Role and Important Properties of a Membrane with Its Recent Advancement in a Microbial Fuel Cell
Abstract
:1. Introduction
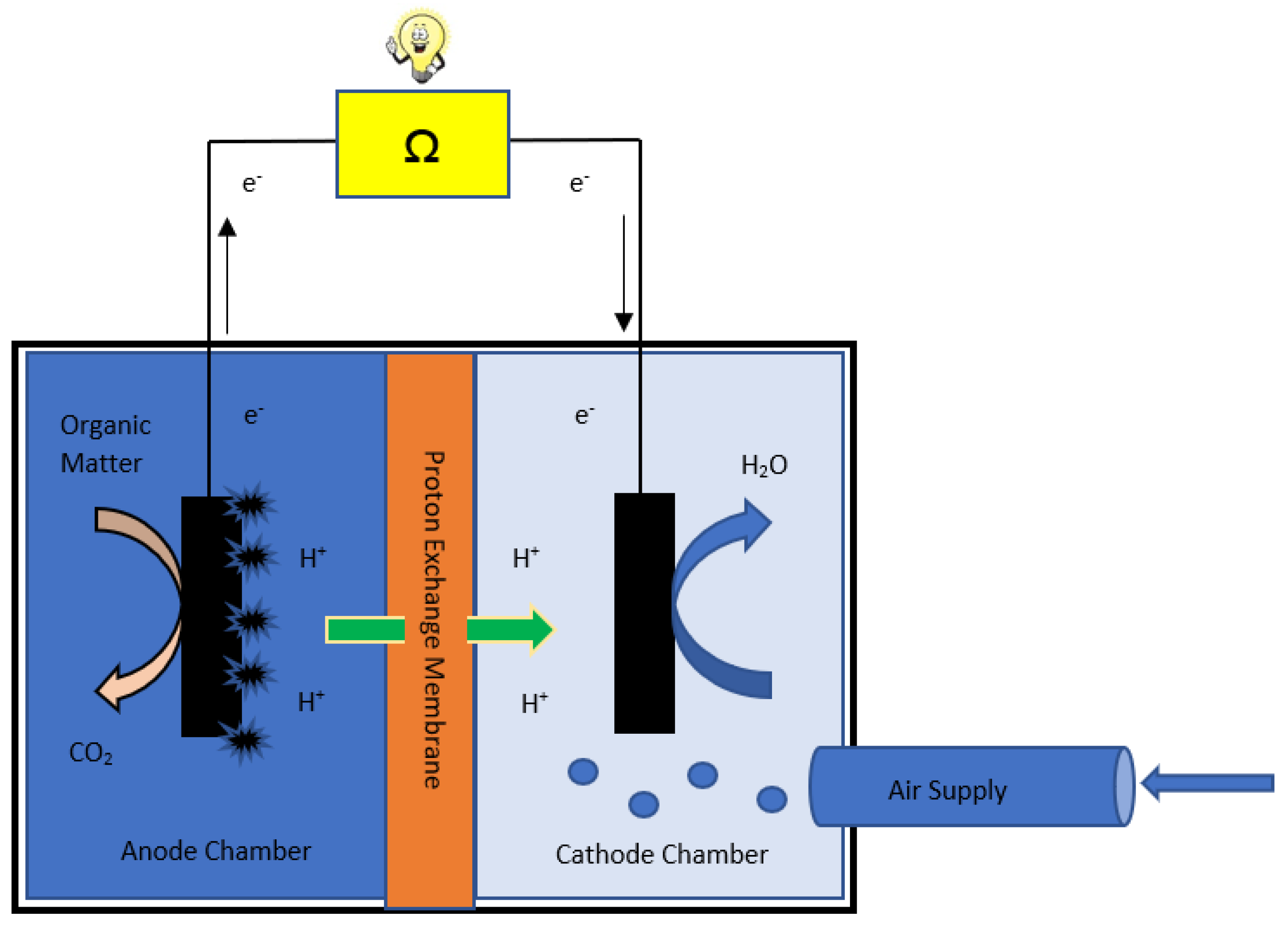
2. Working Principle and Components of MFC
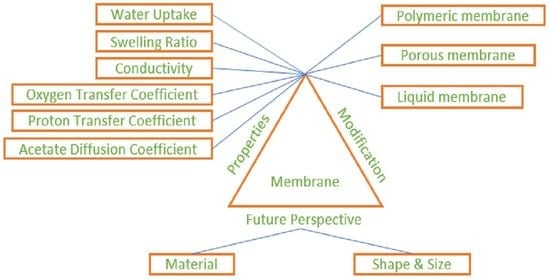
3. Role of Proton Exchange Membrane and Characterization
3.1. Water Uptake
3.2. Swelling Ratio
3.3. Oxygen Transfer Coefficient
3.4. Proton Conductivity
3.5. Substrate (Acetate) Diffusion Coefficient
4. State of the Art and Modification of the Membrane
4.1. Use & Modification of Perfluorinated and Non-Fluorinated Polymeric Membranes
4.2. Porous Membranes
4.3. Liquid Membranes
5. Comparative Analysis of Polymeric Modified Membranes with Nafion
6. Future Perspective
7. Conclusions
- The two most essential membrane characteristics are ion exchange capacity and the mass transfer coefficients for MFC operation. The researchers have made several attempts to find alternative materials compared to expensive Nafion 117. GO/SPEEK and SiO2-S dopped in SSEBS are shown as suitable alternative materials that performed 55% and 107% better than Nafion, respectively.
- Cheap, porous materials such as clay and ceramic are also potential materials for membranes in MFC. The surface of clay membranes can be modified using activated carbon to improve performance. The modified clay membrane performed better than the clay membrane, but clay membranes were not sustainable.
- The ceramic membrane is found to be better than Nafion, but it is not reusable. Due to the large porosity, oxygen diffuses into the anode and adversely affects the anaerobic conditions. The substrate also diffuses into the cathode, inhibiting microbial activity.
- Most published papers show that MFC works fine under controlled laboratory environments using the lab-scale system. But to develop the MFC system for large-scale operations in real applications, several aspects need to be considered. Only then the MFC technology can compete with the existing wastewater treatment and waste to energy technologies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Notation
| V | Volume of the anodic (1) and cathodic (2) chamber (ml) |
| A | Effective surface area of the membrane (cm2) |
| C0 | Saturated oxygen concentration in the cathodic chamber (mg/L) |
| t | Time (h) |
| Ko | Mass Transfer Coefficient of Oxygen (cm/s) |
| Do | Diffusion Coefficient of Oxygen (cm2/s) |
| C | Concentration of oxygen in the anaerobic anode chamber at a time t (mg/L) |
| Lt | Thickness of the membrane (cm) |
| KH | Proton transfer Coefficient (cm/s) |
| DH | Diffusion Coefficient of proton (cm2/s) |
| C1 | concentration of proton (H+) in first chamber (mol/L) |
| C2 | concentration of proton (H+) in second chamber (mol/L) |
