Abstract
Background: Bioenergy attracts much attention due to the global demand for renewable and sustainable energy resources. Waste biomass feedstocks—date pits, coffee waste, and cow dung—require efficient and environmentally friendly waste-management technologies such as pyrolysis. Fast pyrolysis occurs at fast heating rates (10–100 °C/s), generates high bio-oil yields, and is the most widely used process for biofuel generation. The aim of the study is to compare the effect of pyrolysis between single, binary, and ternary feeds on thermal degradation behavior and bio-oil composition. Methods: Thermogravimetric analysis (TGA) was conducted at 30 °C/min from room temperature to 850 °C to understand the thermal degradation behavior of the biomasses. A Pyroprobe® reactor—a micro-scale pyrolyzer—was used to conduct the fast pyrolysis at 500 °C with a heating rate of 10 °C/s, and the volatile contents were quantified using a gas chromatograph–mass spectrometer (GC/MS). Results: The (TGA) showed three main stages of decomposition following dehydration, devolatilization, and char degradation for the different single and multiple feeds. According to the identified compounds, the bio-oil components are broadly identified as aldehydes, amines, aliphatic, aromatics, alcohols, furans, ketones, and acids. The three single-biomass pyrolysis products have four compounds in common, acetic acid and ketone groups (acetic acid, 2-propanone, 1-hydroxy-, benzyl methyl ketone, and 1,2-cyclopentanedione). Conclusion: The bio-oil generated from the feeds comprises great potential for volatiles, diesel, and gasoline production with carbon atoms ranging from C2–C33. Future studies should focus on understanding the effect of procedural parameters, including blending ratio, temperature, and heating rates, on bio-oil composition. Additional molecular techniques should be employed to understand biomass components’ reaction mechanisms to produce useful bio-oil products.
1. Introduction
Bioenergy attracts much attention due to the global demand for renewable and sustainable energy resources. Conventional fossil fuels are increasingly being replaced by biomass-derived energy sources (biofuels); in the United States, the most significant proportion of renewables comprises biofuels [1]. Thermochemically there are four bioenergy production processes: incineration, gasification, hydrothermal liquefaction, and pyrolysis [2]. Pyrolysis is a thermochemical conversion technology that produces bio-oil, which requires further processing and refinement to generate biofuels [2]. In addition, pyrolysis has several advantages when compared to fossil fuels since it produces fewer sulfur and nitrogen emissions while producing fuels [3]. Bio-oil is considered renewable due to its feedstock availability for producing fuels, chemicals, and energy [4]. In addition to bio-oil, biochar and syngas are products from pyrolysis and have a variety of applications. Fast pyrolysis occurs at fast heating rates (10–100 °C/s), generates high bio-oil yields, and is the most widely used process [3]. In comparison to flash pyrolysis (heating rate > 1000 °C/s), the products from fast pyrolysis are deemed to be of better quality, higher thermal stability, and low solid content, among other advantages [5]. Biomass utilization for biofuel generation is integral to future sustainable energy development goals [6]. First-generation biofuel production from energy crops is not preferred due to competition with the food industry [7]. However, biomasses include secondary renewable sources such as waste oils, biomass, and vegetable oils [2].
