Effects of Cofiring Coal and Biomass Fuel on the Pulverized Coal Injection Combustion Zone in Blast Furnaces
Abstract
:1. Introduction
2. Experimental Section
2.1. Coal and Biomass Samples Preparation
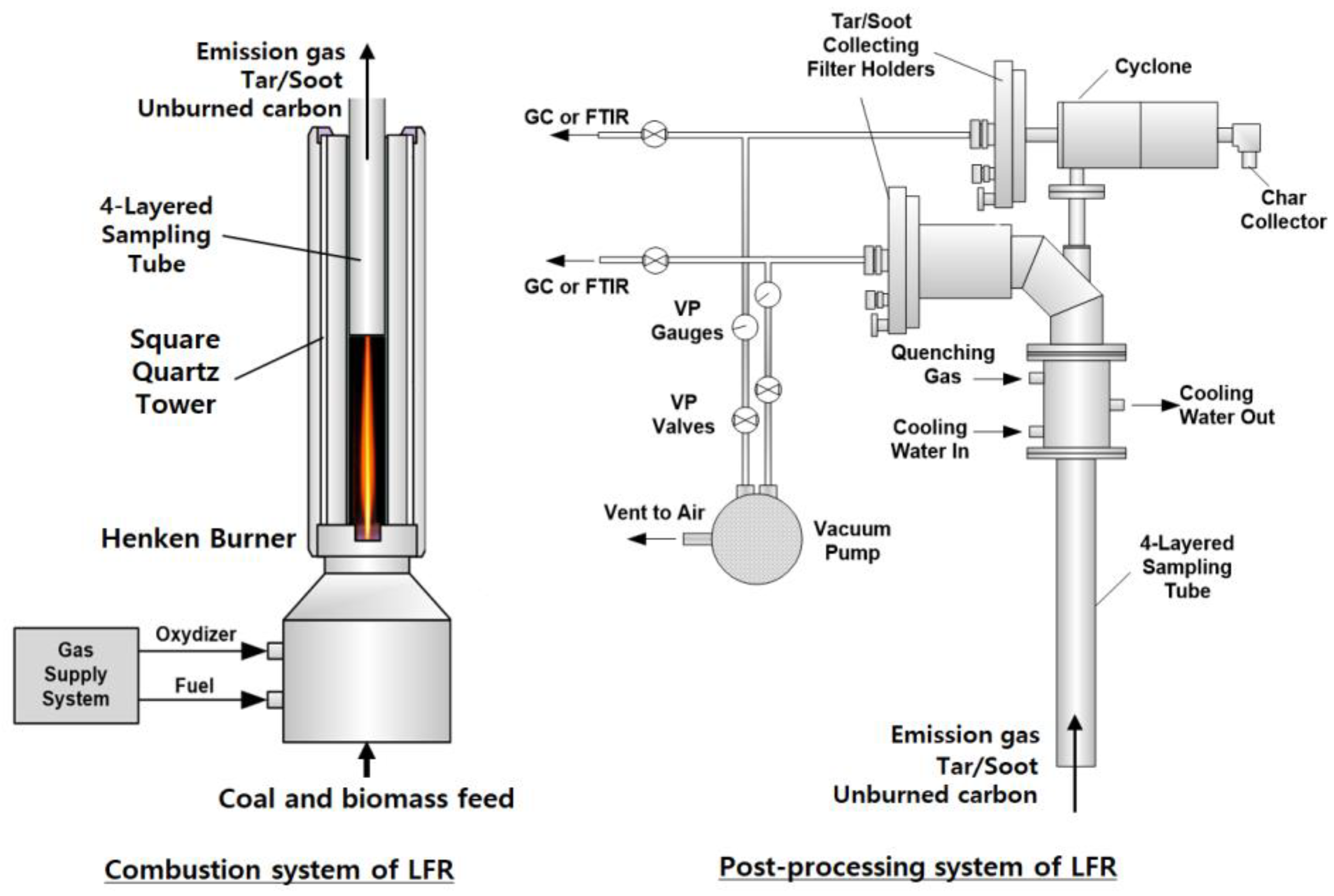
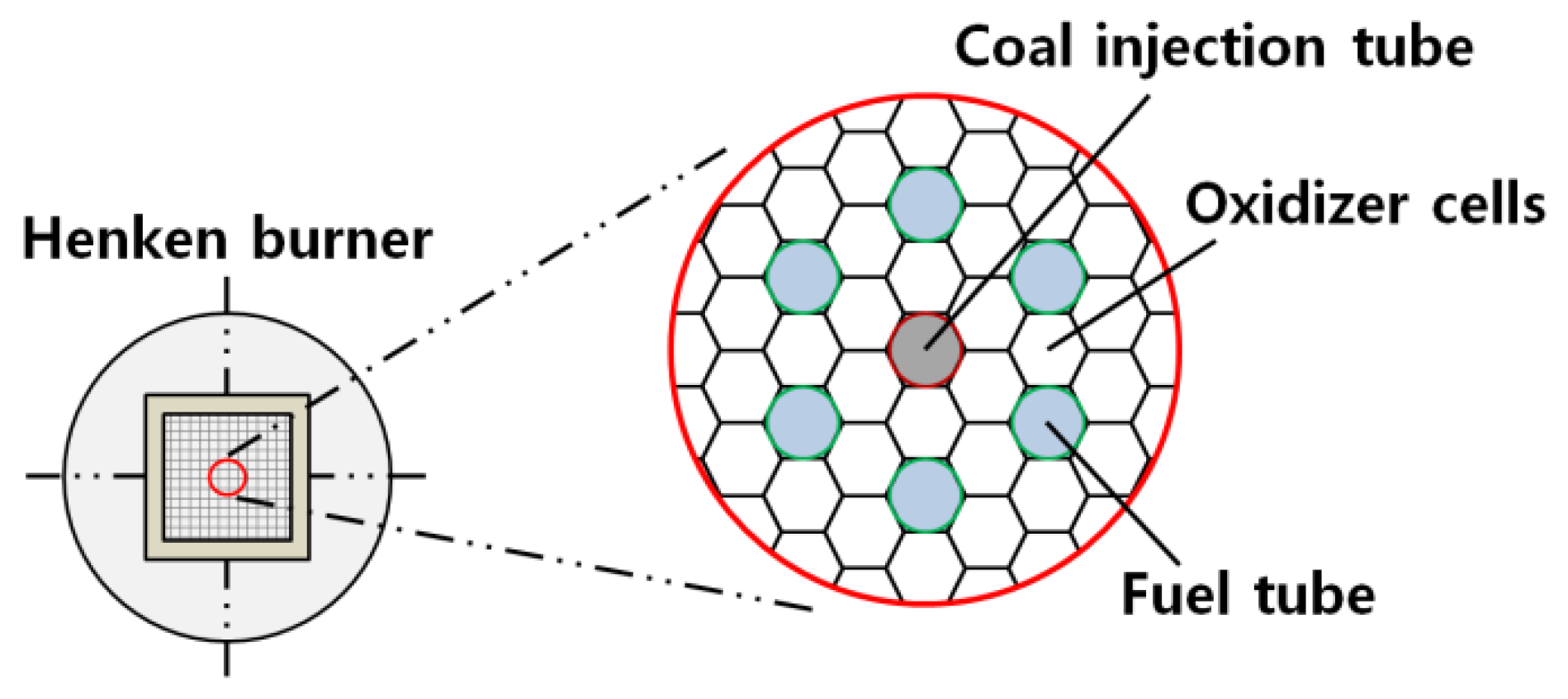
2.2. LFR
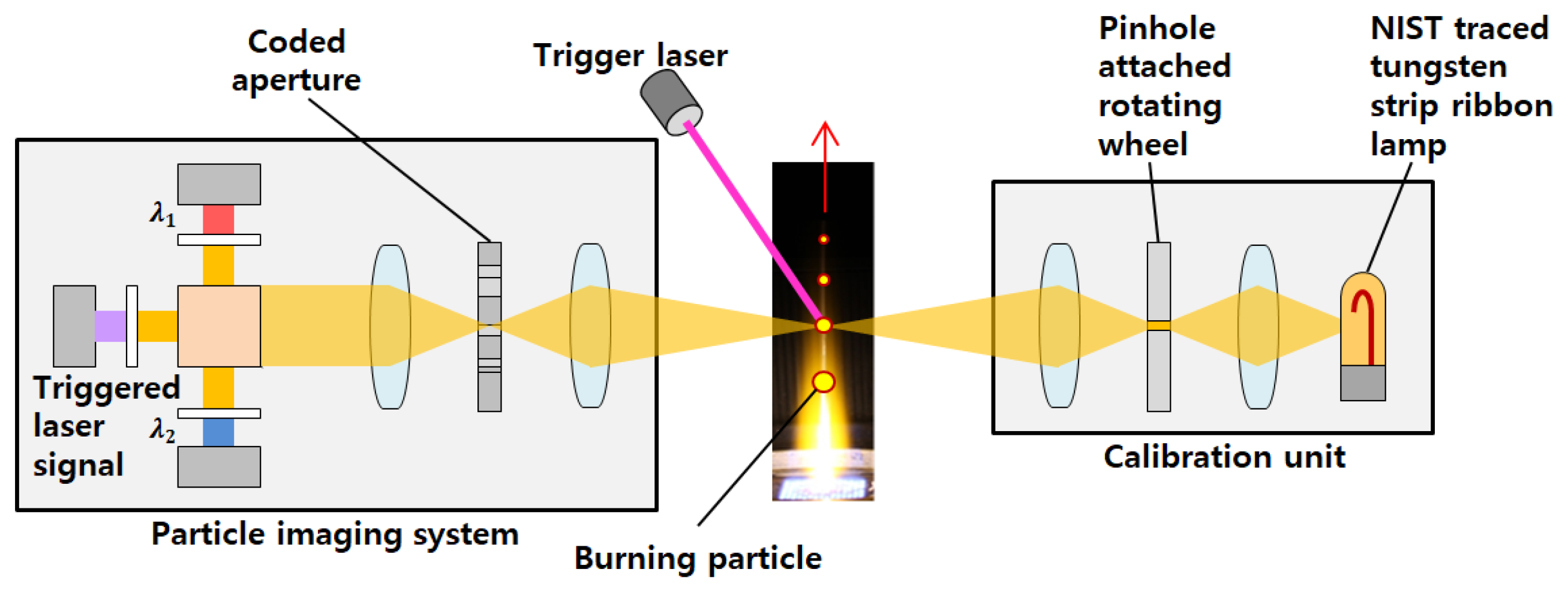
2.3. Measurement of the Burning Coal Particle Temperature and Gas Emission
2.4. Experimental Conditions
3. Results and Discussion
3.1. Coal and Biomass Samples Properties
3.2. Image of the Burning Coal Particle Flame
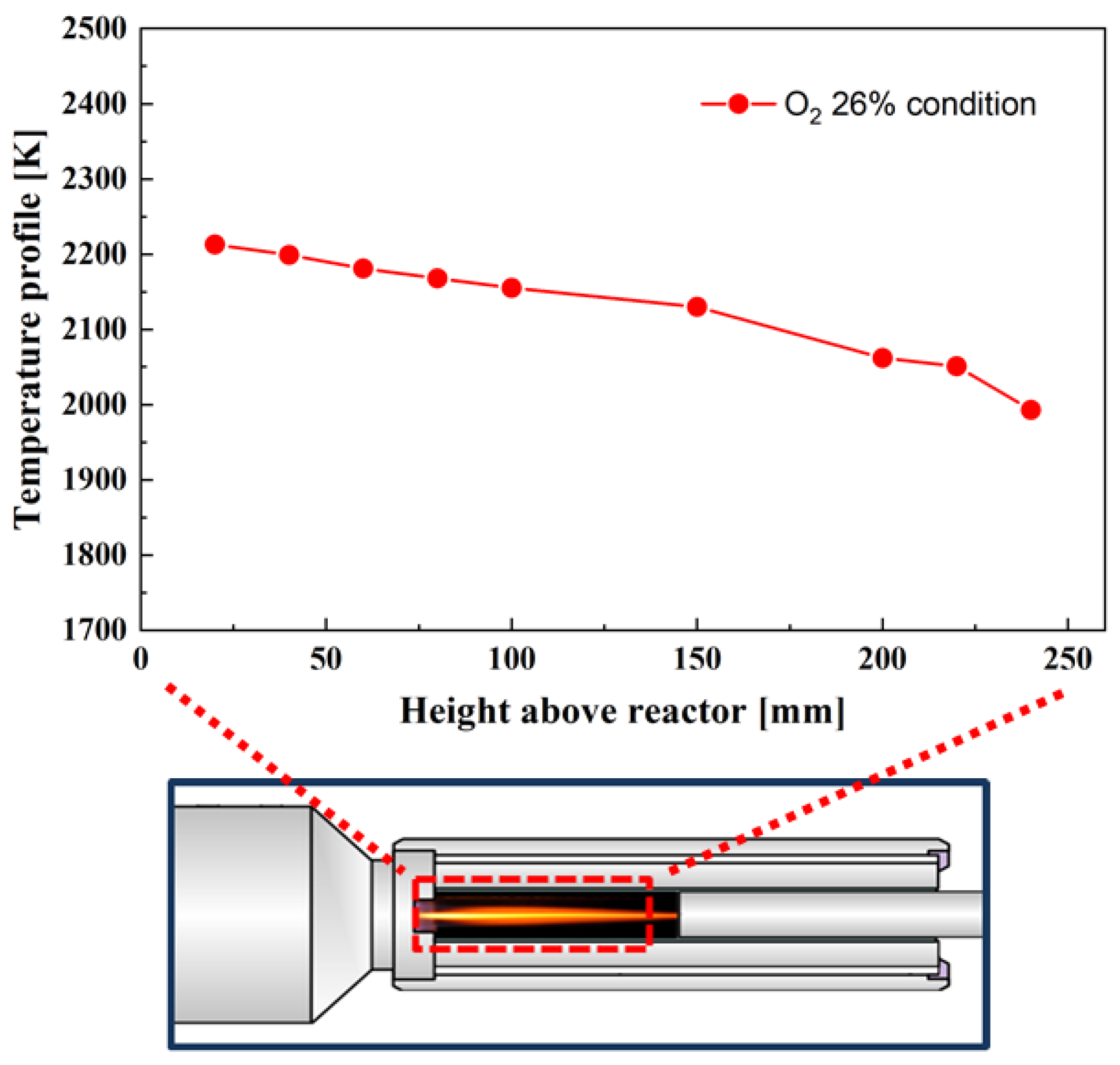
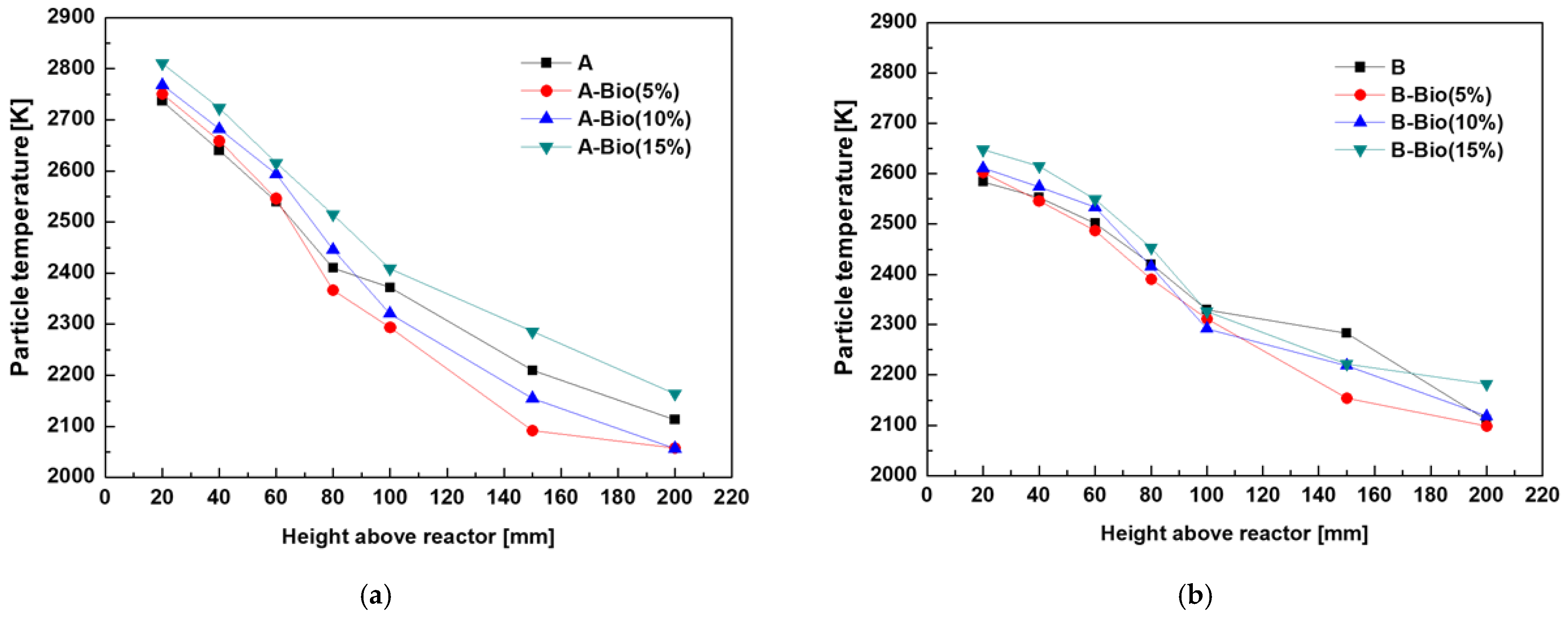
3.3. Distribution of the Burning Coal Particle Temperature
3.4. CO/CO2 Emission Characteristics
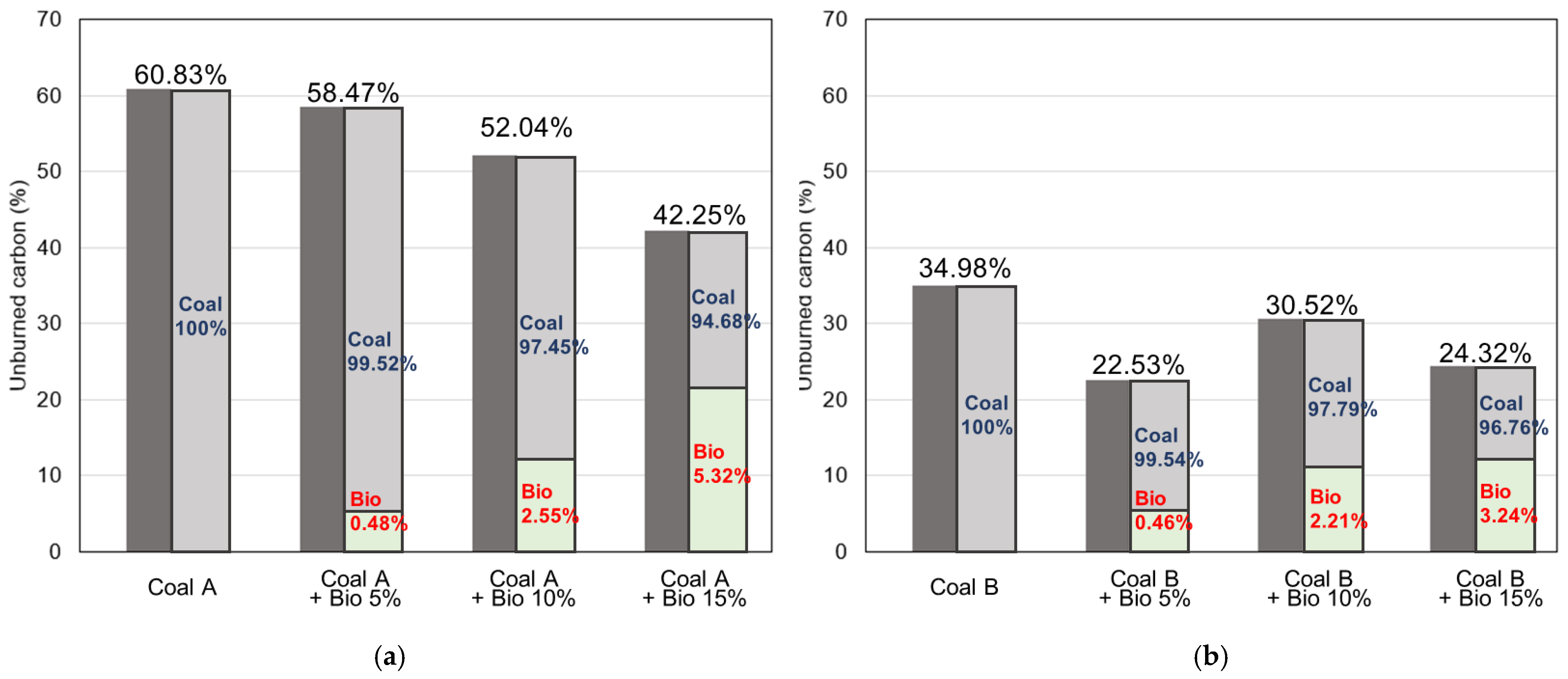
