Study on Physicochemical Properties and Rock-Cracking Mechanism of High-Energy Expansion Agent
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Preparation of High-Energy Expansion Agent
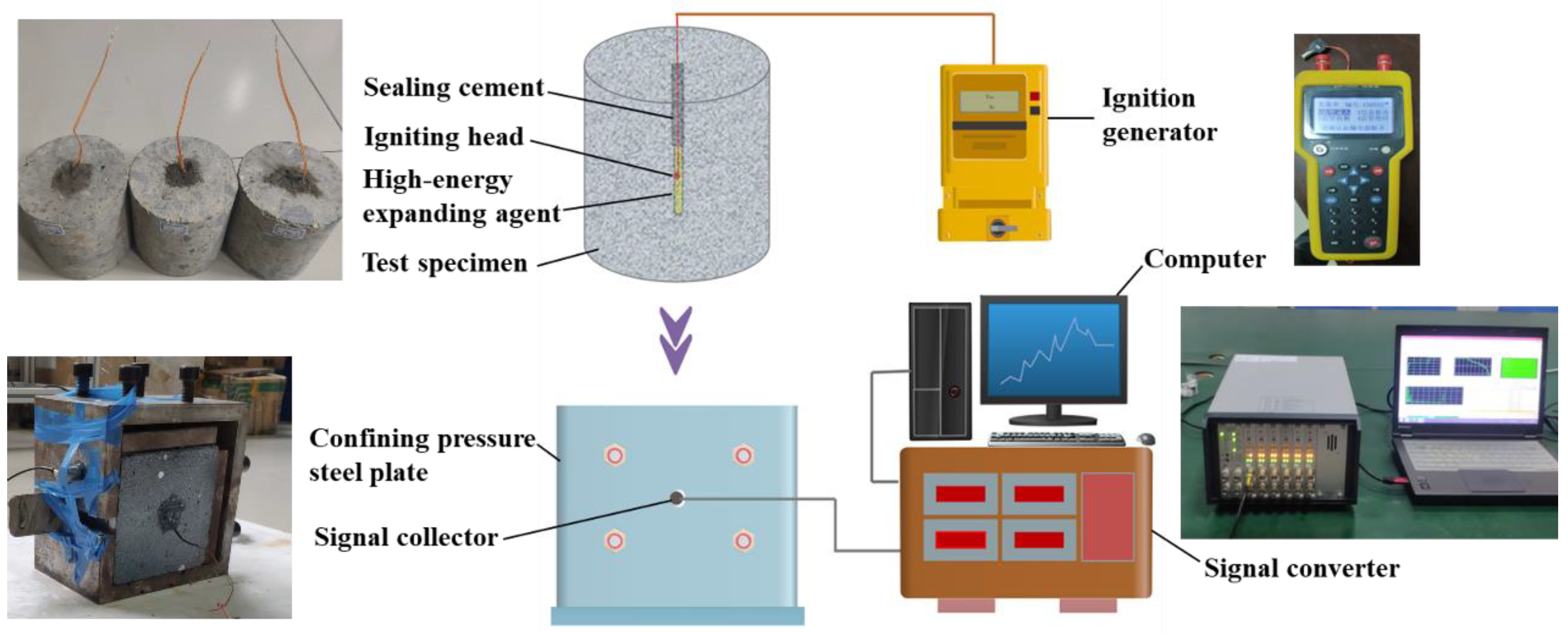
2.2. Testing Method
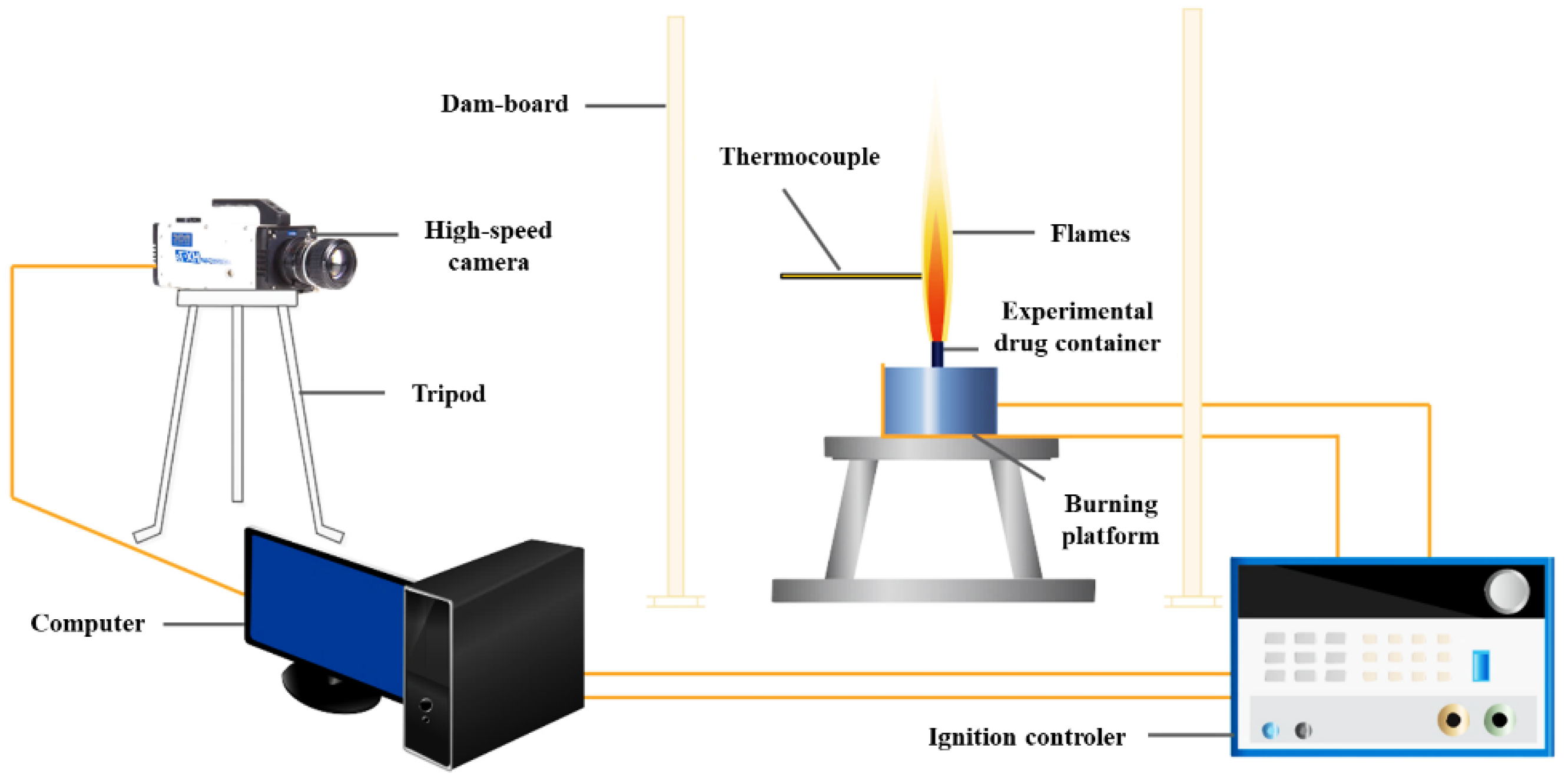
2.2.1. Combustion Flame Characteristic Test
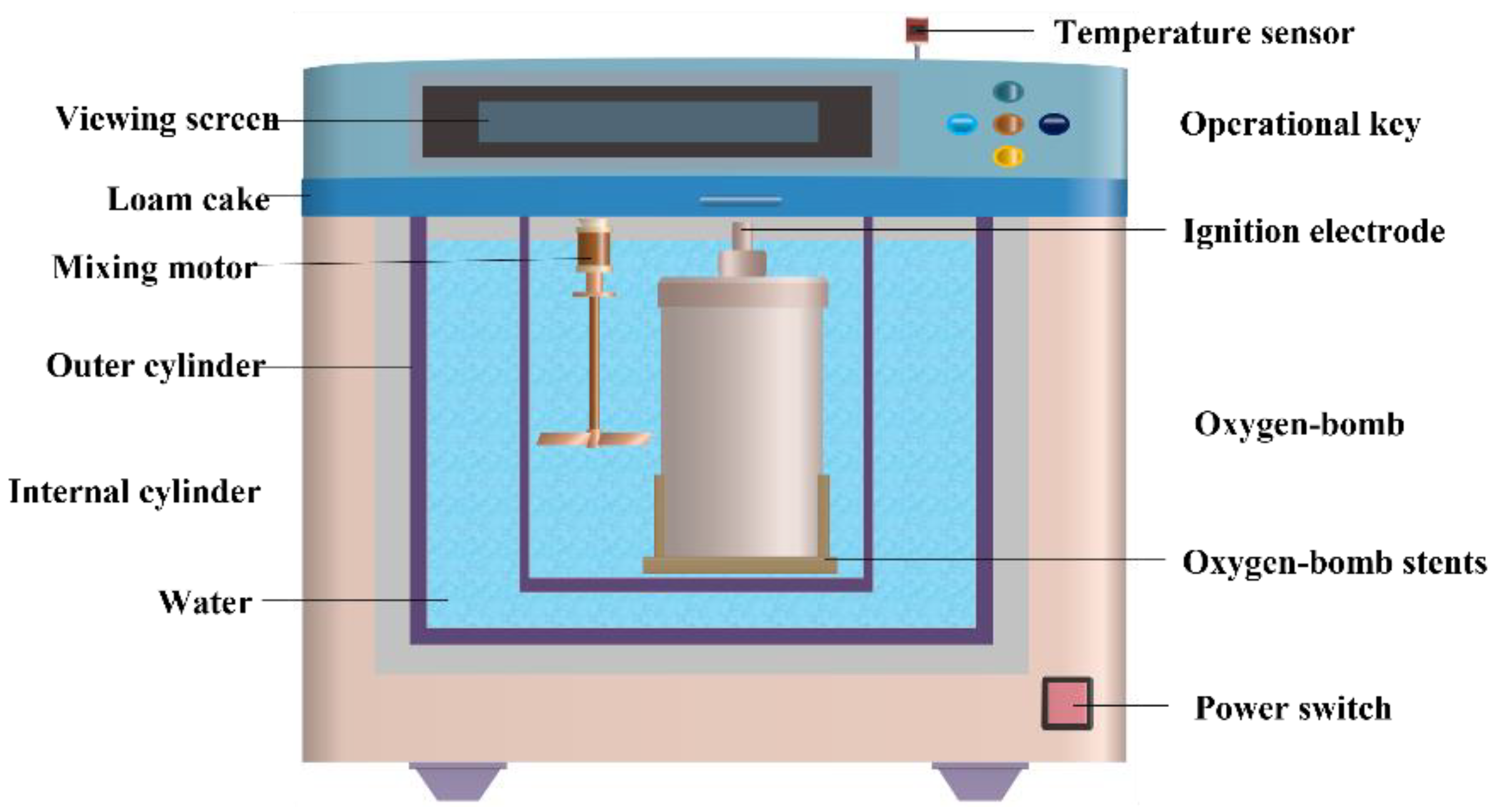
2.2.2. Combustion Heat Test
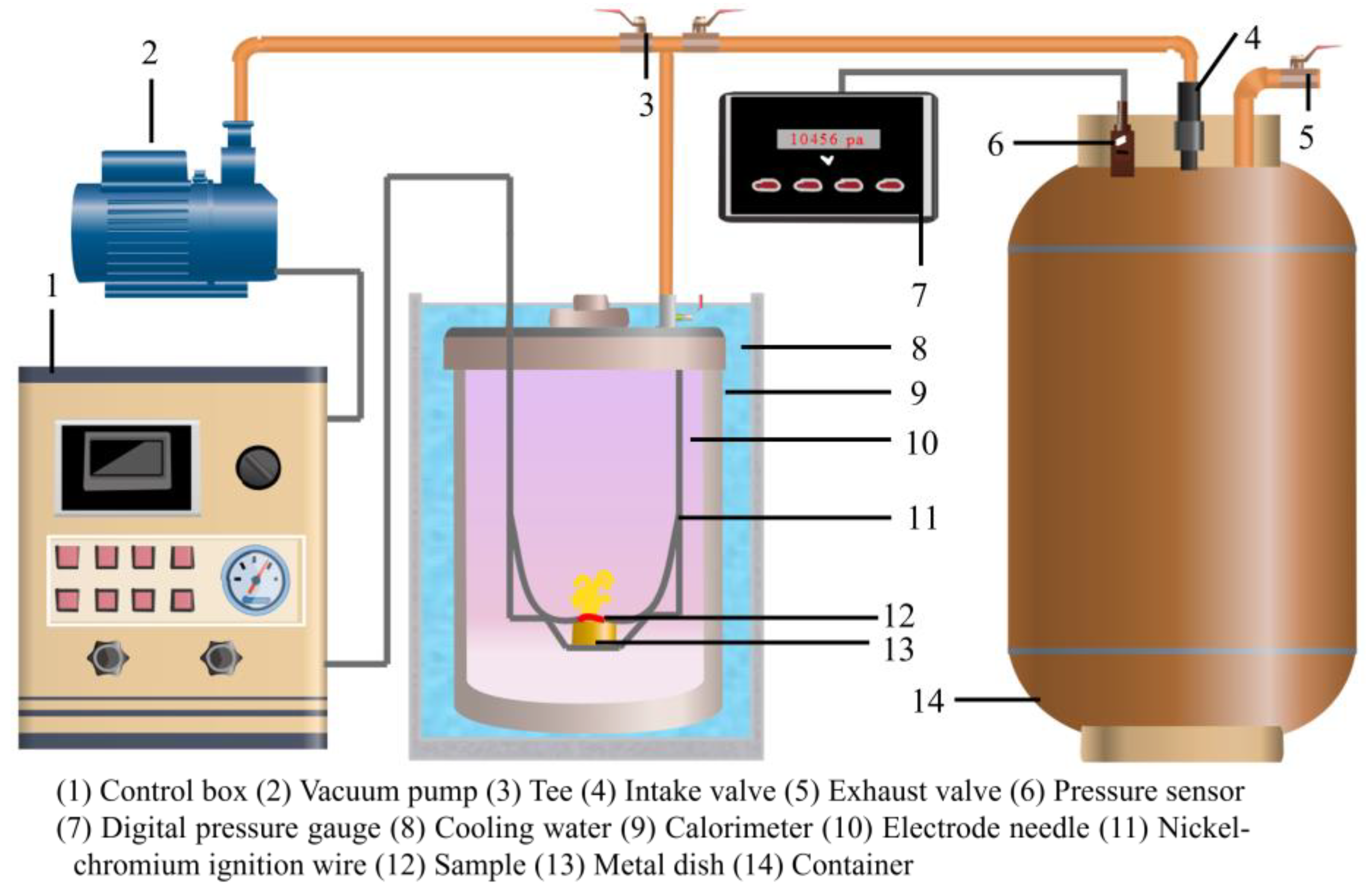
2.2.3. Gas Specific Volume Test
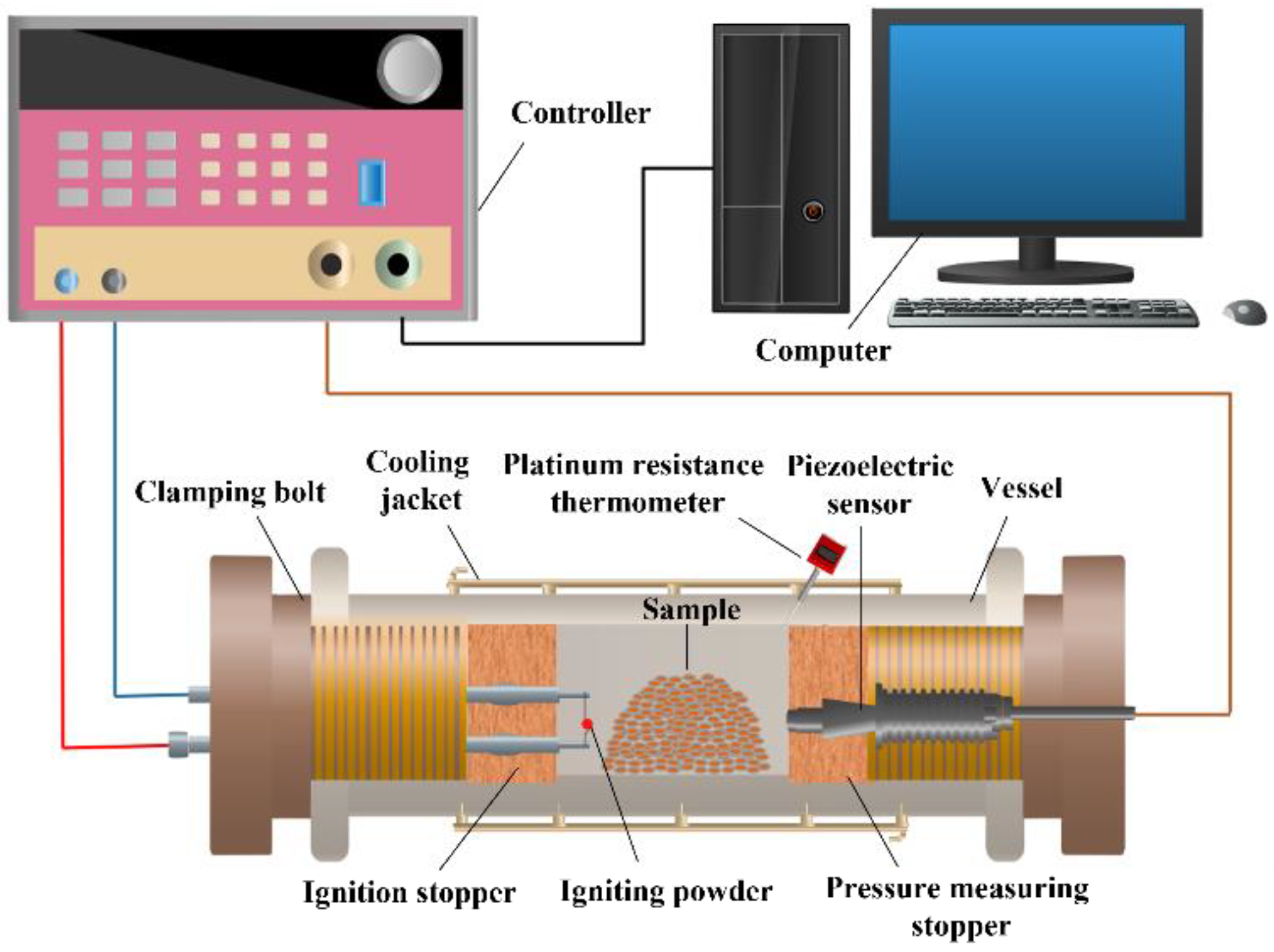
2.2.4. Explosion Pressure Test
3. Results and Discussion
3.1. Physicochemical Properties of High-Energy Expansion Agent
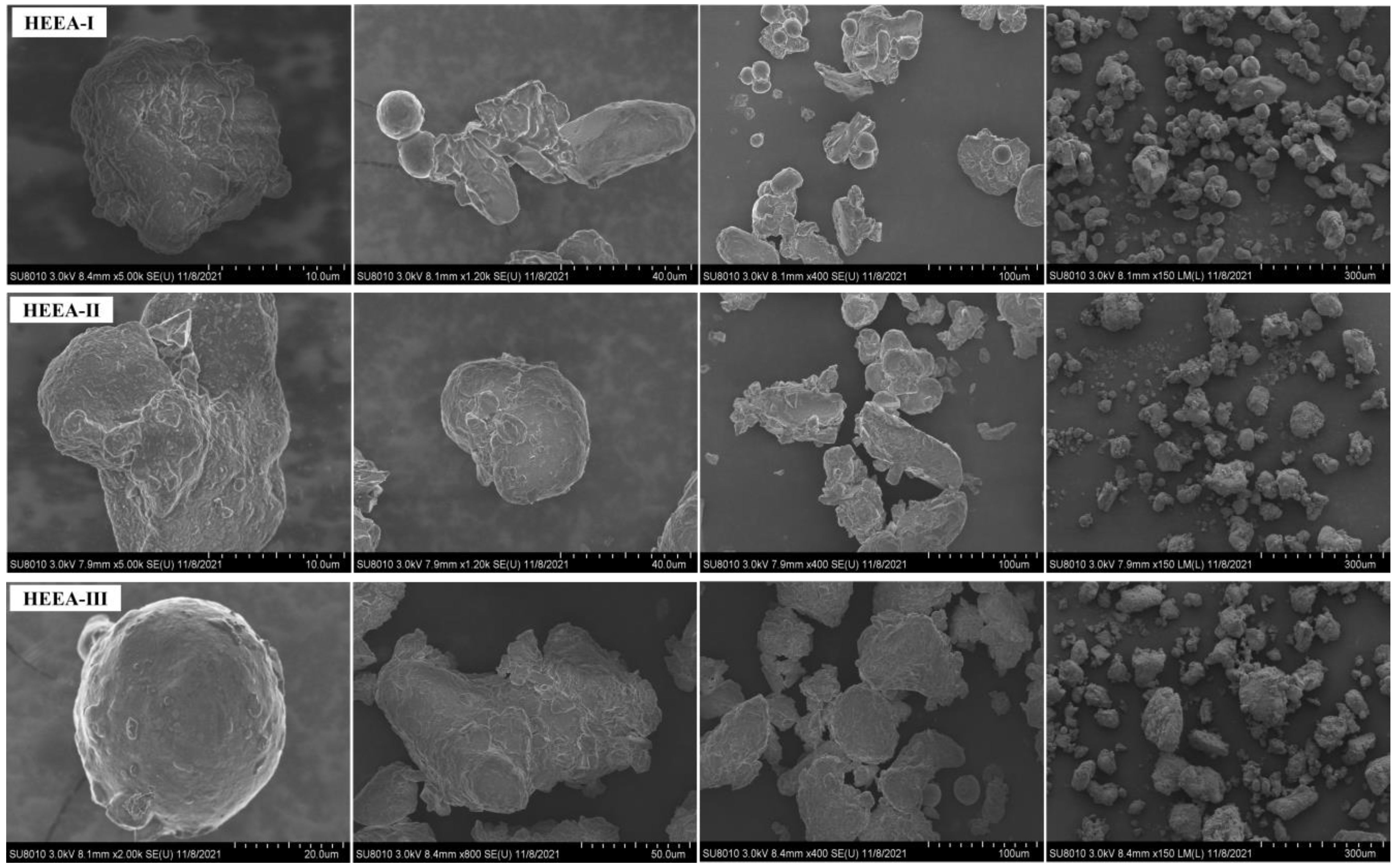
3.1.1. Structure and Morphology of High-Energy Expansion Agents
3.1.2. Particle Size Distribution of High-Energy Expansion Agents
3.1.3. Heat of Combustion and Gas Specific Volume of High-Energy Expansion Agents
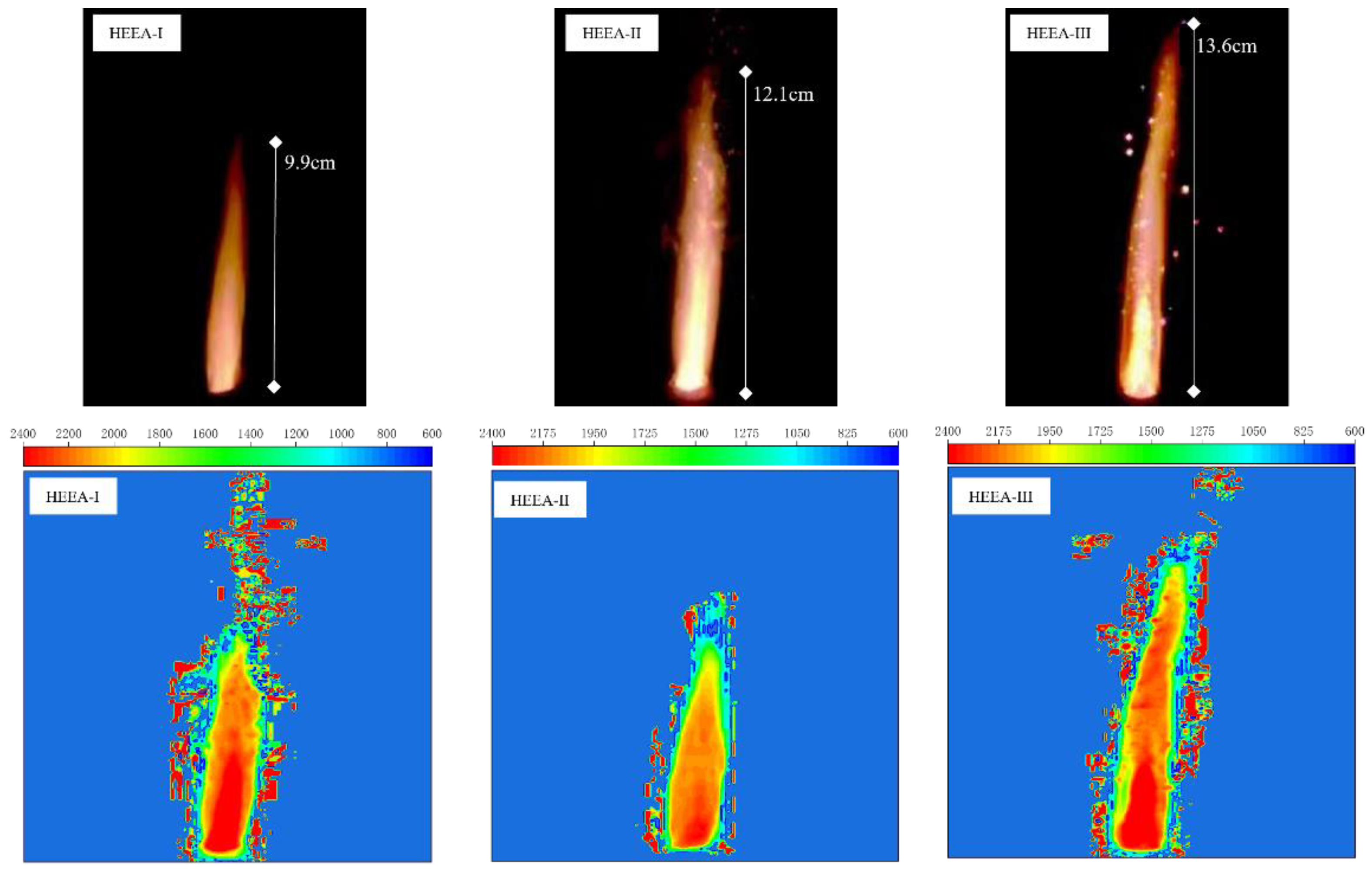
3.1.4. Combustion Flame Characteristics of High-Energy Expansion Agents
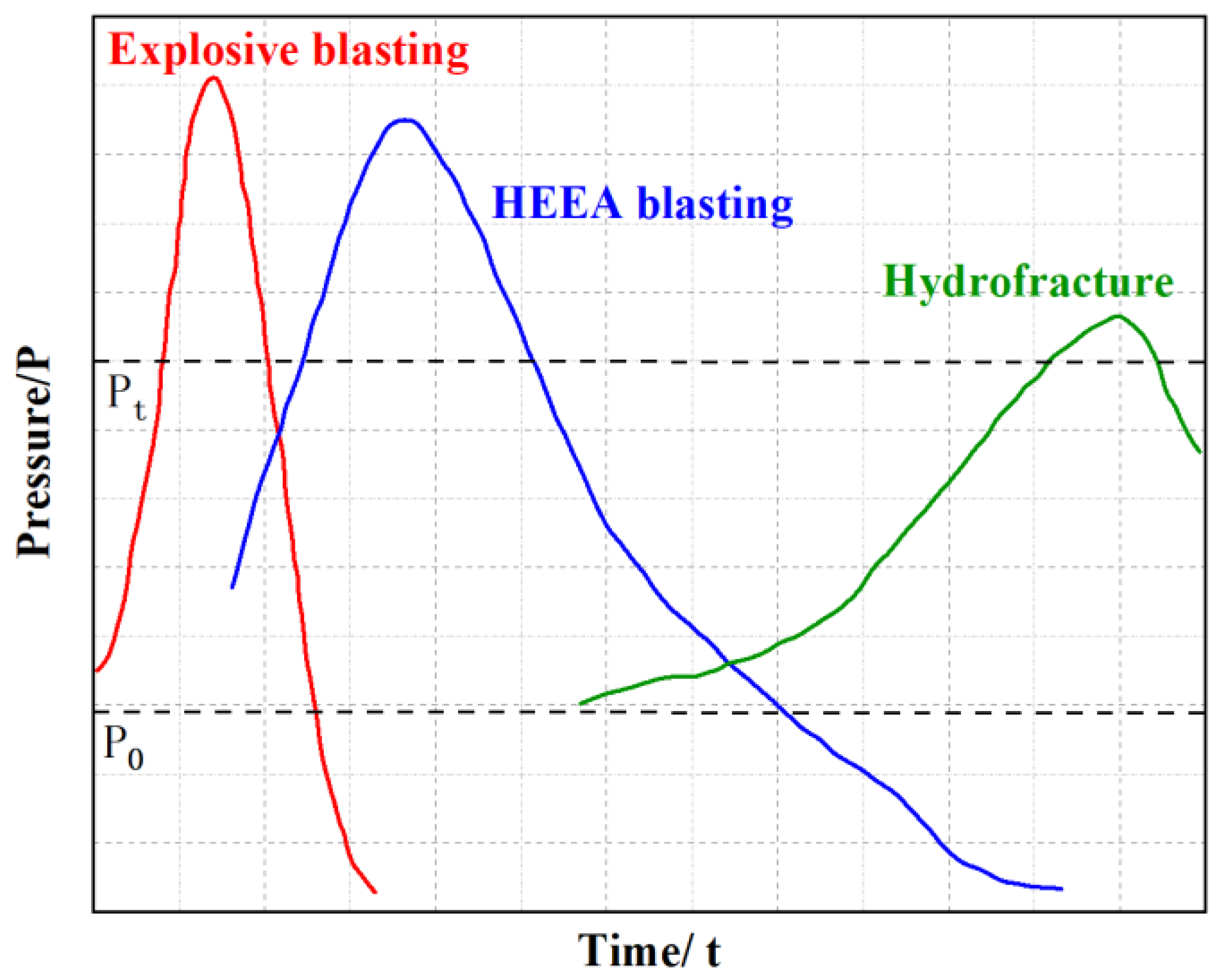
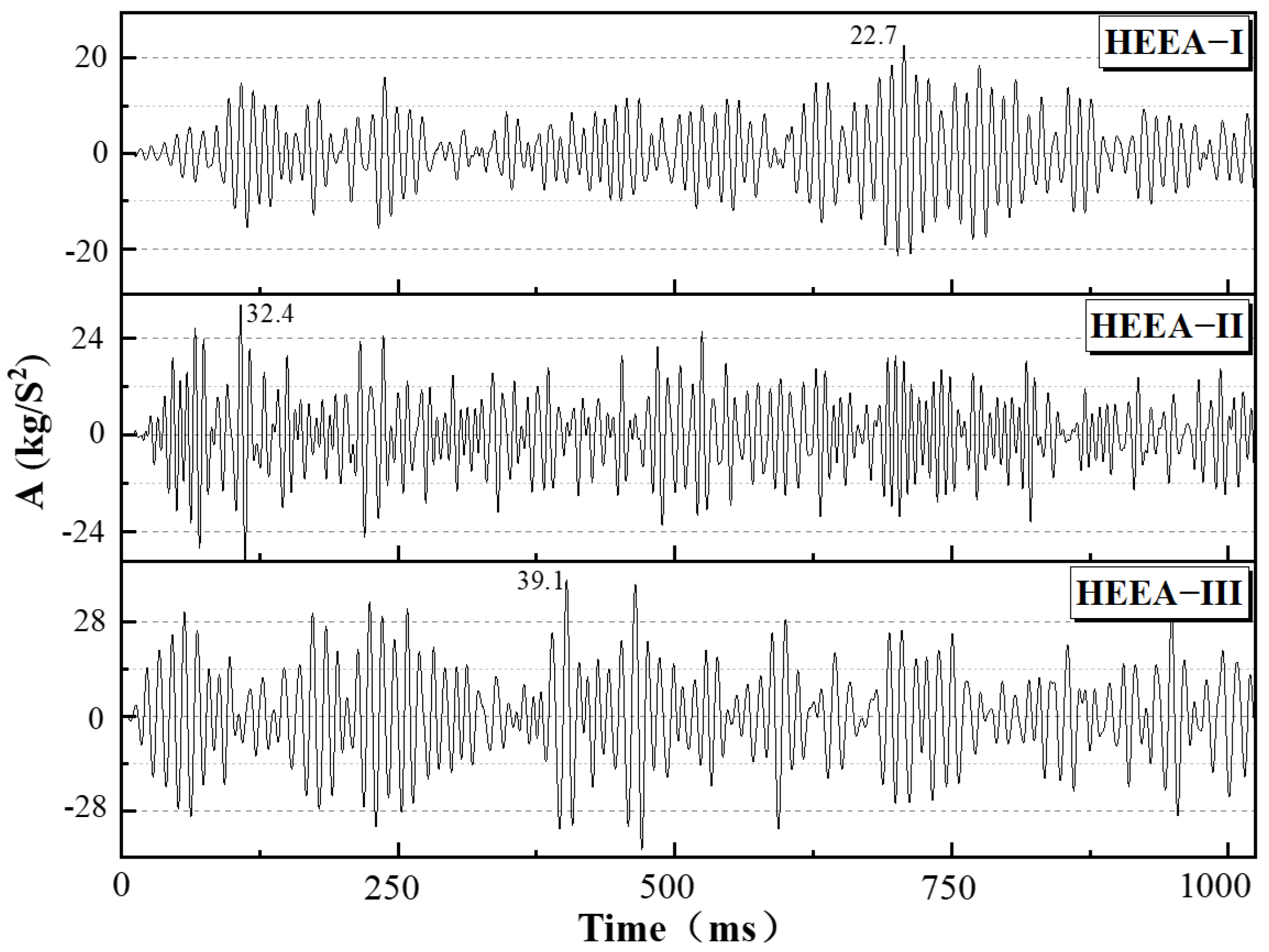