| C22 | concentration of proton (H+) in second chamber at a time interval t (mol/L) |
| KA | Acetate transfer Coefficient (cm/s) |
| DA | Diffusion Coefficient of Acetate (cm2/s) |
| CA | Concentration of acetate in anode chamber (mg/L) |
| CB | Concentration of acetate in cathode chamber at a time interval t (mg/L) |
| ΔG | Gibbs free energy (J/mol) |
| F | Faraday’s constant (C/mol) |
References
- Cohen, B. The Bacterial Culture as an Electrical Half-Cell. J. Bacteriol. 1931, 21, 18–19. [Google Scholar]
- Perna, A.; Kim, S. Pem Fuel Cells and Vanadium Redox Flow Batteries: Two. In Proceedings of the European Fuel Cell Technology & Applications Conference—Piero Lunghi Conference 2017, Naples, Italy, 12–15 December 2017; pp. 15–17. [Google Scholar]
- Zhang, H.; Sun, C. Cost-effective iron-based aqueous redox flow batteries for large-scale energy storage application: A review. J. Power Sources 2021, 493, 229445. [Google Scholar] [CrossRef]
- Gangadharan, P.; Nambi, I.M. Hexavalent chromium reduction and energy recovery by using dual-chambered microbial fuel cell. Water Sci. Technol. 2015, 71, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Park, H.S.; Hyun, M.S.; Chang, I.S.; Kim, M.; Kim, B.H. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzym. Microb. Technol. 2002, 30, 145–152. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, P.K. Recent Development of Polymer Electrolyte Membranes for Fuel Cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef]
- Kaewkannetra, P.; Chiwes, W.; Chiu, T.Y. Treatment of cassava mill wastewater and production of electricity through microbial fuel cell technology. Fuel 2011, 90, 2746–2750. [Google Scholar] [CrossRef]
- Song, T.-S.; Jin, Y.; Bao, J.; Kang, D.; Xie, J. Graphene/biofilm composites for enhancement of hexavalent chromium reduction and electricity production in a biocathode microbial fuel cell. J. Hazard. Mater. 2016, 317, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, U.; Jin, W.; Pervez, A.; Bhatti, Z.A.; Tariq, M.; Shaheen, S.; Iqbal, A.; Mahmood, Q. Anaerobic microbial fuel cell treating combined industrial wastewater: Correlation of electricity generation with pollutants. Bioresour. Technol. 2016, 200, 1–7. [Google Scholar] [CrossRef]
- Yu, D.; Bai, L.; Zhai, J.; Wang, Y.; Dong, S. Toxicity detection in water containing heavy metal ions with a self-powered microbial fuel cell-based biosensor. Talanta 2017, 168, 210–216. [Google Scholar] [CrossRef]
- Mukherjee, S.; Su, S.; Panmanee, W.; Irvin, R.T.; Hassett, D.J.; Choi, S. A microliter-scale microbial fuel cell array for bacterial electrogenic screening. Sens. Actuators A: Phys. 2013, 201, 532–537. [Google Scholar] [CrossRef]
