Redox Cycles, Active Materials, and Reactors Applied to Water and Carbon Dioxide Splitting for Solar Thermochemical Fuel Production: A Review
Abstract
1. Introduction
2. H2O and CO2 Splitting by Direct Thermolysis
- The design of solar reactors able to withstand repeated thermal constraints is challenging, given that the materials that can potentially be thermally-resistant at such high temperatures are costly and not widely available.
- The reverse reaction (recombination of products) may be avoided, either by the means of H2 (or CO) and O2 separation at a very high temperature (thereby avoiding a potentially explosive mixture), or by carrying out a fast quenching of this gaseous species mixture. At present, there does not exist any available technology that would be able to address these points.
- Because radiative heat losses vary with the fourth power of temperature, it is not realistic to achieve high energy conversion efficiencies for this kind of high-temperature process.
- Concentrated solar energy is the only renewable heat source that could be employed to reach such high levels of temperature. However, directly heating a gas (such as H2O or CO2) with solar radiation is difficult although these gas species absorb at given wavelengths a fraction of the incident solar spectrum. Therefore, the process complexity has to be increased, for instance, with the addition of particles for promoting absorption and diffusion of solar radiation.
3. H2O and CO2 Splitting by Thermochemical Redox Cycles
3.1. Context
3.2. Cycles Based on Volatile Oxides
3.2.1. ZnO/Zn Redox Pair
- The reduction step temperature is elevated (above 2000 K).
- The quenching of gases from >2000 K to >1200 K is a key step during the reduction: its efficiency is decisive for the zinc oxidation step (depending on both size of zinc particles and purity) and global process energy efficiency.
- The re-oxidation step is limited by a passivation phenomenon arising from the formation of a ZnO layer at the particle surface.
3.2.2. SnO2/SnO Redox Pair
- The reduction step temperature is below the one of the ZnO/Zn cycle and could be further decreased by a total pressure decrease inside the reaction chamber. In addition, the decrease in the operating temperature directly results in a higher energy efficiency [83] and thus a more favorable economic viability of the process.
- The dissociation rate of the oxide is significant and is less impacted by the gas quenching stage at the reactor outlet in comparison with zinc oxide (a product with 72% purity is obtained, against 48% for ZnO [84]). Indeed, the zinc boiling point is 907 °C, while that of SnO is 1425 °C; then, prompt condensation of the latter is facilitated.