3.2. Explosion Pressure Law of High-Energy Expansion Agents
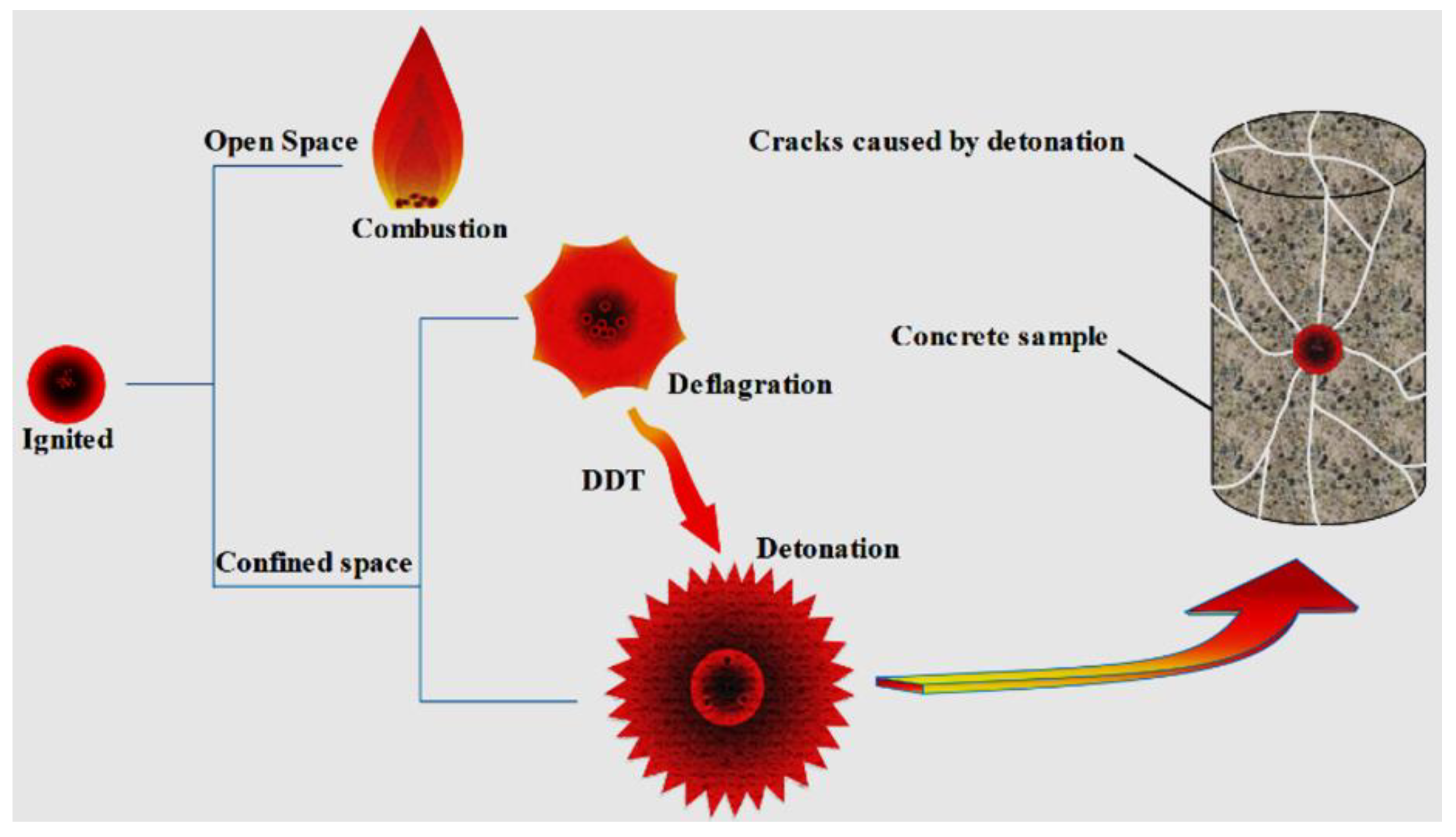
3.3. Damage Characterization of Rocks Fractured by High-Energy Expansion Agents
3.4. Damage Mechanism of Rock Cracked by High-Energy Expansion Agents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Cheng, Z.; Zou, Q.; Li, Z.; Sun, F.; Yang, H.; Lei, Y. Elimination of coal and gas outburst risk of an outburst-prone coal seam using controllable liquid CO2 phase transition fracturing. Fuel 2021, 284, 119091. [Google Scholar] [CrossRef]
- Lu, W.; Chen, M.; Geng, X.; Shu, D.; Zhou, C. A study of excavation sequence and contour blasting method for underground powerhouses of hydropower stations. Tunn. Undergr. Space Technol. 2012, 29, 31–39. [Google Scholar] [CrossRef]
- Onederra, I.A.; Furtney, J.K.; Sellers, E.; Iverson, S. Modelling blast induced damage from a fully coupled explosive charge. Int. J. Rock Mech. Min. Sci. 2013, 58, 73–84. [Google Scholar] [CrossRef]
- Dai, F.; Li, B.; Xu, N.; Zhu, Y. Microseismic early warning of surrounding rock mass deformation in the underground powerhouse of the Houziyan hydropower station, China. Tunn. Undergr. Space Technol. 2017, 62, 64–74. [Google Scholar] [CrossRef]