- He, Y.-R.; Xiao, X.; Li, W.-W.; Cai, P.-J.; Yuan, S.-J.; Yan, F.-F.; He, M.-X.; Sheng, G.-P.; Tong, Z.-H.; Yu, H.-Q. Electricity generation from dissolved organic matter in polluted lake water using a microbial fuel cell (MFC). Biochem. Eng. J. 2013, 71, 57–61. [Google Scholar] [CrossRef]
- Feng, C.; Huang, L.; Yu, H.; Yi, X.; Wei, C. Simultaneous phenol removal, nitrification and denitrification using microbial fuel cell technology. Water Res. 2015, 76, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Sevda, S.; Abu-Reesh, I.M.; Yuan, H.; He, Z. Bioelectricity generation from treatment of petroleum refinery wastewater with simultaneous seawater desalination in microbial desalination cells. Energy Convers. Manag. 2017, 141, 101–107. [Google Scholar] [CrossRef]
- Mohanakrishna, G.; Venkata Mohan, S.; Sarma, P.N. Bio-electrochemical treatment of distillery wastewater in microbial fuel cell facilitating decolorization and desalination along with power generation. J. Hazard. Mater. 2010, 177, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Venkata Mohan, S.; Mohanakrishna, G.; Srikanth, S.; Sarma, P. Harnessing of bioelectricity in microbial fuel cell (MFC) employing aerated cathode through anaerobic treatment of chemical wastewater using selectively enriched hydrogen producing mixed consortia. Fuel 2008, 87, 2667–2676. [Google Scholar] [CrossRef]
- Chae, K.-J.; Choi, M.-J.; Kim, K.-Y.; Ajayi, F.; Park, W.; Kim, C.-W.; Kim, I.S. Methanogenesis control by employing various environmental stress conditions in two-chambered microbial fuel cells. Bioresour. Technol. 2010, 101, 5350–5357. [Google Scholar] [CrossRef]
- Ezziat, L.; ElAbed, A.; Ibnsouda, S.; El Abed, S.; ElAbed, A. Challenges of Microbial Fuel Cell Architecture on Heavy Metal Recovery and Removal From Wastewater. Front. Energy Res. 2019, 7, 1–13. [Google Scholar] [CrossRef]
- Mathuriya, A.S.; Jadhav, D.A.; Ghangrekar, M.M. Architectural adaptations of microbial fuel cells. Appl. Microbiol. Biotechnol. 2018, 102, 9419–9432. [Google Scholar] [CrossRef]
- Kalia, V.C.; Kumar, P. Microbial Applications vol. 1 Bioremediation and Bioenergy; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Das, D. Microbial Fuel Cell; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar]
- Flimban, S.G.A.; Ismail, I.M.I.; Kim, T.; Oh, S.-E. Overview of Recent Advancements in the Microbial Fuel Cell from Fundamentals to Applications: Design, Major Elements, and Scalability. Energies 2019, 12, 3390. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, V.; Simões, M.; Melo, L.; Pinto, A. Overview on the developments of microbial fuel cells. Biochem. Eng. J. 2013, 73, 53–64. [Google Scholar] [CrossRef]
- Gezginci, M.; Uysal, Y. Electricity generation using different substrates and their different concentrations in microbial fuel cell. J. Environ. Prot. Ecol. 2014, 15, 1744–1750. [Google Scholar]