Recent reviews have summarized biomass-based bio-oil production [6,8,9]. There are many ways to analyze bio-oil components from biomass pyrolysis. Chromatographic methods of gas chromatography–mass spectrometry (GC/MS) have been coupled with pyrolysis reactors such as blade-type reactors, conical-spouted bed reactors, multi-zone fixed-bed reactors, centrifugal reactors, fixed-bed tubular reactors, and fluidized-bed reactors [10]. However, Py–GC/MS is one of the most popular means for analyzing fast pyrolysis products due to its versatility, small feed amount, and quick screening of the bio-oil composition [10]. Py–GC/MS has been utilized to understand the bio-oil composition of different microalgae [11], rice straw [12], pinewood, catalysts [13], and switchgrass–pine residues [14]. This study aims at understanding the bio-oil composition of single, binary, and ternary biomass wastes, which may assist in constructing biomass-based prediction models. For the first time, the effect of mixing date pits (DP), coffee waste (CW), and cow dung (CD) on the bio-oil composition are analyzed using Py–GC/MS. Coffee waste, primarily from coffee beverage preparation (one of the most-consumed), is plentiful, and is expected to increase in plenty, with coffee being considered an essential commodity in the world [15]. Another biomass of interest—DP—comes from the date fruits used to generate many products, including juice concentrates, and fermented products generating date fruit wastes, including pits with high nutritional value [16]. The third and key waste feed—cow manure—is produced in huge amounts, and is one of the biggest threats in the world due to its associated greenhouse gas emissions [17].On a national scale, Qatar produces around 210,000 tons every year [18]; assuming about 60–80% of this is volatiles generated, about 150,000 tons of products could be produced every year. Pyrolysis has recently emerged as an alternative to biogas plants, as the processing time is limited to a few minutes, compared to anaerobic digestion which requires 30–40 days on average [19,20]. At the same time, pyrolysis produces syngas (mainly H2 and CO), which is considered a cleaner product as compared to biogas (CH4, CO2) [21]. In addition, the direct application of dung to fertilize soil may cause pollution to soil, air, and groundwater [22]. Therefore, different waste-valorization techniques are considered to create value-added products (such as bio-oil) while managing waste effectively.
2. Materials and Methods
2.1. Raw Materials Procurement and Characterization
Natural DP was acquired from a local company producing seedless date products in Qatar, washed with distilled water, and dried at 105 °C for 24 h before grinding with an electric grinder. Similar processing was conducted for CW and CD after being procured from the local coffee shop and dairy products companies, respectively. The feeds were sieved using a <125 µm sieve (Haver & Boecker OHG, Oelde, Germany). Further characterization of proximate and ultimate analyses and quantification of the raw materials were conducted for the single, binary (1:1), and ternary (1:1:1) mixtures.
The proximate analysis of the samples was conducted using a thermogravimetric analyzer (Discover SDT650, TA Instruments, New Castle, DE, USA). The standard procedure of ASTM D7582-12 was utilized to understand the samples’ ash, fixed carbon, moisture, and carbon content. Approximately 9 ± 0.1 mg of the samples were heated from room temperature to 105 °C in an inert nitrogen environment for 30 min to evaluate the moisture content. The temperature was increased at a heating rate of 30 °C/min to 950 °C with a residence time of seven minutes under isothermal conditions to quantify the volatile content. The final stage used oxygen to combust the residual samples for 10 min which helps calculate the ash content. The fixed carbon was quantified using the difference between the initial mass and the sum of volatiles, moisture, and ash. The elemental or ultimate analyses of the feeds were conducted by following the standard procedure of ASTM D 3176-8 to quantify the carbon, hydrogen, nitrogen, oxygen, sulfur, and ash in each sample of feedstock using a EuroVector EA3000 CHNS elemental analyzer. A mass of 0.5 to 1.5 mg of each feed biomass was fed into the analyzer, and the oxygen content was calculated by the mass weight and ash content difference from the proximate analyses.
2.2. Thermal Degradation Using Thermogravimetric Analyses (TGA)
The thermal degradation studies of the samples were conducted using a thermogravimetric analyzer (Discovery SDT650, TA Instruments, New Castle, DE, USA) for the single, binary, and ternary feeds. The analysis was conducted at a 30 °C/min heating rate from room temperature to 850 °C. The binary and ternary mixtures employed the feeds at 1:1 and 1:1:1 ratios, respectively, and the runs were performed under an N2 atmosphere purged at 100 mL/min. The runs were triplicated to ensure reproducibility and accuracy of results. The data from the TGA and derivate thermogravimetric (DTG) curves obtained from the analysis help understand how the feeds behave during pyrolysis.