- Wang, H.; Li, G.; Shen, Z.; Tian, S.; Sun, B.; He, Z.; Lu, P. Experiment on rock breaking with supercritical carbon dioxide jet. J. Pet. Sci. Eng. 2015, 127, 305–310. [Google Scholar] [CrossRef]
- Zhuang, L.; Zang, A. Laboratory hydraulic fracturing experiments on crystalline rock for geothermal purposes. Earth-Sci. Rev. 2021, 216, 103580. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, W.; Su, X.; Li, J.; Li, Z.; Wen, L.; Chang, J. Numerical study of the impact mechanism of decoupling charge on blasting-enhanced permeability in low-permeability sandstones. Int. J. Rock Mech. Min. Sci. 2018, 106, 300–310. [Google Scholar] [CrossRef]
- Li, Z.; Yu, S.; Zhu, W.; Feng, G.; Xu, J.; Guo, Y.; Qi, T. Dynamic loading induced by the instability of voussoir beam structure during mining below the slope. Int. J. Rock Mech. Min. Sci. 2020, 132, 104343. [Google Scholar] [CrossRef]
- Shang, J.; Zhao, Z.; Aliyu, M.M. Stresses induced by a demolition agent in non-explosive rock fracturing. Int. J. Rock Mech. Min. Sci. 2018, 107, 172–180. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, J.; Zhai, H.; Fu, G.; Tian, L.; Liu, S. CO2 gas fracturing: A novel reservoir stimulation technology in low permeability gassy coal seams. Fuel 2017, 203, 197–207. [Google Scholar] [CrossRef]
- Li, Q.Y.; Chen, G.; Luo, D.Y.; Ma, H.P.; Liu, Y. An experimental study of a novel liquid carbon dioxide rock-breaking technology. Int. J. Rock Mech. Min. Sci. 2020, 128, 104244. [Google Scholar] [CrossRef]
- He, Y.; Flynn, S.L.; Folkerts, E.J.; Zhang, Y.; Ruan, D.; Alessi, D.S.; Martin, J.W.; Goss, G.G. Chemical and toxicological characterizations of hydraulic fracturing flowback and produced water. Water Res. 2017, 114, 78–87. [Google Scholar] [CrossRef]
- Luek, J.L.; Gonsior, M. Organic compounds in hydraulic fracturing fluids and wastewaters: A review. Water Res. 2017, 123, 536–548. [Google Scholar] [CrossRef]
- Zhigach, A.N.; Leipunskii, I.O.; Pivkina, A.N.; Muravyev, N.V.; Monogarov, K.A.; Kuskov, M.L.; Afanasenkova, E.S.; Berezkina, N.G.; Pshechenkov, P.A.; Bragin, A.A. Aluminum/HMX nanocomposites: Synthesis, microstructure, and combustion. Combust. Explos. Shock Waves 2015, 51, 100–106. [Google Scholar] [CrossRef]