- Eventually, SnO is a reactive compound for steam reduction.
3.3. Cycles Based on Non-Volatile Oxides
3.4. Cycles Based on Non-Stoichiometric Oxides
3.4.1. Cycles Based on Ferrites
3.4.2. Cycles Based on Ceria
- The ceria formulations can further be optimized (incorporation of several new dopants, utilization of composite materials [136], etc.).
- When the material is used in the form of a powder, the considered synthesis methods warranting high-temperature material resistance must be preferred.
- When the reacting material is structured (porous architecture), thermally-resistant supports (substrates) and efficient coating methods are needed. Alternatively, original methods need to be developed for the elaboration of architectured porous structures (e.g., biomimetics, 3D printing, etc.) [137,138,139,140,141,142].
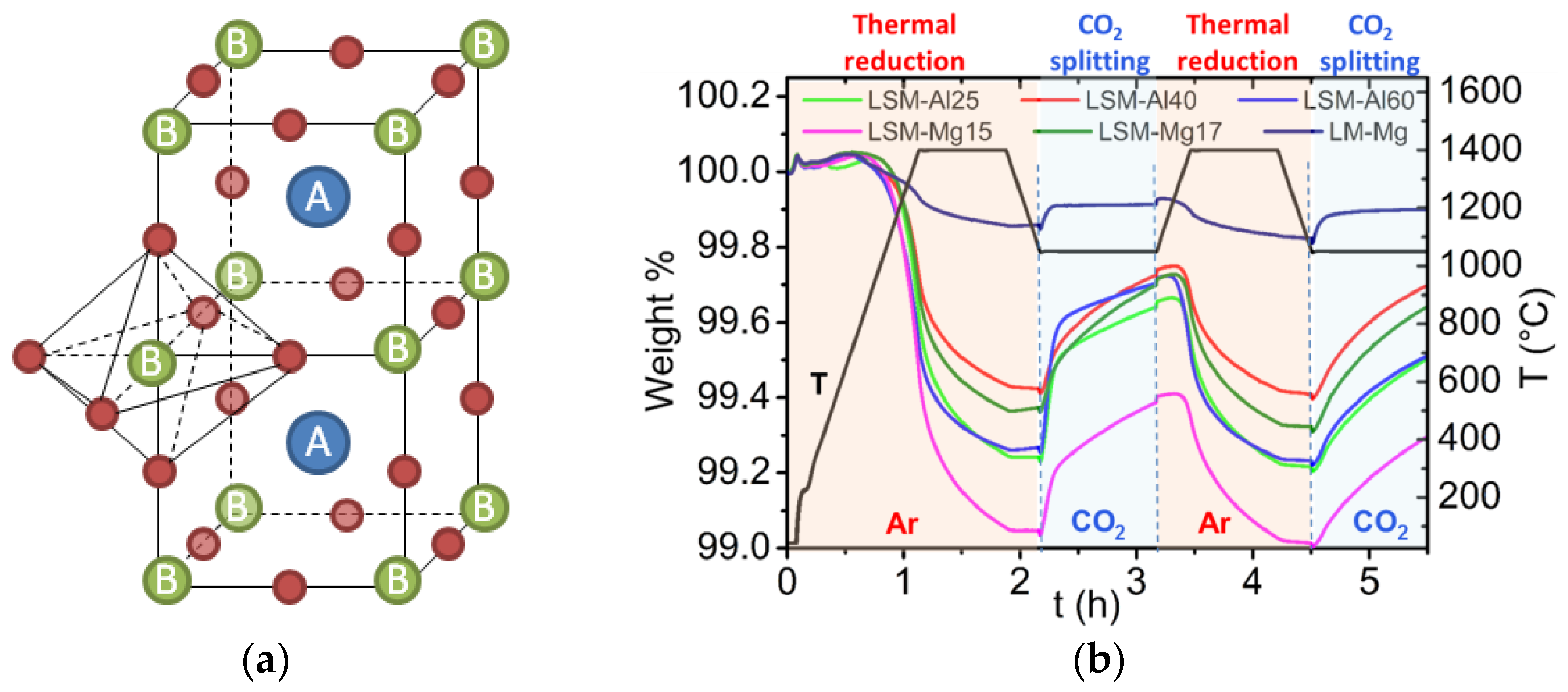
3.4.3. Cycles Based on Perovskites
4. Solar Reactor Technologies Developed for Redox Cycles
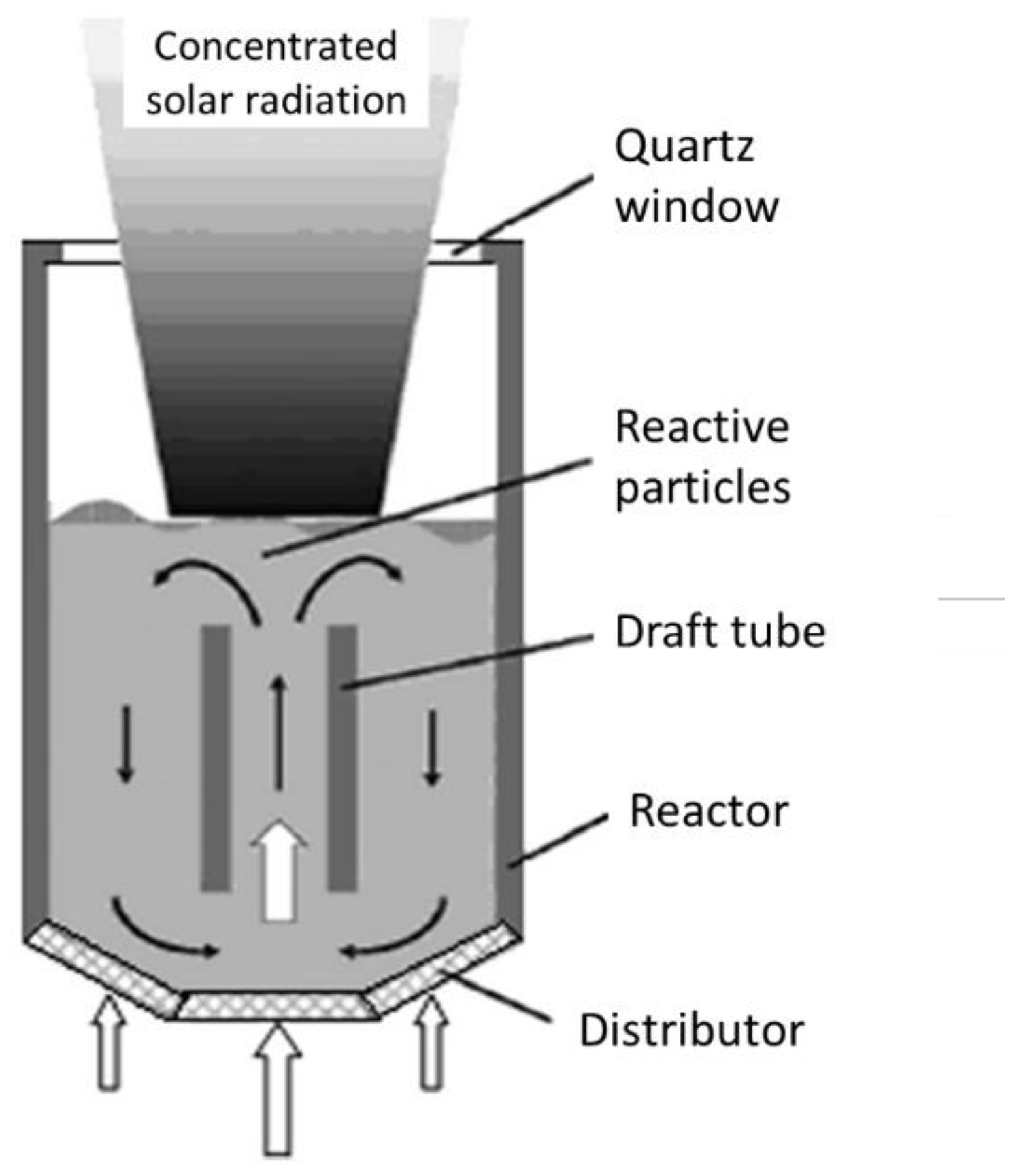
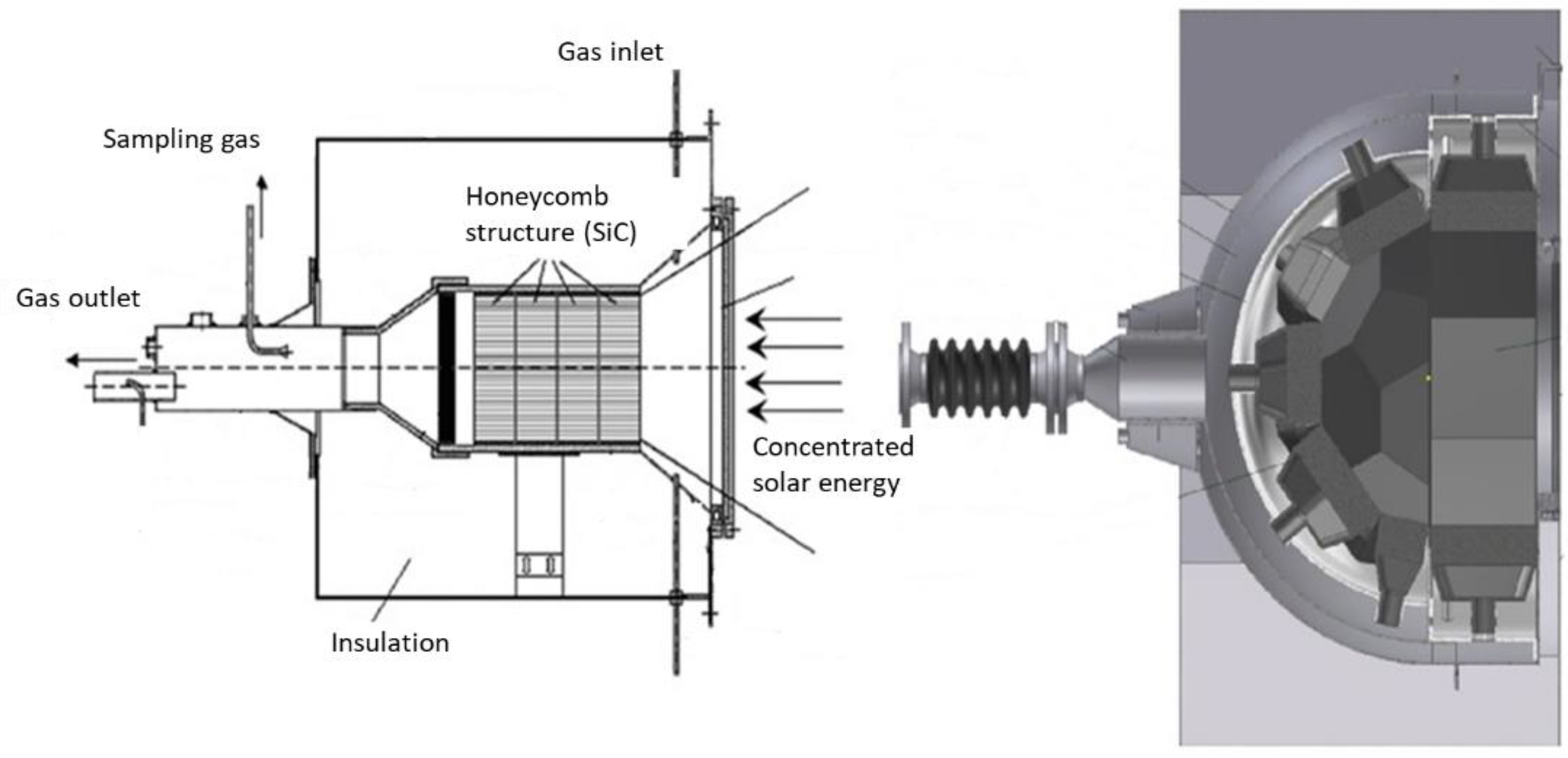
4.1. Single-Chamber Solar Reactors
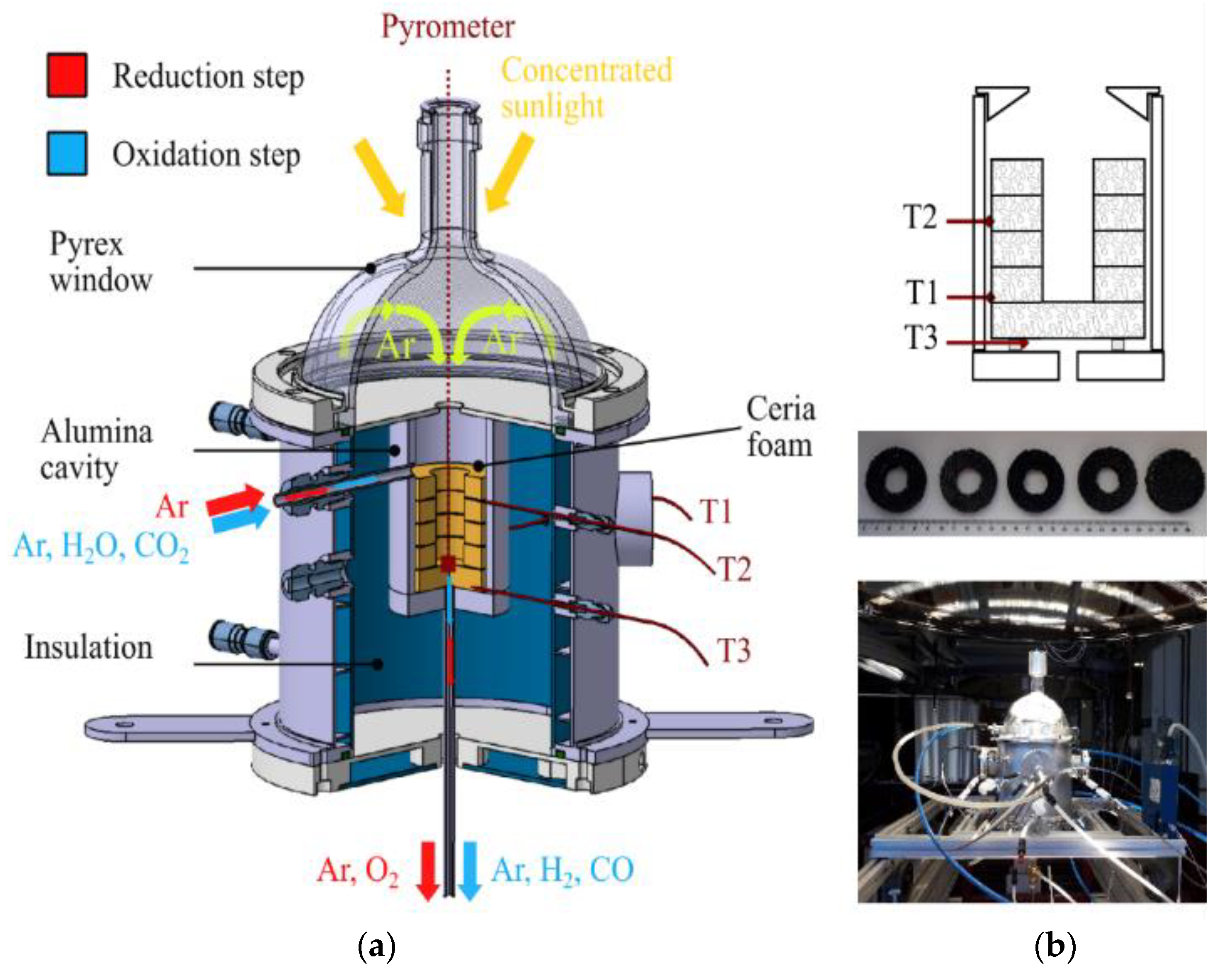
4.2. Decoupled Reactors for Separated Reduction and Oxidation Steps
5. Conclusions
- The volatile oxide cycles offer the advantages of relatively high specific fuel productivity due to discrete oxidation state transitions (leading to high oxygen amounts being released and recovered at each cycle), and of their specific surface area being renewed during each cycle thanks to the vaporization/condensation mechanism. However, it is difficult to prevent products’ recombination during gas cooling in the reduction step. ZnO/Zn and SnO2/SnO thermochemical cycles are the most attractive (with potential maximum fuel productivity up to ~12.3 mmol/g for ZnO and 6.7 mmol/g for SnO2).
- Non-volatile oxide cycles mainly relate to non-stoichiometric oxides. They allow a lower reduction step temperature, absence of phase change during cycling, and the possibility to carry out the two reactions of the cycle in the same reactor without any reactant transfer. However, they feature a lower mass-specific fuel productivity (in the range 0.1–0.4 mmol/g) than for the stoichiometric oxides (due to a limited amount of oxygen being exchanged during solid-state reactions, as represented by the lattice oxygen sub-stoichiometry δ), a possible loss of specific surface area during the cycling (due to sintering), and the need to optimize the formulations and the methods for the materials synthesis.
