1. Introduction
The development of industry, the construction of housing and the rapid growth of enterprises processing agricultural products necessitate a sharp increase in the production of heat and electricity. In the past few decades, the growing demand for heat and electricity was mainly met through the construction of new coal-fired power plants and boiler houses [
1]. However, coal combustion is considered one of the largest sources of greenhouse gas emissions in the atmosphere. These emissions can be significantly reduced by replacing coal with biomass, as biomass is considered a CO
2-neutral fuel [
2]. In addition, synthesis gas can be obtained from biomass, which can be used to produce environmentally friendly liquid fuels [
3].
Among the sources of biomass, biowastes are distinguished, which are formed during the processing of biomass and the production of food from it (straw, rice husks, waste from the production of olive and sunflower oil, etc.).
Sunflower (
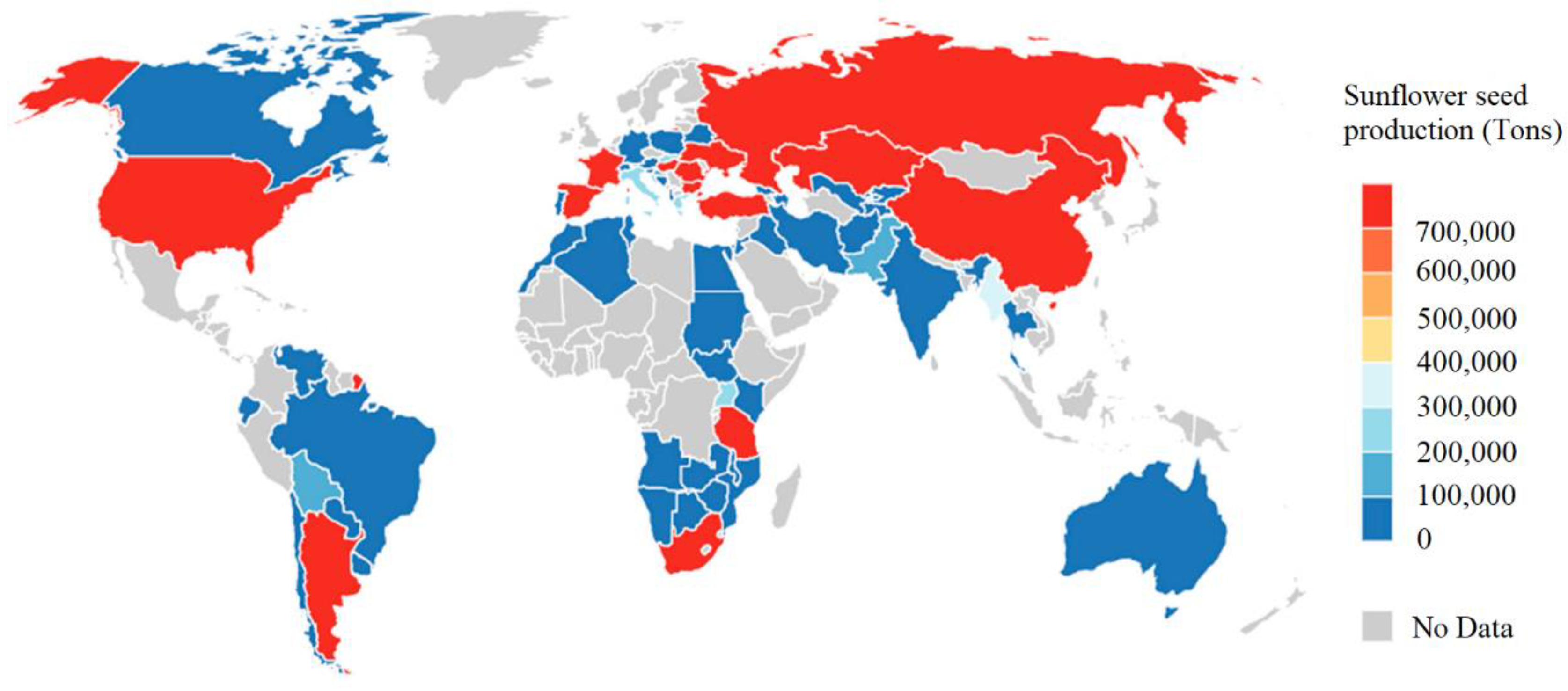
Helianthus annuus) is a herbaceous crop of the Compositae family, widely cultivated throughout the world due to its relatively short growth cycle, high drought tolerance and adaptation to different soil conditions. The leading producing countries of sunflower and sunflower derivatives are the Russian Federation, Ukraine and Argentina, which produce about half of the world’s sunflower crop.