3.5. Char–CO2 Gasification Reaction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, H.; Kim, M.; Kim, H.; Park, S. Decomposition Analysis of CO2 Emission from Electricity Generation: Comparison of OECD Countries before and after the Financial Crisis. Energies 2020, 13, 3522. [Google Scholar] [CrossRef]
- Deb Abhi, T.; Norouzi, O.; Macdermid-Watts, K.; Heidari, M.; Tasnim, S.; Dutta, A. Miscanthus to Biocarbon for Canadian Iron and Steel Industries: An Innovative Approach. Energies 2021, 14, 4493. [Google Scholar] [CrossRef]
- Toktarova, A.; Karlsson, I.; Rootzén, J.; Göransson, L.; Odenberger, M.; Johnsson, F. Pathways for Low-Carbon Transition of the Steel Industry—A Swedish Case Study. Energies 2020, 13, 3840. [Google Scholar] [CrossRef]
- Perpiñán, J.; Bailera, M.; Romeo, L.M.; Peña, B.; Eveloy, V. CO2 recycling in the iron and steel industry via power-to-gas and oxy-fuel combustion. Energies 2021, 14, 7090. [Google Scholar] [CrossRef]
- Lacroix, P.; Dauwels, G.; Dufresne, P.; Godijn, R.; Perini, P.G.; Stricker, K.P.; Virtala, J. High blast furnaces productivity operations with low coke rates in the European Union. Rev. Métallurgie 2001, 98, 259–268. [Google Scholar] [CrossRef]
- Machado, J.G.M.S.; Osório, E.; Vilela, A.C.F.; Babich, A.; Senk, D.; Gudenau, H.W. Reactivity and conversion behaviour of Brazilian and imported coals, charcoal and blends in view of their Injection into Blast Furnaces. Steel Res. Int. 2010, 81, 9–16. [Google Scholar] [CrossRef]
- Babich, A.; Senk, D.; Fernandez, M. Charcoal behaviour by its injection into the modern blast furnace. ISIJ Int. 2010, 50, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Du, S.W.; Chen, W.H.; Lucas, J.A. Pretreatment of biomass by torrefaction and carbonization for coal blend used in pulverized coal injection. Bioresour. Technol. 2014, 161, 333–339. [Google Scholar] [CrossRef]
