Application and Validation of Analytical Software (SQX) for Semi-Quantitative Determination of the Main Chemical Composition of Solid, Bulk and Powder Fuel Samples by Wavelength Dispersive X-ray Fluorescence Technique
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussions
3.1. Optimization of Sample Preparation for X-ray Measurements
3.1.1. Optimization of the Grinding Procedure
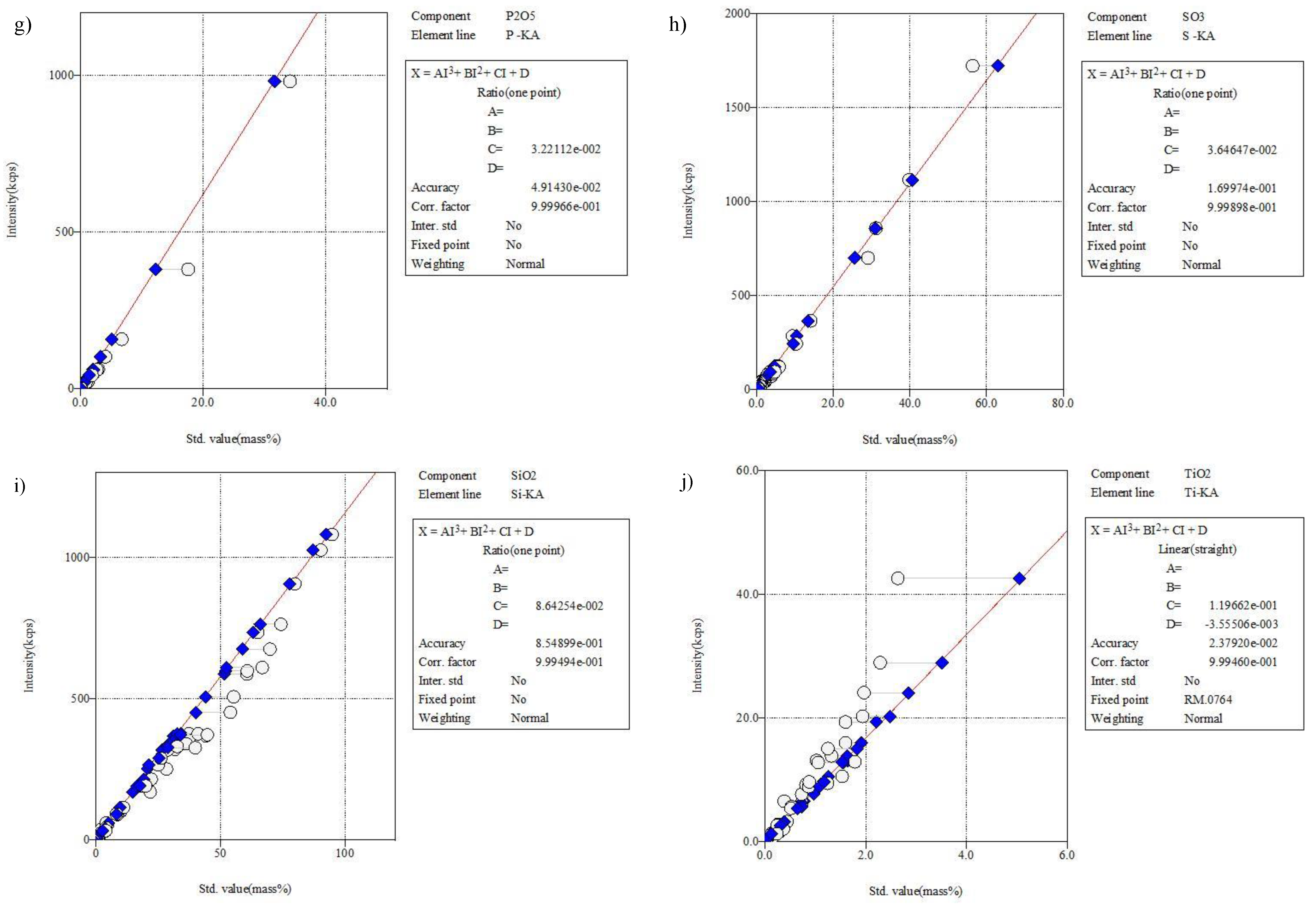
3.2. Development of a Method for Determining the Main Chemical Composition of Solid Waste with the Use of the WDXRF Technique with Sample Preparation for X-ray Measurements by Pressing with a Binding Agent—Calibration-Based Method
3.2.1. The Problem of Obtaining a Durable Tablet for Multiple X-ray Measurements with the Use of Rigaku ZSX Primus II Spectrometer
3.2.2. Method of Preparing Calibration Standards and Test Samples for X-ray Measurement by Pressing with Binding Material
3.2.3. Selection and Measurement of Standards to Obtain Calibration Curves
3.2.4. The Principle of Semi-Quantitative Analysis and Optimization of the Method of Performing the Determination in Terms of the Correctness of the Obtained Results
3.2.5. The Effect of the Type and Amount of Binding Agent on the Result of the SQX Software Analysis
3.2.6. Estimation of the Reproducibility of the Tablet Preparation Procedure for the Standardless Analysis and the Repeatability of the Results Obtained Using the SQX Software
3.2.7. Results Accuracy Estimation of the Determination of the Main Chemical Composition of Solid Samples with the Use of the SQX Software for Semi-Quantitative Analysis of the Rigaku ZSX Primus II Wavelength Dispersive X-ray Fluorescence Spectrometer
- -
- Range 1: below < 0.1%;
- -
- Range 2: between 0.1% and 1.0%;
- -
- Range 3: between 1% and 10%;
- -
- Range 4: above 10%.