2.3. Py-GC-MS Setup for Bio-Oil Production and Analysis
The pyrolysis products of different feeds were analyzed by Pyroprobe (CDS 6200) equipped for GC–MS (Shimadzu GCMS-QP2020 NX), Kyoto, Japan). A weight quantity of the biomass samples (0.9 mg) was transferred into the cylindrical quartz tube; for binary and ternary feeds, the samples were in the ratios 1:1 and 1:1:1, respectively. The initial temperature was set as 40 °C with a residence time of 100 s. The samples were pyrolyzed at 500 °C for 100 s at a heating rate of 10 °C/second. Helium was used as a carrier gas at a flow rate of 215 mL/min. The GC–MS was equipped with a Restek Rxi-5ms column with a split ratio of 20:1. The GC–MS was run in a scan mode at a detector voltage of 0.7 kV in the mass range of 20–400 m/z. The scan speed was 555 amu/s. The initial column oven temperature was 100 °C for 5 min, and then raised to 250 °C at a heating rate of 10 °C/s. The mass spectra of the products were compared with the NIST library, and the compounds with the highest similarity were considered. This study reported the bio-oil composition as GC–MS peak area percentages, and compounds with areas higher than 2% were reported. The peak area values indicate a given compound’s quantity, and the relative peak areas show the relative content in the product’s composition.
3. Results and Discussion
3.1. Proximate and Elemental Analyses of Biomass Feeds
The starting materials are non-conventional solid materials of biomass resources and required characterization of proximate and elemental analyses as reported in Table 1. The characterization is useful since the thermochemical process of pyrolysis converts the biomass resources. The volatile content was lowest for CD (~58%) and highest for CW (~76%), which reflects the range of potential bio-oil and syngas generation across the biomasses. Furthermore, the ash and moisture contents are highest and lowest, respectively, for CD, and almost the same for DP and CW. The carbon, hydrogen, and oxygen contents are lowest for CD. The three elemental compositions in DP and CD are similar in range. However, the nitrogen content is highest for DP and lowest for CW. When the samples are mixed equally to form binary and ternary blends, the proximate and ultimate analyses show that they are within the range of values for the single biomasses. Therefore, the ternary mixtures are composed of 69% volatiles and 20% fixed carbon.

Table 1.
Proximate and elemental analyses of studied biomasses.
3.2. Thermal Degradation Behavior during Pyrolysis of Single, Binary, and Ternary Samples
The thermal degradation results are shown in Table 2, and the TGA and DTG curves are shown in Figure 1. The thermal degradation behavior of the three biomasses is defined through three stages. The first stage, evaporation, occurs from room temperature to P1Ti (initial major decomposition temperature or stage II). The second stage is the major weight-loss region—also known as devolatilization—the temperatures of which are mentioned in Table 2. Stage II demonstrates some lignin, cellulose, hemicellulose, protein, and fat degradation. The final stage comprises the char and residual lignin degradation that occurs from P1TF to the final experimental temperature (850 °C). CW degradation occurs at a broader temperature range than the other biomasses in the devolatilization stage, which could be explained by it having the highest volatile content, as well as the low nitrogen composition (Table 1), as the nitrogen content requires higher temperature (Ti) to initiate its devolatilization as that of oxygen and hydrogen. The total weight loss follows the order DP > CW > CD%. CD having the highest residual waste percentage is also demonstrated by the high ash yield reflected in the proximate analysis results. Additionally, although the peak temperature is the earliest for DP, its weight-loss rate is also the highest. Similarly, the peak is at a higher temperature for CD, which has the lowest weight-loss rate.

Table 2.
Thermal degradation behavior of single, binary, and ternary resulting in significant weight loss.