Funding
Data Availability Statement
Conflicts of Interest
References
- Safari, F.; Dincer, I. A Review and Comparative Evaluation of Thermochemical Water Splitting Cycles for Hydrogen Production. Energy Convers. Manag. 2020, 205, 112182. [Google Scholar] [CrossRef]
- Rozzi, E.; Minuto, F.D.; Lanzini, A.; Leone, P. Green Synthetic Fuels: Renewable Routes for the Conversion of Non-Fossil Feedstocks into Gaseous Fuels and Their End Uses. Energies 2020, 13, 420. [Google Scholar] [CrossRef]
- Mao, Y.; Gao, Y.; Dong, W.; Wu, H.; Song, Z.; Zhao, X.; Sun, J.; Wang, W. Hydrogen Production via a Two-Step Water Splitting Thermochemical Cycle Based on Metal Oxide—A Review. Appl. Energy 2020, 267, 114860. [Google Scholar] [CrossRef]
- Davis, S.J.; Lewis, N.S.; Shaner, M.; Aggarwal, S.; Arent, D.; Azevedo, I.L.; Benson, S.M.; Bradley, T.; Brouwer, J.; Chiang, Y.-M.; et al. Net-Zero Emissions Energy Systems. Science 2018, 360, eaas9793. [Google Scholar] [CrossRef] [PubMed]
- Fedunik-Hofman, L.; Bayon, A.; Donne, S.W. Comparative Kinetic Analysis of CaCO3/CaO Reaction System for Energy Storage and Carbon Capture. Appl. Sci. 2019, 9, 4601. [Google Scholar] [CrossRef]
- Ji, G.; Yang, H.; Memon, M.Z.; Gao, Y.; Qu, B.; Fu, W.; Olguin, G.; Zhao, M.; Li, A. Recent Advances on Kinetics of Carbon Dioxide Capture Using Solid Sorbents at Elevated Temperatures. Appl. Energy 2020, 267, 114874. [Google Scholar] [CrossRef]
- Cheng, W.-H.; de la Calle, A.; Atwater, H.A.; Stechel, E.B.; Xiang, C. Hydrogen from Sunlight and Water: A Side-by-Side Comparison between Photoelectrochemical and Solar Thermochemical Water-Splitting. ACS Energy Lett. 2021, 6, 3096–3113. [Google Scholar] [CrossRef]
- Boretti, A. Technology Readiness Level of Solar Thermochemical Splitting Cycles. ACS Energy Lett. 2021, 6, 1170–1174. [Google Scholar] [CrossRef]
- Carrillo, R.J.; Scheffe, J.R. Advances and Trends in Redox Materials for Solar Thermochemical Fuel Production. Sol. Energy 2017, 156, 3–20. [Google Scholar] [CrossRef]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S.J.; Saravanan, A. A Review on Photochemical, Biochemical and Electrochemical Transformation of CO2 into Value-Added Products. J. CO2 Util. 2019, 33, 131–147. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. An Overview of Solar Decarbonization Processes, Reacting Oxide Materials, and Thermochemical Reactors for Hydrogen and Syngas Production. Int. J. Hydrogen Energy 2020, 45, 25783–25810. [Google Scholar] [CrossRef]
- Krenzke, P.T.; Fosheim, J.R.; Davidson, J.H. Solar Fuels via Chemical-Looping Reforming. Sol. Energy 2017, 156, 48–72. [Google Scholar] [CrossRef]
- Bulfin, B.; Vieten, J.; Agrafiotis, C.; Roeb, M.; Sattler, C. Applications and Limitations of Two Step Metal Oxide Thermochemical Redox Cycles; a Review. J. Mater. Chem. A 2017, 5, 18951–18966. [Google Scholar] [CrossRef]
- Li, S.; Wheeler, V.M.; Kumar, A.; Venkataraman, M.B.; Muhich, C.L.; Hao, Y.; Lipiński, W. Thermodynamic Guiding Principles for Designing Nonstoichiometric Redox Materials for Solar Thermochemical Fuel Production: Ceria, Perovskites, and Beyond. Energy Technol. 2021, 10, 2000925. [Google Scholar] [CrossRef]
- Romero, M.; Steinfeld, A. Concentrating Solar Thermal Power and Thermochemical Fuels. Energy Environ. Sci. 2012, 5, 9234. [Google Scholar] [CrossRef]
- Jensen, R.; Lyman, J.; King, J.; Guettler, R. Solar Reduction of CO2. U.S. Patent US6066187A, 23 May 2000. [Google Scholar]
- Bhatta, S.; Nagassou, D.; Mohsenian, S.; Trelles, J.P. Photo-Thermochemical Decomposition of Carbon-Dioxide in a Direct Solar Receiver-Reactor. Sol. Energy 2019, 178, 201–214. [Google Scholar] [CrossRef]
- Bhatta, S.; Nagassou, D.; Trelles, J.P. Solar Photo-Thermochemical Reactor Design for Carbon Dioxide Processing. Sol. Energy 2017, 142, 253–266. [Google Scholar] [CrossRef]
- Funk, J.E.; Reinstrom, R.M. Energy Requirements in Production of Hydrogen from Water. Ind. Eng. Chem. Proc. Des. Dev. 1966, 5, 336–342. [Google Scholar] [CrossRef]
- Abanades, S.; Charvin, P.; Flamant, G.; Neveu, P. Screening of Water-Splitting Thermochemical Cycles Potentially Attractive for Hydrogen Production by Concentrated Solar Energy. Energy 2006, 31, 2805–2822. [Google Scholar] [CrossRef]
- Wijewardane, S. Inventions, Innovations and New Technologies—Solar Thermochemical Fuels. Sol. Compass 2022, 2, 100024. [Google Scholar] [CrossRef]
- Agrafiotis, C.; Roeb, M.; Sattler, C. A Review on Solar Thermal Syngas Production via Redox Pair-Based Water/Carbon Dioxide Splitting Thermochemical Cycles. Renew. Sustain. Energy Rev. 2015, 42, 254–285. [Google Scholar] [CrossRef]
- Muhich, C.L.; Ehrhart, B.D.; Al-Shankiti, I.; Ward, B.J.; Musgrave, C.B.; Weimer, A.W. A Review and Perspective of Efficient Hydrogen Generation via Solar Thermal Water Splitting: A Review and Perspective of Efficient Hydrogen Generation. WIREs Energy Environ. 2016, 5, 261–287. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Lemort, F.; Flamant, G. Analysis of Solar Chemical Processes for Hydrogen Production from Water Splitting Thermochemical Cycles. Energy Convers. Manag. 2008, 49, 1547–155610. [Google Scholar] [CrossRef]
- Graf, D.; Monnerie, N.; Roeb, M.; Schmitz, M.; Sattler, C. Economic Comparison of Solar Hydrogen Generation by Means of Thermochemical Cycles and Electrolysis. Int. J. Hydrogen Energy 2008, 33, 4511–4519. [Google Scholar] [CrossRef]
- Yadav, D.; Banerjee, R. A Review of Solar Thermochemical Processes. Renew. Sustain. Energy Rev. 2016, 54, 497–532. [Google Scholar] [CrossRef]
- Moser, M.; Pecchi, M.; Fend, T. Techno-Economic Assessment of Solar Hydrogen Production by Means of Thermo-Chemical Cycles. Energies 2019, 12, 352. [Google Scholar] [CrossRef]
- Onigbajumo, A.; Swarnkar, P.; Will, G.; Sundararajan, T.; Taghipour, A.; Couperthwaite, S.; Steinberg, T.; Rainey, T. Techno-Economic Evaluation of Solar-Driven Ceria Thermochemical Water-Splitting for Hydrogen Production in a Fluidized Bed Reactor. J. Clean. Prod. 2022, 371, 133303. [Google Scholar] [CrossRef]
- Ma, Z.; Davenport, P.; Saur, G. System and Technoeconomic Analysis of Solar Thermochemical Hydrogen Production. Renew. Energy 2022, 190, 294–308. [Google Scholar] [CrossRef]
- Prats-Salvado, E.; Monnerie, N.; Sattler, C. Synergies between Direct Air Capture Technologies and Solar Thermochemical Cycles in the Production of Methanol. Energies 2021, 14, 4818. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. CO2-Based Energy Vectors for the Storage of Solar Energy. Greenhouse Gas Sci. Technol. 2011, 1, 21–35. [Google Scholar] [CrossRef]
- Dry, M.E. The Fischer–Tropsch Process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Espinoza, R.L.; Steynberg, A.P.; Jager, B.; Vosloo, A.C. Low Temperature Fischer–Tropsch Synthesis from a Sasol Perspective. Appl. Catal. A Gen. 1999, 186, 13–26. [Google Scholar] [CrossRef]
- Steynberg, A.P.; Espinoza, R.L.; Jager, B.; Vosloo, A.C. High Temperature Fischer–Tropsch Synthesis in Commercial Practice. Appl. Catal. A Gen. 1999, 186, 41–54. [Google Scholar] [CrossRef]
