent. The pH sensitivity at different temperatures and the alkali resistance of the SAP are evaluated. Furthermore, styrene–butadiene latex is added to the polymerization solution to modulate the SAP’s water absorption capabilities.
2. Materials and Methods
2.1. Materials
Class G oil well cement was provided by Jiahua Special Cement Co., Ltd. The chemical composition of the cement was tested using X-ray fluorescence (XRF), as shown in Table 1. The phase composition of the cement was estimated using quantitative Rietveld refinement, as shown in Table 2. The monomers N,N-Dimethylacrylamide (DMAA, 98%) and Diallyldimethylammonium chloride (DMDAAC, 60%), the cross-linking agent Methylene-bis-acrylamide (MBA, 99%), and the co-initiator N,N,N’,N’-Tetramethylethylenediamine (TEMED, 99%) were purchased from Shanghai Macklin Biochemical Co., Ltd., Shanghai, China. Sodium hydroxide (NaOH, 95%) and initiator ammonium persulfate (APS, 98%) were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Styrene–butadiene latex (SBL, 50wt.% solid content) was purchased from Shandong Jiaying Chemical Technology Co., Ltd., Jinan, China. Distilled water was used throughout the experiments.

Table 1.
Chemical composition of Class G oil well cement (wt.%).

Table 2.
Phase composition of Class G oil well cement (wt.%).
2.2. Synthesis of p(DMAA-co-DMDAAC) SAPs
The method used in this paper to prepare pH-sensitive SAP was aqueous solution polymerization. Distilled water, monomers (DMAA and DMDAAC), and cross-linking agent (MBA) were added in sequence to the reaction vessel. The solution was stirred for 10 min until MBA was completely dissolved. To remove the air, nitrogen was injected into the solution. The initiator (APS) solution was added dropwise. A co-initiator (TEMED) was added 5 min later. The solution was stirred for another 10 min and then left to react at 30 °C for 8 h. The product was removed from the vessel and was dried in a vacuum drying oven at 80 °C for 24 h, and then pulverized and passed through a 50-mesh sieve to obtain a pH-sensitive SAP.
2.3. Preparation of Cement Slurry Filtrate
Cement slurry filtrate (CSF) was prepared using a static fluid loss test based on standard API RP 10B-2: 2013 [32]. A total of 650 g cement and 286 g water (W/C = 0.44) were mixed following the oil well cement slurry preparation procedure [33]. The cement slurry was poured into the atmospheric consistometer, and then heated to 90 °C at a heating rate of 3 °C/min. After the atmospheric consistometer was heated to 90 °C, the cement slurry was stirred for another 20 min. Then, the cement slurry was poured into a fluid-loss cell to filter the pore solution. A pressure of 6.9 MPa nitrogen was applied. The obtained CSF was used to test the properties of the SAP.
2.4. Water Absorption Rate
The SAP’s absorption rate was the weight of water absorbed divided by its own weight. A certain mass of SAP powder was weighed and denoted as . The SAP powder was placed into a nylon bag. represented the total mass of the SAP powder and the nylon bag. The nylon bag was then immersed in the test liquid. At regular intervals, the nylon bag was removed, and the water on its surface was absorbed using absorbent paper. The entire mass of the nylon bag and the SAP after water absorption was weighted and recorded as . The absorption rate (G, g/g) of the SAP was calculated using Equation (1).
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
The FTIR spectra of p(DMAA-co-DMDAAC) SAPs were recorded on a Bio-Rad FTS135 FTIR spectroscope. SAP powder and diluent (KBr, spectral pure) were fully ground, and a tablet press was used to prepared the test sample. The wavelength range was 4000–400 .
3. Results and Discussion
3.1. Optimization of SAP Preparation
In the paper, the pH sensitivity of SAP was evaluated using the ratio of the absorption rate of SAP in water () to the absorption rate in NaOH solution with pH = 12 (). The larger was, the stronger the pH sensitivity of SAP was. The preparation of SAP was optimized, including the monomer ratio, cross-linking agent dosage, and monomer concentration.