Figure 1 shows the geographical distribution of world sunflower production [
1].
The mass of sunflower husks is 45–60% of the mass of seeds, depending on the sunflower variety [
4]. The sunflower husk is separated from the kernel to improve seed pressing and increase the yield of sunflower oil. Sunflower seed husks are a by-product of sunflower oil production. It is widely used for the production of animal feed, but its use for energy production is limited [
5], despite the fact that sunflower husks have a relatively high calorific value.
The high energy content of this by-product makes it attractive for use as a fuel for heat and power generation. However, the relatively high content of alkaline elements in the husk during its combustion causes the formation of ash and fuel agglomerates, ash deposits and corrosion of the convective heating surfaces of boilers [
6]. These processes are affected by the inorganic composition of the biofuel, as well as the design of the boiler and its operating conditions (changes in the temperature of gases as they move through the boiler) [
6]. First of all, alkali (K, Na) and alkaline earth metals (Ca, Mg), as well as Si, S, Al, P and Cl, which are present in significant amounts in biomass, have a very strong effect on the boiler fouling process [
6].
We encountered the problems that arise when burning sunflower husks when commissioning a fluidized bed boiler, which was developed and built by Head Specialized Design Bureau for a complex of equipment for the microclimate (Brest, Republic of Belarus).
In 2021, a boiler with a fluidized bed furnace was designed and put into operation. According to the calculations, the boiler was supposed to produce 12 tons of steam per hour with a pressure of 1.3 MPa. As fuel in the boiler, pellets from sunflower husks were to be burned, the characteristics of which are presented in
Table 1.
Sunflower husk ash had the following characteristics: initial shrinkage temperature, SST–700 °C, deformation temperature, DT–850 °C, hemisphere temperature, HT–1100 °C, spreading temperature, FT > 1100 °C.
Therefore, when burning pellets from sunflower husks in a fluidized bed, the temperature was maintained at no higher than 750 °C.
When testing the boiler, the design characteristics of its operation were achieved; however, after three days of continuous operation, there were big problems with maintaining the nominal capacity and efficiency of the boiler.
The reasons for the drop in boiler efficiency when burning pellets from sunflower husks are analyzed below.
First, agglomeration in a fluidized bed of an inert material was established, which consisted of particles of quartz sand with a diameter of 0.4–1.0 mm. Sand particles were fused with husk ash, resulting in the formation of large (3 to 5 mm in diameter) and smaller (2 to 3 mm in diameter) balls, a sectional view of which is shown in
Figure 2.
The chemical composition of the ash in the balls and the chemical composition of the ash of the shell of the balls, determined according to [
12,
13], are indicated in
Table 2.
The chemical composition of the ash in the balls extracted from the fluidized bed differs from the ash of the initial pellets only by a significantly higher content of silicon oxide. This is due to the fact that the basis of the balls was particles of quartz sand.
Rapidly growing ash deposits were also found on the fire tubes and the tube sheet of the boiler heat exchanger. The chemical composition of these deposits is shown in
Table 3.
The deposits on the fire tubes were quite loose, while the deposits on the heat exchanger tube sheet were dense.
The rapid growth of ash deposits sharply reduced the efficiency of the boiler, and the agglomeration of ash in the bed and the formation of balls prevented normal fluidization. It was not possible to quickly remove the balls from the bed. In order for the boiler to continue to operate normally, the steam output of the boiler had to be reduced to 8 t/h.
The results obtained during the testing of the boiler require a solution to the problem of the rapid growth of ash deposits on the convective heating surfaces of the boiler and the prevention of agglomeration in the fluidized bed.
There are several methods for solving these problems.