4. Conclusions
- Rigaku software for semi-quantitative analysis provided by the manufacturer with its wavelength dispersive X-ray fluorescence ZSX Primus II spectrometer is an ideal analytical tool for determining the chemical composition, including 10 main oxides: SiO2, Al2O3, Fe2O3, CaO, MgO, Na2O, K2O, SO3, TiO2 and P2O5 of any unknown solid, bulk and powder samples.
- The uncertainty of determination of the above-mentioned oxides based on measurements of 24 certified reference materials estimated at the validation stage of the method after dividing the entire range of its applicability into four content sub-ranges is: 68.11% for contents below 0.1%; 22.53% in the content range 0.1–1%; 14.40% for the results between 1–10%; and 7.13% for the results above 10%.
- Rigaku SQX software enables direct analysis of the initial samples as well as samples mixed and then pressed with the binding agent since, at the stage of calculating the determined contents, the software deals with the correction of matrix effects very well and efficiently. The effectiveness of the mathematical conversion algorithm is demonstrated by the small differences between the results obtained for the different binding agents tested and mixed at different weight ratios with coal ash and soil samples selected for this purpose.
- The preparation of the sample for X-ray measurements by pressing with a binding agent and then the measurement of the obtained tablet itself using the SQX software are not significant sources of determination error. This is evidenced by the small differences between the maximum and minimum results obtained from the measurement of 7 tablets and for all 10 determined oxides. The calculated values of standard deviations range from 0.0088% for P2O5 and 0.0103% for TiO2 to 0.0588% for CaO and 0.0654% for Fe2O3. The values of coefficients of variation (RSD) do not exceed even 1.5% and are the lowest for SiO2 and Al2O3 (0.12% and 0.20%, respectively) and the largest for SO3 (1.32%) and for Na2O (1.49%).
- The ease of sample preparation for X-ray measurement, the low cost of analysis and the short time leading to correct results make the SQX software a valuable, useful and even necessary analytical tool for any chemical laboratory equipped with a WDXRF spectrometer.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shaltout, A.; Welz, B.; Ibrahim, M. Influence of the grain size on the quality of standardless WDXRF analysis of river Nile sediments. Microchem. J. 2011, 99, 356–363. [Google Scholar] [CrossRef]
- Chuparina, E.V.; Chubarov, V.M.; Paradina, L.P. A comparative determination of major components in coal power plant wastes by wavelength dispersive X-ray fluorescence using pellet and fused bead specimens. Appl. Radiat. Isot. 2019, 152, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, O.; Margui, E.; Queralt, I. Multielemental analysis of dried residue from metal-bearing waters by wavelength dispersive X-ray fluorescence spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2009, 64, 184–190. [Google Scholar] [CrossRef]
- Zolotajkin, M.; Ciba, J.; Kluczka, J.; Skwira, M.; Smolinski, A. Mobile aluminium in the mountain forest soil of Barania Góra range (Silesian Beskids, Poland). Water Air Soil Pollut. 2011, 216, 571–580. [Google Scholar] [CrossRef]
- Zołotajkin, M.; Smoliński, A.; Ciba, J.; Kluczka, J.; Skwira, M. Comparison of the Chemical Properties of Forest Soil from the Silesian Beskid, Poland. J. Chem. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Marieta, C.; Guerrero, A.; Leon, I. Municipal solid waste incineration fly ash to produce eco-friendly binders for sustainable building construction. Waste Manag. 2021, 120, 114–124. [Google Scholar] [CrossRef]
- An, J.; Jeong, B.; Jeong, S.; Nam, K. Diffusive gradients in thin films technique coupled to X-ray fluorescence spectrometry for the determination of bioavailable arsenic concentrations in soil. Spectrochim. Acta Part B At. Spectrosc. 2020, 164, 105752. [Google Scholar] [CrossRef]
- Figueiredo, A.; Fernandes, T.; Costa, I.M.; Gonçalves, L.; Brito, J. Feasibility of wavelength dispersive X-ray fluorescence spec-trometry for the determination of metal impurities in pharmaceutical products and dietary supplements in view of regulatory guidelines. J. Pharm. Biomed. 2016, 122, 52–58. [Google Scholar] [CrossRef]
- Amorim, F.A.C.; Costa, V.C.; da Silva, E.G.P.; de Castro Lima, D.; de Jesus, R.M.; de Almeida Bezerra, M. Multivariate opti-mization of simple procedure for determination of Fe and Mg in cassava starch employing slurry sampling and FAAS. Food Chem. 2017, 227, 41–47. [Google Scholar] [CrossRef]