3.1.1. Effect of Monomer Ratio on SAP’s Absorption Rate
The monomer weight ratios of DMAA to DMDAAC were set as 10:9, 10:12, 10:15, and 10:18, respectively. The fixed cross-linking agent (MBA) was added in an amount of 0.715% by weight of the sum of DMAA and DMDAAC, and the fixed monomer concentration was 35%. Four SAPs with different monomer ratios were prepared, and the absorption rates in water (pH = 7) and in NaOH solution (pH = 12) at 90 °C were tested, as shown in Figure 1. From Figure 1, the absorption rates of the four SAPs increased gradually with time. The saturated water absorption of the four SAPs in water requires 90 min, but it takes 30 min in NaOH solution (pH = 12). This is most likely owing to their lower absorption rates in NaOH solution (pH = 12) compared to those in water.
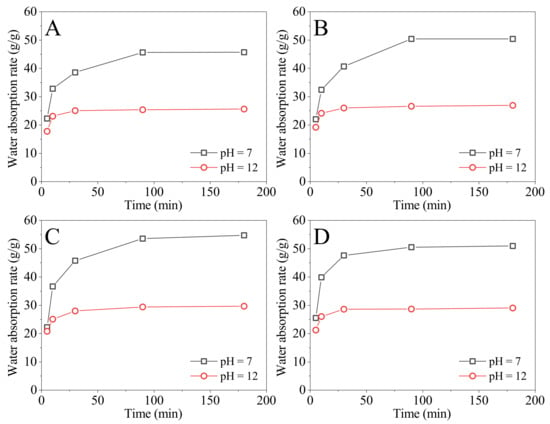
Figure 1.
The absorption rates of SAPs prepared with different monomer ratios tested at 90 °C in water (pH = 7), and in NaOH solution (pH = 12) varied with time; the ratios of DMAA to DMDAAC are (A) 10:9, (B) 10:12, (C) 10:15, and (D) 10:18.
The four SAPs are considered to be saturated with water at 180 min, from Figure 1. The saturated absorption rates and the pH-sensitive index () are summarized in Table 3. From Table 3, the saturated absorption rate of the SAPs in water and in NaOH solution (pH = 12) first increases and subsequently declines as the amount of DMDAAC increases. When the monomer ratio of DMAA to DMDAAC is 10:15, the saturated absorption rates of the SAP in water and NaOH solution (pH = 12) are the highest, which are 54.74 g/g and 29.65 g/g, respectively. In addition, increases initially and subsequently reduces as the amount of DMDAAC in the monomers increases, reaching a maximum when the ratio of DMAA to DMDAAC is 10:12. The maximum is 1.87. Therefore, the preferred monomer ratio is 10:12, as pH sensitivity is the desired property of SAP.

Table 3.
The saturated absorption rates of SAPs prepared with different monomer ratios tested at 90 °C in water (pH = 7), and in NaOH solution (pH = 12), and the pH-sensitive index.
3.1.2. Effect of MBA Dosage on SAP’s Absorption Rate
The cross-linking agent (MBA) was added in amounts of 0.143%, 0.429%, 0.715%, and 1.001% by weight of the sum of DMAA and DMDAAC, respectively. The monomer weight ratio of DMAA and DMDAAC was fixed at 10:18, and the fixed monomer concentration was 35%. Four SAPs with different MBA dosages were prepared, and the absorption rates in water (pH = 7) and in NaOH solution (pH = 12) at 90 °C were tested, as shown in Figure 2. From Figure 2, the saturation water absorption of the SAP in water and in NaOH solution (pH = 12) requires 180 min when MBA dosage is 0.1%. This is due to the SAP’s greater absorption rate at a low cross-linking level. When the dosages of MBA are 0.429% to 1.001%, the saturation water absorption of the SAPs in water and in NaOH solution (pH = 12) requires 90 and 30 min, respectively.
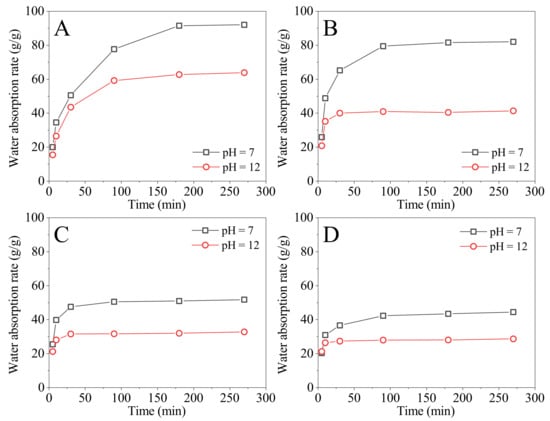
Figure 2.