- Abanades, S. Metal Oxides Applied to Thermochemical Water-Splitting for Hydrogen Production Using Concentrated Solar Energy. ChemEngineering 2019, 3, 63. [Google Scholar] [CrossRef]
- Scheffe, J.R.; Steinfeld, A. Oxygen Exchange Materials for Solar Thermochemical Splitting of H2O and CO2: A Review. Mater. Today 2014, 17, 341–348. [Google Scholar] [CrossRef]
- Xiao, L.; Wu, S.-Y.; Li, Y.-R. Advances in Solar Hydrogen Production via Two-Step Water-Splitting Thermochemical Cycles Based on Metal Redox Reactions. Renew. Energy 2012, 41, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Sun, X.; Song, Q.; Yang, Z.; Wang, H.; Duan, Y. A Critical Review on Integrated System Design of Solar Thermochemical Water-Splitting Cycle for Hydrogen Production. Int. J. Hydrogen Energy 2022, in press. [CrossRef]
- Oudejans, D.; Offidani, M.; Constantinou, A.; Albonetti, S.; Dimitratos, N.; Bansode, A. A Comprehensive Review on Two-Step Thermochemical Water Splitting for Hydrogen Production in a Redox Cycle. Energies 2022, 15, 3044. [Google Scholar] [CrossRef]
- Roeb, M.; Neises, M.; Monnerie, N.; Call, F.; Simon, H.; Sattler, C.; Schmücker, M.; Pitz-Paal, R. Materials-Related Aspects of Thermochemical Water and Carbon Dioxide Splitting: A Review. Materials 2012, 5, 2015–2054. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Loutzenhiser, P.G.; Hischier, I.; Steinfeld, A. CO2 Splitting via Two-Step Solar Thermochemical Cycles with Zn/ZnO and FeO/Fe3O4 Redox Reactions: Thermodynamic Analysis. Energy Fuels 2008, 22, 3544–3550. [Google Scholar] [CrossRef]
- Falter, C.; Sizmann, A. Solar Thermochemical Hydrogen Production in the USA. Sustainability 2021, 13, 7804. [Google Scholar] [CrossRef]
- Falter, C.; Scharfenberg, N.; Habersetzer, A. Geographical Potential of Solar Thermochemical Jet Fuel Production. Energies 2020, 13, 802. [Google Scholar] [CrossRef]
- Falter, C.; Valente, A.; Habersetzer, A.; Iribarren, D.; Dufour, J. An Integrated Techno-Economic, Environmental and Social Assessment of the Solar Thermochemical Fuel Pathway. Sustain. Energy Fuels 2020, 4, 3992–4002. [Google Scholar] [CrossRef]
- Steinfeld, A. Solar Hydrogen Production via a Two-Step Water-Splitting Thermochemical Cycle Based on Zn/ZnO Redox Reactions. Int. J. Hydrogen Energy 2002, 27, 611–619. [Google Scholar] [CrossRef]
- Loutzenhiser, P.G.; Meier, A.; Steinfeld, A. Review of the Two-Step H2O/CO2-Splitting Solar Thermochemical Cycle Based on Zn/ZnO Redox Reactions. Materials 2010, 3, 4922–4938. [Google Scholar] [CrossRef]
- Schunk, L.O.; Steinfeld, A. Kinetics of the Thermal Dissociation of ZnO Exposed to Concentrated Solar Irradiation Using a Solar-Driven Thermogravimeter in the 1800-2100 K Range. AIChE J. 2009, 55, 1497–1504. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S. Design and Operation of a Solar-Driven Thermogravimeter for High Temperature Kinetic Analysis of Solid–Gas Thermochemical Reactions in Controlled Atmosphere. Sol. Energy 2014, 105, 225–235. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S. Investigation of Thermal and Carbothermal Reduction of Volatile Oxides (ZnO, SnO2, GeO2, and MgO) via Solar-Driven Vacuum Thermogravimetry for Thermochemical Production of Solar Fuels. Thermochim. Acta 2015, 605, 86–94. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S. Kinetic Analysis of High-Temperature Solid–Gas Reactions by an Inverse Method Applied to ZnO and SnO2 Solar Thermal Dissociation. Chem. Eng. J. 2013, 217, 139–149. [Google Scholar] [CrossRef]
- Perkins, C.; Lichty, P.; Weimer, A.W. Determination of Aerosol Kinetics of Thermal ZnO Dissociation by Thermogravimetry. Chem. Eng. Sci. 2007, 62, 5952–5962. [Google Scholar] [CrossRef]
- Perkins, C.; Lichty, P.R.; Weimer, A.W. Thermal ZnO Dissociation in a Rapid Aerosol Reactor as Part of a Solar Hydrogen Production Cycle. Int. J. Hydrogen Energy 2008, 33, 499–510. [Google Scholar] [CrossRef]
- Chambon, M.; Abanades, S.; Flamant, G. Solar Thermal Reduction of ZnO and SnO2 Characterization of the Recombination Reaction with O2. Chem. Eng. Sci. 2010, 65, 3671–3680. [Google Scholar] [CrossRef]
- Ernst, F.; Steinfeld, A.; Pratsinis, S. Hydrolysis Rate of Submicron Zn Particles for Solar H2 Synthesis. Int. J. Hydrogen Energy 2009, 34, 1166–1175. [Google Scholar] [CrossRef]
- Abanades, S. Thermogravimetry Analysis of CO2 and H2O Reduction from Solar Nanosized Zn Powder for Thermochemical Fuel Production. Ind. Eng. Chem. Res. 2012, 51, 741–750. [Google Scholar] [CrossRef]
- Weibel, D.; Jovanovic, Z.R.; Gálvez, E.; Steinfeld, A. Mechanism of Zn Particle Oxidation by H2O and CO2 in the Presence of ZnO. Chem. Mater. 2014, 26, 6486–6495. [Google Scholar] [CrossRef] [PubMed]
- Chambon, M.; Abanades, S.; Flamant, G. Kinetic Investigation of Hydrogen Generation from Hydrolysis of SnO and Zn Solar Nanopowders. Int. J. Hydrogen Energy 2009, 34, 5326–5336. [Google Scholar] [CrossRef]
- Lv, M.; Zhou, J.; Yang, W.; Cen, K. Thermogravimetric Analysis of the Hydrolysis of Zinc Particles. Int. J. Hydrogen Energy 2010, 35, 2617–2621. [Google Scholar] [CrossRef]
- Abanades, S.; Chambon, M. CO2 Dissociation and Upgrading from Two-Step Solar Thermochemical Processes Based on ZnO/Zn and SnO2/SnO Redox Pairs. Energy Fuels 2010, 24, 6667–6674. [Google Scholar] [CrossRef]
- Loutzenhiser, P.G.; Barthel, F.; Stamatiou, A.; Steinfeld, A. CO2 Reduction with Zn Particles in a Packed-Bed Reactor. AIChE J. 2011, 57, 2529–2534. [Google Scholar] [CrossRef]
- Loutzenhiser, P.G.; Gálvez, M.E.; Hischier, I.; Stamatiou, A.; Frei, A.; Steinfeld, A. CO2 Splitting via Two-Step Solar Thermochemical Cycles with Zn/ZnO and FeO/Fe3O4 Redox Reactions II: Kinetic Analysis. Energy Fuels 2009, 23, 2832–2839. [Google Scholar] [CrossRef]
- Stamatiou, A.; Loutzenhiser, P.G.; Steinfeld, A. Solar Syngas Production via H2O/CO2-Splitting Thermochemical Cycles with Zn/ZnO and FeO/Fe3O4 Redox Reactions. Chem. Mater. 2010, 22, 851–859. [Google Scholar] [CrossRef]
- Melchior, T.; Piatkowski, N.; Steinfeld, A. H2 Production by Steam-Quenching of Zn Vapor in a Hot-Wall Aerosol Flow Reactor. Chem. Eng. Sci. 2009, 64, 1095–1101. [Google Scholar] [CrossRef]
- Venstrom, L.J.; Hilsen, P.; Davidson, J.H. Heterogeneous Oxidation of Zinc Vapor by Steam and Mixtures of Steam and Carbon Dioxide. Chem. Eng. Sci. 2018, 183, 223–230. [Google Scholar] [CrossRef]
- Lindemer, M.D.; Advani, S.G.; Prasad, A.K. Experimental Investigation of Heterogeneous Hydrolysis with Zn Vapor under a Temperature Gradient. Int. J. Hydrogen Energy 2017, 42, 7847–7856. [Google Scholar] [CrossRef]
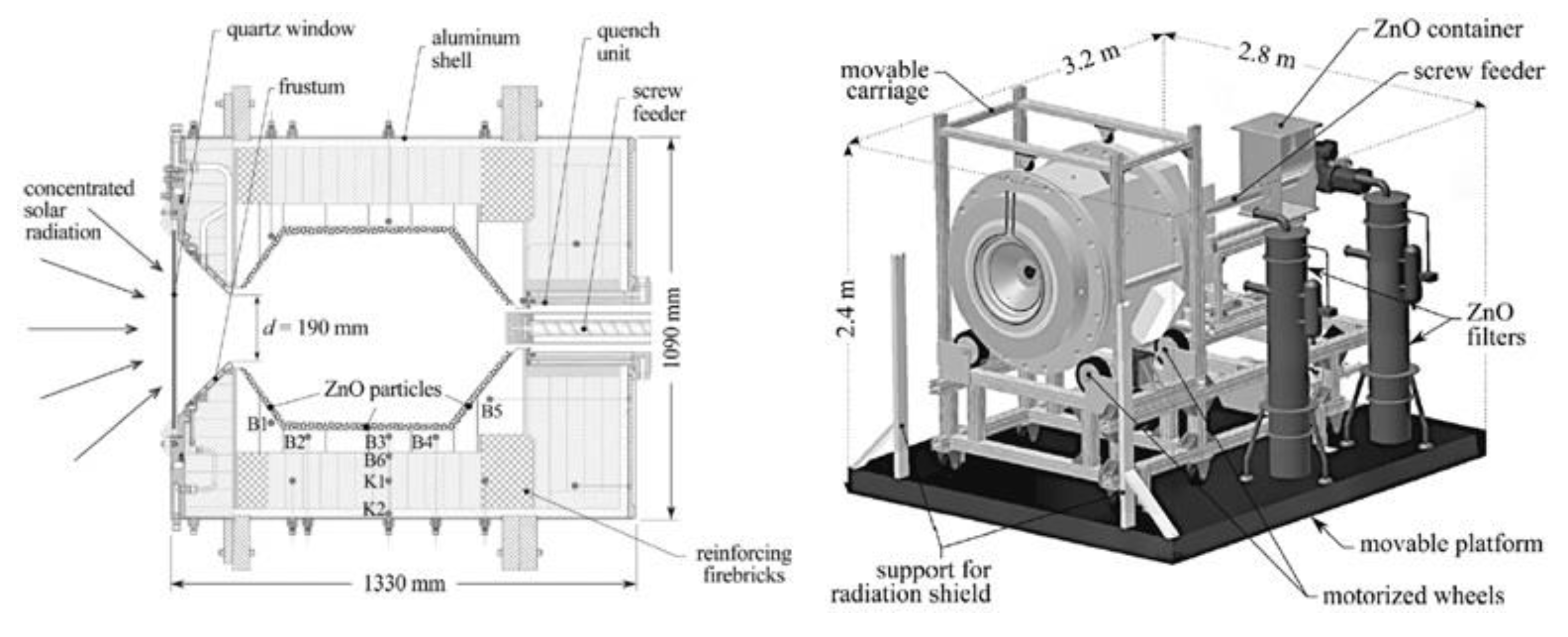
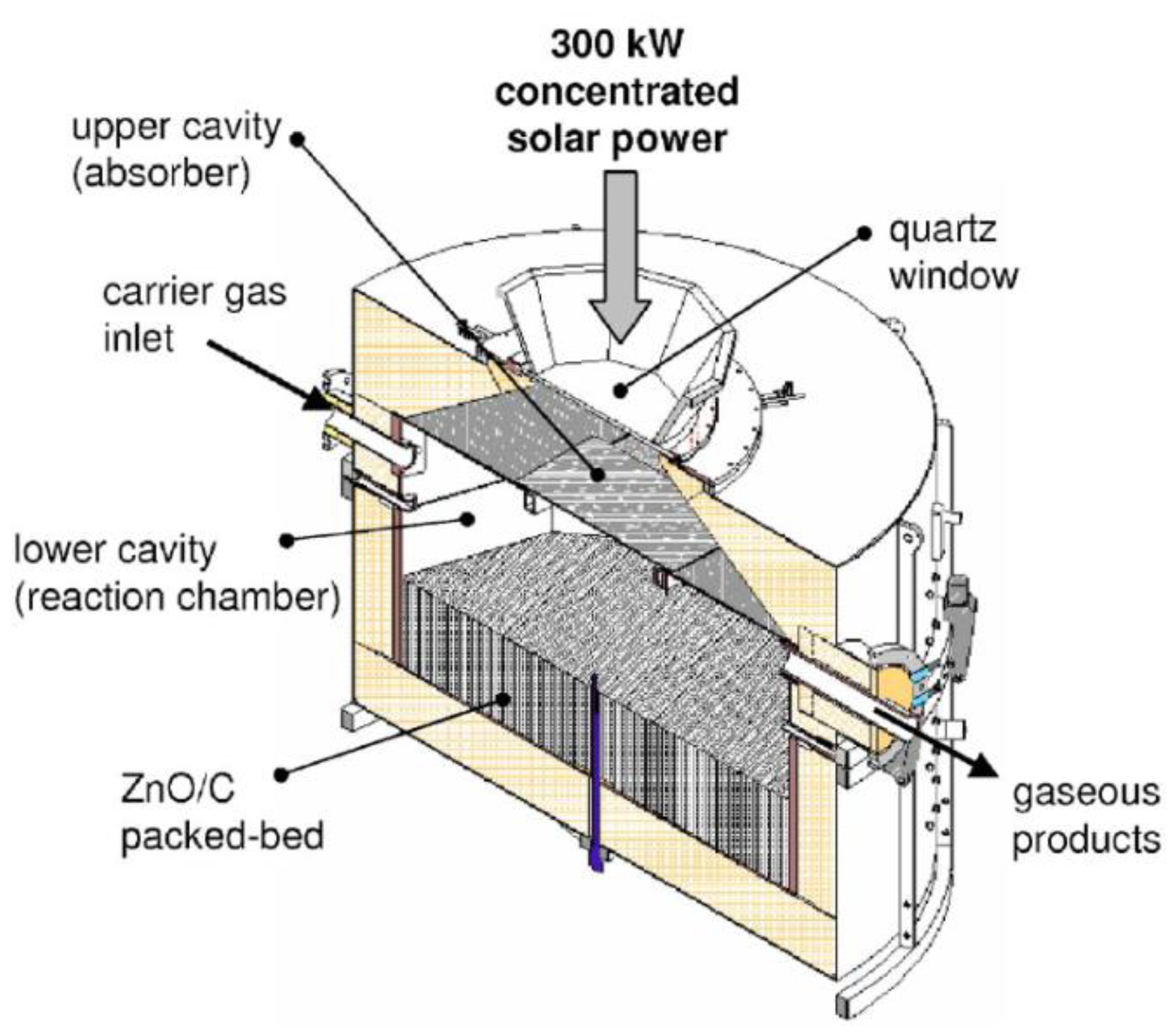
- Wieckert, C.; Frommherz, U.; Kräupl, S.; Guillot, E.; Olalde, G.; Epstein, M.; Santén, S.; Osinga, T.; Steinfeld, A. A 300 kW Solar Chemical Pilot Plant for the Carbothermic Production of Zinc. J. Sol. Energy Eng. 2007, 129, 190–196. [Google Scholar] [CrossRef]