- Yan, T.; Ren, H.; Liu, J.; Jiao, Q. Facile preparation and synergetic energy releasing of nano-Al@RDX@Viton hollow microspheres. Chem. Eng. J. 2020, 379, 122333. [Google Scholar] [CrossRef]
- Sun, H.; Li, X.; Wu, P.; Song, C.; Yang, Y. Preparation and Properties of RDX/Aluminum Composites by Spray-Drying Method. J. Nanomater. 2020, 2020, 1083267. [Google Scholar] [CrossRef]
- Hu, H.; Chen, L.; Yan, J.; Feng, H.; Xiao, C.; Song, P. Effect of Aluminum Powder on Underwater Explosion Performance of CL-20 Based Explosives. Propellants Explos. Pyrotech. 2019, 44, 837–843. [Google Scholar] [CrossRef]
- Li, X.; Pei, H.; Zhang, X.; Zheng, X. Effect of Aluminum Particle Size on the Performance of Aluminized Explosives. Propellants Explos. Pyrotech. 2020, 45, 807–813. [Google Scholar] [CrossRef]
- Kuznetsova, N.; Zhgun, D.; Golovanevskiy, V. Plasma blasting of rocks and rocks-like materials: An analytical model. Int. J. Rock Mech. Min. Sci. 2022, 150, 104986. [Google Scholar] [CrossRef]