- Jafary, T.; Ghoreyshi, A.A.; Najafpour, G.D. The Effect of Substrate Concentration on the Electrical Performance of Microbial Fuel Cell (MFC). In Proceedings of the International Conference on Environment 2010, Penag, Malaysia, 13–15 December 2010. [Google Scholar]
- Rahimnejad, M.; Bakeri, G.; Najafpour, G.; Ghasemi, M.; Oh, S.-E. A review on the effect of proton exchange membranes in microbial fuel cells. Biofuel Res. J. 2014, 1, 7–15. [Google Scholar] [CrossRef]
- Gautam, D.; Anjum, S.; Ikram, S. Proton Exchange Membrane (PEM) in Fuel Cells: A Review. IUP J. Chem. 2010, III, 51–81. [Google Scholar]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial Fuel Cells: Methodology and Technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Dharmalingam, S.; Kugarajah, V.; Sugumar, M. Membranes for Microbial Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ramirez-nava, J.; Mart, M.; Hern, G. The Implications of Membranes Used as Separators in Microbial Fuel Cells. Membranes 2021, 11, 738. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Złotorowicz, A.; Nawn, G.; Negro, E.; Bertasi, F.; Pagot, G.; Vezzù, K.; Pace, G.; Guarnieri, M.; Di Noto, V. [Nafion/(WO3)x] hybrid membranes for vanadium redox flow batteries. Solid State Ionics 2018, 319, 110–116. [Google Scholar] [CrossRef]
- Mokhtarian, N.; Ghasemi, M.; Daud, W.R.W.; Ismail, M.; Najafpour, G.; Alam, J. Improvement of Microbial Fuel Cell Performance by Using Nafion Polyaniline Composite Membranes as a Separator. J. Fuel Cell Sci. Technol. 2013, 10, 041008. [Google Scholar] [CrossRef]
- Merino-Jimenez, I.; Obata, O.; Pasternak, G.; Gajda, I.; Greenman, J.; Ieropoulos, I. Effect of microbial fuel cell operation time on the disinfection efficacy of electrochemically synthesised catholyte from urine. Process. Biochem. 2020, 101, 294–303. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Investigation of Nafion series membranes on the performance of iron-chromium redox flow battery. Int. J. Energy Res. 2019. [CrossRef]
- Sugumar, M.; Kugarajah, V.; Dharmalingam, S. Optimization of operational factors using statistical design and analysis of nanofiller incorporated polymer electrolyte membrane towards performance enhancement of microbial fuel cell. Process. Saf. Environ. Prot. 2021. [Google Scholar] [CrossRef]
- Ghosh, P.; Ramachandran, M.; Kumar, V. Review on Proton Exchange Membranes and its Application in Microbial Fuel Cells. Int. J. Mech. Eng. Technol. 2018, 9, 1632–1641. Available online: http://www.iaeme.com/IJMET/index.asp1632; http://www.iaeme.com/ijmet/issues.asp?JType=IJMET&VType=09&IType=13; http://www.iaeme.com/IJMET/issues; http://www.iaeme.com/IJMET/index.asp1633 (accessed on 21 December 2021).