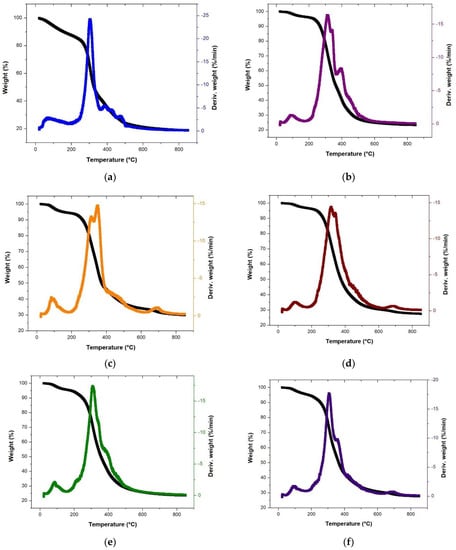
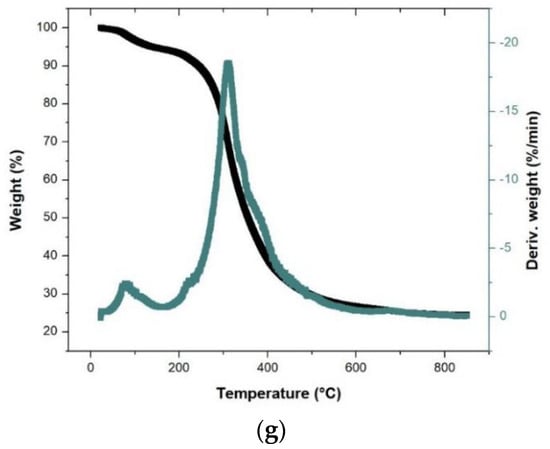
Figure 1.
TGA and DTG curves for the single DP (a), CW (b), and CD (c), binary CW:CD (d), DP:CW (e), and DP:CD (f), and ternary DP:CD:CW (g) biomass waste feeds.
The degradation ranges in Stage II increased in the binary and ternary mixtures, with the onset and end temperatures varying considerably from the single feed’s pyrolysis results. The peak temperatures for the mixtures range between 307–315 °C. The TGA graphs show similar curves for three individual biomasses and mixed feeds. The DTG graphs show that the Stage II peaks are not distinctly separate for any biomass, with embedded peaks representing the degradation of the different biomass pseudo-components. While the embedded peaks are easily visible for the single-feeds (Figure 1a–c), the binary and ternary mixtures show a single peak with minimal embedded peaks; this reflects the thorough mixing of the samples and degradation of pseudo-components in Stage II. The DTG curves also show tiny peaks in Stages I and III, reflecting the moisture content and the char degradation steps, respectively. The next section discusses the bio-oil composition (a significant portion of the volatile yield) during fast pyrolysis.
3.3. Bio-Oil Composition
The volatiles generated during the fast pyrolysis of single, binary, and ternary wastes were analyzed using Py/GC–MS. The products are conventional compounds, unlike biomass feeds. The identified compounds with >80% similarity to those from the NIST library and areas higher than 2% are presented in Table 3. The number of identified compounds for DP, CW, CD, CW:CD, DP:CW, DP:CD, and DP:CD:CW are 14, 13, 11, 9, 12, 15, and 10, respectively. According to the identified compounds, the bio-oil components are broadly identified as aldehydes, amines, aliphatic compounds, aromatics, alcohols, furans, ketones, and acids (Figure 2a). Recent studies report that similar functional groups have been identified by fast biomass pyrolysis in several other studies using oilfield sludge [23], pecan nutshell [24], jatropha waste [25], and wheat stalk [26]. The finding is also consistent with an earlier study which stated that, despite the biomass feed, the bio-oil consists of acids, alcohols, phenols, aldehydes, ketones, aromatics, and furans, regardless of the type of feedstock [27]. The three single-biomass pyrolysis products have four compounds in common, acetic acid and ketone groups (acetic acid, 2-Propanone, 1-hydroxy-, Benzyl methyl ketone, and 1,2-Cyclopentanedione). Acetic acid is known to be a main bio-oil component in spent coffee grounds [15], date palm biomass [28], and cattle manure [29]. Furthermore, the results show that DP and CW have seven of the categorized functional compounds, but CD has six (Figure 2b). The binary feeds share six compounds, and the ternary feeds comprise three compounds similar to single and binary feeds. The area percentage reflects the quantity of the compounds, and Figure 2b shows that the highest amounts are for the acids or ketones, depending on the feed. The ketones are the majority for all the feeds except the ternary mixture (acids comprise 32.91%, very close to the ketone content of 32.89).

