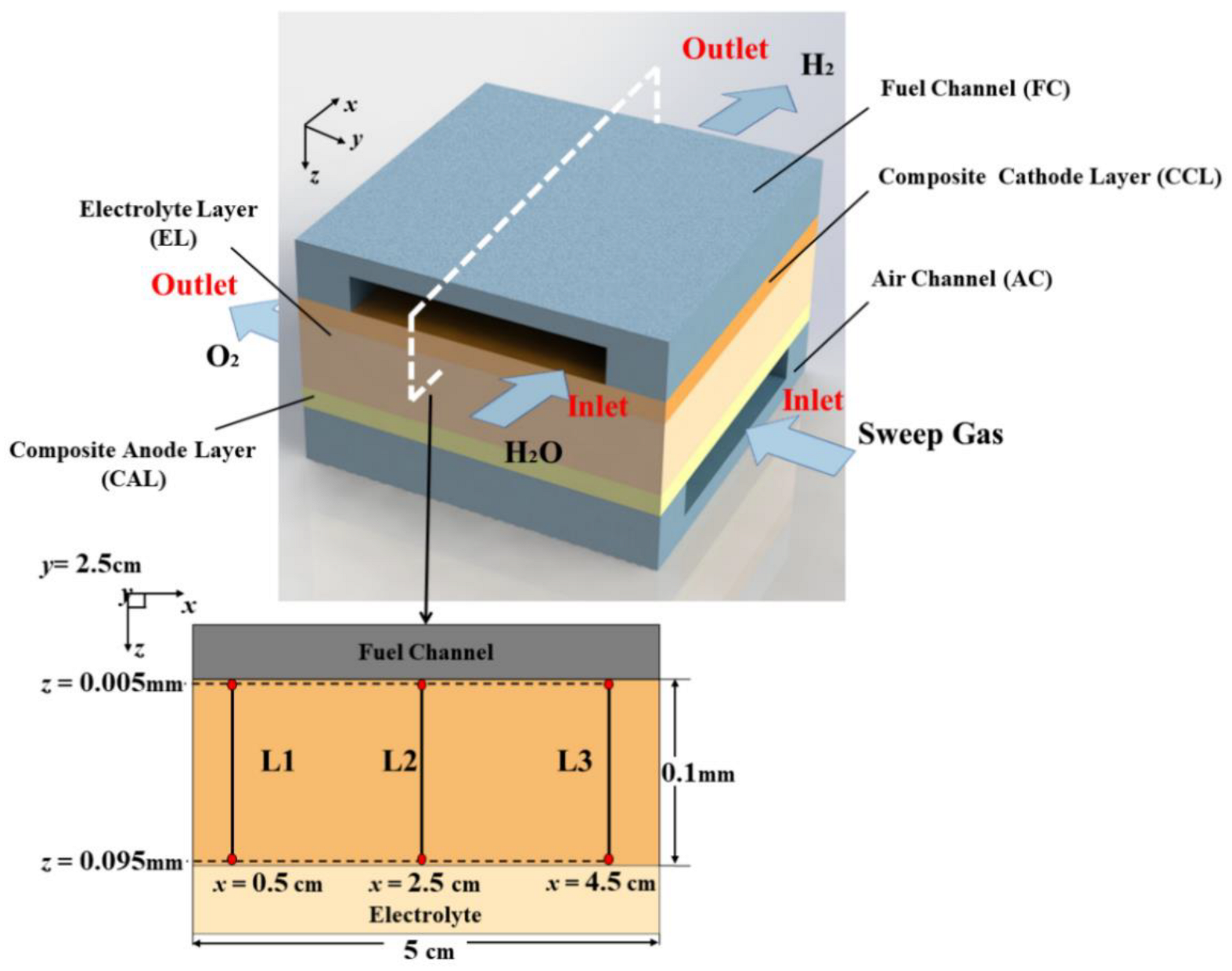
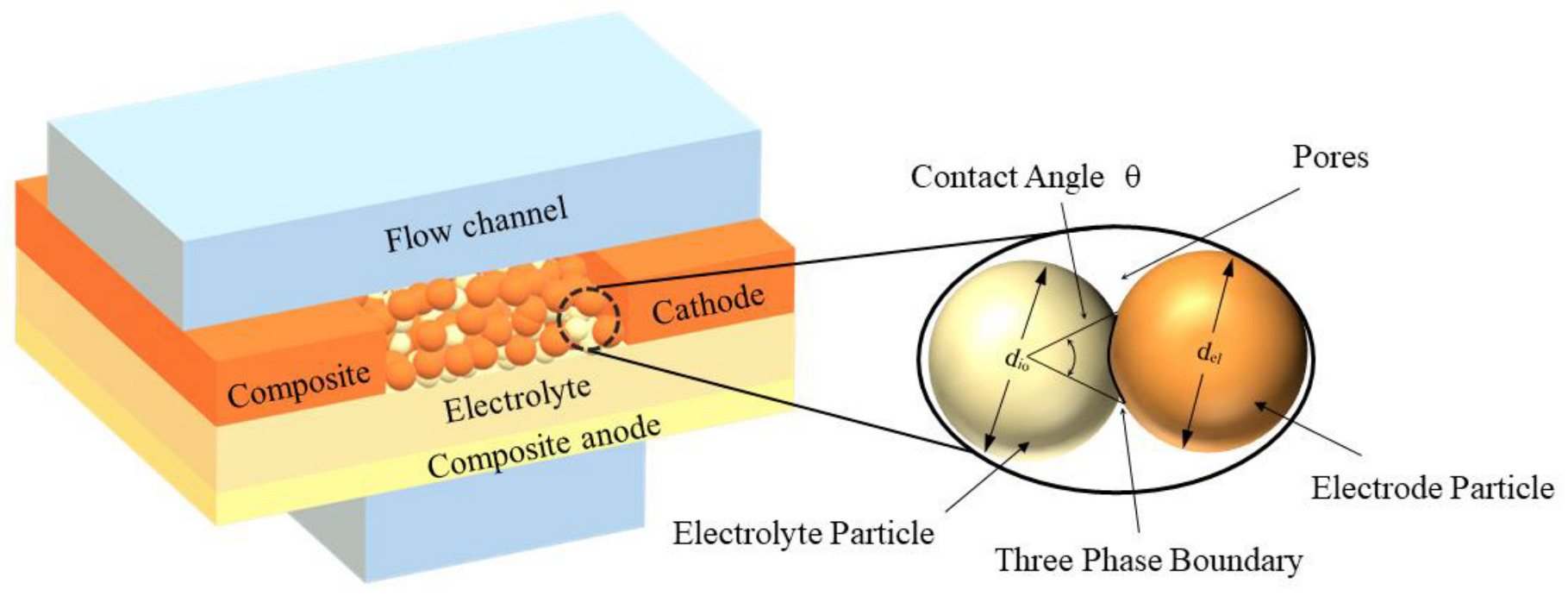
1. Introduction
In the current context of vigorously developing green and sustainable energy, hydrogen energy is considered a key energy carrier due to its unique and excellent performance characteristics. In general, electrolytic hydrogen production has advantages of cleanliness and high purity compared with other current industrial hydrogen production methods. It is the most promising technology to achieve large-scale hydrogen production [
1,
2]. The technology of hydrogen production through electrolysis using a solid oxide electrolysis cell (SOEC) under high temperature conditions was first proposed by Doenitz in 1980 [
3], which differed from other conventional electrolysis cells in their working mechanisms and material characteristics. Compared with the low-temperature proton exchange membrane (PEM) electrolysis cell and alkaline electrolysis cell, SOEC has higher electrolytic efficiency [
4,
5].
In response to limitations of the SOEC under high temperature operating conditions, such as material fabrication processes and performance degradation of the electrolysis cell [
6], an increasing number of scholars have started to investigate intermediate-temperature SOECs (IT-SOECs) and fuel cells (IT-SOFCs) with operating temperatures between 723 and 1073 K [
7,
8,
9]. However, from a thermodynamic viewpoint, the decrease in the operating temperature also inevitably has a negative impact on the cell performance [
10]. In the technological development of IT-SOECs or IT-SOFCs, many scholars have focused on developing new electrode materials or improving the electrode structure to expand the triple phase boundary (TPB) area while obtaining more stable electrochemical reactions and multi-component diffusion processes inside the SOEC [
6,
11]. Thus, many studies have been carried out on the composite electrode.
The composite electrode is a multi-phase material made by mixing electrode materials with electrolyte materials. Due to the structural features of the composite material, the TPB area is effectively enlarged [
12,
13]. The yttria-stabilized zirconia (YSZ), gadolinium-doped ceria (GDC), scandium-ceria-stabilized-zirconia (ScSZ), cobalt-samarium-doped ceria (Co-SDC), and other electrolyte materials that are combined with nickel (Ni) to form the composite electrode have received extensive attention [
1,
14].