- Bridgeman, T.G.; Jones, J.M.; Shield, I.; Williams, P.T. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Wijayanta, A.T.; Alam, M.S.; Nakaso, K.; Fukai, J.; Kunitomo, K.; Shimizu, M. Combustibility of biochar injected into the raceway of a blast furnace. Fuel Proc. Technol. 2014, 117, 53–59. [Google Scholar] [CrossRef]
- Borrego, A.G.; Osório, E.; Casal, M.D.; Vilela, A.C.F. Coal char combustion under a CO2-rich atmosphere: Implications for pulverized coal injection in a blast furnace. Fuel Proc. Technol. 2008, 89, 1017–1024. [Google Scholar] [CrossRef]
- Molina, A.; Shaddix, C.R. Ignition and devolatilization of pulverized bituminous coal particles during oxygen/carbon dioxide coal combustion. Proc. Combust. Inst. 2007, 31, 1905–1912. [Google Scholar] [CrossRef]
- Saastamoinen, J.J.; Aho, M.J.; Hämäläinen, J.P.; Hernberg, R.; Joutsenoja, T. Pressurized pulverized fuel combustion in different concentrations of oxygen and carbon dioxide. Energy Fuels 1996, 10, 121–133. [Google Scholar] [CrossRef]
- Rathnam, R.K.; Elliott, L.K.; Wall, T.F.; Liu, Y.; Moghtaderi, B. Differences in reactivity of pulverised coal in air (O2/N2) and oxy-fuel (O2/CO2) conditions. Fuel Proc. Technol. 2009, 90, 797–802. [Google Scholar] [CrossRef]
- Žajdlík, R.; Markoš, J.; Remiarová, Ľ.J.B. Single coal char particle combustion in the carbon dioxide atmosphere. Chem. Pap. 2000, 54, 467–472. [Google Scholar]
- Rodriguez, M.; Raiko, R. Effect of O2 and CO2 Content on Particle Surface Temperature and Size of Coal Char during Combustion. Proc. Finn. Swed. Flame Days 2009, 3B. Available online: http://www.ffrc.fi/FlameDays_2009/3B/RodriguezPaper.pdf (accessed on 5 July 2021).