Pre-washing biomass with hot water is considered a promising method for improving the fuel characteristics of biomass, which does not require the use of chemical additives [
14,
15]. The process of washing with water is carried out at temperatures of up to 240 °C; the duration of the process reaches one hour [
15]. With such processing of samples of poplar, miscanthus, corn stalks and millet, it was possible to increase the heat of combustion of biofuel by 1–12% [
15]. It is noted [
16] that as a result of water washing for 1 h at 80 °C, the content of chemical elements in the ash is significantly reduced: potassium–by 93%, sodium–by 96%, phosphorus–by 85% and chlorine–by 97%.
The disadvantage of the washing process is the need to use high-pressure reactors, as well as the need to dry the biomass after treatment. At the same time, the calorific value of biofuel increases only by 2–12%.
Torrefaction of biomass after water washing seems to be a promising technology in terms of removing problematic elements, such as alkali metal chlorides or sulfur compounds. After such a combined treatment, the amount of winter straw slag formed during the gasification of this biomass at a temperature of 950 °C decreased [
17].
However, such a combined pretreatment of biomass complicates and increases the cost of obtaining high-quality fuels comparable in their fuel characteristics to fossil fuels.
However, there are no data in the literature on how the torrefaction process affects the behavior of biomass ash and whether it is possible to limit oneself to only one torrefaction of biomass (without water washing) in order to reduce the rate of formation of ash deposits on the heat exchange surfaces of the boiler or prevent agglomeration when burning biomass in a fluidized bed.
The torrefaction process can be carried out in a nitrogen environment or in an environment of gaseous torrefaction products.
Superheated steam can also be used as a gas medium in which the torrefaction process is carried out [
18,
19,
20]. In such a biomass pre-treatment, it would probably be possible to combine water washing and torrefaction processes, which would dramatically simplify the process. In addition, the torrefaction process proceeds at a steam pressure close to the atmospheric level, which simplifies and reduces the cost of the design of the reactor for the preliminary heat treatment of biomass.
The purpose of this work was to study the properties of sunflower husk ash subjected to torrefaction in a fluidized bed in an environment of superheated steam.
The results of this treatment are compared with the results of studying the properties of sunflower husk ash subjected to torrefaction in an environment of gaseous torrefaction products.
