Heat Storage and Release Performance of Cascade Phase Change Units for Solar Heating in a Severe Cold Region of China
Abstract
1. Introduction
2. Modelling
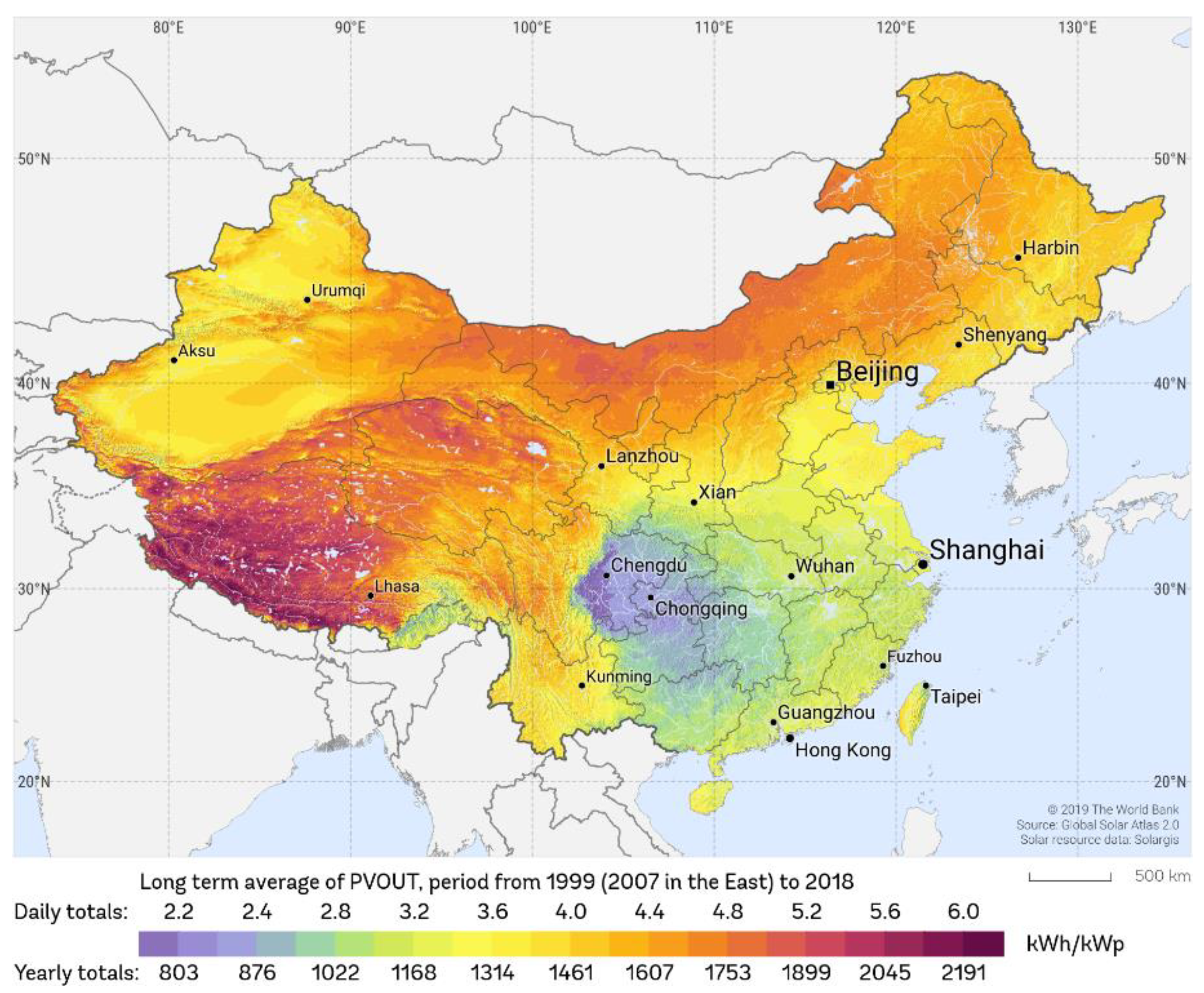
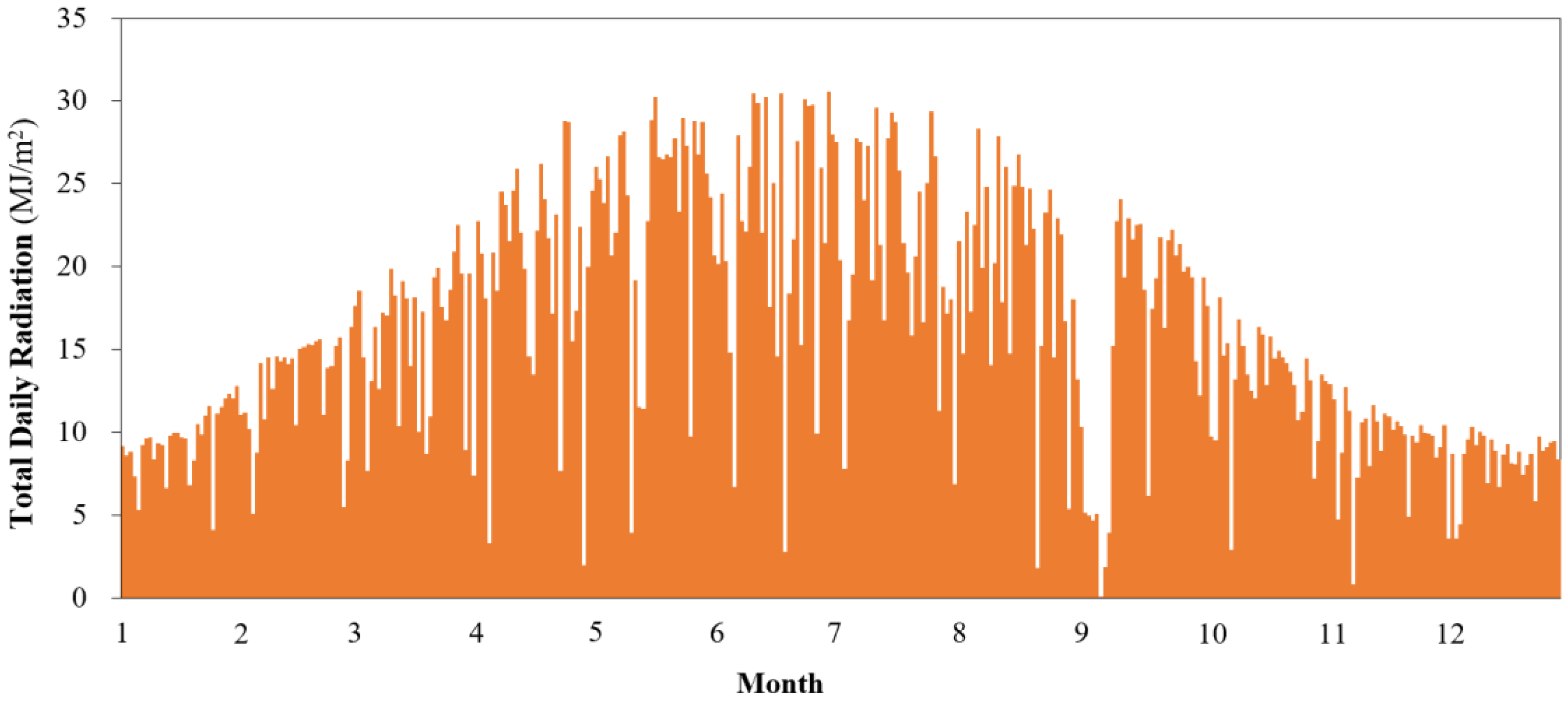
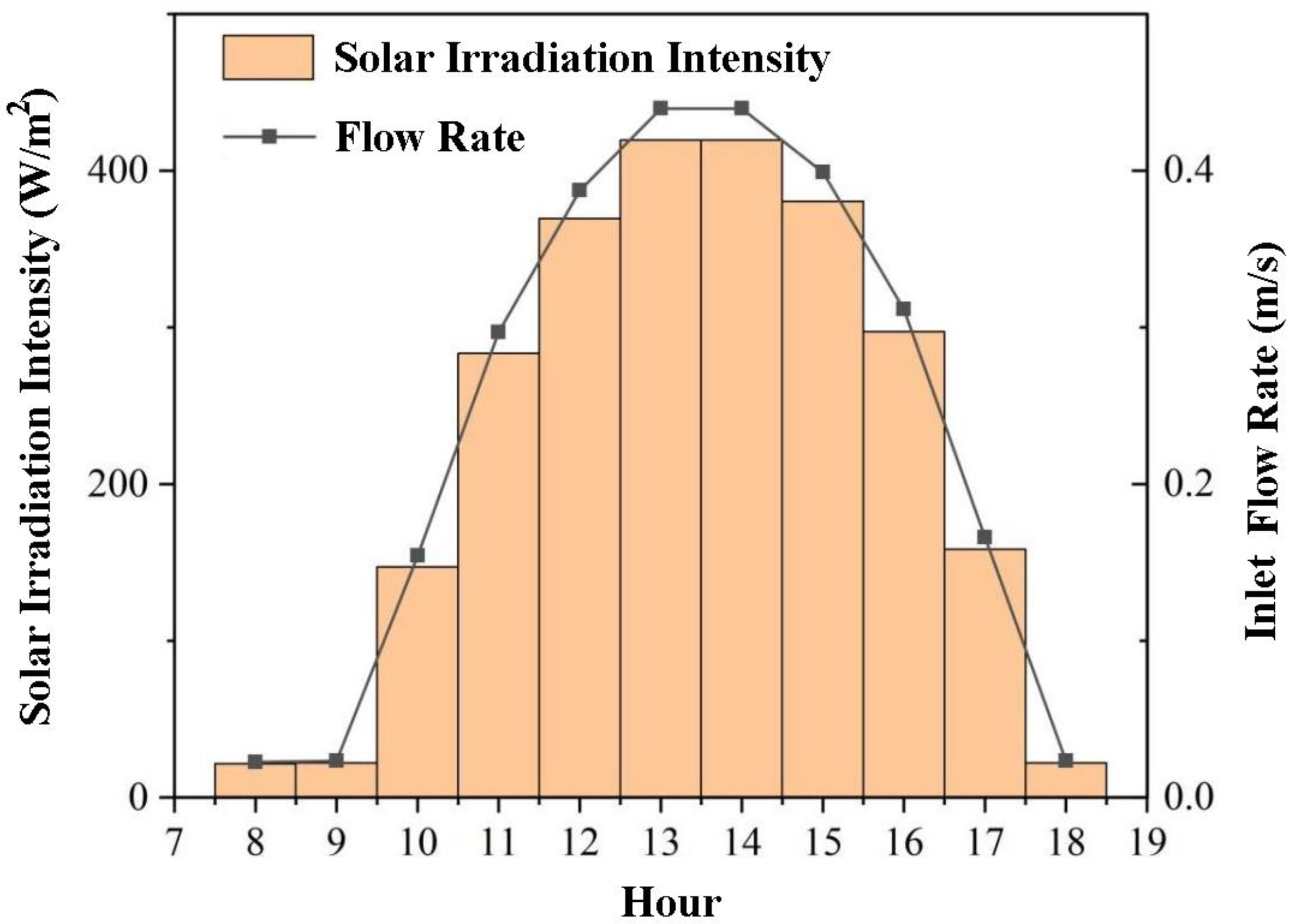
2.1. Solar Irradiation Data
2.2. Materials Combination Schemes
- (1)
- The phase change temperature of the selected materials should be lower than the inlet temperature of the HTF. The phase change temperature of the material in the first stage unit should be higher than that in the second stage unit;
- (2)
- A gradient is formed by the phase change temperature difference of the specified material combinations;
- (3)
- The chosen materials should be common PCM in solar thermal utilisation systems and meet the most basic principles of phase change material screening, such as meeting thermal storage parameter requirements, good economy, environmental protection, and so on.