- Augusto, A.D.S.; Barsanelli, P.L.; Pereira, F.M.V.; Pereira-Filho, E.R. Calibration strategies for the direct determination of Ca, K, and Mg in commercial samples of powdered milk and solid dietary supplements using laser-induced breakdown spectroscopy (LIBS). Food Res. Int. 2017, 94, 72–78. [Google Scholar] [CrossRef]
- Andrade, D.F.; Pereira-Filho, R. Direct determination of contaminants and major and minor nutrients in solid fertilizers using laser-induced breakdown spectroscopy (LIBS). J. Agric. Food Chem. 2016, 64, 7890–7898. [Google Scholar] [CrossRef] [PubMed]
- Togdanovic, T.; Ujevic, I.; Sedak, M.; Listeš, E.; Šimat, V.; Petricevic, S.; Poljak, V. As, Cd, Hg and Pb in four edible shellfish species from breeding and harvesting areas along the eastern Adriatic Coast, Croatia. Food Chem. 2014, 146, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Smolinski, A.; Stempin, M.; Howaniec, N. Determination of rare earth elements in combustion ashes from selected Polish coal mines by wavelength dispersive X-ray fluorescence spectrometry. Spectrochim. Acta B At. Spectrosc. 2016, 116, 63–74. [Google Scholar] [CrossRef]
- Kasper, C.; Schlegel, P.; Ruiz-Ascacibar, I.; Stoll, P.; Bee, G. Accuracy of predicting chemical body composition of growing pigs using dual-energy X-ray absorptiometry. Animal 2021, 15, 100307. [Google Scholar] [CrossRef]
- Kipper, M.; Marcoux, M.; Andretta, I.; Pomar, C. Assessing the accuracy of measurements obtained by dual-energy X-ray absorptiometry on pig carcasses and primal cuts. Meat Sci. 2019, 148, 79–87. [Google Scholar] [CrossRef]
- Ghidotti, M.; Papoci, S.; Dumitrascu, C.; Zdiniakova, T.; Fiamegos, Y.; Gutiñas, M.B.D.L.C. ED-XRF as screening tool to help customs laboratories in their fight against fraud. State-of-the-art. Talanta Open 2021, 3, 100040. [Google Scholar] [CrossRef]
- Kleeberg, R. State-of-the-Art and Trends in Quantitative Phase Analysis of Geological and Raw Materials. 2009. Available online: https://doi.org/10.1524/9783486992588-012 (accessed on 10 July 2022).
- Smolinski, A.; Zolotajkin, M.; Ciba, J.; Dydo, P.; Kluczka, J. Robust PLS Regression Models to Predict Aluminum Content in Soils of Beskid Mountains Region. Chemosphere 2009, 76, 565–571. [Google Scholar] [CrossRef]
- Álvarez-Ayuso, E.; Querol, X.; Plana, F.; Alastuey, A.; Moreno, N.; Izquierdo, M.; Font, O.; Moreno, T.; Diez, S.; Vázquez, E.; et al. Environmental, physical and structural characterisation of geopolymer matrixes synthesised from coal (co-)combustion fly ashes. J. Hazard. Mater. 2008, 154, 175–183. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative study on the characteristics of fly ash and bottom ash geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef]
- Huang, Y.; Han, M. The influence of α-Al2O3 addition on microstructure, mechanical and formaldehyde adsorption properties of fly ash-based geopolymer products. J. Hazard. Mater. 2011, 193, 90–94. [Google Scholar] [CrossRef]
- Rompalski, P.; Smoliński, A.; Krztoń, H.; Gazdowicz, J.; Howaniec, N.; Róg, L. Determination of mercury content in hard coal and fly ash using X-ray diffraction and scanning electron microscopy coupled with chemical analysis. Arab. J. Chem. 2019, 12, 3927–3942. [Google Scholar] [CrossRef]
- Pérez-Bernal, J.; Amigo, J.; Fernandez-Torres, R.; Bello, M.; Callejón-Mochón, M. Trace-metal distribution of cigarette ashes as marker of tobacco brands. Forensic Sci. Int. 2011, 204, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Fiamegos, Y.; Papoci, S.; Dumitrascu, C.; Ghidotti, M.; Zdiniakova, T.; Ulberth, F.; de la Calle, G.M.B. Are the elemental fingerprints of organic and conventional food different? ED-XRF as screening technique. J. Food Compos. Anal. 2021, 99, 103854. [Google Scholar] [CrossRef] [PubMed]
- Ahrenholz, B.; Tölke, J.; Lehmann, P.; Peters, A.; Kaestner, A.; Krafczyk, M.; Durner, W. Prediction of capillary hysteresis in a porous material using lattice-Boltzmann methods and comparison to experimental data and a morphological pore network model. Adv. Water Resour. 2008, 31, 1151–1173. [Google Scholar] [CrossRef]
- ISO 13909-4:2016; Hard Coal and Coke—Mechanical Sampling—Part 4: Coal—Preparation of Test Samples. ISO: Geneva, Switzerland, 2016.
- Cherkashina, T.; Shtel’Makh, S.; Pashkova, G. Determination of trace elements in calcium rich carbonate rocks by Wavelength Dispersive X-ray Fluorescence Spectrometry for environmental and geological studies. Appl. Radiat. Isot. 2017, 130, 153–161. [Google Scholar] [CrossRef] [PubMed]
- PN EN 15309:2010; Characterization of Waste and Soil—Determination of Elemental Composition by X-ray Fluorescence. Polish Committee for Standardization: Warsaw, Poland, 2013.