The absorption rates of SAPs prepared with different MBA dosages tested at 90 °C in water (pH = 7) and in NaOH solution (pH = 12) varied with time; the MBA dosages are (A) 0.143%, (B) 0.429%, (C) 0.715%, and (D) 1.001%.
The four SAPs are considered to be saturated with water at 270 min, from Figure 2. The saturated absorption rates and the pH-sensitive index () are summarized in Table 4. From Table 4, the saturated absorption rate of the SAPs in water and in NaOH solution (pH = 12) decrease as MBA dosage increases. When the MBA dosage increases from 0.143% to 1.001%, the absorption rate of the SAPs in water reduces from 91.50 to 41.43 g/g, while the absorption rate in NaOH solution (pH = 12) decreases from 62.78 to 25.66 g/g. This is because MBA increases the cross-linking density of the SAP, lowering its absorption rate. increases initially and subsequently reduces as MBA dosage increases, reaching a maximum when the MBA dosage is 0.429%. The maximum is 2.01. Therefore, the preferred MBA dosage is 0.429%.

Table 4.
The saturated absorption rates of SAPs prepared with different MBA dosages tested at 90 °C in water (pH = 7) and in NaOH solution (pH = 12), and the pH-sensitive index.
3.1.3. Effect of Monomer Concentration on SAP’s Absorption Rate
The monomer concentrations were 21.00%, 26.25%, 35.00%, and 52.50%, respectively. The monomer weight ratio of DMAA and DMDAAC was fixed at 10:18, and the fixed cross-linking agent (MBA) was added in an amount of 0.715% by weight of the sum of DMAA and DMDAAC. Four SAPs with different monomer concentrations were prepared, and the absorption rates in water (pH = 7) and in NaOH solution (pH = 12) at 90 °C were tested, as shown in Figure 3. From Figure 3, the saturated water absorption of the four SAPs in water requires 90 min, but it takes 30 min in NaOH solution (pH = 12).
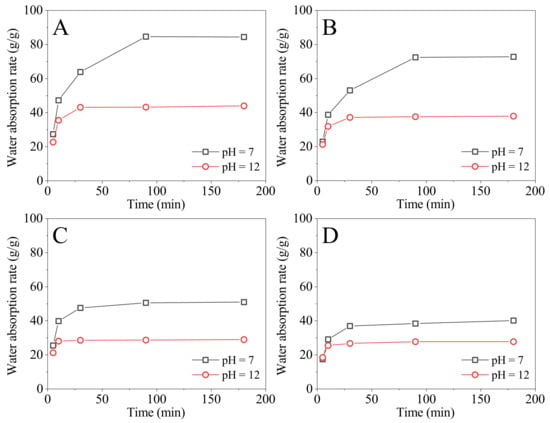
Figure 3.
The absorption rates of SAPs prepared with different monomer concentrations tested at 90 °C in water (pH = 7) and in NaOH solution (pH = 12) varied with time; the monomer concentrations are (A) 21.00%, (B) 26.25%, (C) 35.00%, and (D) 52.50%.
The four SAPs are considered to be saturated with water at 180 min, from Figure 3. The saturated absorption rates and the pH-sensitive index () are summarized in Table 5. From Table 5, the saturated absorption rate of the SAPs in water and in NaOH solution (pH = 12) decrease as monomer concentration increases. When the monomer concentration increases from 21.00% to 52.50%, the absorption rate of the SAPs in water reduces from 83.44 to 40.12 g/g, while the absorption rate in NaOH solution (pH = 12) decreases from 43.97 to 27.11 g/g. increases initially and subsequently reduces as monomer concentration increases, reaching a maximum when the monomer concentration is 26.25%. The maximum is 1.92. Therefore, the preferred monomer concentration is 26.25%.

Table 5.
The saturated absorption rates of SAPs prepared with different monomer concentrations tested at 90 °C in water (pH = 7) and in NaOH solution (pH = 12), and the pH-sensitive index.