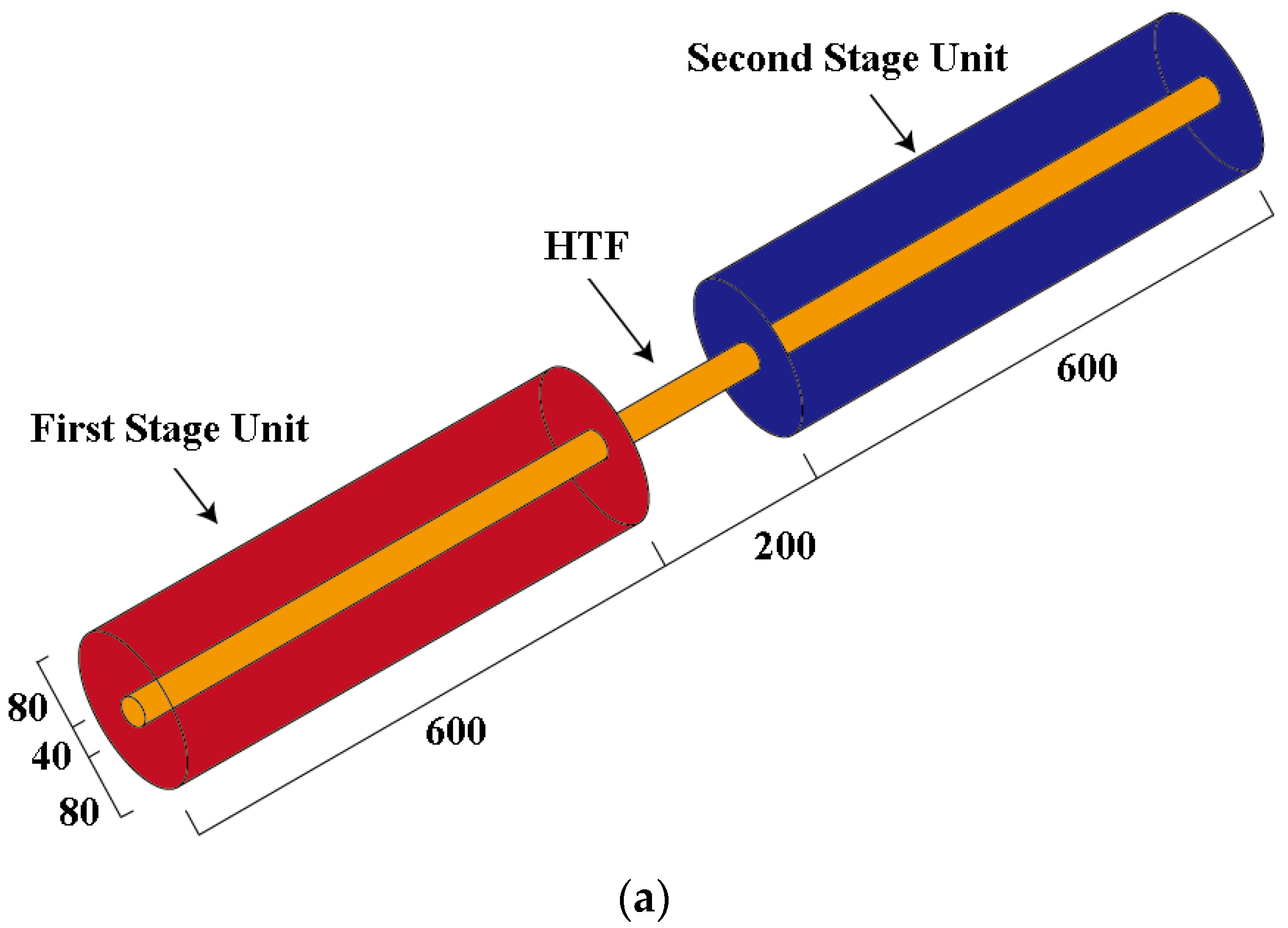
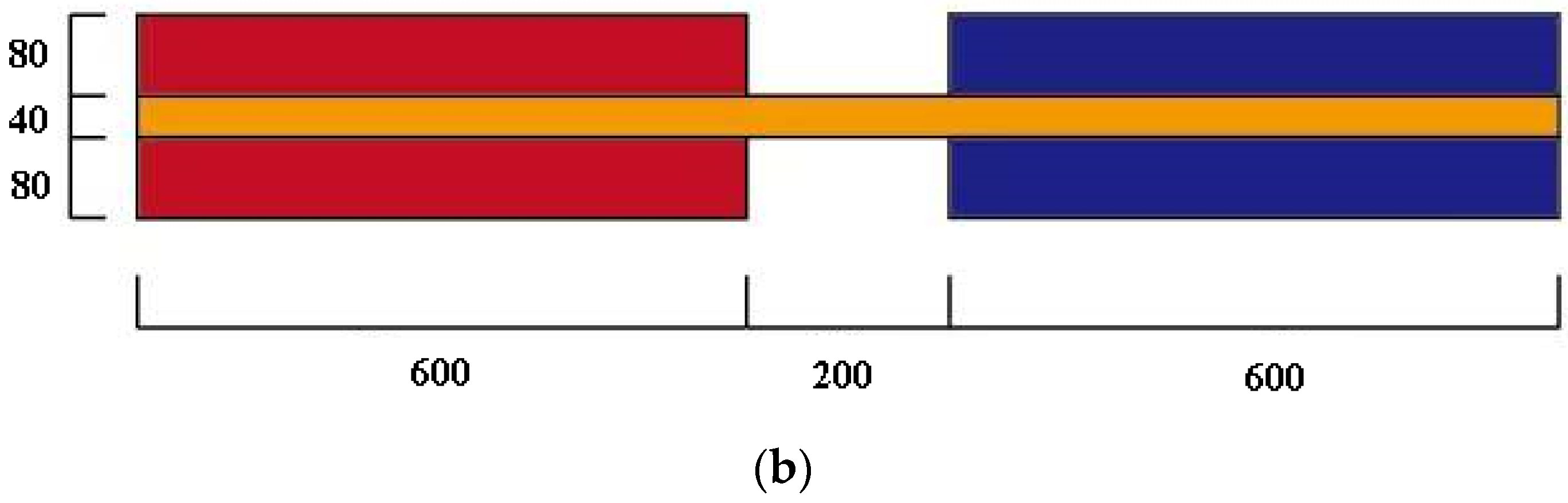
2.3. Physical and Mathematical Model
- (1)
- The shell and tube’s wall thickness is ignored, and the shell wall is adiabatic;
- (2)
- The PCMs are homogeneous and uniformly distributed in the storage unit;
- (3)
- The thermal properties of PCMs are constant.
- (1)
- Mathematical model of HTF and model assumptionsThe following assumptions give unsteady three-dimensional flow models of heat transfer during the melting process of PCM in the cylindrical exchanger enclosure:
- The HTF flow and the liquid PCM are in the laminar pattern;
- The average temperature of inlet corresponds to the HTF inlet value;
- Term of viscous dissipation has been neglected, thus the viscous incompressible flow and the temperature distribution in the annulus are described by Navier-Stokes and thermal energy equations, respectively;
- The density is calculated using the Boussinesq approximation;
- During the transition from the solid to the liquid state, density remains constant;
- Since the term of viscous dissipation has been ignored, Navier–Stokes is used to explain the viscous incompressible flow and thermal energy equations is used to explain temperature distribution in the annulus.