- Kim, Y.-G.; Kim, J.-D.; Lee, B.-H.; Song, J.-H.; Chang, Y.-J.; Jeon, C.-H. Experimental investigation into combustion characteristics of two sub-bituminous coals in O2/N and O2/CO2 environments. Energy Fuels 2010, 24, 6034–6040. [Google Scholar] [CrossRef]
- Tichenor, D.A.; Mitchell, R.E.; Hencken, K.R.; Niksa, S. Simultaneous in situ measurement of the size, temperature and velocity of particles in a combustion environment. Symp. Int. Combust. 1985, 20, 1213–1221. [Google Scholar] [CrossRef]
- Ma, J.L. Soot Formation during Coal Pyrolysis; Brigham Young University: Provo, UT, USA, 1996. [Google Scholar]
- Molina, A. Evolution of Nitrogen during Char Oxidation; University of Utah: Salt Lake City, UT, USA, 2002. [Google Scholar]
- Du, S.-W.; Yeh, C.-P.; Chen, W.-H.; Tsai, C.-H.; Lucas, J.A. Burning characteristics of pulverized coal within blast furnace raceway at various injection operations and ways of oxygen enrichment. Fuel 2015, 143, 98–106. [Google Scholar] [CrossRef]
- Pohlmann, J.G.; Osorio, E.; Vilela, A.C.F.; Borrego, A.G. Reactivity to CO2 of chars prepared in O2/N2 and O2/CO2 mixtures for pulverized coal injection (PCI) in blast furnace in relation to char petrographic characteristics. Int. J. Coal Geol. 2010, 84, 293–300. [Google Scholar] [CrossRef] [Green Version]
- Khatami, R.; Levendis, Y.A. An overview of coal rank influence on ignition and combustion phenomena at the particle level. Combust. Flame 2016, 164, 22–34. [Google Scholar] [CrossRef] [Green Version]
- ASTM Standard D6866-21; Standard Test Methods for Determining the Biobased Content of Solid, Liquid, and Gaseous Samples Using Radiocarbon Analysis. ASTM International: West Conshohocken, PA, USA, 2021.