3.2. Properties of the SAP
According to the above optimization, the optimal DMAA to DMDAAC ratio was 10:12, the optimal MBA dosage was 0.429%, and the optimal monomer concentration was 26.25%. SAP was prepared in the optimal situation, and the pH sensitivity and alkali resistance of the SAP were evaluated.
3.2.1. pH Sensitivity of the SAP
The absorption rates of the SAP at various temperatures and pH values are shown in the Figure 4. From Figure 4, temperature has little effect on the SAP’s absorption rate, which decreases slightly as the temperature decreases from 95 °C to 40 °C at the pH value range of 7 to 10. It has almost no effect on the absorption rate in the pH range of 12 to 13.5.
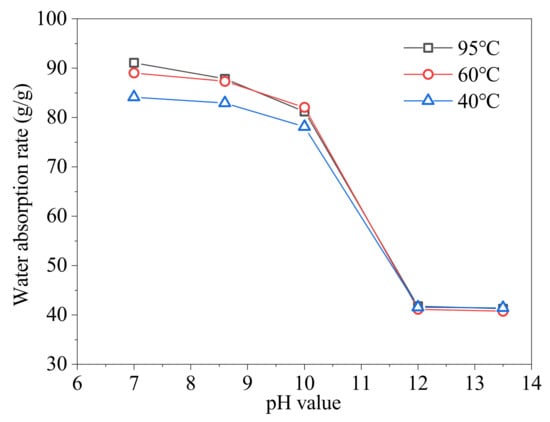
Figure 4.
The absorption rates of the SAP at various temperatures and pH values.
The pH value has a significant impact on the SAP’s absorption rate, which reduces modestly as the pH value increases from 7 to 10, but reduces significantly as the pH value increases from 10 to 12. The SAP’s absorption rate is not much different when the pH value is 12 and 13.5. The curves are divided into two sections: the first section in the pH range of 7–10, and the second section in the pH range of 12–13.5. The SAP’s absorption rate is pH sensitive: it is greater in neutral and mild alkaline solutions than that in strong alkaline solutions. The of the SAP are 2.01, 2.16, and 2.18 at 40 °C, 60 °C, and 95 °C, respectively. The pH sensitivity of the SAP’s absorption rate makes it suitable for oil well cement to realize the self-healing of cement microcracks.
The water absorption capacity of cationic SAP is related to the degree of protonation of amine groups on the molecular chain, as shown in Figure 5. When the aqueous solution is neutral or mild alkaline, the degree of protonation of amine groups on the molecular chain of SAP increases. The electrostatic repulsion between molecules increases, and the molecular chain extends. At the same time, the increase in the ionic strength of SAP leads to an increase in the osmotic pressure of SAP, making it easier for water to enter. As a result, the SAP has a high absorption rate in neutral or mild alkaline solution. However, the degree of protonation of the amine group is weakened in strong alkaline solution, and the intermolecular force is enhanced, resulting in a decrease in the absorption rate of the SAP.
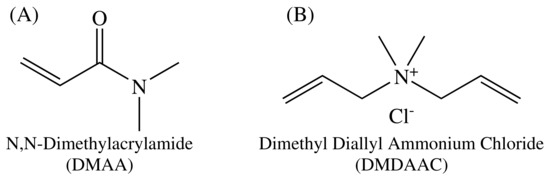
Figure 5.
Molecular structures of (A) DMAA and (B) DMDAAC.
3.2.2. Alkali Resistance of the SAP
The absorption rate of the SAP in CSF at 90 °C was conducted. The SAP was transferred to water after it was saturated with water in CSF. Its continued absorption rate in water was tested and compared to the direct absorption rate in water. The result is shown in Figure 6.
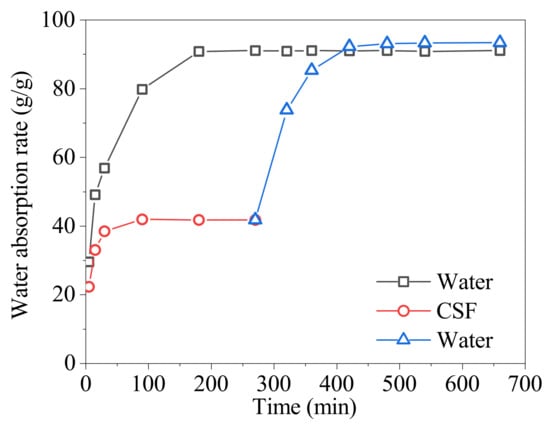
Figure 6.