| Component | Grinding Time, s | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 120 | 180 | 240 | 30 | 60 | 120 | 180 | 240 | |
| Concentration in the Coal Ash Sample, wt% | Concentration in the Soil Sample, wt% | |||||||||
| SiO2 | 42.69 | 45.52 | 47.99 | 48.1 | 47.92 | 81.17 | 88.51 | 91.97 | 91.82 | 92.03 |
| Al2O3 | 26.93 | 24.81 | 23.93 | 23.86 | 24.00 | 8.98 | 5.16 | 3.83 | 3.90 | 3.85 |
| Fe2O3 | 6.28 | 6.93 | 7.51 | 7.49 | 7.54 | 2.71 | 1.43 | 1.01 | 0.992 | 1.00 |
| CaO | 7.31 | 6.95 | 6.74 | 6.71 | 6.72 | 0.569 | 0.332 | 0.263 | 0.261 | 0.257 |
| MgO | 2.49 | 2.81 | 3.02 | 3.04 | 3.02 | 0.626 | 0.365 | 0.239 | 0.24 | 0.242 |
| Na2O | 0.913 | 0.862 | 0.835 | 0.834 | 0.837 | 0.429 | 0.287 | 0.233 | 0.227 | 0.228 |
| K2O | 6.53 | 5.4 | 5.10 | 5.12 | 5.07 | 3.28 | 1.54 | 1.28 | 1.28 | 1.30 |
| SO3 | 3.02 | 2.61 | 2.38 | 2.35 | 2.42 | 0.164 | 0.101 | 0.074 | 0.074 | 0.072 |
| TiO2 | 1.53 | 1.36 | 1.21 | 1.19 | 1.20 | 0.58 | 0.306 | 0.214 | 0.21 | 0.211 |
| P2O5 | 1.36 | 1.19 | 1.02 | 1.03 | 1.04 | 0.198 | 0.099 | 0.056 | 0.058 | 0.055 |
| Measurement No./ Parameter | Component Concentration, wt% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | TiO2 | P2O5 | |
| 1 | 50.244 | 24.035 | 7.489 | 5.222 | 3.014 | 0.835 | 4.306 | 2.072 | 1.106 | 0.922 |
| 2 | 53.743 | 25.654 | 7.460 | 5.309 | 3.115 | 0.937 | 4.437 | 2.152 | 1.092 | 1.001 |
| 3 | 54.727 | 26.099 | 7.465 | 5.372 | 3.128 | 0.964 | 4.482 | 2.166 | 1.087 | 1.017 |
| 4 | 55.437 | 26.408 | 7.474 | 5.396 | 3.136 | 0.976 | 4.512 | 2.183 | 1.087 | 1.027 |
| 5 | 55.920 | 26.612 | 7.470 | 5.428 | 3.157 | 0.999 | 4.534 | 2.191 | 1.089 | 1.040 |
| 6 | 56.342 | 26.865 | 7.456 | 5.423 | 3.171 | 1.011 | 4.555 | 2.202 | 1.082 | 1.050 |
| 7 | 56.696 | 26.986 | 7.466 | 5.443 | 3.164 | 1.009 | 4.575 | 2.207 | 1.087 | 1.058 |
| Average | 54.730 | 26.094 | 7.469 | 5.370 | 3.126 | 0.962 | 4.486 | 2.168 | 1.090 | 1.016 |
| Maximum | 56.696 | 26.986 | 7.489 | 5.443 | 3.171 | 1.011 | 4.575 | 2.207 | 1.106 | 1.058 |
| Minimum | 50.244 | 24.035 | 7.456 | 5.222 | 3.014 | 0.835 | 4.306 | 2.072 | 1.082 | 0.922 |
| Range | 6.452 | 2.951 | 0.033 | 0.221 | 0.157 | 0.176 | 0.269 | 0.135 | 0.024 | 0.136 |
| Standard deviation | 2.0521 | 0.9404 | 0.0100 | 0.0735 | 0.0496 | 0.0572 | 0.0849 | 0.0429 | 0.0071 | 0.0426 |
| Coefficient of variation (RSD), % | 3.75 | 3.60 | 0.13 | 1.37 | 1.59 | 5.95 | 1.89 | 1.98 | 0.65 | 4.19 |
| Tablet No./ Parameter | Component Concentration, wt% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | TiO2 | P2O5 | |
| Tablet 1 | 49.859 | 23.858 | 7.474 | 5.324 | 3.025 | 0.846 | 4.270 | 2.140 | 1.096 | 0.924 |
| Tablet 2 | 49.929 | 23.876 | 7.539 | 5.236 | 3.032 | 0.833 | 4.300 | 2.121 | 1.107 | 0.923 |
| Tablet 3 | 50.201 | 23.995 | 7.464 | 5.229 | 3.013 | 0.834 | 4.298 | 2.076 | 1.102 | 0.929 |
| Tablet 4 | 49.917 | 23.902 | 7.491 | 5.224 | 3.012 | 0.834 | 4.295 | 2.054 | 1.111 | 0.909 |
| Tablet 5 | 50.100 | 23.964 | 7.443 | 5.207 | 3.016 | 0.837 | 4.306 | 2.076 | 1.103 | 0.920 |
| Tablet 6 | 50.110 | 24.002 | 7.474 | 5.247 | 3.023 | 0.835 | 4.313 | 2.084 | 1.110 | 0.926 |
| Tablet 7 | 49.900 | 23.879 | 7.511 | 5.220 | 3.016 | 0.827 | 4.290 | 2.085 | 1.106 | 0.921 |