- Kräupl, S.; Steinfeld, A. Operational Performance of a 5-KW Solar Chemical Reactor for the Co-Production of Zinc and Syngas. J. Sol. Energy Eng. 2003, 125, 124–126. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Thermochemical Performance Assessment of Solar Continuous Methane-Driven ZnO Reduction for Co-Production of Pure Zinc and Hydrogen-Rich Syngas. Chem. Eng. J. 2022, 429, 132356. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Solar-Driven Chemical Looping Methane Reforming Using ZnO Oxygen Carrier for Syngas and Zn Production in a Cavity-Type Solar Reactor. Catalysts 2020, 10, 1356. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Combined ZnO Reduction and Methane Reforming for Co-Production of Pure Zn and Syngas in a Prototype Solar Thermochemical Reactor. Fuel Process. Technol. 2021, 211, 106572. [Google Scholar] [CrossRef]
- Osinga, T.; Frommherz, U.; Steinfeld, A.; Wieckert, C. Experimental Investigation of the Solar Carbothermic Reduction of ZnO Using a Two-Cavity Solar Reactor. J. Sol. Energy Eng. 2004, 126, 633–637. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Solar Carbo-Thermal and Methano-Thermal Reduction of MgO and ZnO for Metallic Powder and Syngas Production by Green Extractive Metallurgy. Processes 2022, 10, 154. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Solar Metallurgy for Sustainable Zn and Mg Production in a Vacuum Reactor Using Concentrated Sunlight. Sustainability 2020, 12, 6709. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Solar Chemical Looping Gasification of Biomass with the ZnO/Zn Redox System for Syngas and Zinc Production in a Continuously-Fed Solar Reactor. Fuel 2018, 215, 66–79. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S. Thermodynamic and Experimental Investigation of Solar-Driven Biomass Pyro-Gasification Using H2O, CO2, or ZnO Oxidants for Clean Syngas and Metallurgical Zn Production. Processes 2021, 9, 687. [Google Scholar] [CrossRef]
- Osinga, T.; Olalde, G.; Steinfeld, A. Solar Carbothermal Reduction of ZnO: Shrinking Packed-Bed Reactor Modeling and Experimental Validation. Ind. Eng. Chem. Res. 2004, 43, 7981–7988. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Lemont, F.; Flamant, G. Experimental Study of SnO2/SnO/Sn Thermochemical Systems for Solar Production of Hydrogen. AIChE J. 2008, 54, 2759–2767. [Google Scholar] [CrossRef]
- Abanades, S.; Charvin, P.; Lemont, F.; Flamant, G. Novel Two-Step SnO2/SnO Water-Splitting Cycle for Solar Thermochemical Production of Hydrogen. Int. J. Hydrogen Energy 2008, 33, 6021–6030. [Google Scholar] [CrossRef]
- Abanades, S. CO2 and H2O Reduction by Solar Thermochemical Looping Using SnO2/SnO Redox Reactions: Thermogravimetric Analysis. Int. J. Hydrogen Energy 2012, 37, 8223–8231. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S.; Jumas, J.-C.; Olivier-Fourcade, J. Characterization of Two-Step Tin-Based Redox System for Thermochemical Fuel Production from Solar-Driven CO2 and H2O Splitting Cycle. Ind. Eng. Chem. Res. 2014, 53, 5668–5677. [Google Scholar] [CrossRef]
- Levêque, G.; Abanades, S. Thermodynamic and Kinetic Study of the Carbothermal Reduction of SnO2 for Solar Thermochemical Fuel Generation. Energy Fuels 2014, 28, 1396–1405. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Kumar, A.; Sutar, P. Thermodynamic Analysis of Solar Driven SnO2/SnO Based Thermochemical Water Splitting Cycle. Energy Convers. Manag. 2017, 135, 226–235. [Google Scholar] [CrossRef]
- Chambon, M.; Abanades, S.; Flamant, G. Thermal Dissociation of Compressed ZnO and SnO2 Powders in a Moving-Front Solar Thermochemical Reactor. AIChE J. 2011, 57, 2264–2273. [Google Scholar] [CrossRef]
- Nakamura, T. Hydrogen Production from Water Utilizing Solar Heat at High Temperatures. Sol. Energy 1977, 19, 467–475. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Flamant, G.; Lemort, F. Two-Step Water Splitting Thermochemical Cycle Based on Iron Oxide Redox Pair for Solar Hydrogen Production. Energy 2007, 32, 1124–1133. [Google Scholar] [CrossRef]
- Abanades, S.; Villafan-Vidales, H.I. CO2 and H2O Conversion to Solar Fuels via Two-Step Solar Thermochemical Looping Using Iron Oxide Redox Pair. Chem. Eng. J. 2011, 175, 368–375. [Google Scholar] [CrossRef]
- Abanades, S.; Villafan-Vidales, I. CO2 Valorisation Based on Fe3O4/FeO Thermochemical Redox Reactions Using Concentrated Solar Energy. Int. J. Energy Res. 2013, 37, 598–608. [Google Scholar] [CrossRef]
- Tamaura, Y.; Ueda, Y.; Matsunami, J.; Hasegawa, N.; Nezuka, M.; Sano, T.; Tsuji, M. Solar Hydrogen Production by Using Ferrites. Sol. Energy 1999, 65, 55–57. [Google Scholar] [CrossRef]
- Coker, E.N.; Ambrosini, A.; Miller, J.E. Compositional and Operational Impacts on the Thermochemical Reduction of CO2 to CO by Iron Oxide/Yttria-Stabilized Zirconia. RSC Adv. 2021, 11, 1493–1502. [Google Scholar] [CrossRef]
- Abanades, S.; Flamant, G. Thermochemical Hydrogen Production from a Two-Step Solar-Driven Water-Splitting Cycle Based on Cerium Oxides. Sol. Energy 2006, 80, 1611–1623. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Beche, E.; Lemont, F.; Flamant, G. Hydrogen Production from Mixed Cerium Oxides via Three-Step Water-Splitting Cycles. Solid State Ion. 2009, 180, 1003–1010. [Google Scholar] [CrossRef]
- Le Gal, A.; Abanades, S. Catalytic Investigation of Ceria-Zirconia Solid Solutions for Solar Hydrogen Production. Int. J. Hydrogen Energy 2011, 36, 4739–4748. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Drobek, M.; Ayral, A.; Julbe, A. Recent Progress on Ceria Doping and Shaping Strategies for Solar Thermochemical Water and CO2 Splitting Cycles. AIMS Mater. Sci. 2019, 6, 657–684. [Google Scholar] [CrossRef]
- Call, F.; Roeb, M.; Schmücker, M.; Sattler, C.; Pitz-Paal, R. Ceria Doped with Zirconium and Lanthanide Oxides to Enhance Solar Thermochemical Production of Fuels. J. Phys. Chem. C 2015, 119, 6929–6938. [Google Scholar] [CrossRef]
- Roeb, M.; Säck, J.-P.; Rietbrock, P.; Prahl, C.; Schreiber, H.; Neises, M.; de Oliveira, L.; Graf, D.; Ebert, M.; Reinalter, W.; et al. Test Operation of a 100kW Pilot Plant for Solar Hydrogen Production from Water on a Solar Tower. Sol. Energy 2011, 85, 634–644. [Google Scholar] [CrossRef]
- Fresno, F.; Fernández-Saavedra, R.; Belén Gómez-Mancebo, M.; Vidal, A.; Sánchez, M.; Isabel Rucandio, M.; Quejido, A.J.; Romero, M. Solar Hydrogen Production by Two-Step Thermochemical Cycles: Evaluation of the Activity of Commercial Ferrites. Int. J. Hydrogen Energy 2009, 34, 2918–2924. [Google Scholar] [CrossRef]
- Lorentzou, S.; Dimitrakis, D.; Zygogianni, A.; Karagiannakis, G.; Konstandopoulos, A.G. Thermochemical H2O and CO2 Splitting Redox Cycles in a NiFe2O4 Structured Redox Reactor: Design, Development and Experiments in a High Flux Solar Simulator. Sol. Energy 2017, 155, 1462–1481. [Google Scholar] [CrossRef]
- Kostoglou, M.; Lorentzou, S.; Konstandopoulos, A.G. Improved Kinetic Model for Water Splitting Thermochemical Cycles Using Nickel Ferrite. Int. J. Hydrogen Energy 2014, 39, 6317–6327. [Google Scholar] [CrossRef]
- Guene Lougou, B.; Geng, B.; Jiang, B.; Zhang, H.; Sun, Q.; Shuai, Y.; Qu, Z.; Zhao, J.; Wang, C.-H. Copper Ferrite and Cobalt Oxide Two-Layer Coated Macroporous SiC Substrate for Efficient CO2-Splitting and Thermochemical Energy Conversion. J. Colloid Interface Sci. 2022, 627, 516–531. [Google Scholar] [CrossRef]