- (2)
- Mathematical model of phase change material
2.4. Boundary and Initial Conditions
- (1)
- Boundary conditions
- (2)
- Initial conditions
2.5. Numerical Simulation Method
3. Results and Analysis
3.1. Model Validation
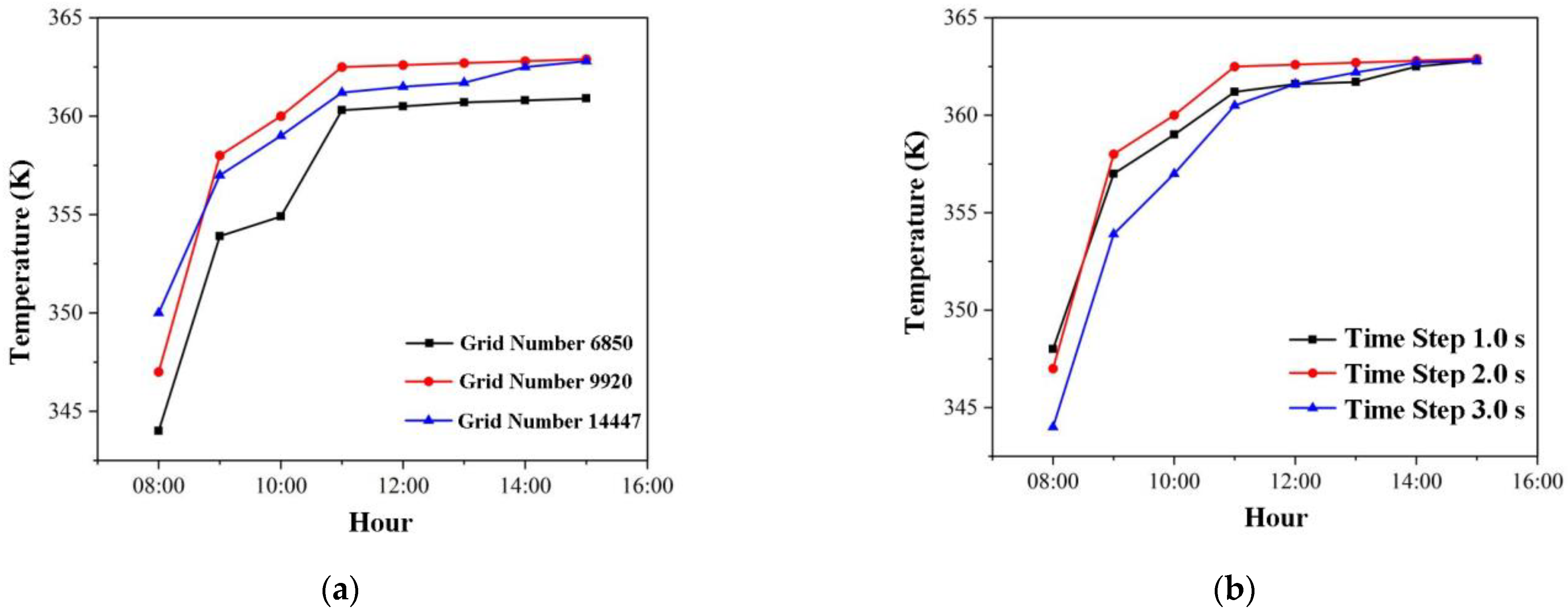
3.1.1. Independence Verification
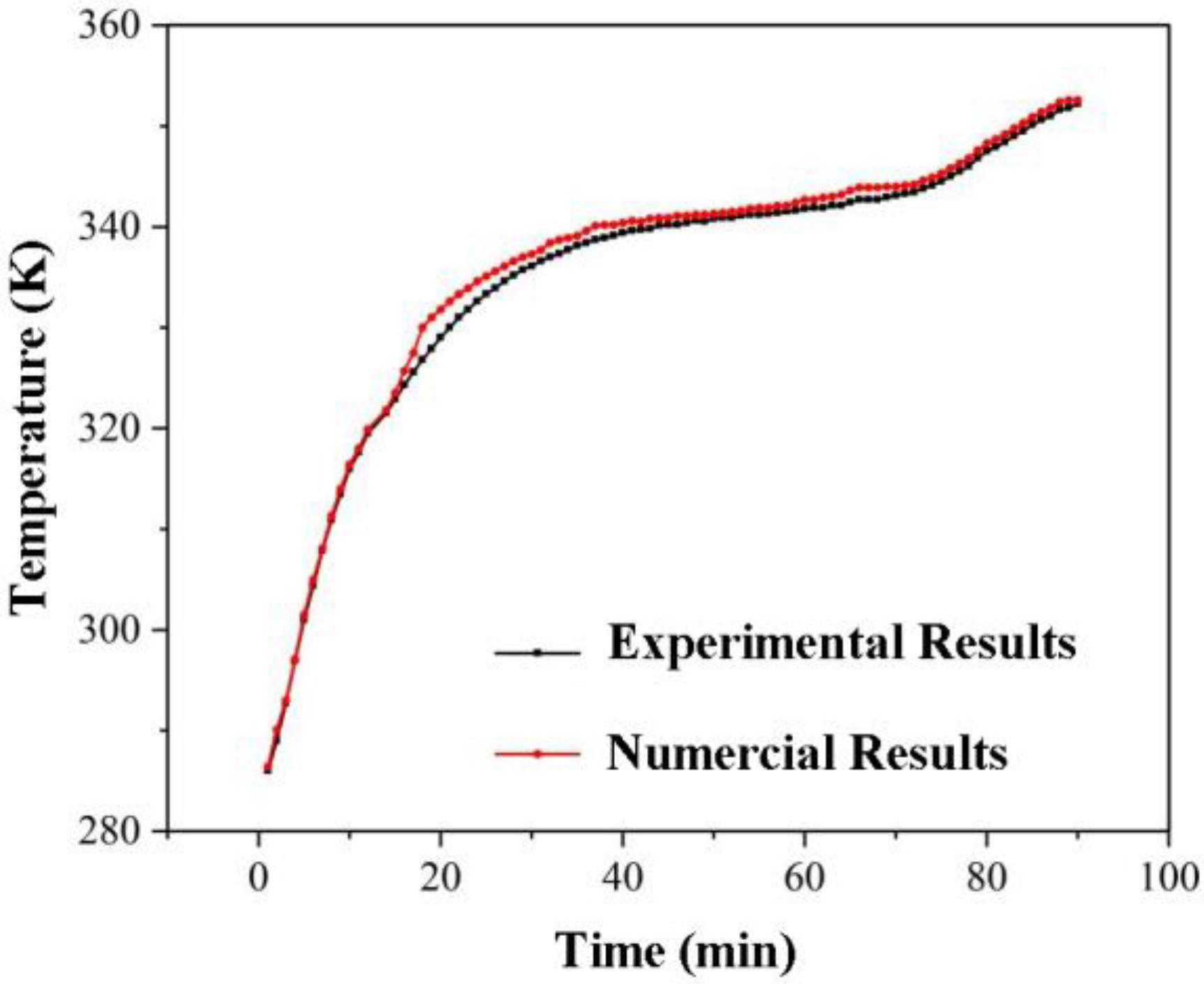
3.1.2. Comparison and Verification with Experimental Results
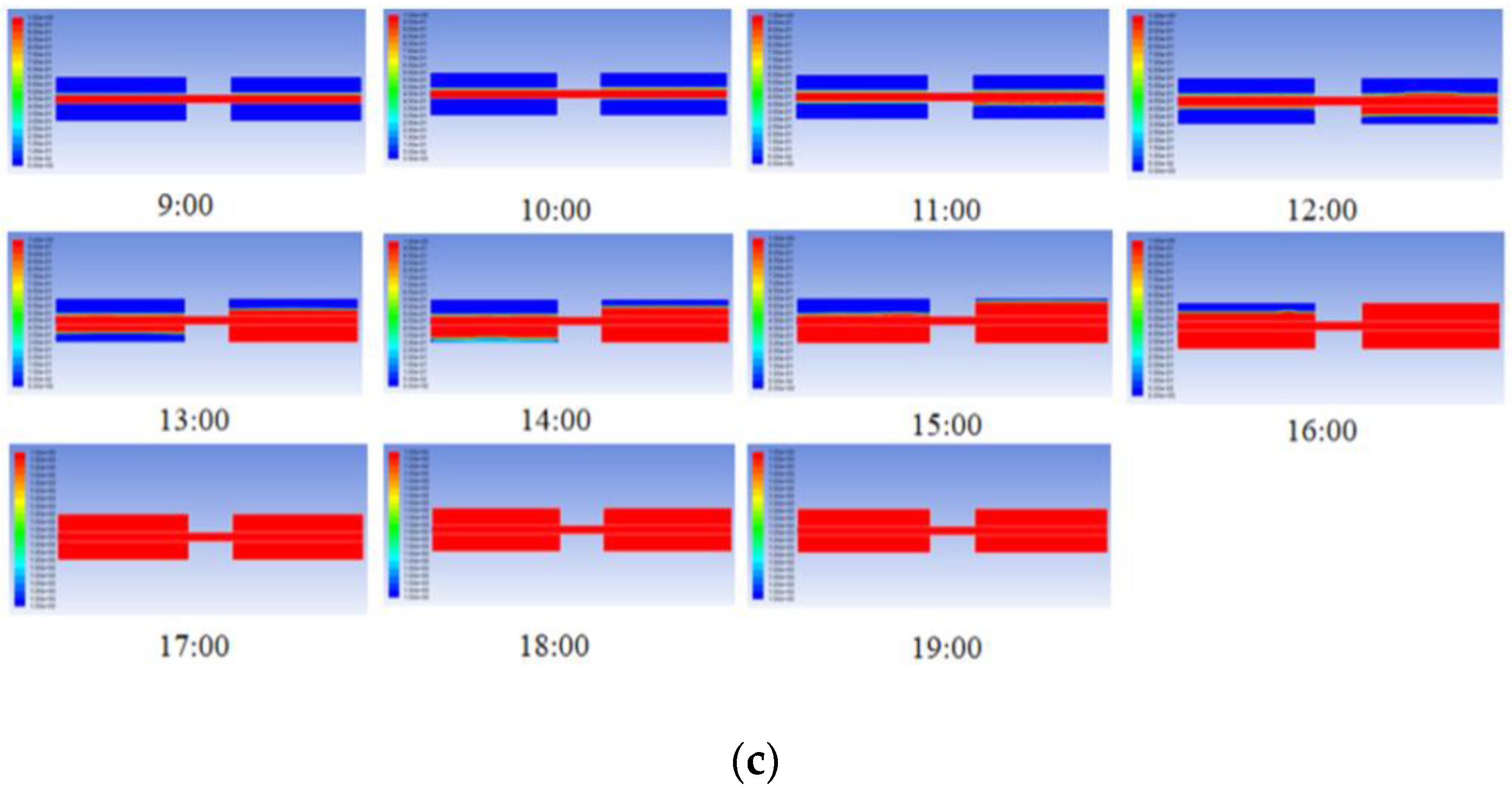
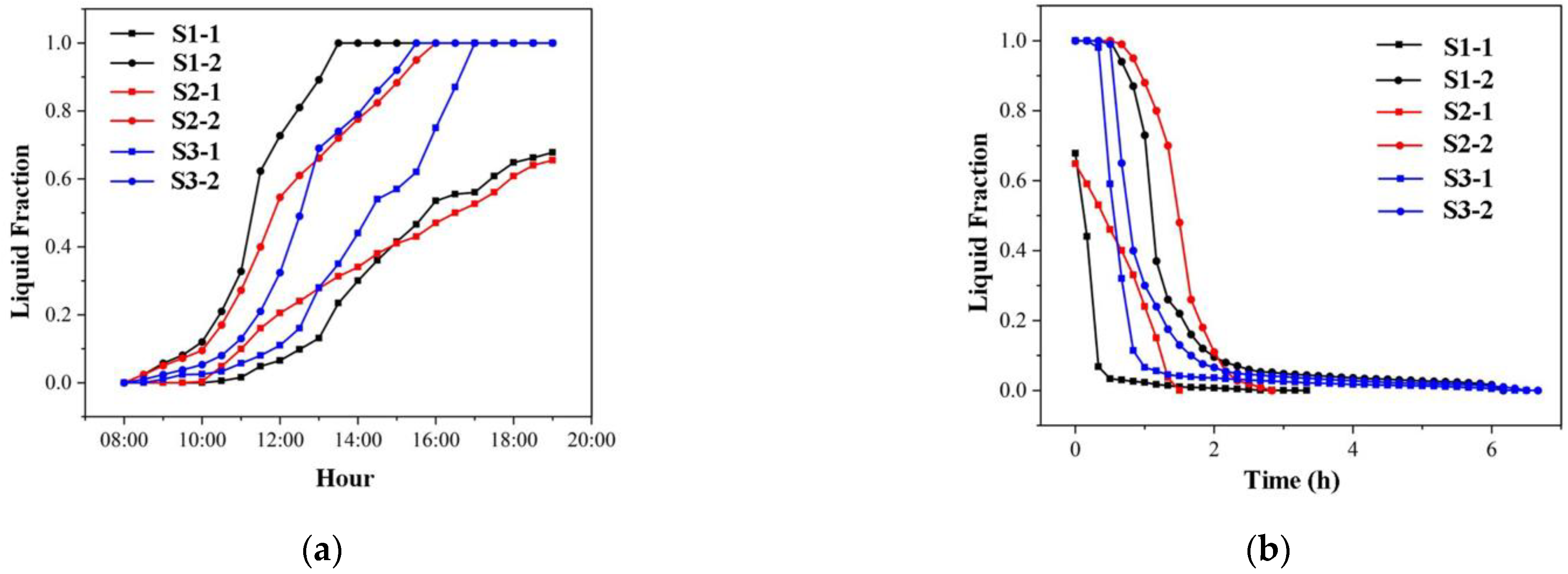
3.2. Heat Storage and Release Performance of Cascade Units
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jradi, M.; Veje, C.; Jorgensen, B.N. Performance analysis of a soil-based thermal energy storage system using solar-driven air-source heat pump for Danish buildings sector. Appl. Therm. Eng. 2017, 114, 360–373. [Google Scholar] [CrossRef]
- Herche, W. Solar energy strategies in the U.S. utility market. Renew. Sustain. Energy Rev. 2017, 77, 590–595. [Google Scholar] [CrossRef]
- Huang, M.; He, W.; Incecik, A.; Gupta, M.K.; Królczyk, G.; Li, Z. Phase change material heat storage performance in the solar thermal storage structure employing experimental evaluation. J. Energy Storage 2022, 46, 103638. [Google Scholar] [CrossRef]
- Zhu, C.; Li, B.; Yan, S.; Luo, Q.; Li, C. Experimental research on solar phase change heat storage evaporative heat pump system. Energy Convers. Manag. 2021, 229, 113683. [Google Scholar] [CrossRef]
- Lin, W.; Ling, Z.; Fang, X.; Zhang, Z. Experimental and numerical research on thermal performance of a novel thermal energy storage unit with phase change material. Appl. Therm. Eng. 2021, 186, 116493. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, R.; Ansu, A.; Goyal, R.; Sarı, A.; Tyagi, V. A comprehensive review on development of eutectic organic phase change materials and their composites for low and medium range thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2021, 223, 110955. [Google Scholar] [CrossRef]