| Average | 50.002 | 23.925 | 7.485 | 5.241 | 3.020 | 0.835 | 4.296 | 2.091 | 1.105 | 0.922 |
| Maximum | 50.201 | 24.002 | 7.539 | 5.324 | 3.032 | 0.846 | 4.313 | 2.140 | 1.111 | 0.929 |
| Minimum | 49.859 | 23.858 | 7.443 | 5.207 | 3.012 | 0.827 | 4.270 | 2.054 | 1.096 | 0.909 |
| Range | 0.342 | 0.144 | 0.096 | 0.117 | 0.020 | 0.019 | 0.043 | 0.086 | 0.015 | 0.020 |
| Standard deviation | 0.1319 | 0.0603 | 0.0317 | 0.0387 | 0.0074 | 0.0059 | 0.0135 | 0.0296 | 0.0051 | 0.0063 |
| Coefficient of variation (RSD), % | 0.26 | 0.25 | 0.42 | 0.74 | 0.24 | 0.71 | 0.31 | 1.42 | 0.46 | 0.69 |
| Chemical Element | Analytical Line | 2θ Degree | Measurement Time, s | Current Parameters of the Tube | Analysing Crystal | Collimator | Detector | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Background One | Peak | Background Two | Peak | Background | Voltage, kV | Current, mA | |||||
| Si | Kα | 106.15 | 109.05 | 111.90 | 20 | 10 | 50 | 60 | PET | Course | PC |
| Al | Kα | 143.35 | 144.61 | 147.85 | 20 | 10 | 50 | 60 | PET | Course | PC |
| Fe | Kα | 56.00 | 57.50 | 58.90 | 20 | 10 | 50 | 60 | LiF200 | Fine | SC |
| Ca | Kα | 110.30 | 113.12 | 115.85 | 20 | 10 | 50 | 60 | LiF200 | Course | PC |
| Mg | Kα | 37.05 | 40.07 | 42.40 | 30 | 14 | 50 | 60 | Rx25 | Course | PC |
| Na | Kα | 45.70 | 48.70 | 51.15 | 30 | 14 | 50 | 60 | Rx25 | Course | PC |
| K | Kα | 133.20 | 136.68 | 139.70 | 20 | 10 | 50 | 60 | LiF200 | Course | PC |
| S | Kα | 107.35 | 110.82 | 113.85 | 20 | 10 | 50 | 60 | Ge | Course | PC |
| Ti | Kα | 84.80 | 86.11 | 87.90 | 20 | 10 | 50 | 60 | LiF200 | Fine | SC |
| P | Kα | 139.50 | 141.19 | 143.85 | 20 | 10 | 50 | 60 | Ge | Course | PC |
| Tablet Name | Mass of the Sample | Mass of the Binding Agent and the Kind of Binding Agent Used |
|---|---|---|
| A | 4.0000 g | 2.0000 g of cellulose |
| B | 3.0000 g | 3.0000 g of cellulose |
| C | 4.0000 g | 2.0000 g of boric acid |
| D | 3.0000 g | 3.0000 g of boric acid |
| E | 5.0000 g | 1.0000 g of wax |
| F | 4.0000 g | 2.0000 g of cellulose + 0.6000 g of graphite |
| G | 4.0000 g | 2.0000 g of boric acid + 0.6000 of graphite |
| Tablet/ Parameter | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | TiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Coal sample | ||||||||||
| Tablet A | 47.22 | 23.97 | 7.41 | 6.77 | 3.10 | 0.831 | 5.16 | 2.47 | 1.21 | 1.03 |
| Tablet B | 46.29 | 24.18 | 7.63 | 6.98 | 3.05 | 0.801 | 5.35 | 2.64 | 1.29 | 1.06 |
| Tablet C | 46.61 | 24.24 | 7.26 | 6.88 | 3.26 | 0.856 | 5.16 | 2.43 | 1.46 | 1.05 |
| Tablet D | 47.19 | 25.24 | 6.74 | 6.41 | 3.40 | 0.907 | 4.89 | 2.32 | 1.22 | 0.99 |
| Tablet E | 45.35 | 24.91 | 7.84 | 7.21 | 2.85 | 0.810 | 5.37 | 2.48 | 1.39 | 1.07 |
| Tablet F | 46.74 | 24.35 | 7.62 | 6.98 | 2.98 | 0.822 | 5.37 | 2.66 | 1.27 | 1.08 |
| Tablet G | 46.73 | 24.72 | 7.25 | 6.74 | 3.23 | 0.831 | 5.11 | 2.46 | 1.27 | 1.06 |
| Total number of tablets | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Average | 46.59 | 24.52 | 7.39 | 6.85 | 3.12 | 0.84 | 5.20 | 2.49 | 1.30 | 1.05 |
| Maximum | 47.22 | 25.24 | 7.84 | 7.21 | 3.40 | 0.91 | 5.37 | 2.66 | 1.46 | 1.08 |
| Minimum | 45.35 | 23.97 | 6.74 | 6.41 | 2.85 | 0.80 | 4.89 | 2.32 | 1.21 | 0.99 |