2. Materials and Methods
The crushed sunflower husk was subjected to the study, the characteristics of which are presented in
Table 3. This husk differs from the husk, the combustion of pellets from which is described in the introduction.
The following instruments were used for the analysis: a low-temperature laboratory furnace SNOL 67/350, a laboratory electric furnace SNOL 10/11-V, a TruSpec (ICP-OES) analyzer of carbon, hydrogen, nitrogen and sulfur, and a bomb calorimeter AKB–1.
The analyses were carried out according to standard methods [
14,
15,
16,
17,
18].
The oxygen content was determined by calculation as the difference between 100% and the percentage of ash, moisture, sulfur, carbon, hydrogen and nitrogen in the biomass.
The yield of the annealed husk mass (at a temperature of 1000 °C), determined according to [
15], was 20% (mass), and the yield of carbonates was 8.7% (mass).
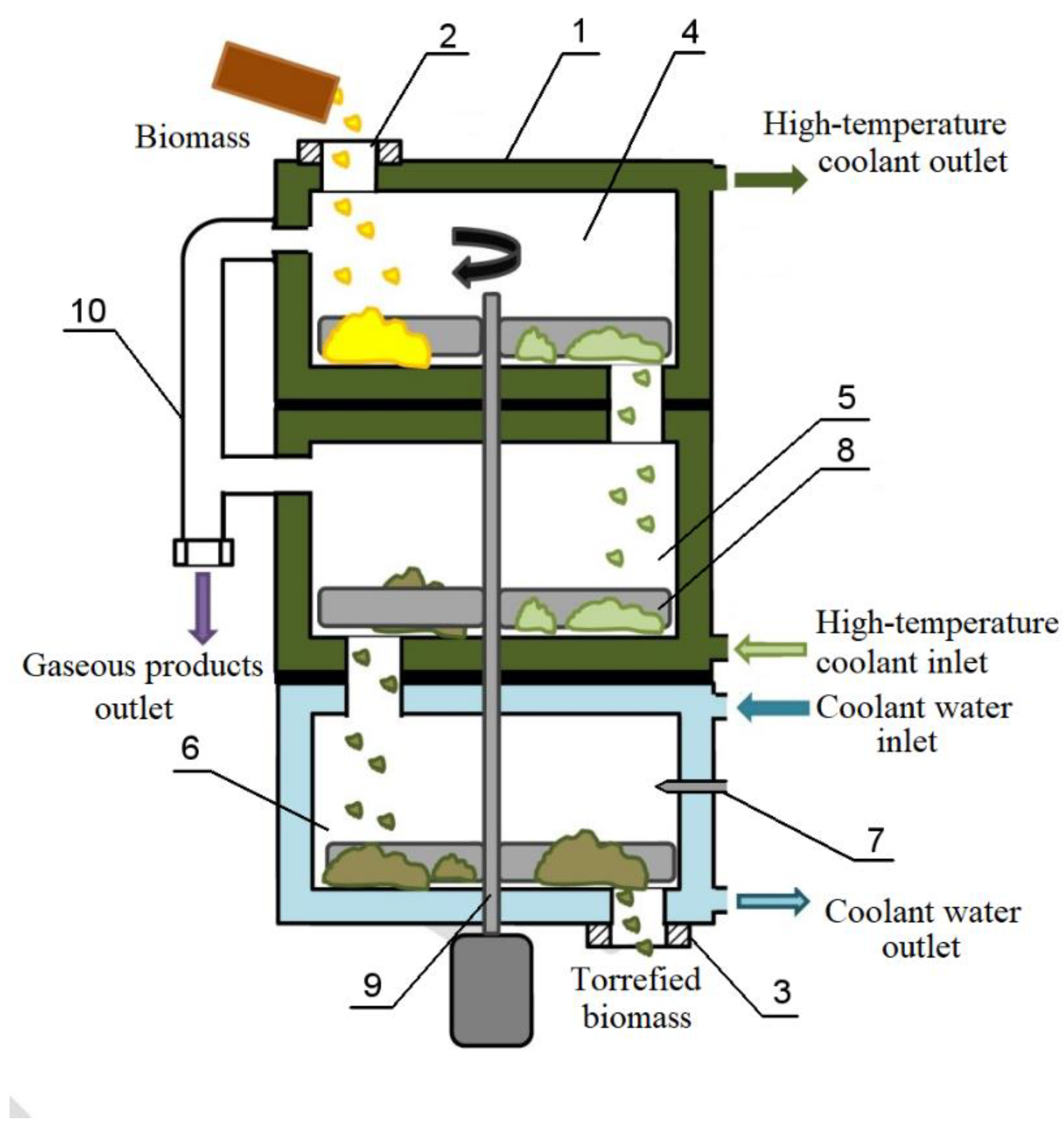
2.1. Reactor for the Torrefaction of Biomass in an Environment of Gaseous Products of Torrefaction
The scheme of the reactor for biomass torrefaction in an environment of gaseous torrefaction products is shown in
Figure 3.
This reactor works as follows: 1—torrefaction reactor vessel, 2—initial biomass loading unit, 3—torrefied biomass unloading unit, 4,5—plates for torrefaction, 6—plate for cooling torrefied biomass, 7—nozzle for cooling water supply, 8—agitator, 9—shaft electrically driven agitators, 10—pipeline for removal of gaseous torrefaction products.
The high-temperature coolant (TLV-330 thermal oil), heated in a special boiler to a temperature of 300 °C, is fed into reactor jacket 1.
After the high-temperature coolant is supplied to the reactor, the internal volume of the reactor is heated. After reaching a temperature inside the reactor above 220 °C, a portion of the initial biomass is loaded into reactor 1. The biomass is loaded into reactor 1 manually through the loading unit of the initial biomass 2. After loading, unit 2 is closed. Due to the operation of mixer 8, mounted on shaft 9, the biomass moves through plates 4 and 5, which are equipped with jackets to receive high-temperature coolant. In this case, biomass torrefaction occurs in an environment of gaseous torrefaction products generated from biomass when it is heated by a high-temperature coolant. These gaseous products are a mixture of carbon dioxide and carbon monoxide. Torrefied biomass is unloaded from reactor 1 through node 3. In an environment of gaseous torrefaction products, the process is carried out at a temperature of up to 250 °C. The duration of the torrefaction process is 45 min.