For understanding and analyzing the complex coupling process of electrochemical reactions, flow, and multi-component diffusion in composite electrodes, numerical research has more advantages compared with experimental research [
15,
16,
17]. Grondin et al. [
16] developed a one-dimensional (1D) model to simulate the cathodic kinetic behavior of a composite cathode (Ni-ceramic) in SOEC and discussed the effects of chemical and electrochemical kinetic steps on the polarization curves. It is worth noting that the composite electrode is the main place where the above complex coupling process occurs. The composite electrode structure on the mesoscopic scale, including particle diameter, the volume fraction of electrode or electrolyte phase, and so on, will also affect the electrochemical reactions, flow, and multi-component diffusion process inside the electrode [
18].
Therefore, many scholars have adopted different mesoscale mathematical models to study the species transport phenomena and reactions in the microporous structure of composite electrodes. Moussaoui et al. [
19] adopted a so-called 3D Gaussian ‘random field model’ to carry out a mechanistic study about the relationship between the structural properties and performance of the electrode. Laurencin et al. [
13] similarly reconstructed single-phase La
1−xSr
xCo
yFe
1−yO
3−δ (LSCF) electrodes as well as composite electrodes LSCF-CGO. It was found that the LSCF and ceria-doped gadolinium oxide (CGO) composites exhibited higher performance than the LSCF single-phase electrodes. Ren et al. [
20] developed a microkinetic model to investigate the reduction mechanism of CO
2 at the TPB surface based on Ni-SDC. Density functional theory (DFT) was also used to analyze the rate-controlling step in different surface models. Schneider et al. [
21] used a discrete element method (DEM) to study the influence of parameters, including electrode composition, thickness, and density, on the resistance and effective conductivity of the electrode. Gaiselmann et al. [
22] quantitatively analyzed the effects of different sinter temperatures and different pore former contents in production processes on microstructures, using stochastic microstructural models. Deseure et al. [
23] also used a one-dimensional flooded homogeneous model and microscopic approach to conclude that the grain size determined the rate-controlling step of the reaction and that optimum porosity was close to 0.3.
Therefore, when using the model to analyze the influence of the macro process on a SOEC unit performance, the corresponding internal structure of the composite electrode cannot be ignored. However, considering the fundamental differences in the scale of the SOEC unit and the internal structure of the composite electrode on the mesoscopic scale, it is difficult for the analysis of the unit performance to describe the composite electrode by mesoscale method [
24,
25,
26,
27,
28,
29,
30]. In some studies, when analyzing the unit performance using macroscale models in different dimensions, some scholars have directly ignored [
15] or adopted constants to reflect the internal structured features of composite electrodes [
31,
32,
33,
34], which certainly affect the accuracy and reliability of the model. Demin et al. [
15] used the 0D equivalent circuit model to simulate and analyze the SOEC of ionic and proton conductive electrolytes in different feeding modes (downstream and countercurrent) while considering the influence of mass transport, but only external structural parameters, such as thickness, were considered. Grondin et al. [
31] built a 2D macroscopic continuity model to describe the electrochemical reactions, mass transport, and temperature distribution inside a SOEC unit. However, this model assumed the internal TPB area to be 1 m
2 m
−3.
Earlier, in fields related to SOFC, some scholars [
35,
36] established a more accurate and easier mathematical model by coupling the percolation theory with the continuity model to solve the above problem. This method can preserve the relationship between the structural features of the composite electrode on the mesoscopic scale and the macroscopic physical processes involved inside the electrode by calculating the interparticle coordination number and percolation threshold in a binary particle random stacking system. Chen et al. [
37] analyzed the effect of various parameters on the performance of the composite cathode inter-layer of the SOFC by combining the equivalent circuit model and percolation theory. Although the electrochemical reactions and multi-component diffusion processes involved in SOEC and SOFC are quite different, they have similar structural parameter or electrode characteristics. Therefore, some scholars [
38,
39] have applied this method to the field of SOEC for hydrogen production. Ni et al. [
38] established a mathematical model of SOEC coupled with percolation theory. In this study, the authors point out that the particle diameter affects the activation overpotential and concentration overpotential and that the volume fraction of the electrode phase affects the overall overpotential. Li et al. [
39] similarly proposed a 1D solid oxide cell (SOC) model, incorporating percolation theory. They investigated the effects of structured features on the mesoscopic scale, including particle diameter and porosity, on the overpotential, ion current density, oxygen partial pressure, and polarization characteristics in both SOEC and SOFC models.