- Cui, S.; Liu, S.; Li, H.; Zhou, F.; Sun, D. Critical parameters investigation of rock breaking by high-pressure foam fracturing method. Energy 2022, 258, 124871. [Google Scholar] [CrossRef]
- Wang, J.; Elsworth, D.; Cao, Y.; Liu, S. Reach and geometry of dynamic gas-driven fractures. Int. J. Rock Mech. Min. Sci. 2020, 129, 104287. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, J.; Li, B.; Zhu, C. Crack propagation patterns and factors controlling complex crack network formation in coal bodies during tri-axial supercritical carbon dioxide fracturing. Fuel 2021, 286, 119381. [Google Scholar] [CrossRef]
- Deng, B.; Yin, G.; Li, M.; Zhang, D.; Lu, J.; Liu, Y.; Chen, J. Feature of fractures induced by hydrofracturing treatment using water and L-CO2 as fracturing fluids in laboratory experiments. Fuel 2018, 226, 35–46. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, Q.; Zhang, Z.; Yang, W.; Gou, S.; Huang, J.; Fan, G. Combustion and catalytic performance of metal-free heat-resistant energetic polymeric materials. Chem. Eng. J. 2020, 399, 125739. [Google Scholar] [CrossRef]
- Ye, R.; Yu, Y.G.; Cao, Y.J. Analysis of Micro-scale Flame Structure of AP/HTPB Base Bleed Propellant Combustion. Def. Technol. 2013, 9, 217–223. [Google Scholar] [CrossRef]
- Zha, K.; Florea, R.; Jansons, M. Soot Evolution with Cyclic Crank-angle-resolved Two-color Thermometry in an Opti-cal Diesel Engine Fueled with Biodiesel Blend and ULSD. J. Eng. Gas Turbines Power 2012, 88, 1620–1628. [Google Scholar]
- Wu, F.; Wei, X.; Chen, Z.; Rahman, S.S.; Pu, C.; Li, X.; Zhang, Y. Numerical simulation and parametric analysis for designing High Energy Gas Fracturing. J. Nat. Gas Sci. Eng. 2018, 53, 218–236. [Google Scholar] [CrossRef]





| Component | Oxidant | Fuel | Combustion Regulator | Adhesive | Plasticizer |
|---|---|---|---|---|---|
| HEEA-I | KClO4 (50.9%) | C-powder (4.4%) CH6N4O3 (42.7%) | Cu2Cr2O5 (1.5%) | HTPB (1%) | C26H50O4 (1.5%) |
| HEEA-II | KClO4 (68.1%) | Si-powder (27.9%) | Cu2Cr2O5 (1.5%) | HTPB (1%) | C26H50O4 (1.5%) |
| HEEA-III | KClO4 (64.8%) | Al-powder (31.2%) | Cu2Cr2O5 (1.5%) | HTPB (1%) | C26H50O4 (1.5%) |