The choice of temperature and duration of the torrefaction process was due to the need to exclude the ignition of biomass in the reactor since sunflower husk, which can contain up to 6% (wt.) of oil is highly flammable, but it is quite difficult to eliminate the combustion of husks in the reactor.
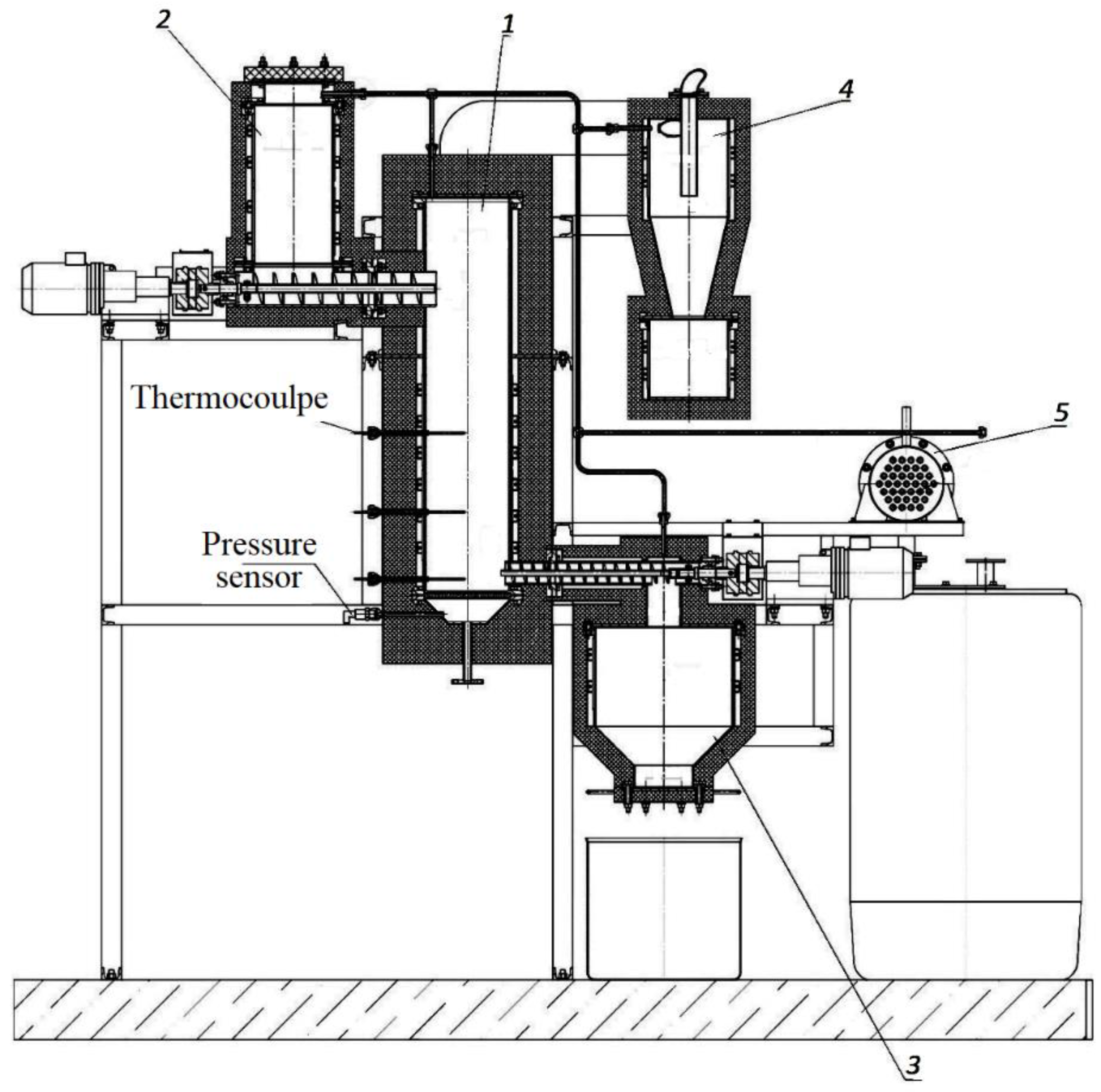
2.2. Installation for Torrefaction of Sunflower Husks in a Fluidized Bed in an Environment of a Superheated Steam
The installation scheme for the torrefaction of sunflower husks in a fluidized bed in an environment of superheated steam is shown in
Figure 4.