| Range | 1.87 | 1.27 | 1.10 | 0.80 | 0.55 | 0.11 | 0.48 | 0.34 | 0.25 | 0.09 |
| Standard deviation | 0.5888 | 0.4202 | 0.3322 | 0.2319 | 0.1723 | 0.0329 | 0.1637 | 0.1103 | 0.0846 | 0.0280 |
| Coefficient of variation (RSD), % | 1.26 | 1.71 | 4.49 | 3.38 | 5.51 | 3.93 | 3.15 | 4.42 | 6.50 | 2.67 |
| Relative error, % | 4.01 | 5.18 | 14.88 | 11.67 | 17.60 | 12.67 | 9.23 | 13.63 | 19.21 | 8.58 |
| Soil sample | ||||||||||
| Tablet A | 92.19 | 3.78 | 1.13 | 0.326 | 0.208 | 0.239 | 1.30 | 0.109 | 0.209 | 0.0582 |
| Tablet B | 92.04 | 3.96 | 1.10 | 0.293 | 0.217 | 0.256 | 1.33 | 0.105 | 0.217 | 0.0660 |
| Tablet C | 91.74 | 4.02 | 1.07 | 0.304 | 0.223 | 0.227 | 1.35 | 0.097 | 0.230 | 0.0613 |
| Tablet D | 91.53 | 4.25 | 1.08 | 0.286 | 0.231 | 0.264 | 1.37 | 0.098 | 0.225 | 0.0642 |
| Tablet E | 91.76 | 4.27 | 1.15 | 0.322 | 0.229 | 0.243 | 1.38 | 0.102 | 0.241 | 0.0629 |
| Tablet F | 92.30 | 3.82 | 1.11 | 0.285 | 0.204 | 0.252 | 1.29 | 0.081 | 0.212 | 0.0554 |
| Tablet G | 92.19 | 3.91 | 1.13 | 0.307 | 0.215 | 0.249 | 1.32 | 0.094 | 0.220 | 0.0578 |
| Total number of tablets | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| Average | 91.96 | 4.00 | 1.11 | 0.30 | 0.22 | 0.25 | 1.33 | 0.10 | 0.22 | 0.06 |
| Maximum | 92.30 | 4.27 | 1.15 | 0.33 | 0.23 | 0.26 | 1.38 | 0.11 | 0.24 | 0.07 |
| Minimum | 91.53 | 3.78 | 1.07 | 0.29 | 0.20 | 0.23 | 1.29 | 0.08 | 0.21 | 0.06 |
| Range | 0.77 | 0.49 | 0.08 | 0.04 | 0.03 | 0.04 | 0.09 | 0.03 | 0.03 | 0.01 |
| Standard deviation | 0.2675 | 0.1798 | 0.0267 | 0.0152 | 0.0094 | 0.0112 | 0.0316 | 0.0084 | 0.0102 | 0.0035 |
| Coefficient of variation (RSD), % | 0.29 | 4.49 | 2.41 | 5.02 | 4.32 | 4.53 | 2.37 | 8.55 | 4.61 | 5.83 |
| Relative error, % | 0.84 | 12.25 | 7.21 | 13.52 | 12.38 | 14.97 | 6.75 | 28.57 | 14.41 | 17.43 |
| Measurement No./ Parameter | Component Concentration, wt% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | TiO2 | P2O5 | |
| 1 | 46.00 | 24.40 | 7.64 | 7.07 | 3.04 | 0.838 | 5.31 | 2.71 | 1.27 | 1.07 |
| 2 | 46.10 | 24.40 | 7.46 | 7.03 | 3.03 | 0.799 | 5.36 | 2.71 | 1.25 | 1.07 |
| 3 | 46.20 | 24.50 | 7.62 | 6.90 | 3.02 | 0.806 | 5.33 | 2.63 | 1.24 | 1.07 |
| 4 | 46.10 | 24.50 | 7.66 | 6.91 | 3.02 | 0.812 | 5.33 | 2.61 | 1.27 | 1.05 |
| 5 | 46.10 | 24.50 | 7.61 | 6.93 | 3.00 | 0.815 | 5.32 | 2.65 | 1.26 | 1.07 |
| 6 | 46.10 | 24.40 | 7.63 | 6.96 | 3.03 | 0.819 | 5.32 | 2.66 | 1.26 | 1.06 |
| 7 | 46.10 | 24.50 | 7.67 | 6.99 | 3.01 | 0.802 | 5.32 | 2.65 | 1.25 | 1.08 |
| Average | 46.10 | 24.46 | 7.61 | 6.97 | 3.02 | 0.813 | 5.33 | 2.66 | 1.26 | 1.07 |
| Maximum | 46.20 | 24.50 | 7.67 | 7.07 | 3.04 | 0.838 | 5.36 | 2.71 | 1.27 | 1.08 |
| Minimum | 46.00 | 24.40 | 7.46 | 6.90 | 3.00 | 0.799 | 5.31 | 2.61 | 1.24 | 1.05 |
| Range | 0.20 | 0.10 | 0.21 | 0.17 | 0.04 | 0.04 | 0.05 | 0.10 | 0.03 | 0.03 |
| Standard deviation | 0.0535 | 0.0495 | 0.0654 | 0.0588 | 0.0125 | 0.0121 | 0.0148 | 0.0351 | 0.0103 | 0.0088 |
| Coefficient of variation (RSD), % | 0.12 | 0.20 | 0.86 | 0.84 | 0.41 | 1.49 | 0.28 | 1.32 | 0.82 | 0.83 |
| Oxide | Content Range in the Standards (CRMs), et% |
|---|---|
| SiO2 | 0.505–90.36 |
| Al2O3 | 0.0419–54.50 |
| Fe2O3 | 0.0154–14.67 |
| CaO | 0.0180–61.87 |
| MgO | 0.0120–18.00 |
| Na2O | 0.0070–3.86 |
| K2O | 0.0100–5.80 |