Table 3.
Identified bio-oil compounds and family for the different feeds during fast pyrolysis.
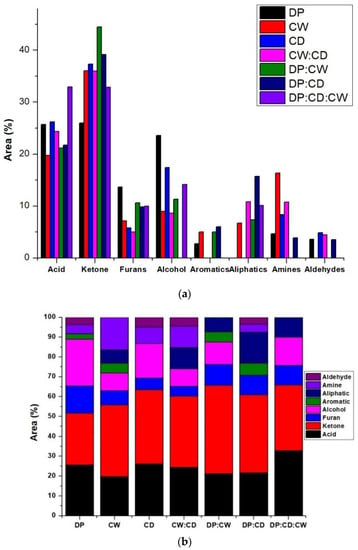
Figure 2.
(a) Identified bio-oil component quantity in different feeds. (b) Ratio of bio-oil components in the feeds.
Several products shown in Table 2 have been identified in other feedstocks. Spent coffee grounds have been known to produce furanmethanol, 1,2-clyclopentanedione, pyridines, and acids [30]. Bio-oil produced from Imperarata cylindrica—a perennial grass—was found to have acetic acid, cyclopentadiene, furan, 2,5-dimethyl-, styrene, furfural, and furanmethanol (among a few others), similar to the feeds in this study [26]. Furans arise from the cleavage of β-1,4-glycosidic bonds in the cellulose content of the biomasses; however, there are limited hydrogen donors to form stable phenolic donors in biomasses as reported for cattle manure [31]. Another study also reported acetic acid, 1, 2-Cyclopentanedione, 2-Propanone, 1-hydroxy-, and Cyclopentanone during rice husk fast pyrolysis [32].
Figure 2b shows that the acid and ketone content increased and reduced, respectively, in the ternary mixtures when compared to single-feeds. Average areas of single-feed furans and alcohol content are seen in the binary and ternary mixtures. While aromatics were missing in the ternary mixture, there was less than 6% in the single (DP and CW) and binary mixtures (DP:CW and DP:CD). Furthermore, the presence of aliphatic compounds was highest in mixtures where CW was present. The CW single pyrolysis also produced 5% aromatics. Amines are present in the single-feed bio-oil composition but not in the DP:CW or DP:CD:CW samples. The absence could mean that it is present in less than the 2% peak area, but mixing reduced the number of amines. A similar trend is found for aldehydes; although only DP and CW have less than 5% of aldehyde, the ternary and binary DP:CW had an absence of aldehydes. Co-pyrolysis improved the ketone and acid content, with the highest ranges in DP:CW (44% area) and DP:CD:CW (32%), respectively. Since furan compounds are lower in CW and CD, the binary mixtures with DP improved the furan content. The alcohol content was reduced in the binary and ternary mixtures in relation to DP and CD. The results from this study reflect the complex nature of bio-oil composition during co-pyrolysis of biomass feeds. However, despite the complicated bio-oil composition during co-pyrolysis, the volatile, diesel, and gasoline potential can be approximately estimated by their atomic carbon numbers between C1–C3, C4–C10, and C11–C22, respectively [23,33]. Other than acetic acid and 2-Propanone, 1-hydroxy- (found in all feeds’ bio-oil composition), all other compounds have carbon atoms higher than 4, which suggests great potential for diesel and gasoline production.