- Breslau, B.R.; Miller, I.F. A hydrodynamic model for electroosmosis. Ind. Eng. Chem. Fundam. 1971, 10, 554–565. [Google Scholar] [CrossRef]
- De Grotthuss, C.J.T. Sur la décomposition de l’eau et des corps qu’elle tient en dissolution à l’aide de l’électricité galvanique. Ann. Chim. 1806, 58, 54–73. [Google Scholar]
- Miskan, M.; Ismail, M.; Ghasemi, M.; Jahim, J.M.; Nordin, D.; Abu Bakar, M.H. Characterization of membrane biofouling and its effect on the performance of microbial fuel cell. Int. J. Hydrogen Energy 2016, 41, 543–552. [Google Scholar] [CrossRef]
- Xu, J.; Sheng, G.-P.; Luo, H.-W.; Li, W.-W.; Wang, L.-F.; Yu, H.-Q. Fouling of proton exchange membrane (PEM) deteriorates the performance of microbial fuel cell. Water Res. 2012, 46, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P.N.; Dharmalingam, S. Effect of cation transport of SPEEK—Rutile TiO2 electrolyte on microbial fuel cell performance. J. Membr. Sci. 2015, 492, 518–527. [Google Scholar] [CrossRef]
- González-Pabón, M.J.; Figueredo, F.; Martínez-Casillas, D.C.; Cortón, E. Characterization of a new composite membrane for point of need paper-based micro-scale microbial fuel cell analytical devices. PLoS ONE 2019, 14, e0222538. [Google Scholar] [CrossRef]
- Tiwari, B.; Noori, T.; Ghangrekar, M. A novel low cost polyvinyl alcohol-Nafion-borosilicate membrane separator for microbial fuel cell. Mater. Chem. Phys. 2016, 182, 86–93. [Google Scholar] [CrossRef]
- Dawkhar, S.A.; Attar, S.J.; Rikame, S.S. Fabrication of Cation Exchange Membrane for Microbial Fuel Cells. Int. J. Recent Technol. Eng. 2019, 8, 769–773. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Nandy, A.; Kundu, P.P. A nanocomposite membrane composed of incorporated nano-alumina within sulfonated PVDF-co-HFP/Nafion blend as separating barrier in a single chambered microbial fuel cell. RSC Adv. 2016, 6, 23571–23580. [Google Scholar] [CrossRef]
- Zhang, Y.; Ting, J.W.Y.; Rohan, R.; Cai, W.; Li, J.; Xu, G.; Chen, Z.; Lin, A.; Cheng, H. Fabrication of a proton exchange membrane via blended sulfonimide functionalized polyamide. J. Mater. Sci. 2014, 49, 3442–3450. [Google Scholar] [CrossRef] [Green Version]
- Tao, H.-C.; Sun, X.-N.; Xiong, Y. A novel hybrid anion exchange membrane for high performance microbial fuel cells. RSC Adv. 2014, 5, 4659–4663. [Google Scholar] [CrossRef]
- Sivasankaran, A.; Sangeetha, D.; Ahn, Y.-H. Nanocomposite membranes based on sulfonated polystyrene ethylene butylene polystyrene (SSEBS) and sulfonated SiO2 for microbial fuel cell application. Chem. Eng. J. 2016, 289, 442–451. [Google Scholar] [CrossRef]
- Ayyaru, S.; Letchoumanane, P.; Dharmalingam, S.; Stanislaus, A.R. Performance of sulfonated polystyrene–ethylene–butylene–polystyrene membrane in microbial fuel cell for bioelectricity production. J. Power Sources 2012, 217, 204–208. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Nandy, A.; Kundu, P.P. Fabrication of laminated and coated Nafion 117 membranes for reduced mass transfer in microbial fuel cells. RSC Adv. 2016, 6, 21526–21534. [Google Scholar] [CrossRef]