| SO3 | 0.0022–2.64 |
| TiO2 | 0.0080–2.69 |
| P2O5 | 0.0090–3.07 |
| Oxide | Content Certified, wt.% | Content Determined with the Use of the Semi-Quantitative Method, wt.% | Relative Error of the Semi-Quantitative Method, % | Content Determined with the Use of the Calibration Method, wt.% | Relative Error of the Calibration Method, % | Content Certified, wt.% | Content Determined with the Use of the Semi-Quantitative Method, wt.% | Relative Error of the Semi-Quantitative Method, % | Content Determined with the Use of the Calibration Method, wt.% | Relative Error of the Calibration Method, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Mixed standards: RM.0764–RM.0054 (ratio 1:1) | Mixed standards: RM.0093–RM.0061 (ratio 1:1) | |||||||||
| SiO2 | 31.775 | 27.528 | 13.37 | 31.34 | 1.37 | 31.775 | 27.528 | 13.37 | 31.34 | 1.37 |
| Al2O3 | 11.715 | 10.381 | 11.39 | 11.96 | 2.09 | 11.715 | 10.381 | 11.39 | 11.96 | 2.09 |
| Fe2O3 | 2.578 | 2.644 | 2.56 | 3.29 | 27.62 | 2.578 | 2.644 | 2.56 | 3.29 | 27.62 |
| CaO | 20.53 | 25.815 | 25.74 | 21.26 | 3.56 | 20.53 | 25.815 | 25.74 | 21.26 | 3.56 |
| MgO | 10.268 | 10.584 | 3.08 | 10.33 | 0.60 | 10.268 | 10.584 | 3.08 | 10.33 | 0.60 |
| Na2O | 0.175 | 0.1323 | 24.40 | 0.166 | 5.14 | 0.175 | 0.1323 | 24.40 | 0.166 | 5.14 |
| K2O | 0.632 | 0.733 | 15.98 | 0.654 | 3.48 | 0.632 | 0.733 | 15.98 | 0.654 | 3.48 |
| SO3 | 0.192 | 0.294 | 53.13 | 0.165 | 14.06 | 0.192 | 0.294 | 53.13 | 0.165 | 14.06 |
| TiO2 | 0.435 | 0.485 | 11.49 | 0.436 | 0.23 | 0.435 | 0.485 | 11.49 | 0.436 | 0.23 |
| P2O5 | 0.658 | 0.58 | 11.85 | 0.548 | 16.72 | 0.658 | 0.58 | 11.85 | 0.548 | 16.72 |
| RM.0126: NIST 1881a—Portlant Cement | RM.0764: NCS DC 70310—Carbonate Rock | |||||||||
| SiO2 | 22.26 | 18.935 | 14.94 | 21.55 | 3.19 | 8.25 | 7.488 | 9.24 | 7.96 | 3.52 |
| Al2O3 | 7.06 | 5.872 | 16.83 | 7.39 | 4.67 | 0.10 | 0.141 | 41.00 | 0.168 | 68.00 |
| Fe2O3 | 3.09 | 3.036 | 1.75 | 3.66 | 18.45 | 0.057 | 0.0831 | 45.79 | 0.057 | 0.00 |
| CaO | 57.58 | 62.01 | 7.69 | 56.27 | 2.28 | 33.07 | 36.024 | 8.93 | 34.01 | 2.84 |
| MgO | 2.981 | 2.136 | 28.35 | 2.82 | 5.40 | 18.00 | 16.135 | 10.36 | 18.01 | 0.06 |
| Na2O | 0.199 | 0.165 | 17.09 | 0.185 | 7.04 | 0.026 | 0.0156 | 40.00 | 0.02 | 23.08 |
| K2O | 1.228 | 1.454 | 18.40 | 1.27 | 3.42 | 0.01 | 0.0227 | 127.00 | 0.015 | 50.00 |
| SO3 | 3.366 | 3.823 | 13.58 | 3.31 | 1.66 | 0.01 | 0.081 | 710.00 | 0.018 | 80.00 |
| TiO2 | 0.3663 | 0.367 | 0.19 | 0.412 | 12.48 | 0.003 | 0.00 | 100.00 | 0.003 | 0.00 |
| P2O5 | 0.1459 | 0.131 | 10.21 | 0.142 | 2.67 | 0.124 | 0.114 | 8.06 | 0.128 | 3.23 |
| Oxide | Parameter | Range 1 | Range 2 | Range 3 | Range 4 |
|---|---|---|---|---|---|
| SiO2 | No. of standards in the range | 0 | 1 | 8 | 15 |
| MRE for the semi-quantitative method | --- | 10.10 | 7.66 | 7.74 | |
| MRE for the method based on calibration | --- | 15.64 | 6.13 | 1.68 | |
| Al2O3 | No. of standards in the range | 1 | 6 | 4 | 13 |
| MRE for the semi-quantitative method | 70.41 | 63.08 | 15.51 | 5.17 | |
| MRE for the method based on calibration | 15.57 | 29.25 | 4.60 | 1.12 | |
| Fe2O3 | No. of standards in the range | 4 | 3 | 15 | 2 |
| MRE for the semi-quantitative method | 23.48 | 19.64 | 5.12 | 2.25 | |