- Cho, H.S.; Gokon, N.; Kodama, T.; Kang, Y.H.; Lee, H.J. Improved Operation of Solar Reactor for Two-Step Water-Splitting H2 Production by Ceria-Coated Ceramic Foam Device. Int. J. Hydrogen Energy 2015, 40, 114–124. [Google Scholar] [CrossRef]
- Gokon, N.; Hasegawa, T.; Takahashi, S.; Kodama, T. Thermochemical Two-Step Water-Splitting for Hydrogen Production Using Fe-YSZ Particles and a Ceramic Foam Device. Energy 2008, 33, 1407–1416. [Google Scholar] [CrossRef]
- Gokon, N.; Murayama, H.; Nagasaki, A.; Kodama, T. Thermochemical Two-Step Water Splitting Cycles by Monoclinic ZrO2-Supported NiFe2O4 and Fe3O4 Powders and Ceramic Foam Devices. Sol. Energy 2009, 83, 527–537. [Google Scholar] [CrossRef]
- Chueh, W.C.; Haile, S.M. A Thermochemical Study of Ceria: Exploiting an Old Material for New Modes of Energy Conversion and CO2 Mitigation. Philos. Trans. R. Soc. A 2010, 368, 3269–3294. [Google Scholar] [CrossRef]
- Chueh, W.C.; Haile, S.M. Ceria as a Thermochemical Reaction Medium for Selectively Generating Syngas or Methane from H2O and CO2. ChemSusChem 2009, 2, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Chueh, W.C.; Falter, C.; Abbott, M.; Scipio, D.; Furler, P.; Haile, S.M.; Steinfeld, A. High-Flux Solar-Driven Thermochemical Dissociation of CO2 and H2O Using Nonstoichiometric Ceria. Science 2010, 330, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Bulfin, B.; Call, F.; Lange, M.; Lübben, O.; Sattler, C.; Pitz-Paal, R.; Shvets, I.V. Thermodynamics of CeO2 Thermochemical Fuel Production. Energy Fuels 2015, 29, 1001–1009. [Google Scholar] [CrossRef]
- Lu, Y.; Zhu, L.; Agrafiotis, C.; Vieten, J.; Roeb, M.; Sattler, C. Solar Fuels Production: Two-Step Thermochemical Cycles with Cerium-Based Oxides. Prog. Energy Combust. Sci. 2019, 75, 100785. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Takalkar, G.; Sutar, P.; Kumar, A.; AlMomani, F.; Khraisheh, M. A Decade of Ceria Based Solar Thermochemical H2O/CO2 Splitting Cycle. Int. J. Hydrogen Energy 2019, 44, 34–60. [Google Scholar] [CrossRef]
- Rhodes, N.R.; Bobek, M.M.; Allen, K.M.; Hahn, D.W. Investigation of Long Term Reactive Stability of Ceria for Use in Solar Thermochemical Cycles. Energy 2015, 89, 924–931. [Google Scholar] [CrossRef]
- Takalkar, G.D.; Bhosale, R.R.; Kumar, A.; AlMomani, F.; Khraisheh, M.; Shakoor, R.A.; Gupta, R.B. Transition Metal Doped Ceria for Solar Thermochemical Fuel Production. Sol. Energy 2018, 172, 204–211. [Google Scholar] [CrossRef]
- Takalkar, G.; Bhosale, R.R.; Rashid, S.; AlMomani, F.; Shakoor, R.A.; Al Ashraf, A. Application of Li-, Mg-, Ba-, Sr-, Ca-, and Sn-Doped Ceria for Solar-Driven Thermochemical Conversion of Carbon Dioxide. J. Mater. Sci. 2020, 55, 11797–11807. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Y.; Li, F. Reactivity of Ni, Cr and Zr Doped Ceria in CO2 Splitting for CO Production via Two-Step Thermochemical Cycle. Int. J. Hydrogen Energy 2018, 43, 13754–13763. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Y. Reactivity and Efficiency of Ceria-Based Oxides for Solar CO2 Splitting via Isothermal and Near-Isothermal Cycles. Energy Fuels 2018, 32, 736–746. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Kumar, A.; AlMomani, F.; Ghosh, U.; Al-Muhtaseb, S.; Gupta, R.; Alxneit, I. Assessment of CeZrHfO2 Based Oxides as Potential Solar Thermochemical CO2 Splitting Materials. Ceram. Int. 2016, 42, 9354–9362. [Google Scholar] [CrossRef]
- Bhosale, R.R.; Takalkar, G.D. Nanostructured Co-Precipitated Ce0.9Ln0.1O2 (Ln = La, Pr, Sm, Nd, Gd, Tb, Dy, or Er) for Thermochemical Conversion of CO2. Ceram. Int. 2018, 44, 16688–16697. [Google Scholar] [CrossRef]
- Arifin, D.; Ambrosini, A.; Wilson, S.A.; Mandal, B.; Muhich, C.L.; Weimer, A.W. Investigation of Zr, Gd/Zr, and Pr/Zr—Doped Ceria for the Redox Splitting of Water. Int. J. Hydrogen Energy 2020, 45, 160–174. [Google Scholar] [CrossRef]
- Portarapillo, M.; Russo, D.; Landi, G.; Luciani, G.; Di Benedetto, A. K-Doped CeO2–ZrO2 for CO2 Thermochemical Catalytic Splitting. RSC Adv. 2021, 11, 39420–39427. [Google Scholar] [CrossRef]
- Portarapillo, M.; Landi, G.; Luciani, G.; Imparato, C.; Vitiello, G.; Deorsola, F.A.; Aronne, A.; Di Benedetto, A. Redox Behavior of Potassium Doped and Transition Metal Co-Doped Ce0.75Zr0.25O2 for Thermochemical H2O/CO2 Splitting. RSC Adv. 2022, 12, 14645–14654. [Google Scholar] [CrossRef]
- Le Gal, A.; Abanades, S. Dopant Incorporation in Ceria for Enhanced Water-Splitting Activity during Solar Thermochemical Hydrogen Generation. J. Phys. Chem. C 2012, 116, 13516–13523. [Google Scholar] [CrossRef]
- Le Gal, A.; Abanades, S.; Bion, N.; Le Mercier, T.; Harlé, V. Reactivity of Doped Ceria-Based Mixed Oxides for Solar Thermochemical Hydrogen Generation via Two-Step Water-Splitting Cycles. Energy Fuels 2013, 27, 6068–6078. [Google Scholar] [CrossRef]
- Bulfin, B.; Call, F.; Vieten, J.; Roeb, M.; Sattler, C.; Shvets, I.V. Oxidation and Reduction Reaction Kinetics of Mixed Cerium Zirconium Oxides. J. Phys. Chem. C 2016, 120, 2027–2035. [Google Scholar] [CrossRef]
- Pullar, R.C.; Novais, R.M.; Caetano, A.P.F.; Barreiros, M.A.; Abanades, S.; Oliveira, F.A.C. A Review of Solar Thermochemical CO2 Splitting Using Ceria-Based Ceramics With Designed Morphologies and Microstructures. Front. Chem. 2019, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Gladen, A.C.; Davidson, J.H. The Morphological Stability and Fuel Production of Commercial Fibrous Ceria Particles for Solar Thermochemical Redox Cycling. Sol. Energy 2016, 139, 524–532. [Google Scholar] [CrossRef]
- Keene, D.J.; Lipiński, W.; Davidson, J.H. The Effects of Morphology on the Thermal Reduction of Nonstoichiometric Ceria. Chem. Eng. Sci. 2014, 111, 231–243. [Google Scholar] [CrossRef]
- Venstrom, L.J.; Petkovich, N.; Rudisill, S.; Stein, A.; Davidson, J.H. The Effects of Morphology on the Oxidation of Ceria by Water and Carbon Dioxide. J. Sol. Energy Eng. 2011, 134, 011005. [Google Scholar] [CrossRef]
- Furler, P.; Scheffe, J.; Marxer, D.; Gorbar, M.; Bonk, A.; Vogt, U.; Steinfeld, A. Thermochemical CO2 Splitting via Redox Cycling of Ceria Reticulated Foam Structures with Dual-Scale Porosities. Phys. Chem. Chem. Phys. 2014, 16, 10503–10511. [Google Scholar] [CrossRef]
- Orfila, M.; Sanz, D.; Linares, M.; Molina, R.; Sanz, R.; Marugán, J.; Botas, J.Á. H2 Production by Thermochemical Water Splitting with Reticulated Porous Structures of Ceria-Based Mixed Oxide Materials. Int. J. Hydrogen Energy 2021, 46, 17458–17471. [Google Scholar] [CrossRef]
- Lorentzou, S.; Pagkoura, C.; Zygogianni, A.; Karagiannakis, G.; Konstandopoulos, A.G. Thermochemical Cycles over Redox Structured Reactors. Int. J. Hydrogen Energy 2017, 42, 19664–19682. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Julbe, A.; Jouannaux, J.; Drobek, M.; Ayral, A.; Cartoixa, B. Remarkable Performance of Microstructured Ceria Foams for Thermochemical Splitting of H2O and CO2 in a Novel High–Temperature Solar Reactor. Chem. Eng. Res. Des. 2020, 156, 311–323. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Julbe, A.; Jouannaux, J.; Cartoixa, B. Solar Thermochemical Fuel Production from H2O and CO2 Splitting via Two-Step Redox Cycling of Reticulated Porous Ceria Structures Integrated in a Monolithic Cavity-Type Reactor. Energy 2020, 201, 117649. [Google Scholar] [CrossRef]