- Chae, K.-J.; Choi, M.; Ajayi, F.F.; Park, W.; Chang, I.S.; Kim, I.S. Mass Transport through a Proton Exchange Membrane (Nafion) in Microbial Fuel Cells. Energy Fuels 2007, 22, 169–176. [Google Scholar] [CrossRef]
- Neethu, B.; Bhowmick, G.D.; Ghangrekar, M. A novel proton exchange membrane developed from clay and activated carbon derived from coconut shell for application in microbial fuel cell. Biochem. Eng. J. 2019, 148, 170–177. [Google Scholar] [CrossRef]
- Colomban, P. Proton conductors and their applications: A tentative historical overview of the early researches. Solid State Ionics 2019, 334, 125–144. [Google Scholar] [CrossRef]
- Scott, K. Membranes and separators for microbial fuel cells. In Microbial Electrochemical and Fuel Cells; Elsevier: Amsterdam, The Netherlands, 2016; pp. 153–178. [Google Scholar] [CrossRef]
- Merlo, L.; Ghielmi, A.; Cirillo, L.; Gebert, M.; Arcella, V. Resistance to peroxide degradation of Hyflon® Ion membranes. J. Power Sources 2007, 171, 140–147. [Google Scholar] [CrossRef]
- Leong, J.X.; Daud, W.R.W.; Ghasemi, M.; Ahmad, A.; Ismail, M.; Ben Liew, K. Composite membrane containing graphene oxide in sulfonated polyether ether ketone in microbial fuel cell applications. Int. J. Hydrogen Energy 2015, 40, 11604–11614. [Google Scholar] [CrossRef]
- Kumar, V.; Mondal, S.; Nandy, A.; Kundu, P.P. Analysis of polybenzimidazole and polyvinylpyrrolidone blend membranes as separating barrier in single chambered microbial fuel cells. Biochem. Eng. J. 2016, 111, 34–42. [Google Scholar] [CrossRef]
- Liu, H.; Logan, B. Electricity generation using an air-cathode single chamber microbial fuel cell (MFC) in the absence of a proton exchange membrane. ACS Natl. Meet. B. Abstr. 2004, 228, 4040–4046. [Google Scholar]
- Daud, S.M.; Daud, W.R.W.; Kim, B.H.; Somalu, M.R.; Abu Bakar, M.H.; Muchtar, A.; Jahim, J.M.; Lim, S.S.; Chang, I.S. Comparison of performance and ionic concentration gradient of two-chamber microbial fuel cell using ceramic membrane (CM) and cation exchange membrane (CEM) as separators. Electrochim. Acta 2018, 259, 365–376. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Kujawski, W.; Fatyeyeva, K.; Kujawa, J. A Review on Ionic Liquids-Based Membranes for Middle and High Temperature Polymer Electrolyte Membrane Fuel Cells (PEM FCs). Int. J. Mol. Sci. 2021, 22, 5430. [Google Scholar] [CrossRef] [PubMed]
- Koók, L.; Kaufer, B.; Bakonyi, P.; Rózsenberszki, T.; Rivera, I.; Buitrón, G.; Bélafi-Bakó, K.; Nemestóthy, N. Supported ionic liquid membrane based on [bmim][PF6] can be a promising separator to replace Nafion in microbial fuel cells and improve energy recovery: A comparative process evaluation. J. Membr. Sci. 2019, 570–571, 215–225. [Google Scholar] [CrossRef]