The absorption rate of the SAP in water and in CSF at 90 °C.
From Figure 6, the saturated water absorption of the SAP in water requires 180 min, and the saturated absorption rate () is 91.09 g/g. In addition, the saturated water absorption of the SAP in CSF requires 90 min, and the the saturated absorption rate () is 41.76 g/g. After 270 min, the SAP was saturated with water in CSF and transferred to water. After another 150 min, the SAP was saturated with water once more, and the aturated absorption rate () was 93.35 g/g. There was little difference between and , indicating that CSF has little effect on the water absorption ability of the SAP in water. The SAP is alkali resistant.
Figure 7 shows the FTIR spectrum of the SAP before and after CSF treatment. The black curve is the FTIR spectrum of the SAP before CSF treatment. The unsymmetrical stretching vibrations of the aliphatic C–H bond in the methyl groups correlate to the absorption band at 2930 . The asymmetric bending vibrations of C–H in the methine groups provide the absorption band at 1480 . The stretching vibration of C=O in the DMAA unit causes the absorption bands at 1620 . The stretching vibration of C-N in DMAA and DMDAAC is presented at 1256 and 1141 . The unreacted monomer and crosslinker’s C=C stretch is no longer evident (about 1650 ). The FTIR spectroscopy results indicate that the copolymerization was successful.
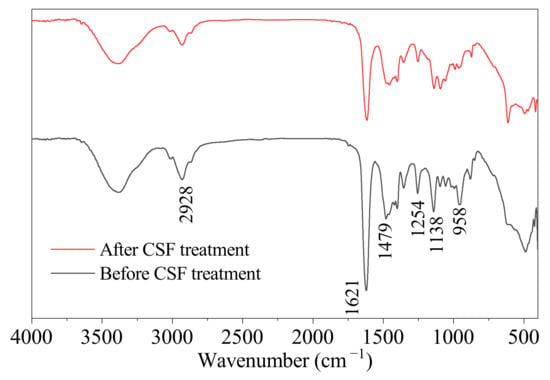
Figure 7.
FTIR spectrum of the SAP before CSF treatment and after CSF treatment for 300 min at 90 °C.
The red curve is the FTIR spectrum of the SAP after CSF treatment for 300 min at 90 °C. The characteristic peaks of the red curve are not much different from those of the black curve, demonstrating that CSF has minimal influence on SAP structure and that SAP is alkali resistant.
3.3. Adjustment of the SAP’s Absorption Rate
According to a previous study [34], the particle size and absorption rate of SAP were factors that affected its self-healing effect. Styrene–butadiene latex (SBL) was added to the polymerization solution to adjust the SAP’s absorption rate. The amount of SBL added was the ratio of the solid phase weight in SBL to the monomer weight. The effect of SBL dosage on SAP’s absorption rate was studied, as shown in Figure 8. From Figure 8, the absorption rate of SAP in water and in CSF at 90 °C decreases with the increasing dosage of SBL. This is mainly because the latex particles are not water absorbent. The absorption rate of the SAP has a good exponential functional relationship with SBL dosage. The functional relationship between the SAP’s absorption rate in water and the SBL dosage is: . The functional relationship between the SAP’s absorption rate in CSF and the SBL dosage is: . Therefore, the SBL dosage can be determined using functional relationships to prepare the SAP with the required properties.
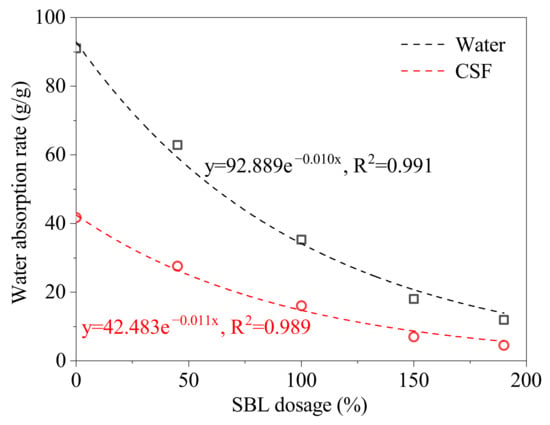
Figure 8.