- Oliveira, F.A.C.; Barreiros, M.A.; Abanades, S.; Caetano, A.P.F.; Novais, R.M.; Pullar, R.C. Solar Thermochemical CO2 Splitting Using Cork-Templated Ceria Ecoceramics. J. CO2 Util. 2018, 26, 552–563. [Google Scholar] [CrossRef]
- Oliveira, F.A.C.; Barreiros, M.A.; Haeussler, A.; Caetano, A.P.F.; Mouquinho, A.I.; Oliveira e Silva, P.M.; Novais, R.M.; Pullar, R.C.; Abanades, S. High Performance Cork-Templated Ceria for Solar Thermochemical Hydrogen Production via Two-Step Water-Splitting Cycles. Sustain. Energy Fuels 2020, 4, 3077–3089. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Costa Oliveira, F.A.; Barreiros, M.A.; Caetano, A.P.F.; Novais, R.M.; Pullar, R.C. Solar Redox Cycling of Ceria Structures Based on Fiber Boards, Foams, and Biomimetic Cork-Derived Ecoceramics for Two-Step Thermochemical H2O and CO2 Splitting. Energy Fuels 2020, 34, 9037–9049. [Google Scholar] [CrossRef]
- Malonzo, C.D.; De Smith, R.M.; Rudisill, S.G.; Petkovich, N.D.; Davidson, J.H.; Stein, A. Wood-Templated CeO2 as Active Material for Thermochemical CO Production. J. Phys. Chem. C 2014, 118, 26172–26181. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Julbe, A.; Jouannaux, J.; Cartoixa, B. Two-Step CO2 and H2O Splitting Using Perovskite-Coated Ceria Foam for Enhanced Green Fuel Production in a Porous Volumetric Solar Reactor. J. CO2 Util. 2020, 41, 101257. [Google Scholar] [CrossRef]
- Abanades, S.; Haeussler, A.; Julbe, A. Synthesis and Thermochemical Redox Cycling of Porous Ceria Microspheres for Renewable Fuels Production from Solar-Aided Water-Splitting and CO2 Utilization. Appl. Phys. Lett. 2021, 119, 023902. [Google Scholar] [CrossRef]
- Abanades, S.; Haeussler, A.; Julbe, A. Thermochemical Solar-Driven Reduction of CO2 into Separate Streams of CO and O2 via an Isothermal Oxygen-Conducting Ceria Membrane Reactor. Chem. Eng. J. 2021, 422, 130026. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S. Additive Manufacturing and Two-Step Redox Cycling of Ordered Porous Ceria Structures for Solar-Driven Thermochemical Fuel Production. Chem. Eng. Sci. 2021, 246, 116999. [Google Scholar] [CrossRef]
- Abanades, S.; Haeussler, A. Two-Step Thermochemical Cycles Using Fibrous Ceria Pellets for H2 Production and CO2 Reduction in Packed-Bed Solar Reactors. Sustain. Mater. Technol. 2021, 29, e00328. [Google Scholar] [CrossRef]
- Parvanian, A.M.; Salimijazi, H.; Shabaninejad, M.; Troitzsch, U.; Kreider, P.; Lipiński, W.; Saadatfar, M. Thermochemical CO2 Splitting Performance of Perovskite Coated Porous Ceramics. RSC Adv. 2020, 10, 23049–23057. [Google Scholar] [CrossRef]
- Hoes, M.; Ackermann, S.; Theiler, D.; Furler, P.; Steinfeld, A. Additive-Manufactured Ordered Porous Structures Made of Ceria for Concentrating Solar Applications. Energy Technol. 2019, 7, 1900484. [Google Scholar] [CrossRef]
- Bayon, A.; de la Calle, A.; Ghose, K.K.; Page, A.; McNaughton, R. Experimental, Computational and Thermodynamic Studies in Perovskites Metal Oxides for Thermochemical Fuel Production: A Review. Int. J. Hydrogen Energy 2020, 45, 12653–12679. [Google Scholar] [CrossRef]
- Vieten, J.; Bulfin, B.; Huck, P.; Horton, M.; Guban, D.; Zhu, L.; Lu, Y.; Persson, K.A.; Roeb, M.; Sattler, C. Materials Design of Perovskite Solid Solutions for Thermochemical Applications. Energy Environ. Sci. 2019, 12, 1369–1384. [Google Scholar] [CrossRef]
- Kubicek, M.; Bork, A.H.; Rupp, J.L.M. Perovskite Oxides—A Review on a Versatile Material Class for Solar-to-Fuel Conversion Processes. J. Mater. Chem. A 2017, 5, 11983–12000. [Google Scholar] [CrossRef]
- Ezbiri, M.; Takacs, M.; Theiler, D.; Michalsky, R.; Steinfeld, A. Tunable Thermodynamic Activity of LaxSr1−x MnyAl1−yO3−δ (0 ≤ x ≤ 1, 0 ≤ y ≤ 1) Perovskites for Solar Thermochemical Fuel Synthesis. J. Mater. Chem. A 2017, 5, 4172–4182. [Google Scholar] [CrossRef] [PubMed]
- Emery, A.A.; Saal, J.E.; Kirklin, S.; Hegde, V.I.; Wolverton, C. High-Throughput Computational Screening of Perovskites for Thermochemical Water Splitting Applications. Chem. Mater. 2016, 28, 5621–5634. [Google Scholar] [CrossRef]
- Gao, X.; Liu, G.; Zhu, Y.; Kreider, P.; Bayon, A.; Gengenbach, T.; Lu, T.; Liu, Y.; Hinkley, J.; Lipiński, W.; et al. Earth-Abundant Transition Metal Oxides with Extraordinary Reversible Oxygen Exchange Capacity for Efficient Thermochemical Synthesis of Solar Fuels. Nano Energy 2018, 50, 347–358. [Google Scholar] [CrossRef]
- Gao, K.; Liu, X.; Jiang, Z.; Zheng, H.; Song, C.; Wang, X.; Tian, C.; Dang, C.; Sun, N.; Xuan, Y. Direct Solar Thermochemical CO2 Splitting Based on Ca- and Al-Doped SmMnO3 Perovskites: Ultrahigh CO Yield within Small Temperature Swing. Renew. Energy 2022, 194, 482–494. [Google Scholar] [CrossRef]
- Gao, K.; Liu, X.; Wang, T.; Zhu, Z.; Li, P.; Zheng, H.; Song, C.; Xuan, Y.; Li, Y.; Ding, Y. Sr-Doped SmMnO3 Perovskites for High-Performance near-Isothermal Solar Thermochemical CO2-to-Fuel Conversion. Sustain. Energy Fuels 2021, 5, 4295–4310. [Google Scholar] [CrossRef]
- Liu, X.; Wang, T.; Gao, K.; Meng, X.; Xu, Q.; Song, C.; Zhu, Z.; Zheng, H.; Hao, Y.; Xuan, Y. Ca- and Ga-Doped LaMnO3 for Solar Thermochemical CO2 Splitting with High Fuel Yield and Cycle Stability. ACS Appl. Energy Mater. 2021, 4, 9000–9012. [Google Scholar] [CrossRef]
- Naik, J.M.; Ritter, C.; Bulfin, B.; Steinfeld, A.; Erni, R.; Patzke, G.R. Reversible Phase Transformations in Novel Ce-Substituted Perovskite Oxide Composites for Solar Thermochemical Redox Splitting of CO2. Adv. Energy Mater. 2021, 11, 2003532. [Google Scholar] [CrossRef]
- Ezbiri, M.; Takacs, M.; Stolz, B.; Lungthok, J.; Steinfeld, A.; Michalsky, R. Design Principles of Perovskites for Solar-Driven Thermochemical Splitting of CO2. J. Mater. Chem. A 2017, 5, 15105–15115. [Google Scholar] [CrossRef] [PubMed]
- Gálvez, M.E.; Jacot, R.; Scheffe, J.; Cooper, T.; Patzke, G.; Steinfeld, A. Physico-Chemical Changes in Ca, Sr and Al-Doped La–Mn–O Perovskites upon Thermochemical Splitting of CO 2 via Redox Cycling. Phys. Chem. Chem. Phys. 2015, 17, 6629–6634. [Google Scholar] [CrossRef] [PubMed]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Julbe, A. Non-Stoichiometric Redox Active Perovskite Materials for Solar Thermochemical Fuel Production: A Review. Catalysts 2018, 8, 611. [Google Scholar] [CrossRef]
- Demont, A.; Abanades, S.; Beche, E. Investigation of Perovskite Structures as Oxygen-Exchange Redox Materials for Hydrogen Production from Thermochemical Two-Step Water-Splitting Cycles. J. Phys. Chem. C 2014, 118, 12682–12692. [Google Scholar] [CrossRef]
- Haeussler, A.; Julbe, A.; Abanades, S. Investigation of Reactive Perovskite Materials for Solar Fuel Production via Two-Step Redox Cycles: Thermochemical Activity, Thermodynamic Properties and Reduction Kinetics. Mater. Chem. Phys. 2022, 276, 125358. [Google Scholar] [CrossRef]