The installation consists of a fluidized bed torrefaction reactor 1, a raw biomass hopper 2, a biochar hopper 3, a cyclone 4 for cleaning the steam-gas flow from solid particles, and a steam-gas mixture condenser 5.
Figure 4 does not show a steam boiler or steam heater. Electric heaters are installed on the side wall of the reactor.
During the experiments, the following temperatures were maintained: in hopper 2, hopper 3, and in cyclone 4—110–120 °C; in the reactor, 1—300 °C.
As shown by our previous experiments [
20], at a temperature of 300 °C, the process of biomass torrefaction proceeds most intensively. On the other hand, as the temperature rises, the biomass gasification process begins, the purpose of which, as is known, is to produce combustible gases. The goal of the torrefaction process is to obtain solid fuel with improved characteristics.
The superheated steam temperature did not exceed 310 °C, and the excess steam pressure did not exceed 0.07 MPa. During the experiment, non-condensable gases (carbon dioxide, carbon monoxide, hydrogen and methane) were continuously sampled behind heat exchanger 5 using the Vario Plus Industrial Syngaz gas analyzer. The experiment was terminated after the concentration of these substances decreased to the values that were registered before the start of the biomass supply to reactor 1. After that, reactor 1 was purged with cold nitrogen and the cooled biochar was unloaded from the hopper for analysis.
3. Results and Discussion
The characteristics of the initial sunflower husk (RM) used in the torrefaction experiments are presented in
Table 4. This table also presents the characteristics of biochar obtained by torrefaction of sunflower husks in a fluidized bed in an environment of superheated steam (BC 1) and the characteristics of biochar obtained as a result of torrefaction of sunflower husks in an environment of gaseous torrefaction products in a heart-type reactor (BC 2).
Comparison of the data in
Table 3 shows that as a result of torrefaction in a fluidized bed in an environment of superheated steam, the moisture content of the biomass decreased by 2.03 times, the sulfur content decreased by 2 times, the carbon content increased by 1.24 times, the hydrogen content decreased by 1.3 times, the nitrogen content increased by 1.89 times, the oxygen content decreased by 2.02 times, the volatile matter output decreased by 1.76 times, and the net calorific value increased by 23%.
The ash yield of biochar obtained as a result of torrefaction in an environment of superheated steam, determined according to [
15], was 24.4% (mass), and the yield of carbon was 17.7% (mass).
As a result of torrefaction in an environment of gaseous torrefaction products in a heart-type reactor, the moisture content of treated biomass decreased by 2.52 times, the sulfur content decreased by 2 times, the carbon content increased by 1.13 times, the hydrogen content increased by 1.04 times, the nitrogen content almost did not change, the oxygen content decreased by 1.09 times, the yield of volatile substances decreased by 1.03 times, the net calorific value increased by 14.8%.
Consequently, in terms of the heat of combustion and the yield of volatile substances, torrefaction in an environment of superheated steam makes it possible to obtain higher-quality fuel than the process of torrefaction in an environment of gaseous torrefaction products.
The chemical composition of the initial husk ash (RM) and biochar ash (BC 1, BC 2) is shown in
Table 5.
As shown in
Table 5, as a result of torrefaction of sunflower husks in a fluidized bed in an environment of superheated steam, the following changes in the chemical composition of ash occurred: the content of SiO
2 increased by 6.9 times, the content of TiO
2 increased by 3.5 times, the content of Al
2O
3 increased by 2.33 times, Fe
2O
3 content increased 2.42 times, CaO content increased 3.12 times, MgO content decreased 1.58 times, K
2O content decreased 2.15 times, Na
2O content decreased 1.24 times, P
2O
5 content increased 1.19 times, SO
3 content decreased 6.56 times, Cl content decreased 1.9 times.
As follows from
Table 5, as a result of torrefaction of sunflower husks in an environment of gaseous torrefaction products, the following changes in the chemical composition of ash occurred: the content of SiO
2 increased by 2.35 times, the content of TiO
2 increased by 8.5 times, the content of Al
2O
3 increased by 1.92 times, and the content of Fe
2O
3 increased by 16.65 times, the content of CaO almost did not change, and MgO decreased by 1.12 times, the content of K
2O decreased by 1.15 times, the content of Na
2O decreased by 1.47 times, the content of P
2O
5 decreased by 1.27 times, the content of SO
3 and Cl almost did not change.