4. Conclusions
The studied biomasses and their mixtures have great potential for bio-oil production due to their total volatile content and bio-oil compositions. The TGA’s thermal degradation data show three primary decomposition stages following evaporation, devolatilization, and char degradation from room temperatures to 850 C. This study reports bio-oil compositions from fast pyrolysis using Py–GC/MS with peak areas higher than 2%. The number of compounds for DP, CW, CD, CW:CD, DP:CW, DP:CD, and DP:CD:CW were 14, 13, 11, 9, 12, 15, and 10, respectively. According to the identified compounds, the bio-oil components were broadly identified as aldehydes, amines, aliphatic compounds, aromatics, alcohols, furans, ketones, and acids. Only three of the bio-oil compounds are shared in all seven feeds; therefore, the complexity of the effect of mixing biomasses requires more investigation, including molecular techniques, such as FTIR analysis, and biomass-component analysis. Further studies on the effects of parameters such as temperature, residence time, and blending ratios are necessary to make appropriate conclusions and prepare for bioenergy applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en15197409/s1.
Author Contributions
Conceptualization, S.M. and G.M.; methodology, S.M. and N.R.; formal analysis, S.M. and M.A.; writing—S.M.; writing—review and editing, S.M., M.A., N.R., T.A.-A. and G.M.; supervision, G.M.; funding acquisition, T.A.-A. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was made possible by NPRP—Standard (NPRP-S) 11 cycle grant—NPRP11S-0117-180328 from the Qatar National Research Fund (a member of Qatar Foundation). The findings herein reflect the work, and are solely the responsibility, of the authors.
Data Availability Statement
The TGA data is contained within the supplementary material.
Acknowledgments
The authors acknowledge Qatar Foundation and Hamad Bin Khalifa University for the support in conducting this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hansen, S.; Mirkouei, A.; Diaz, L.A. A Comprehensive State-of-Technology Review for Upgrading Bio-Oil to Renewable or Blended Hydrocarbon Fuels. Renew. Sustain. Energy Rev. 2020, 118, 109548. [Google Scholar] [CrossRef]
- Haghighat, M.; Majidian, N.; Hallajisani, A.; Samipourgiri, M. Production of Bio-Oil from Sewage Sludge: A Review on the Thermal and Catalytic Conversion by Pyrolysis. Sustain. Energy Technol. Assess. 2020, 42, 100870. [Google Scholar] [CrossRef]
- Gupta, S.; Mondal, P.; Borugadda, V.B.; Dalai, A.K. Advances in Upgradation of Pyrolysis Bio-Oil and Biochar towards Improvement in Bio-Refinery Economics: A Comprehensive Review. Environ. Technol. Innov. 2021, 21, 101276. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Progress of the Applications of Bio-Oil. Renew. Sustain. Energy Rev. 2020, 134, 110124. [Google Scholar] [CrossRef]
- Kumar, V.; Nanda, M. Biomass Pyrolysis-Current Status and Future Directions. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 2914–2921. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the Lignocellulosic Biomass Pyrolysis for Biofuel Production toward Environmental Sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, N.; Li, H.; Deng, W.; Cheng, Y.; Wang, Y.; Liu, Y.; Cobb, K.; Lei, H.; Chen, P.; et al. Recent Advances in Improving Lignocellulosic Biomass-Based Bio-Oil Production. J. Anal. Appl. Pyrolysis 2020, 149, 104845. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, S.; Uzoejinwa, B.B.; Zheng, A.; Wang, Q.; Huang, J.; Abomohra, A.E.-F. A State-of-the-Art Review on Dual Purpose Seaweeds Utilization for Wastewater Treatment and Crude Bio-Oil Production. Energy Convers. Manag. 2020, 222, 113253. [Google Scholar] [CrossRef]