The effect of SBL dosage on SAP’s absorption rate at 90 °C.
4. Conclusions
The study results in the following conclusions in this paper:
- (1)
- The preparation of SAP was optimized: the optimal DMAA to DMDAAC ratio was 10:12, the optimal MBA dosage was 0.429%, and the optimal monomer concentration was 26.25%.
- (2)
- The SAP’s absorption rate had good pH sensitivity. The of the SAP were 2.01, 2.16, and 2.18 at 40 °C, 60 °C, and 95 °C, respectively. The pH sensitivity of the SAP’s absorption rate made it suitable for oil well cement to realize the self-healing of cement microcracks.
- (3)
- The secondary water absorption of the SAP after soaking in CSF, and the FTIR result showed that CSF had minimal influence on SAP structure, and that SAP is alkali resistant.
- (4)
- Styrene–butadiene latex can be used to adjust SAP’s absorption rate. The SAP’s absorption rate had a good exponential functional relationship with SBL dosage. The SBL dosage can be determined using functional relationships to prepare a SAP with the required properties.
Author Contributions
Conceptualization, L.Z. (Lin Zhao) and J.Y.; methodology, L.Z. (Lihui Zheng) and N.L.; validation, H.W.; formal analysis, L.Z. (Lin Zhao) and N.L.; investigation, J.Y. and C.W.; resources, H.W.; data curation, L.Z. (Lin Zhao) and N.L.; writing—original draft preparation, L.Z. (Lin Zhao) and C.W.; writing—review and editing, L.Z. (Lihui Zheng) and N.L.; visualization, C.W.; supervision, J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This research did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SHC | Self-healing cement |
| SAP | Superabsorbent polymer |
| DMAA | N,N-Dimethylacrylamide |
| DMDAAC | Diallyldimethylammonium chloride |
| XRF | X-ray fluorescence |
| MBA | Methylene-bis-acrylamide |
| TEMED | N,N,N’,N’-Tetramethylethylenediamine |
| NaOH | Sodium hydroxide |
| APS | Ammonium persulfate |
| SBL | Styrene–butadiene latex |
| CSF | Cement slurry filtrate |
| FTIR | Fourier transform infrared spectroscopy |
References
- Liu, H.; Bu, Y.; Zhou, A.; Du, J.; Zhou, L.; Pang, X. Silica sand enhanced cement mortar for cementing steam injection well up to 380 °C. Constr. Build. Mater. 2021, 308, 125142. [Google Scholar] [CrossRef]
- Guo, S.; Bu, Y.; Zhou, A.; Du, J.; Cai, Z. A three components thixotropic agent to enhance the thixotropic property of natural gas well cement at high temperatures. J. Nat. Gas Sci. Eng. 2020, 84, 103699. [Google Scholar] [CrossRef]
- Albuhairi, D.; Di Sarno, L. Low-Carbon Self-Healing Concrete: State-of-the-Art, Challenges and Opportunities. Buildings 2022, 12, 1196. [Google Scholar] [CrossRef]
- Cavanagh, P.H.; Johnson, C.R.; Roy-Delage, L.; DeBruijn, G.G.; Cooper, I.; Guillot, D.J.; Bulte, H.; Dargaud, B. Self-healing cement-novel technology to achieve leak-free wells. In Proceedings of the SPE/IADC Drilling Conference, Amsterdam, The Netherlands, 20–22 February 2007. [Google Scholar] [CrossRef]
- Darbe, R.P.; Karcher, J.; Pewitt, K. Dynamic Test Evaluates the Effectiveness of Self-Healing Cement Systems in the Downhole Environment. In Proceedings of the Middle East Drilling Technology Conference & Exhibition, Manama, Bahrain, 26–28 October 2009. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Yang, R.; Zhang, Y.; Jiang, L.; Wang, Z.; Wang, Q.; Liu, W.; Zeng, M.; Zhou, S.; et al. Oil swellable polymer modified cement paste: Expansion and crack healing upon oil absorption. Constr. Build. Mater. 2016, 114, 98–108. [Google Scholar] [CrossRef]