Table 6 presents data on the content of trace elements in the ash of the initial husk (RM) and in the ash of the obtained biochars (BC 1 and BC 2). The content of the trace elements was determined according to methods [
12,
13].
As a result of torrefaction in an environment of superheated steam, the content of microelements in biochar ash changed as follows: the content of vanadium remained almost unchanged, the content of manganese increased by 1.9 times, the content of copper decreased by 1.3 times, the content of nickel decreased by 2.43 times, the content of strontium increased by 2.36 times, the content of chromium decreased by 9.61 times, the content of zinc increased by 3.84 times, the content of lead decreased by 2.04 times, the content of arsenic did not change.
When burning biochar obtained from sunflower husks as a result of torrefaction in an environment of superheated steam, one can expect lower emissions of nickel, copper, chromium and lead compounds but larger emissions of manganese, strontium and zinc compounds in comparison with the combustion of the initial husk.
As a result of torrefaction in an environment of gaseous torrefaction products in a heart-type reactor in biochar ash, the content of microelements changed as follows: the content of vanadium did not change, the content of manganese increased by 2.25 times, the content of copper increased by 1.18 times, the content of nickel almost did not change, the content of strontium almost did not change, the content of chromium decreased by 1.5 times, the content of zinc increased by 3.25 times, the content of lead increased by 1.21 times, the content of arsenic did not change.
When burning biochar obtained as a result of torrefaction of sunflower husks in a plate reactor in an environment of gaseous torrefaction products, in comparison with the initial husk, one can expect less chromium emissions; emissions of manganese, copper, zinc and lead compounds would increase.
Let us now discuss possible problems during combustion of biochar obtained as a result of torrefaction of sunflower husks, associated with slagging of the furnace, the formation of deposits on convective heating surfaces or the formation of agglomerates when these biochars are burned in fluidized bed furnaces.
The process of forming slag agglomerates, in addition to the chemical composition of biofuel ash, is influenced by a large number of factors. For example, when burning biofuel in a fluidized bed, the process of formation of agglomerates is affected by the chemical composition of the inert material, the temperature of the bed, the fractional composition of the biofuel, the ratio between the air supplied to the fluidized bed and the total air consumption for combustion, etc. [
22].
A number of indicators have been developed to assess the slagging ability of fuel [
9,
23]. These include the ratio of basic and acidic compounds (B/A) [
23]:
where each oxide is represented by a mass fraction in the ash (wt.%).
It has been shown that acidic compounds increase the overall melting point, while basic compounds have the opposite effect. The ash is considered to have a low slagging tendency at B/A < 0.5, medium at 0.5 < B/A < 1, high at 1 < B/A < 1.75 and strong at B/A above 1.75 [
24].
The agglomeration index for biomass combustion in a fluidized bed was calculated according to [
16]:
According to [
16], agglomeration in a fluidized bed is observed if BAI takes values less than 0.15.
This index was proposed in [
22] to assess the risk of contamination of convective heating surfaces of boilers during biofuel combustion.
A low tendency toward ash growth is expected at Fu < 0.6, high at Fu values up to 40, and very high at Fu values above 40.
When calculating according to Equation (1), it was found: B/A = 36.29 for the initial husk; for biochar obtained by torrefaction in a plate reactor in an environment of gaseous torrefaction products B/A = 15.65; for biochar obtained as a result of torrefaction in an environment of superheated steam in a fluidized bed, B/A = 4.99. That is, the torrefaction of sunflower husk, according to the calculations, does not exclude the possibility of slagging during the combustion of this biofuel but reduces the likelihood of slagging by 2.31–7.27 times.
On the other hand, when calculating according to Equation (2), the following was found:
For the initial husk BAI = 0.01 (agglomeration in the fluidized bed should be observed during the combustion of this type of biofuel). For biochar obtained by torrefaction in a plate reactor in an environment of gaseous torrefaction products, BAI = 0.206 (agglomeration in a fluidized bed should not be observed when this type of biofuel is burned). For biochar obtained as a result of torrefaction in an environment of superheated steam in a fluidized bed, BAI = 0.055 (agglomeration in a fluidized bed should be observed when this type of biofuel is burned).