- Wang, J.; Ouyang, Y.; Chen, G. Experimental study on charging processes of a cylindrical heat storage capsule employing multiple-phase-change materials. Int. J. Energy Res. 2001, 25, 439–447. [Google Scholar] [CrossRef]
- Kousksou, T.; Strub, F.; Lasvignottes, J.C.; Jamil, A.; Bédécarrats, J.P. Second law analysis of latent thermal storage for solar system. Sol. Energy Mater. Sol. Cells 2007, 91, 1275–1281. [Google Scholar] [CrossRef]
- Fang, M.; Chen, G.M. Effects of different multiple pcms on the performance of a latent thermal energy storage system. Appl. Therm. Eng. 2006, 27, 994–1000. [Google Scholar] [CrossRef]
- Gong, Z.X.; Mujumdar, A.S. Thermodynamic optimization of the thermal process in energy storage using multiple phase change materials. Appl. Therm. Eng. 1997, 17, 1067–1083. [Google Scholar] [CrossRef]
- Chiu, J.; Martin, V. Multistage latent heat cold thermal energy storage design analysis. Appl. Energy 2013, 112, 1438–1445. [Google Scholar] [CrossRef]
- El Mghari, H.; Idrissi, A.; El Amraoui, R. Cascaded latent heat thermal energy storage device with longitudinal fins: Numerical investigation of melting process and thermal performance analysis. J. Energy Storage 2022, 53, 105199. [Google Scholar] [CrossRef]
- Elfeky, K.E.; Ahmed, N.; Wang, Q. Numerical comparison between single PCM and multi-stage PCM based high temperature thermal energy storage for CSP tower plants. Appl. Therm. Eng. 2018, 139, 609–622. [Google Scholar] [CrossRef]
- Peiró, G.; Gasia, J.; Miró, L.; Cabeza, L.F. Experimental evaluation at pilot plant scale of multiple PCMs (cascaded) vs. single PCM configuration for thermal energy storage. Renew. Energy 2015, 83, 729–736. [Google Scholar] [CrossRef]