The above studies on the structural influences of the composite electrode mainly focused on comparisons of overall electrochemical performance. However, the corresponding composite electrode structure evidently also has an effect on the process of flow and multi-component diffusion in the IT-SOEC unit. Taking only the electrochemical behavior into account cannot ensure that the influence of the composite electrode structure will be fully reflected. Meanwhile, the processes of flow and mass transport inside the composite electrode are also important factors to optimize the performance of IT-SOEC. However, there are few systematical studies on the analysis of variation in the flow and mass transfer process with composite electrode structure. Therefore, the effect of composite electrode structure on IT-SOEC performance from a perspective of flow and multi-component diffusion processes needs to be further explored. In this study, we developed a 3D multi-scale model. The effects of the composite electrode structure on the mesoscopic scale and external operating pressures on electrochemical performance were investigated. Furthermore, we studied the influence of the composite electrode structure on the multi-component diffusion process in the different positions of composite electrodes.
In this paper, the adopted method and 3D multi-scale mathematical model of IT-SOEC unit are introduced in
Section 2. In
Section 3, we validate our mathematical model using experimental data. Finally, in
Section 4, we study the influence of operating pressure, volume fraction of the electrode phase, and particle diameter on the electrochemical reaction rate, overpotential, hydrogen molecular diffusion flux, hydrogen concentration gradient, hydrogen convection diffusion flux, and hydrogen mole fraction.
This aim of this study is not only to propose a 3D multi-scale model to describe the IT-SOEC with a composite electrode, but to also provide guidance for performance optimization, design, and manufacturing of the IT-SOEC unit through a comprehensive performance-based parameter analysis.
4. Results and Discussion
The effect of macro-operating pressures on the performance of IT-SOEC was analyzed by performance indices of the hydrogen mole fraction distribution, overpotential, and electrochemical reaction rate using the multi-scale model. The corresponding results were then compared with those of other scholars to further verify the effectiveness of the multi-scale model. Finally, from mesoscopic perspective, the effect of the mesostructural parameters on the performance of IT-SOEC was studied. The parametric analysis of performance indices, such as hydrogen molecular diffusion/convective flux, overpotential, and electrochemical reaction rate, was carried out under different electrode phase volume fractions and particle diameters in the composite electrode.
4.1. Effects of the Operating Pressure on IT-SOEC Electrolysis Performance
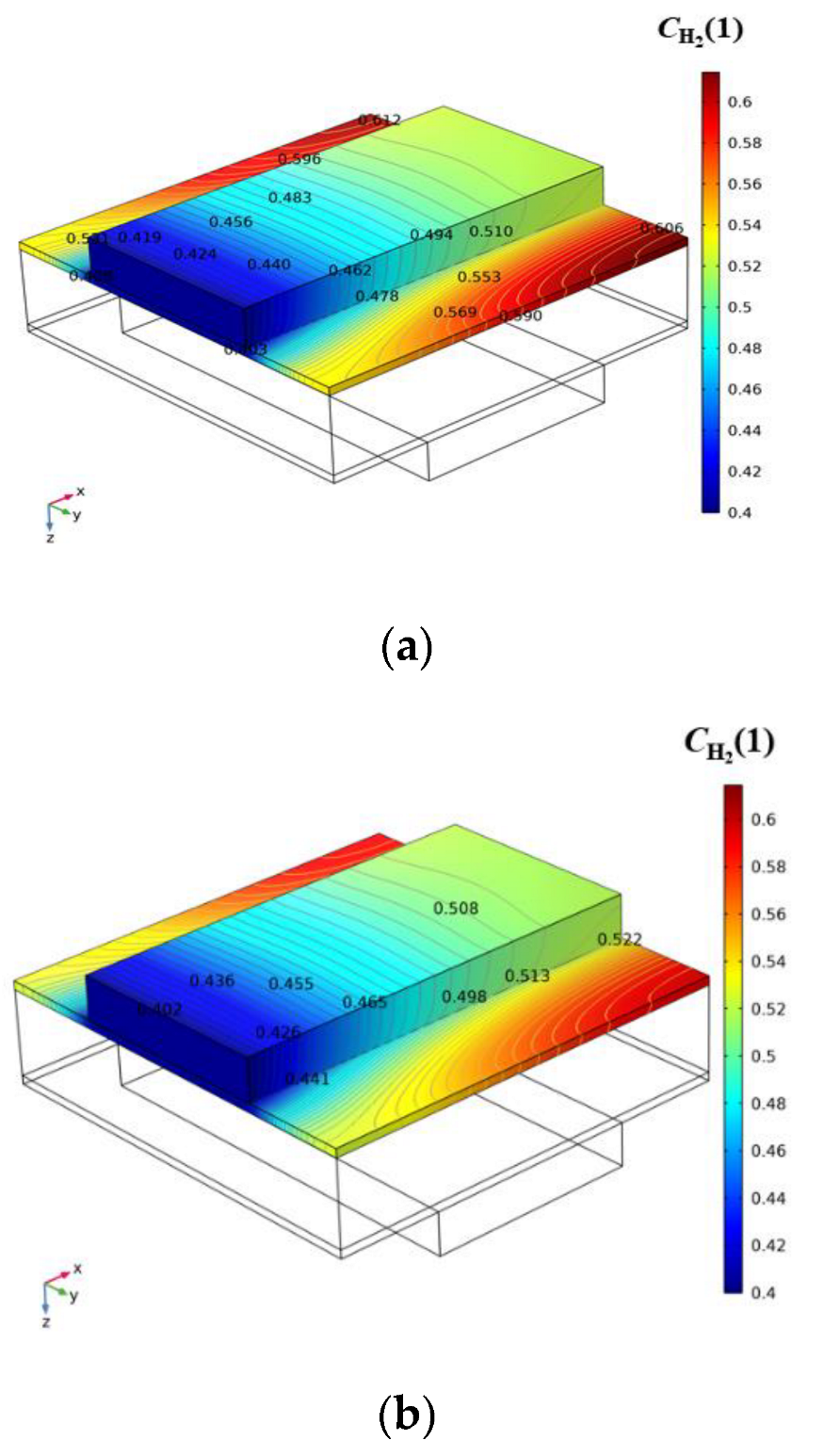
Figure 4a,b shows the distribution of the hydrogen mole fraction in the fuel channel and inside the composite cathode under the operating pressure of 1 and 10 atm, respectively. It can be seen from the figures that under any operating pressure, along the direction of the flow channel, the hydrogen mole fraction is gradually increasing. However, there is a large accumulation of hydrogen inside the composite electrode and the value of the hydrogen mole fraction reaches a maximum at the edges. This suggests that the mass transport process of hydrogen inside the composite electrode cannot be neglected.