As a result of the calculations according to Equation (3), the following was obtained:
For the initial husk Fu = 1448.7 (very high probability of formation of ash deposits on the convective heating surfaces of the boiler), for biochar obtained by torrefaction in a plate reactor in an environment of gaseous torrefaction products, Fu = 542.65 (very high probability of ash deposits on the convective heating surfaces of the boiler), for biochar obtained by torrefaction in a plate reactor in an environment of gaseous torrefaction products, Fu = 94.31 (very high probability of ash deposits on the convective heating surfaces of the boiler). On the other hand, it is obvious that torrefaction of sunflower husks reduces the likelihood of ash deposits on the convective heating surfaces of the boiler by 2.1–12.2 times.
It is noted in [
23] that the indices calculated from dependences (1)–(3) sometimes give conflicting predictions. For example, a forecast based on the B/A ratio for softwood chips shows extremely high pollution, while a forecast for the same fuel based on the pollution index shows low pollution, which is more correlated with the practice of using this biofuel. All criteria show a high tendency for slagging and fouling for commonly used fuels, such as wood chips, while on the other hand, they show a low trend for fuels with a low ash melting point (straw pellets).
In [
24], it was proposed a relatively reliable alternative to prognostic criteria–a triple diagram, shown in
Figure 5. Oxides were divided into three groups based on works [
24,
25]. Chlorine and some oxides, such as SO
3 and TiO
2, were excluded and only the most common oxides were selected to reduce the required analysis time. The two compounds, CaO and MgO, were grouped together, as they are often found in high melting point samples and show a positive correlation with the melting point of the ash. SiO
2 has been grouped with two other metal compounds, Al
2O
3 and Fe
2O
3, which are often found in small amounts. Finally, the two alkali compounds K
2O and Na
2O were grouped with P
2O
5 as they were found to be the most common oxides in low melting samples.
The chart was further divided into three separate areas. Area (1): Samples found in this area have a high ash onset temperature and therefore should not cause fouling problems when burned. This area includes woody biomass (spruce, pine, poplar, willow), both in the form of chips, including bark, and in the form of pellets. Area (2): This is the transition area between areas of high and low ash deformation start temperature (contaminated wood, wood waste, paper, sewage sludge, rice husks, etc.). Area (3) includes agricultural waste: straw of wheat, rye, barley, oats, corn), grasses, sugarcane bagasse, sunflower husks and seeds, chicken droppings and cattle manure. Biowastes in area (3) show a high slagging and pollution propensity and therefore cannot be used without additives or co-burning with less problematic fuels.
1—initial sunflower husk, 2—biochar obtained from sunflower husk, as a result of its torrefaction in the medium of superheated steam, 3—biochar obtained from sunflower husk as a result of its torrefaction in a plate reactor in an environment of gaseous torrefaction products, 4—washed citrus ash wood [
16], 5—ash of the initial citrus wood [
16], 6—miscanthus ash [
25], 7—miscanthus ash subjected to hydrothermal carbonization at a temperature of 160 °C [
25], 8—miscanthus ash subjected to hydrothermal carbonization at a temperature of 180 °C [
25], 9—miscanthus ash subjected to hydrothermal carbonization at a temperature of 200 °C [
25]
In
Figure 5, the diagram shows data for the ash of the initial sunflower husk and for the ash of biochar obtained by two torrefaction methods. As can be seen in
Figure 5, the fuel characteristics of biochar obtained as a result of torrefaction in an environment of superheated steam, in terms of boiler slagging and contamination of convective heating surfaces, are close to wood waste.
Figure 5 also shows data on the ash of washed and unwashed citrus wood [
6], as well as on the ash of the initial miscanthus and the ash of miscanthus subjected to hydrothermal carbonization at temperatures of 160 °C, 180 °C and 200 °C [
25].
As can be seen in
Figure 5, water washing, as well as torrefaction in an environment of superheated steam, can significantly improve the fuel characteristics of biomass in terms of boiler slagging and fouling of convective heating surfaces.
Hydrothermal carbonization cannot do this, despite the fact that, as a result of hydrothermal carbonization, the content of potassium compounds in miscanthus ash decreased by 10 times [
25].