- Adine, H.A.; El Qarnia, H. Numerical analysis of the thermal behaviour of a shell and tube heat storage unit using phase change materials. Appl. Math. Model. 2008, 33, 2132–2144. [Google Scholar] [CrossRef]
- Tamme, R.; Taut, U.; Streuber, C.; Kalfa, H. Energy storage development for solar thermal processes. Sol. Energy Mater. 1991, 24, 386–396. [Google Scholar] [CrossRef]
- Steinmann, W.; Tamme, R. Latent heat storage for solar steam systems. J. Sol. Energy Eng. 2008, 130, 1004–1005. [Google Scholar] [CrossRef]
- Michels, H.; Pitz-Paal, R. Cascaded latent heat storage for parabolic trough solar power plants. Sol. Energy 2007, 81, 829–837. [Google Scholar] [CrossRef]
- Domanski, R.; Fellah, G. Exergy analysis for the evaluation of a thermal storage system employing PCMS with different melting temperatures. Appl. Therm. Eng. 1996, 16, 907–919. [Google Scholar] [CrossRef]
- Gokon, N.; Nakano, D.; Inuta, S.; Kodama, T. High-temperature carbonate/MgO composite materials as thermal storage media for double-walled solar reformer tubes. Sol. Energy 2008, 82, 1145–1153. [Google Scholar] [CrossRef]
- SolarGIS. Available online: https://solargis.com/maps-and-gis-data/download/China (accessed on 6 November 2018).
- The Meteorological Information Center of the China Meteorological Administration also Has the Meteorological Data Room. Special Meteorological Data Set for Building Environment Analysis in China; China Construction Industry Press: Beijing, China, 2005. [Google Scholar]
- Liu, Q.; Bai, Z.; Sun, J.; Yan, Y.; Gao, Z.; Jin, H. Thermodynamics investigation of a solar power system integrated oil and molten salt as heat transfer fluids. Appl. Therm. Eng. 2016, 93, 967–977. [Google Scholar] [CrossRef]


| Scheme 1 | S1 | S2 | S3 | |||
|---|---|---|---|---|---|---|
| Material | Stearic Acid | Lauric Acid | Paraffin (C28) | Paraffin (C16) | Palmitic Acid | Polyethyle-ne Glycol |