Comparing the distribution of the hydrogen mole fraction under the conditions of 1 and 10 atm, we found that with an increase in operating pressure, the local accumulation of hydrogen inside the electrode edge was reduced to a certain extent. The appearance of this phenomenon exerts a certain impact on the kinetic characteristics of the electrode and electrochemical reactions. Interestingly, when the operating pressure increased from 1 to 10 atm, the hydrogen accumulated in the cathode edge decreased by 3.63%. The increase in pressure only reduced the hydrogen mole fraction at the outlet of the flow channel by 0.522%, under these external conditions. Therefore, the increase in operating pressure had no significant effect on the hydrogen output, but greatly improved the hydrogen accumulation phenomenon inside the cathode.
Figure 5a shows the relationship between the overpotential and operating pressure along the characteristic line L2. We found that the overpotential reached a maximum near the electrolyte boundary, regardless of the operating pressure. This may have been because the component mass transfer process became a rate-determining step. The concentration overpotential increased as the diffusion of the vapor into the electrode and diffusion of the generated hydrogen out of the electrode became more difficult to carry out.
Further analysis illustrated that, as the pressure increased, the overpotential inside the composite electrode adjacent to the electrolyte boundary as well as the overall electrode showed a tendency to decrease. These results corroborate the findings of Bernadet et al. [
43], who found that an increase in pressure improved the mass transport of vapor and hydrogen in the porous medium to some extent, and improved diffusion conditions may inhibit the increase in overpotential. Du et al. [
34] also reported that an increase in pressure weakens electrochemical polarization, and thus, reduces the activation overpotential. In summary, these results show that an increase in pressure leads to a decrease in overpotential in the composite cathode.
To further investigate the effect of operating pressure on the electrochemical performance of the electrolysis cell, the electrolysis reaction rate in the volumetric percolation TPB unit area,
RH2, was introduced as an indicator of the electrolysis reaction rate inside the composite cathode, which can be calculated from the following equation:
Figure 5b shows the effect of operating pressure on
RH2 in the composite electrode along the characteristic line L2. Evidently, the maximum value of
RH2 under each operating pressure is obtained near the electrolyte boundary. This suggests that the electrolysis reaction is the most intense in the electrode region near the electrolyte boundary. Chen et al. [
37] pointed out that in the case of equivalent electric particle loading, the region near the electrolyte boundary is identified as the electrochemically active zone (EAZ). Interestingly, from
Figure 5b, the effect of pressure on the electrolysis reaction rate was observed to be different in the regions near and away from the electrolyte. This indicates that the operating pressure affects the positioning of the region covered by the reaction. In the region close to the electrolyte, the pressure has a facilitating effect on the electrolysis reaction. However, in the region far away from the electrolyte, the electrolysis reaction is inhibited. When the operating pressure was increased from 1 to 10 atm, the electrolysis reaction rate in the near region, with a higher ionic current density inside the composite electrode, increased by 47.13% and, in the region away from the electrolyte, was reduced by 66.15%. Generally, the region near the electrolyte has a higher ionic current density and electrolytic reaction rate [
38]. It was also observed that when the position of electrode was greater than 0.07 mm, the electrolysis reaction rate increased.