- Grams, J. Chromatographic Analysis of Bio-Oil Formed in Fast Pyrolysis of Lignocellulosic Biomass. Rev. Anal. Chem. 2020, 39, 65–77. [Google Scholar] [CrossRef]
- Li, G.; Ji, F.; Bai, X.; Zhou, Y.; Dong, R.; Huang, Z. Comparative Study on Thermal Cracking Characteristics and Bio-Oil Production from Different Microalgae (Chlorella pyrenoidosa and Schizochytrium limacinum) Biomass by Py-GC/MS. Int. J. Agric. Biol. Eng. 2019, 12, 208–213. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Liu, Y.; Cen, K.; Cao, X.; Ma, Z.; Li, Y. Comparative Study on the Pyrolysis Behaviors of Rice Straw under Different Washing Pretreatments of Water, Acid Solution, and Aqueous Phase Bio-Oil by Using TG-FTIR and Py-GC/MS. Fuel 2019, 252, 1–9. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sarker, M.; Chai, M.; Li, C.; Liu, R.; Cai, J. Potentiality of Combined Catalyst for High Quality Bio-Oil Production from Catalytic Pyrolysis of Pinewood Using an Analytical Py-GC/MS and Fixed Bed Reactor. J. Energy Inst. 2020, 93, 1737–1746. [Google Scholar] [CrossRef]
- Edmunds, C.W.; Molina, E.A.R.; André, N.; Hamilton, C.; Park, S.; Fasina, O.; Adhikari, S.; Kelley, S.S.; Tumuluru, J.S.; Rials, T.G.; et al. Blended Feedstocks for Thermochemical Conversion: Biomass Characterization and Bio-Oil Production from Switchgrass-Pine Residues Blends. Front. Energy Res. 2018, 6, 79. [Google Scholar] [CrossRef]
- Primaz, C.T.; Schena, T.; Lazzari, E.; Caramão, E.B.; Jacques, R.A. Influence of the Temperature in the Yield and Composition of the Bio-Oil from the Pyrolysis of Spent Coffee Grounds: Characterization by Comprehensive Two Dimensional Gas Chromatography. Fuel 2018, 232, 572–580. [Google Scholar] [CrossRef]
- Niazmand, R. Date Wastes and By-Products: Chemistry, Processing, and Utilization. In Handbook of Fruit Wastes and By-Products; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2022; p. 18. ISBN 9781003164463. [Google Scholar]
- Tallou, A.; Haouas, A.; Jamli, M.Y.; Khadija, A.; Soumia, A.; Faissal, A. Review on Cow Manure as Renewable Energy. In Smart Village Technology; Patnaik, S., Siddhartha, S., Mahmoud, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 341–352. ISBN 978-3-030-37793-9. [Google Scholar]
- Alherbawi, M.; McKay, G.; Mackey, H.R.; Al-Ansari, T. Multi-Biomass Refinery Siting: A GIS Geospatial Optimisation Approach. Chem. Eng. Trans. 2022, 92, 73–78. [Google Scholar] [CrossRef]
- Giwa, A.S.; Xu, H.; Chang, F.; Zhang, X.; Ali, N.; Yuan, J.; Wang, K. Pyrolysis Coupled Anaerobic Digestion Process for Food Waste and Recalcitrant Residues: Fundamentals, Challenges, and Considerations. Energy Sci. Eng. 2019, 7, 2250–2264. [Google Scholar] [CrossRef]
- Castellano-Hinojosa, A.; Armato, C.; Pozo, C.; González-Martínez, A.; González-López, J. New Concepts in Anaerobic Digestion Processes: Recent Advances and Biological Aspects. Appl. Microbiol. Biotechnol. 2018, 102, 5065–5076. [Google Scholar] [CrossRef]