| MRE for the method based on calibration | 16.06 | 23.66 | 10.67 | 1.17 | |
| CaO | No. of standards in the range | 1 | 5 | 9 | 9 |
| MRE for the semi-quantitative method | 51.17 | 23.96 | 33.83 | 8.69 | |
| MRE for the method based on calibration | 25.00 | 10.95 | 13.48 | 2.13 | |
| MgO | No. of standards in the range | 3 | 9 | 10 | 2 |
| MRE for the semi-quantitative method | 29.68 | 17.34 | 15.81 | 13.07 | |
| MRE for the method based on calibration | 22.12 | 10.19 | 4.05 | 6.13 | |
| Na2O | No. of standards in the range | 8 | 11 | 4 | 0 |
| MRE for the semi-quantitative method | 26.05 | 15.69 | 9.06 | --- | |
| MRE for the method based on calibration | 31.79 | 3.21 | 2.86 | --- | |
| K2O | No. of standards in the range | 4 | 9 | 11 | 0 |
| MRE for the semi-quantitative method | 86.14 | 18.86 | 21.48 | --- | |
| MRE for the method based on calibration | 20.48 | 10.43 | 2.84 | --- | |
| SO3 | No. of standards in the range | 4 | 12 | 7 | 0 |
| MRE for the semi-quantitative method | 298.50 | 28.75 | 16.50 | --- | |
| MRE for the method based on calibration | 67.05 | 9.98 | 2.54 | --- | |
| TiO2 | No. of standards in the range | 7 | 9 | 8 | 0 |
| MRE for the semi-quantitative method | 69.78 | 10.13 | 8.01 | --- | |
| MRE for the method based on calibration | 16.61 | 5.53 | 1.67 | --- | |
| P2O5 | No. of standards in the range | 7 | 12 | 4 | 0 |
| MRE for the semi-quantitative method | 16.61 | 19.43 | 9.21 | --- | |
| MRE for the method based on calibration | 19.86 | 9.75 | 7.00 | --- |
| Content Range, wt% | Number of Results in the Range | Mean Relative Error of Determination, % | |
|---|---|---|---|
| SQX Semi-Quantitative Method | Calibration Method | ||
| <0.1 | 39 | 68.11 | 26.43 |
| 0.1–1 | 77 | 22.53 | 10.71 |
| 1–10 | 80 | 14.40 | 6.14 |
| >10 | 41 | 7.13 | 1.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smoliński, A.; Stempin, M.; Howaniec, N. Application and Validation of Analytical Software (SQX) for Semi-Quantitative Determination of the Main Chemical Composition of Solid, Bulk and Powder Fuel Samples by Wavelength Dispersive X-ray Fluorescence Technique. Energies 2022, 15, 7311. https://doi.org/10.3390/en15197311
Smoliński A, Stempin M, Howaniec N. Application and Validation of Analytical Software (SQX) for Semi-Quantitative Determination of the Main Chemical Composition of Solid, Bulk and Powder Fuel Samples by Wavelength Dispersive X-ray Fluorescence Technique. Energies. 2022; 15(19):7311. https://doi.org/10.3390/en15197311
Chicago/Turabian StyleSmoliński, Adam, Marek Stempin, and Natalia Howaniec. 2022. "Application and Validation of Analytical Software (SQX) for Semi-Quantitative Determination of the Main Chemical Composition of Solid, Bulk and Powder Fuel Samples by Wavelength Dispersive X-ray Fluorescence Technique" Energies 15, no. 19: 7311. https://doi.org/10.3390/en15197311
APA StyleSmoliński, A., Stempin, M., & Howaniec, N. (2022). Application and Validation of Analytical Software (SQX) for Semi-Quantitative Determination of the Main Chemical Composition of Solid, Bulk and Powder Fuel Samples by Wavelength Dispersive X-ray Fluorescence Technique. Energies, 15(19), 7311. https://doi.org/10.3390/en15197311