| Fuel | Proximate Analysis (* db., wt.%) | Fuel Ratio | Ultimate Analysis (** daf., wt.%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Moi | VM | Ash | FC | FC/VM | C | H | O | N | S | |
| Coal A | 5.02 | 40.27 | 9.88 | 44.83 | 1.11 | 65.68 | 4.67 | 28.43 | 0.51 | 0.71 |
| Coal B | 6.70 | 12.99 | 7.47 | 72.84 | 5.61 | 75.70 | 4.46 | 18.87 | 0.83 | 0.14 |
| Biomass | 2.67 | 79.68 | 4.19 | 13.46 | 0.17 | 47.52 | 5.18 | 45.54 | 1.23 | 0.53 |
| Coal A (balance) + Biomass (blended, wt.%) | ||||||||||
| Bio 5% | 4.90 | 42.24 | 9.60 | 43.26 | 1.02 | 64.77 | 4.70 | 29.29 | 0.55 | 0.70 |
| Bio 10% | 4.79 | 44.21 | 9.31 | 41.69 | 0.94 | 63.86 | 4.72 | 30.14 | 0.58 | 0.69 |
| Bio 15% | 4.67 | 46.18 | 9.03 | 40.12 | 0.87 | 62.96 | 4.75 | 31.00 | 0.62 | 0.68 |
| Coal B (balance) + Biomass (blended, wt.%) | ||||||||||
| Bio 5% | 6.50 | 16.32 | 7.31 | 69.87 | 4.28 | 74.29 | 4.50 | 20.20 | 0.85 | 0.16 |
| Bio 10% | 6.30 | 19.66 | 7.14 | 66.90 | 3.40 | 72.88 | 4.53 | 21.54 | 0.87 | 0.18 |
| Bio 15% | 6.10 | 22.99 | 6.98 | 63.93 | 2.78 | 71.47 | 4.57 | 22.87 | 0.89 | 0.20 |
| Parameter | Coal A (Balance, wt.%) | Coal B (Balance, wt.%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Coal A | Bio 5% | Bio 10% | Bio 15% | Coal B | Bio 5% | Bio 10% | Bio 15% | |
| pMC (%) | * n.a. | 0.51 | 2.68 | 5.60 | n.a. | 0.48 | 2.33 | 3.41 |
| δ13C (‰) | n.a. | −25.63 | −25.03 | −23.98 | n.a. | −25.84 | −25.21 | −24.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.-M.; Choi, J.H.; Jeon, C.-H.; Lim, D.-H. Effects of Cofiring Coal and Biomass Fuel on the Pulverized Coal Injection Combustion Zone in Blast Furnaces. Energies 2022, 15, 655. https://doi.org/10.3390/en15020655
Kim G-M, Choi JH, Jeon C-H, Lim D-H. Effects of Cofiring Coal and Biomass Fuel on the Pulverized Coal Injection Combustion Zone in Blast Furnaces. Energies. 2022; 15(2):655. https://doi.org/10.3390/en15020655
Chicago/Turabian StyleKim, Gyeong-Min, Jae Hyung Choi, Chung-Hwan Jeon, and Dong-Ha Lim. 2022. "Effects of Cofiring Coal and Biomass Fuel on the Pulverized Coal Injection Combustion Zone in Blast Furnaces" Energies 15, no. 2: 655. https://doi.org/10.3390/en15020655
APA StyleKim, G.-M., Choi, J. H., Jeon, C.-H., & Lim, D.-H. (2022). Effects of Cofiring Coal and Biomass Fuel on the Pulverized Coal Injection Combustion Zone in Blast Furnaces. Energies, 15(2), 655. https://doi.org/10.3390/en15020655