Therefore, to increase the electrolysis reaction rate in the composite electrode by increasing the operating pressure of the IT-SOEC, it is necessary to control the area near the electrolyte region, i.e., the thickness of the composite electrode, within an appropriate range. In other words, the smaller the thickness of the composite electrode, the more obvious the gain effect of operating pressure on electrode performance. However, it has also been pointed out in the literature [
37] that when the thickness of the composite electrode is less than the corresponding thickness of the EAZ, the activation overpotential will inevitably increase, which affects the economy of SOEC.
According to the above results, the effectiveness of the multi-scale model was validated by predicting performance changes of IT-SOEC with composite electrodes at the macroscale. Meanwhile, it should be noted that almost no electrolysis reaction occurred in the composite electrode near the flow channel, regardless of the operating pressure value. Therefore, when the ability to change the external conditions is limited, it is necessary to optimize the internal structure of the composite electrode to improve the overall performance of the SOEC. This will be discussed in detail in the following section.
4.2. Effect of Composite Electrode Structure on the Comprehensive Performance of IT-SOEC
In this section, through the structural parametric analysis of the volume fraction of the electrode phase and the particle diameter within the composite electrode, the effects of different structural features on the electrochemical reaction rate, applied voltage, and diffusion/convection flux of the IT-SOEC unit are discussed.
4.2.1. Effect of Volume Fraction of Electrode Phase
When investigating the effect of different electrode phase volume fractions within the composite electrode, the gas phase volume fraction ψp was taken as 0.335. According to Equations (26), (33) and (34), when d = 1 within the composite cathode, the electrode and electrolyte particle percolation thresholds are ψel = 0.47 and 1 − ψp − ψio = 0.195, respectively.
Therefore,
ψel < 0.195 is the case of low electrode particle loading and
ψel > 0.47 is the case of high electrode particle loading. Chen et al. [
12] discussed in their study that either case is detrimental to electrode performance and has strict requirements on the percolation TPB area. Therefore, we discuss the cases when 0.195 <
ψel < 0.47 for 0.2, 0.25, 0.3, 0.35, and 0.4, respectively. When the volume fraction of the electrode phase is 0.665, the composite electrode is a pure electron conductor porous electrode.
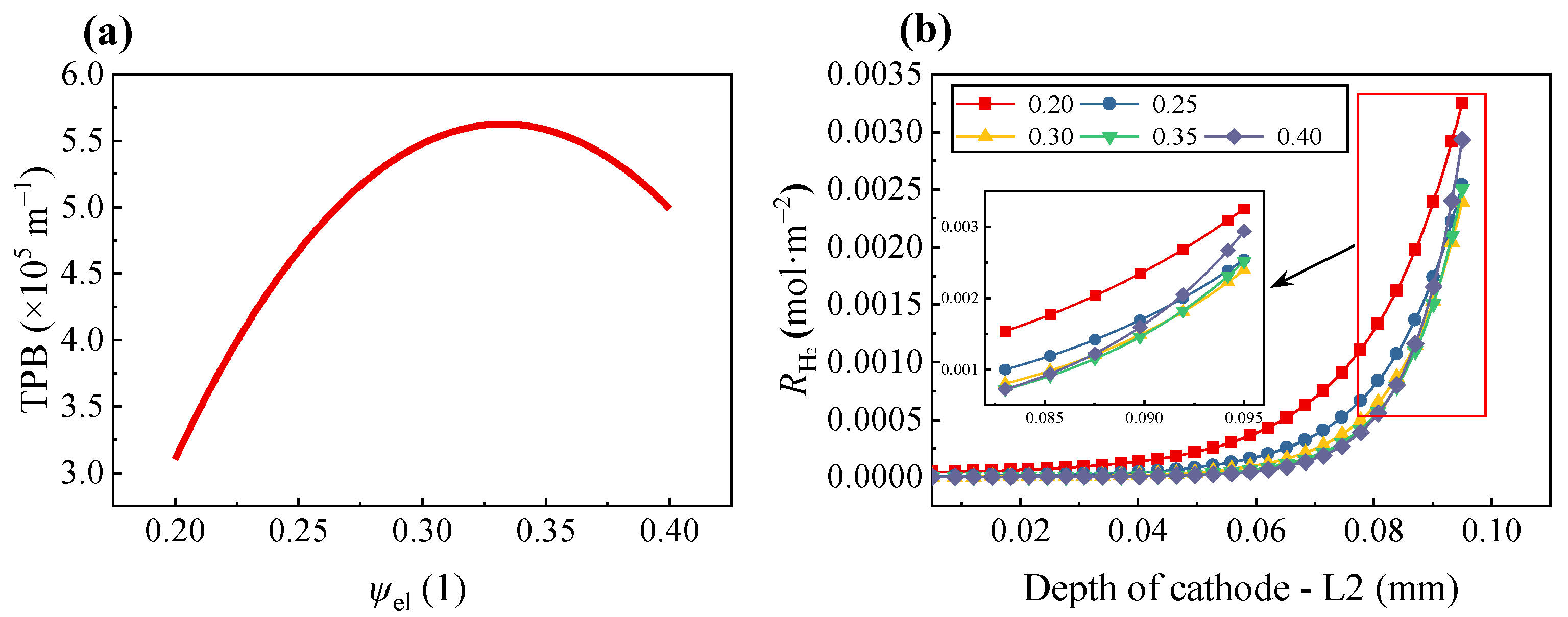
As shown in
Figure 6a, as
ψel gradually increases, the volumetric percolation TPB area also increases, reaching a maximum at
ψel = 0.348. However, when
ψel > 0.348, the composite electrode gradually transits to a high electrode particle loading structure and the volumetric percolation TPB area gradually decreases with the increase in