- Wang, C.; Bu, Y.; Guo, S.; Lu, Y.; Sun, B.; Shen, Z. Self-healing cement composite: Amine- and ammonium-based pH-sensitive superabsorbent polymers. Cem. Concr. Compos. 2019, 96, 154–162. [Google Scholar] [CrossRef]
- Hu, M.; Guo, J.; Du, J.; Liu, Z.; Li, P.; Ren, X.; Feng, Y. Development of Ca2+-based, ion-responsive superabsorbent hydrogel for cement applications: Self-healing and compressive strength. J. Colloid Interface Sci. 2019, 538, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Souradeep, G.; Kua, H.W. Encapsulation Technology and Techniques in Self-Healing Concrete. J. Mater. Civ. Eng. 2016, 28, 04016165. [Google Scholar] [CrossRef]
- Lv, L.; Yang, Z.; Chen, G.; Zhu, G.; Han, N.; Schlangen, E.; Xing, F. Synthesis and characterization of a new polymeric microcapsule and feasibility investigation in self-healing cementitious materials. Constr. Build. Mater. 2016, 105, 487–495. [Google Scholar] [CrossRef]
- Dong, B.; Fang, G.; Wang, Y.; Liu, Y.; Hong, S.; Zhang, J.; Lin, S.; Xing, F. Performance recovery concerning the permeability of concrete by means of a microcapsule based self-healing system. Cem. Concr. Compos. 2017, 78, 84–96. [Google Scholar] [CrossRef]
- Mao, W.; Litina, C.; Al-Tabbaa, A. Development and Application of Novel Sodium Silicate Microcapsule-Based Self-Healing Oil Well Cement. Materials 2020, 13, 456. [Google Scholar] [CrossRef]
- Papaioannou, S.; Amenta, M.; Kilikoglou, V.; Gournis, D.; Karatasios, I. Synthesis and integration of cement-based capsules modified with sodium silicate for developing self-healing cements. Constr. Build. Mater. 2022, 316, 125803. [Google Scholar] [CrossRef]
- Liu, M.; Hu, M.; Li, P.; Zhang, H.; Zhao, J.; Guo, J. A new application of fluid loss agent in enhancing autogenous healing ability and improving mechanical properties of oil well cement. Cem. Concr. Compos. 2022, 128, 104419. [Google Scholar] [CrossRef]
- Peng, Z.; Yu, C.; Feng, Q.; Zheng, Y.; Huo, J.; Liu, X. Preparation and application of microcapsule containing sodium potassium tartrate for self-healing of cement. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Richhariya, G.; Dora, D.; Parmar, K.; Pant, K.; Singhal, N.; Lal, K.; Kundu, P. Development of Self-Healing Cement Slurry through the Incorporation of Dual-Encapsulated Polyacrylamide for the Prevention of Water Ingress in Oil Well. Materials 2020, 13, 2921. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Bu, Y.; Sanjayan, J.G.; Nazari, A.; Shen, Z. The application of coated superabsorbent polymer in well cement for plugging the microcrack. Constr. Build. Mater. 2016, 104, 72–84. [Google Scholar] [CrossRef]
- Sun, D.; Wenxu, M.; Jikun, M.; Yan, J.; Qianjin, M.; Yali, W.; Jianfeng, W.; Lan, M.; Wang, Z.; Cui, S.; et al. The synthesis of DMTDA microcapsules and investigation of self-healing cement paste through an isocyanate-amine system. Cem. Concr. Compos. 2021, 122, 104132. [Google Scholar] [CrossRef]
- Ren, J.; Wang, X.; Li, D.; Han, N.; Dong, B.; Xing, F. Temperature adaptive microcapsules for self-healing cementitious materials. Compos. Part B Eng. 2021, 223, 109138. [Google Scholar] [CrossRef]
- de Souza Oliveira, A.; da Fonseca Martins Gomes, O.; Ferrara, L.; de Moraes Rego Fairbairn, E.; Toledo Filho, R.D. An overview of a twofold effect of crystalline admixtures in cement-based materials: From permeability-reducers to self-healing stimulators. J. Build. Eng. 2021, 41, 102400. [Google Scholar] [CrossRef]