- Ivars-Barceló, F.; Zuliani, A.; Fallah, M.; Mashkour, M.; Rahimnejad, M.; Luque, R. Novel Applications of Microbial Fuel Cells in Sensors and Biosensors. Appl. Sci. 2018, 8, 1184. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, D.A.; Das, I.; Ghangrekar, M.M.; Pant, D. Moving towards practical applications of microbial fuel cells for sanitation and resource recovery. J. Water Process. Eng. 2020, 38, 101566. [Google Scholar] [CrossRef]
- Banu, J.R.; Kavitha, S. Various Sludge Pretreatments: Their Impact on Biogas Generation. In Waste Biomass Management—A Holistic Approach; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Florio, C.; Nastro, R.A.; Flagiello, F.; Minutillo, M.; Pirozzi, D.; Pasquale, V.; Ausiello, A.; Toscano, G.; Jannelli, E.; Dumontet, S. Biohydrogen production from solid phase-microbial fuel cell spent substrate: A preliminary study. J. Clean. Prod. 2019, 227, 506–511. [Google Scholar] [CrossRef]
- Sun, Y. Performance Investigation of Batch Mode Microbial Fuel Cells Fed With High Concentration of Glucose. Biomed. J. Sci. Tech. Res. 2018, 3, 001–006. [Google Scholar] [CrossRef]
- Pannell, T.C.; Goud, R.K.; Schell, D.; Borole, A. Effect of fed-batch vs. continuous mode of operation on microbial fuel cell performance treating biorefinery wastewater. Biochem. Eng. J. 2016, 116, 85–94. [Google Scholar] [CrossRef] [Green Version]
- De Juan, A.; Nixon, B. Technical Evaluation of The Microbial Fuel Cell Technology in Wastewater Applications. J. Sustain. Energy Eng. 2015, 1, 1–18. [Google Scholar] [CrossRef]
- Abdallah, M.; Feroz, S.; Alani, S.; Sayed, E.T.; Shanableh, A. Continuous and scalable applications of microbial fuel cells: A critical review. Rev. Environ. Sci. Bio/Technology 2019, 18, 543–578. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Mungray, A.K.; Arkatkar, A.; Kumar, S.S. Recent advancement in scaling-up applications of microbial fuel cells: From reality to practicability. Sustain. Energy Technol. Assessments 2021, 45, 101226. [Google Scholar] [CrossRef]
- Gajda, I.; Greenman, J.; Ieropoulos, I.A. Recent advancements in real-world microbial fuel cell applications. Curr. Opin. Electrochem. 2018, 11, 78–83. [Google Scholar] [CrossRef]
- Fan, Y.; Sharbrough, E.; Liu, H. Quantification of the Internal Resistance Distribution of Microbial Fuel Cells. Environ. Sci. Technol. 2008, 42, 8101–8107. [Google Scholar] [CrossRef]
- Logan, B.E.; Zikmund, E.; Yang, W.; Rossi, R.; Kim, K.-Y.; Saikaly, P.E.; Zhang, F. Impact of Ohmic Resistance on Measured Electrode Potentials and Maximum Power Production in Microbial Fuel Cells. Environ. Sci. Technol. 2018, 52, 8977–8985. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kang, Y.S.; Choi, J.; Yeon, J.H.; Seol, C.; Nam, L.V.; Choi, M.; Kim, S.M.; Yoo, S.J. Prism patterned TiO2 layers/Nafion® composite membrane for elevated temperature/low relative humidity fuel cell operation. J. Ind. Eng. Chem. 2020, 90, 327–332. [Google Scholar] [CrossRef]