ψel. This phenomenon can be attributed to the increase in electrode particles at a certain electrode volume; most of the electrode particles form corresponding percolation clusters, but the electrolyte particles only appear in some short connection networks near the electrolyte. This results in the TPB belonging to percolation clusters that are too small and percolated only near the electrolyte, similar with the results obtained by Chen et al. [
37]. According to Equations (26), (20), and (21), the increases in
ψio will lead to an increase in the effective electrolyte conductivity
δeff,s within the composite electrode.
Figure 6b shows the relationship between the value of
ψel and
RH2 in the composite electrode along the characteristic line L2. Overall, it was found that, regardless of the value of
ψel, the most intense electrolysis reaction occurred in the region near the electrolyte and the electrolysis reaction rate was decreased in the region away from the electrolyte. When the electrode position was greater than 0.07 mm, the electrolysis reaction rate was significantly increased.
Figure 6b also indicates that when
ψel decreases, the region where the electrolysis reaction occurs is closer to the flow channel and the electrolysis reaction rate also becomes more intense. Therefore, in the range of the percolation threshold, an increase in
ψio improved the electrolytic performance of the composite electrode. For example, at 0.06 mm inside the electrode, when
ψel was 0.25 and 0.4, the
RH2 was 1.747 × 10
−4 and 5.53 × 10
−5 mol m
−2, respectively. The relative difference between them was 68.34%. Meanwhile, the growth rate of
RH2 corresponding to
ψel = 0.4 was larger in the region near the electrolyte and
RH2 was only smaller than that corresponding to the
ψel = 0.2. So, by comparing
Figure 6a,b, we can observe that the effective electrolyte conductivity of composite electrode plays a more important role in the reaction rate than the value of volumetric percolation TPB area.
The composite electrode structure on the mesoscopic scale also had a significant influence on the flow and mass transport processes inside the IT-SOEC unit.
Figure 7a–c shows the relationship between the hydrogen molecular diffusion flux
jH2 and the electrode volume fraction
ψel at different positions inside the composite cathode along the characteristic lines L1, L2, and L3. As shown in the figures, regardless of the value of
ψel in the front (L1), middle (L2), and end (L3) of the composite cathode, being situated closer to the electrolyte (when the position of the electrode is greater than 0.04 mm) is the main determining factor of
jH2. Our results indicated that being positioned closer to the electrolyte or having a thicker electrode increased the resistance to the diffusion of hydrogen, making it more difficult to carry out the diffusion process. Therefore, a proper reduction in the electrode thickness is also beneficial for the mass transport process.