- Peng, Z.; Xia, X.; Feng, Q.; Zheng, Y.; Yu, C.; Yang, Q.; Liu, X. Performance of Permeable Crystalline Self-Healing Agent Onmicro-Cracks of Oil Well Cement. Arab. J. Sci. Eng. 2022, 47, 6073–6084. [Google Scholar] [CrossRef]
- de Souza Oliveira, A.; Toledo Filho, R.D.; de Moraes Rego Fairbairn, E.; de Oliveira, L.F.C.; da Fonseca Martins Gomes, O. Microstructural characterization of self-healing products in cementitious systems containing crystalline admixture in the short- and long-term. Cem. Concr. Compos. 2022, 126, 104369. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, C.; Wu, Z.; Zhang, G.; Mei, K.; Gao, Q.; Cheng, X. Study on the effect of CaCO3 whiskers on carbonized self-healing cracks of cement paste: Application in CCUS cementing. Constr. Build. Mater. 2022, 321, 126368. [Google Scholar] [CrossRef]
- Luo, M.; Qian, C.-X.; Li, R.Y. Factors affecting crack repairing capacity of bacteria-based self-healing concrete. Constr. Build. Mater. 2015, 87, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Jiang, G.; Fang, C.; Sun, J.; Zheng, S.; Liu, H.; Leusheva, E.; Morenov, V.; Nikolaev, N. Field Application of Microbial Self-Healing Cement Slurry in Chunguang 17-14 Well. Energies 2021, 14, 1544. [Google Scholar] [CrossRef]
- Kumar Jogi, P.; Vara Lakshmi, T. Self healing concrete based on different bacteria: A review. Mater. Today Proc. 2021, 43, 1246–1252. [Google Scholar] [CrossRef]
- Wang, C.; Bu, Y.; Liu, H.; Guo, S. Preparation and characterization of core-shell oil absorption materials stabilized by modified fumed silica. J. Polym. Eng. 2017, 37, 391–399. [Google Scholar] [CrossRef]
- Wang, C.; Bu, Y.; Zhao, L. Properties and self-healing behavior of oil absorbent microspheres modified cement. Smart Mater. Struct. 2017, 26, 095010. [Google Scholar] [CrossRef]
- Zhang, R.; Mao, X.; Zhao, Z. Synthesis of Oil-Swelling Material and Evaluation of Its Self-Healing Effect in Cement Paste. Polym. Plast. Technol. Mater. 2019, 58, 618–629. [Google Scholar] [CrossRef]
- Johnson, C.; Gai, A.; Ioan, T.; Landa, J.; Gervasi, G.; Bourgeois, B.; Bouteldja, M. Self-Healing Cement for Long-Term Safe Exploitation of Gas Wells: A New Technology Case Study. In Proceedings of the IPTC International Petroleum Technology Conference, Beijing, China, 26–28 March 2019. [Google Scholar] [CrossRef]
- Nafikova, S.; Bugrayev, A.; Taoutaou, S.; Baygeldiyev, G.; Akhmetzianov, I.; Gurbanov, G.; Eliwa, I. Elimination of the Sustained Casing Pressure using Self-Healing Cement in Turkmenistan Section of the Caspian Sea. In Proceedings of the SPE Annual Technical Conference and Exhibition, Calgary, AB, Canada, 30 September–2 October 2019. [Google Scholar] [CrossRef]
- API Spec 10-B; Recommended Practice for Testing Well Cements. American Petroleum Institute, Washington, DC, USA, 2013.
- Wang, C.; Wang, L.; Yao, X.; Du, J.; Zhai, W.; Guo, S.; Zhou, A. The effect of rutin on the early-age hydration of oil well cement at varying temperatures. Cem. Concr. Compos. 2022, 128, 104438. [Google Scholar] [CrossRef]
- Wang, C.; Bu, Y.; Shen, Z. Study on the Mechanism of Expansive Self-Healing Additives for Oil Well Cement (in Chinese). Drill. Fluid Complet. Fluid 2018, 35, 98–102. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).