| Test Specimen | Density (g/cm3) | Uniaxial Compressive Strength (MPa) | Uniaxial Tensile Strength (MPa) | Elastic Modulus (GPa) | Poisson’s Ratio | Internal Friction Angle (°) | Cohesion (MPa) |
|---|---|---|---|---|---|---|---|
| Concrete | 2.3 | 42.6 | 5.2 | 13.6 | 0.12 | 34.87 | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Cai, L.; Shi, J.; Wei, X. Study on Physicochemical Properties and Rock-Cracking Mechanism of High-Energy Expansion Agent. Energies 2022, 15, 7156. https://doi.org/10.3390/en15197156
Zhang Y, Cai L, Shi J, Wei X. Study on Physicochemical Properties and Rock-Cracking Mechanism of High-Energy Expansion Agent. Energies. 2022; 15(19):7156. https://doi.org/10.3390/en15197156
Chicago/Turabian StyleZhang, Yansong, Li Cai, Jing Shi, and Xiangrui Wei. 2022. "Study on Physicochemical Properties and Rock-Cracking Mechanism of High-Energy Expansion Agent" Energies 15, no. 19: 7156. https://doi.org/10.3390/en15197156
APA StyleZhang, Y., Cai, L., Shi, J., & Wei, X. (2022). Study on Physicochemical Properties and Rock-Cracking Mechanism of High-Energy Expansion Agent. Energies, 15(19), 7156. https://doi.org/10.3390/en15197156