From these findings shown in
Figure 6b, we can conclude that a small
ψel increases the electrolysis reaction rate and the area of the EAZ region to a certain extent. However,
Figure 7a–c shows that a decrease in
ψel is unfavorable to the effective diffusion of the mass inside the electrode and the location of the reduced diffusion flux also occurs earlier. When the position is greater than 0.04 mm, the difference in
jH2 is significant. Along the characteristic line L3, at 0.06 mm inside the electrode, when
ψel = 0.2 and
ψel = 0.25, the
jH2 in the composite electrode was 0.072 and 0.076 mg m
−2, respectively. When
ψel = 0.4, the
jH2 had the maximum value of 0.078 mg m
−2.
jH2 decreased by 8.3% for
ψel = 0.2, compared with
ψel = 0.4. However,
jH2 increased by 5.6% when
ψel = 0.25, compared with
ψel = 0.2.
As shown in Equation (5), in addition to the density of the working medium and the effective diffusion coefficient, the driving force of the diffusion process mainly depended on the concentration gradient of each mass at the corresponding position.
Figure 7d–f shows the relationship between the concentration gradient of hydrogen and
ψel at different positions inside the composite cathode. Similarly, regardless of the value of
ψel, the hydrogen concentration gradient showed a decreasing trend near the electrolyte. As
ψel increased, the concentration gradient at the same position inside the composite electrode also increased to a certain extent. This phenomenon mainly occurred in the electrode region far from the flow channel.
The comparison between the molecular diffusion flux and concentration gradient corresponding to characteristic lines L1, L2, and L3 in
Figure 7a–f reveals a decreasing trend. Thus, as the electrolysis process proceeds, the molecular diffusion process inside the electrode gradually weakens from the inlet to the outlet side; however, there is also a trend toward a more uniform distribution of hydrogen.
When considering the effect of
ψel on the mass transport inside the electrode, we need to consider not only the molecular diffusion flux driven by the concentration gradient, but also the convective flux.
Figure 7g–i shows the relationship between the convective flux of hydrogen at different positions inside the composite cathode and different values of
ψel. Similar to the relationships of
jH2 shown in
Figure 7a–c, in regions closer to the electrolyte, it is more difficult for the mass transport process driven by convection in the electrode to occur, especially when the position is greater than 0.04 mm. However, in the region away from the flow channel, it is conducive to the development of the convection, as
ψel increases. As this study was carried out at a given initial velocity and could be considered as forced convection, there was not much difference between the convective flux at the different locations in L1, L2, and L3.
4.2.2. Effect of the Particle Diameter
To investigate the effect of the particle diameter on IT-SOEC performance, we used percolated TPB size, applied voltage, and maximum value of the hydrogen mole fraction inside the composite electrode as indicators. The corresponding parameters are presented in
Table 6. The value of the ratio
d indirectly reflects the uniformity of the particle diameter distribution within the composite electrode. The overall particle diameter level within the composite electrode is also reflected by the combination of
d and particle diameter
del.
According to the mesostructure model described in
Section 2.2.4, the description of the volumetric percolation TPB area was nonlinear.
Figure 8a reflects the effect of different
del and
d on the volumetric percolation TPB area. We found that, for the same value of
d, there was a monotonically decreasing relationship between
del and the TPB area. However, regardless of the value of
del, the TPB area did not vary monotonically with
d. A larger value was achieved at
d = 0.75. When
d = 1.5 and
del = 0.5 μm, the TPB area was minimal; thus, we can infer that a larger particle diameter will limit the TPB area. So, to increase the TPB area, it is necessary to reduce the particle diameter and keep the size difference between the electrode and electrolyte particles at an optimal range.
Figure 8b depicts the relationship between the applied voltage of the IT-SOEC unit,
del and
d. The variation in the applied voltage also reflects the economics of the electrolysis process. As shown in
Figure 8b, the applied voltage decreases as
del decreases. When
d is closer to 1, the particle diameter is more homogeneous, and the applied voltage is closer to the minimum value. For example, when
del = 0.5 μm and the electrolyte particle diameter was 1.5 times the electrode particle diameter, the applied voltage reached a maximum value and was at its worst, economically. For the appearance of such a phenomenon, it can be seen from
Figure 8a that the smaller the particle diameter of the electrode and electrolyte and the more uniform the size of both particles, the greater the percentage of TPB belonging to the percolation clusters. This allows for effective electrolysis reactions. Ni et al. [
38] also pointed out in their research that when the particle diameter is less than a certain value, the concentration overpotential becomes the main constraint for the performance enhancement of the SOEC. This also indicates that the diffusion process of the reactants becomes the rate-determining step of the electrolysis reaction.
Figure 8c also demonstrates the effects of
del and
d on the hydrogen production rate per unit electrode volume. The results showed that properly reducing the particle diameter (0.2–0.3 μm) was more conducive to hydrogen production inside the electrode. Meanwhile, there was also an optimal range of 0.8–1 for
d to obtain the maximum hydrogen production rate.