| Density/kg·m−3 | 913 | 867 | 780 | 776 | 989 | 1200 |
| Specific Heat/J·(kg·K)−1 | 2175 | 2300 | 2120 | 2500 | 2480 | 2300 |
| Thermal Conductivity/W·(m·K)−1 | 0.216 | 0.147 | 0.151 | 0.118 | 0.160 | 0.190 |
| Latent Heat/kJ·kg−1 | 201.8 | 173.8 | 253.0 | 141.9 | 222.0 | 181.4 |
| Phase Change Temperature/K | 341 | 318 | 334 | 320 | 332 | 324 |
| Time | 8:00 | 9:00 | 10:00 | 11:00 | 12:00 | 13:00 |
| Total Radiation Intensity on Horizontal Plane/W·m−2 | 21.6 | 22.2 | 147.2 | 283.3 | 369.4 | 419.4 |
| Inlet velocity/m s−1 | 0.023 | 0.023 | 0.154 | 0.297 | 0.387 | 0.440 |
| Time | 14:00 | 15:00 | 16:00 | 17:00 | 18:00 | -- |
| Total Radiation Intensity on Horizontal Plane/W·m−2 | 419.4 | 380.6 | 297.2 | 158.3 | 22.2 | -- |
| Inlet velocity/m s−1 | 0.440 | 0.399 | 0.316 | 0.166 | 0.023 | -- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, Z.; Jin, G.; Cuce, E.; Jin, J.; Guo, S. Heat Storage and Release Performance of Cascade Phase Change Units for Solar Heating in a Severe Cold Region of China. Energies 2022, 15, 7421. https://doi.org/10.3390/en15197421
Zhang L, Liu Z, Jin G, Cuce E, Jin J, Guo S. Heat Storage and Release Performance of Cascade Phase Change Units for Solar Heating in a Severe Cold Region of China. Energies. 2022; 15(19):7421. https://doi.org/10.3390/en15197421
Chicago/Turabian StyleZhang, Li, Zhihui Liu, Guang Jin, Erdem Cuce, Jing Jin, and Shaopeng Guo. 2022. "Heat Storage and Release Performance of Cascade Phase Change Units for Solar Heating in a Severe Cold Region of China" Energies 15, no. 19: 7421. https://doi.org/10.3390/en15197421
APA StyleZhang, L., Liu, Z., Jin, G., Cuce, E., Jin, J., & Guo, S. (2022). Heat Storage and Release Performance of Cascade Phase Change Units for Solar Heating in a Severe Cold Region of China. Energies, 15(19), 7421. https://doi.org/10.3390/en15197421