- Tayibi, S.; Monlau, F.; Bargaz, A.; Jimenez, R.; Barakat, A. Synergy of Anaerobic Digestion and Pyrolysis Processes for Sustainable Waste Management: A Critical Review and Future Perspectives. Renew. Sustain. Energy Rev. 2021, 152, 111603. [Google Scholar] [CrossRef]
- Font-Palma, C. Methods for the Treatment of Cattle Manure—A Review. C 2019, 5, 27. [Google Scholar] [CrossRef]
- Ma, Z.; Xie, J.; Gao, N.; Quan, C. Pyrolysis Behaviors of Oilfield Sludge Based on Py-GC/MS and DAEM Kinetics Analysis. J. Energy Inst. 2019, 92, 1053–1063. [Google Scholar] [CrossRef]
- Mumbach, G.D.; Alves, J.L.F.; da Silva, J.C.G.; Di Domenico, M.; Arias, S.; Pacheco, J.G.A.; Marangoni, C.; Machado, R.A.F.; Bolzan, A. Prospecting Pecan Nutshell Pyrolysis as a Source of Bioenergy and Bio-Based Chemicals Using Multicomponent Kinetic Modeling, Thermodynamic Parameters Estimation, and Py-GC/MS Analysis. Renew. Sustain. Energy Rev. 2022, 153, 111753. [Google Scholar] [CrossRef]
- Kaewpengkrow, P.R.; Atong, D.; Sricharoenchaikul, V. Bio-Fuel Production from Catalytic Fast Pyrolysis of Jatropha Wastes Using Pyroprobe GC/MS and Drop Tube Pyrolyzer. J. Anal. Appl. Pyrolysis 2022, 165, 105574. [Google Scholar] [CrossRef]
- Iqbal, T.; Lu, Q.; Dong, C.Q.; Zhou, M.X.; Arain, Z.; Ali, Z.; Khan, I.; Hussain, Z.; Abbas, A. A Study of Product Distribution under Fast Pyrolysis of Wheat Stalk While Producing Bio-Oil. In Proceedings of the 2018 International Conference on Computing, Mathematics and Engineering Technologies (iCoMET), Sukkur, Pakistan, 3–4 March 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Bensidhom, G.; Arabiourrutia, M.; Ben Hassen Trabelsi, A.; Cortazar, M.; Ceylan, S.; Olazar, M. Fast Pyrolysis of Date Palm Biomass Using Py-GCMS. J. Energy Inst. 2021, 99, 229–239. [Google Scholar] [CrossRef]
- He, S.; Cao, C.; Wang, J.; Yang, J.; Cheng, Z.; Yan, B.; Pan, Y.; Chen, G. Pyrolysis Study on Cattle Manure: From Conventional Analytical Method to Online Study of Pyrolysis Photoionization Time-of-Flight Mass Spectrometry. J. Anal. Appl. Pyrolysis 2020, 151, 104916. [Google Scholar] [CrossRef]
- Krause, M.C.; Moitinho, A.C.; Ferreira, L.F.R.; de Souza, R.L.; Krause, L.C.; Caramão, E.B. Production and Characterization of the Bio-Oil Obtained by the Fast Pyrolysis of Spent Coffee Grounds of the Soluble Coffee Industry. J. Braz. Chem. Soc. 2019, 30, 1608–1615. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Liu, J.; Evrendilek, F.; Xie, W.; He, Y.; Buyukada, M. Comparative (Co-)Pyrolytic Performances and by-Products of Textile Dyeing Sludge and Cattle Manure: Deeper Insights from Py-GC/MS, TG-FTIR, 2D-COS and PCA Analyses. J. Hazard. Mater. 2021, 401, 123276. [Google Scholar] [CrossRef]
- Hidayat, S.; Abu Bakar, M.S.; Yang, Y.; Phusunti, N.; Bridgwater, A.V. Characterisation and Py-GC/MS Analysis of Imperata Cylindrica as Potential Biomass for Bio-Oil Production in Brunei Darussalam. J. Anal. Appl. Pyrolysis 2018, 134, 510–519. [Google Scholar] [CrossRef]
- Liu, C.; Duan, X.; Chen, Q.; Chao, C.; Lu, Z.; Lai, Q.; Megharaj, M. Investigations on Pyrolysis of Microalgae Diplosphaera Sp. MM1 by TG-FTIR and Py-GC/MS: Products and Kinetics. Bioresour. Technol. 2019, 294, 122126. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).