| Membrane Type | Substrate | Membrane Width (cm) | Water Uptake % | Proton Conductivity (S/cm) | O2 Transfer Coefficient (cm/s) | MFC Type | Power/Current Output | Ref |
|---|---|---|---|---|---|---|---|---|
| Nafion and SPEEK in TiO2 | Glucose | 0.018 | 21.83 | 0.187 × 10−2 | 2.2 × 10−6 | SCMFC | 5.7 W/m3 | [41] |
| SSEBS | Acetate | 40 | 0.75 × 10−5 | - | SCMFC | 1209.7 mW/m2 | [49] | |
| SPEEK & GO SPEEK | Acetate | 0.02 & 0.02 | 146.69 & 85.36 | 0.108 × 10−3 & 0.148 × 10−3 | 1.38 × 10−6 & 1.15 × 10−6 | Dual chamber | 812 and 902 mW/m2 | [57] |
| Ceramic membrane & Cation exchange membrane | Acetate | 0.25 & 0.018 | - | - | - | Dual chamber | 1790 and 1225 mW/m2 | [60] |
| Supported Ionic liquid membrane (SILM) | Acetate | - | - | - | - | Dual Chamber | 179 mW/m2 | [62] |
| Clay and activated carbon-based membrane-derived coconut shell | Acetate | - | 19.4 | - | 1.3 × 10−4 | Dual chamber | 246 mW/m2 | [52] |
| Nafion 117 | Domestic sludge | 0.02 | 22 | 2 × 10−2 | 1.6 × 10−4 | SCMFC | 262 mW/m2 | [59] |
| SPSEBS | Glucose | 0.018 | 164 | 0.382 | 0.36 × 10−4 | SCMFC | 600 mW/m2 | [50] |
| Polybenzimidazole and polyvinylpyrrolidone blended membrane | - | - | 35.4 | 1.2 × 10−3 | - | - | 231.8 mW/m2 | [58] |
| Sulfonated PVDF coated Nafion 117 | Phosphate | 0.0213 | 14.2 | 5.91 × 10−3 | 9.4 × 10−5 | SCMFC | 446.45 mW/m2 | [51] |
| Sulfonated PVDF-co-HFP/Nafion blended with nano alumina | Phosphate | 0.02 | 24% | 3.57 × 10−2 | 9.27 × 10−4 | SCMFC | 541.52 mW/m2 | [46] |
| Characteristics | Nafion 117 | 7.5% TiO2 Dopped in SPEEK | 7.5% SiO2-S Dopped in SSEBS | GO SPEEK | Ref |
|---|---|---|---|---|---|
| Proton conductivity (S/cm) | 2 × 10−2 | 0.187 × 10−2 | 3.21 × 10−2 | 3.21 × 10−2 | [41,49,53,57] |
| Water uptake % | 22 | 21.83 | 210 | 85.36 | [41,49,53,57] |
| Oxygen transfer coefficient, Ko (cm/s) | 1.6 × 10−4 | 2.2 × 10−6 | 0.75 × 10−5 | 1.15 × 10−6 | [41,49,53,57] |
| Ion exchange capacity (meq/gm) | 0.982 | 1.98 | 3.01 | - | [41,49,53,57] |
| Power Density (mW/m2) | 290 | 5.7 W/m3 | 1209 | 902 | [41,49,53,57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, A.; Calay, R.K.; Eregno, F.E. Role and Important Properties of a Membrane with Its Recent Advancement in a Microbial Fuel Cell. Energies 2022, 15, 444. https://doi.org/10.3390/en15020444
Banerjee A, Calay RK, Eregno FE. Role and Important Properties of a Membrane with Its Recent Advancement in a Microbial Fuel Cell. Energies. 2022; 15(2):444. https://doi.org/10.3390/en15020444
Chicago/Turabian StyleBanerjee, Aritro, Rajnish Kaur Calay, and Fasil Ejigu Eregno. 2022. "Role and Important Properties of a Membrane with Its Recent Advancement in a Microbial Fuel Cell" Energies 15, no. 2: 444. https://doi.org/10.3390/en15020444
APA StyleBanerjee, A., Calay, R. K., & Eregno, F. E. (2022). Role and Important Properties of a Membrane with Its Recent Advancement in a Microbial Fuel Cell. Energies, 15(2), 444. https://doi.org/10.3390/en15020444