- Jouannaux, J.; Haeussler, A.; Drobek, M.; Ayral, A.; Abanades, S.; Julbe, A. Lanthanum Manganite Perovskite Ceramic Powders for CO2 Splitting: Influence of Pechini Synthesis Parameters on Sinterability and Reactivity. Ceram. Int. 2019, 45, 15636–15648. [Google Scholar] [CrossRef]
- Sai Gautam, G.; Stechel, E.B.; Carter, E.A. Exploring Ca–Ce–M–O (M = 3d Transition Metal) Oxide Perovskites for Solar Thermochemical Applications. Chem. Mater. 2020, 32, 9964–9982. [Google Scholar] [CrossRef]
- Barcellos, D.R.; Coury, F.G.; Emery, A.; Sanders, M.; Tong, J.; McDaniel, A.; Wolverton, C.; Kaufman, M.; O’Hayre, R. Phase Identification of the Layered Perovskite CexSr2–xMnO4 and Application for Solar Thermochemical Water Splitting. Inorg. Chem. 2019, 58, 7705–7714. [Google Scholar] [CrossRef]
- McDaniel, A.H. Renewable Energy Carriers Derived from Concentrating Solar Power and Nonstoichiometric Oxides. Curr. Opin. Green Sustain. Chem. 2017, 4, 37–43. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Dey, S. Generation of H2 and CO by Solar Thermochemical Splitting of H2O and CO2 by Employing Metal Oxides. J. Solid State Chem. 2016, 242, 107–115. [Google Scholar] [CrossRef]
- Dey, S.; Naidu, B.S.; Rao, C.N.R. Beneficial Effects of Substituting Trivalent Ions in the B-Site of La0.5Sr0.5Mn1−xAxO3 (A = Al, Ga, Sc) on the Thermochemical Generation of CO and H2 from CO2 and H2O. Dalton Trans. 2016, 45, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; He, J.; Mastronardo, E.; Baldassarri, B.; Wolverton, C.; Haile, S.M. Favorable Redox Thermodynamics of SrTi0.5Mn0.5O3−δ in Solar Thermochemical Water Splitting. Chem. Mater. 2020, 32, 9335–9346. [Google Scholar] [CrossRef]
- Qian, X.; He, J.; Mastronardo, E.; Baldassarri, B.; Yuan, W.; Wolverton, C.; Haile, S.M. Outstanding Properties and Performance of CaTi0.5Mn0.5O3−δ for Solar-Driven Thermochemical Hydrogen Production. Matter 2021, 4, 688–708. [Google Scholar] [CrossRef]
- Demont, A.; Abanades, S. High Redox Activity of Sr-Substituted Lanthanum Manganite Perovskites for Two-Step Thermochemical Dissociation of CO2. RSC Adv. 2014, 4, 54885–54891. [Google Scholar] [CrossRef]
- Kildahl, H.; Cao, H.; Ding, Y. Thermochemical Splitting of Carbon Dioxide by Lanthanum Manganites-Understanding the Mechanistic Effects of Doping. Energy Storage Sav. 2022, in press. [CrossRef]
- Bork, A.H.; Povoden-Karadeniz, E.; Carrillo, A.J.; Rupp, J.L.M. Thermodynamic Assessment of the Solar-to-Fuel Performance of La0.6Sr0.4Mn1-YCryO3-Delta Perovskite Solid Solution Series. Acta Mater. 2019, 178, 163–172. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Kim, K.J.; Hood, Z.D.; Bork, A.H.; Rupp, J.L.M. La0.6Sr0.4Cr0.8Co0.2O3 Perovskite Decorated with Exsolved Co Nanoparticles for Stable CO2 Splitting and Syngas Production. ACS Appl. Energy Mater. 2020, 3, 4569–4579. [Google Scholar] [CrossRef]
- Sawaguri, H.; Gokon, N.; Hayashi, K.; Iwamura, Y.; Yasuhara, D. Two-Step Thermochemical CO2 Splitting Using Partially-Substituted Perovskite Oxides of La0.7Sr0.3Mn0.9X0.1O3 for Solar Fuel Production. Front. Energy Res. 2022, 10, 872959. [Google Scholar] [CrossRef]
- Gager, E.; Frye, M.; McCord, D.; Scheffe, J.; Nino, J.C. Reticulated Porous Lanthanum Strontium Manganite Structures for Solar Thermochemical Hydrogen Production. Int. J. Hydrogen Energy 2022, 47, 31152–31164. [Google Scholar] [CrossRef]
- Sastre, D.; Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Exploring the Redox Behavior of La0.6Sr0.4Mn1−xAlxO3 Perovskites for CO2-Splitting in Thermochemical Cycles. Top. Catal. 2017, 60, 1108–1118. [Google Scholar] [CrossRef]
- Luciani, G.; Landi, G.; Aronne, A.; Di Benedetto, A. Partial Substitution of B Cation in La0.6Sr0.4MnO3 Perovskites: A Promising Strategy to Improve the Redox Properties Useful for Solar Thermochemical Water and Carbon Dioxide Splitting. Sol. Energy 2018, 171, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Al-Mamun, M.; Liu, P.; Wang, Y.; Yang, H.G.; Zhao, H. Notable Hydrogen Production on LaxCa1−xCoO3 Perovskites via Two-Step Thermochemical Water Splitting. J. Mater. Sci. 2018, 53, 6796–6806. [Google Scholar] [CrossRef]
- Orfila, M.; Linares, M.; Molina, R.; Botas, J.Á.; Sanz, R.; Marugán, J. Perovskite Materials for Hydrogen Production by Thermochemical Water Splitting. Int. J. Hydrogen Energy 2016, 41, 19329–19338. [Google Scholar] [CrossRef]
- Takacs, M.; Hoes, M.; Caduff, M.; Cooper, T.; Scheffe, J.R.; Steinfeld, A. Oxygen Nonstoichiometry, Defect Equilibria, and Thermodynamic Characterization of LaMnO3 Perovskites with Ca/Sr A-Site and Al B-Site Doping. Acta Mater. 2016, 103, 700–710. [Google Scholar] [CrossRef]
- Demont, A.; Abanades, S. Solar Thermochemical Conversion of CO2 into Fuel via Two-Step Redox Cycling of Non-Stoichiometric Mn-Containing Perovskite Oxides. J. Mater. Chem. A 2015, 3, 3536–3546. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Insights into the Redox Performance of Non-Stoichiometric Lanthanum Manganite Perovskites for Solar Thermochemical CO2 Splitting. ChemistrySelect 2016, 1, 4449–4457. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Experimental Screening of Perovskite Oxides as Efficient Redox Materials for Solar Thermochemical CO2 Conversion. Sustain. Energy Fuels 2018, 2, 843–854. [Google Scholar] [CrossRef]
- Heo, S.J.; Sanders, M.; O’Hayre, R.; Zakutayev, A. Double-Site Substitution of Ce into (Ba, Sr)MnO3 Perovskites for Solar Thermochemical Hydrogen Production. ACS Energy Lett. 2021, 6, 3037–3043. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Bork, A.H.; Moser, T.; Sediva, E.; Hood, Z.D.; Rupp, J.L.M. Modifying La0.6Sr0.4MnO3 Perovskites with Cr Incorporation for Fast Isothermal CO2-Splitting Kinetics in Solar-Driven Thermochemical Cycles. Adv. Energy Mater. 2019, 9, 1803886. [Google Scholar] [CrossRef]
- McDaniel, A.H.; Miller, E.C.; Arifin, D.; Ambrosini, A.; Coker, E.N.; O’Hayre, R.; Chueh, W.C.; Tong, J. Sr- and Mn-Doped LaAlO3−δ for Solar Thermochemical H2 and CO Production. Energy Environ. Sci. 2013, 6, 2424. [Google Scholar] [CrossRef]
- Şanlı, S.B.; Pişkin, B. Effect of B-Site Al Substitution on Hydrogen Production of La0.4Sr0.6Mn1-XAlx (X = 0.4, 0.5 and 0.6) Perovskite Oxides. Int. J. Hydrogen Energy 2022, 47, 19411–19421. [Google Scholar] [CrossRef]
- Cooper, T.; Scheffe, J.R.; Galvez, M.E.; Jacot, R.; Patzke, G.; Steinfeld, A. Lanthanum Manganite Perovskites with Ca/Sr A-Site and Al B-Site Doping as Effective Oxygen Exchange Materials for Solar Thermochemical Fuel Production. Energy Technol. 2015, 3, 1130–1142. [Google Scholar] [CrossRef]
- Carrillo, R.J.; Scheffe, J.R. Beyond Ceria: Theoretical Investigation of Isothermal and Near-Isothermal Redox Cycling of Perovskites for Solar Thermochemical Fuel Production. Energy Fuels 2019, 33, 12871–12884. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Solid-State Reoxidation Kinetics of A/B-Site Substituted LaMnO3 During Solar Thermochemical CO2 Conversion. Energy Technol. 2021, 9, 2000885. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Correlating Oxygen Mobility with Thermochemical CO2-Splitting Efficiency in A-Site Substituted Manganite Perovskites. Sustain. Energy Fuels 2021, 5, 4570–4574. [Google Scholar] [CrossRef]
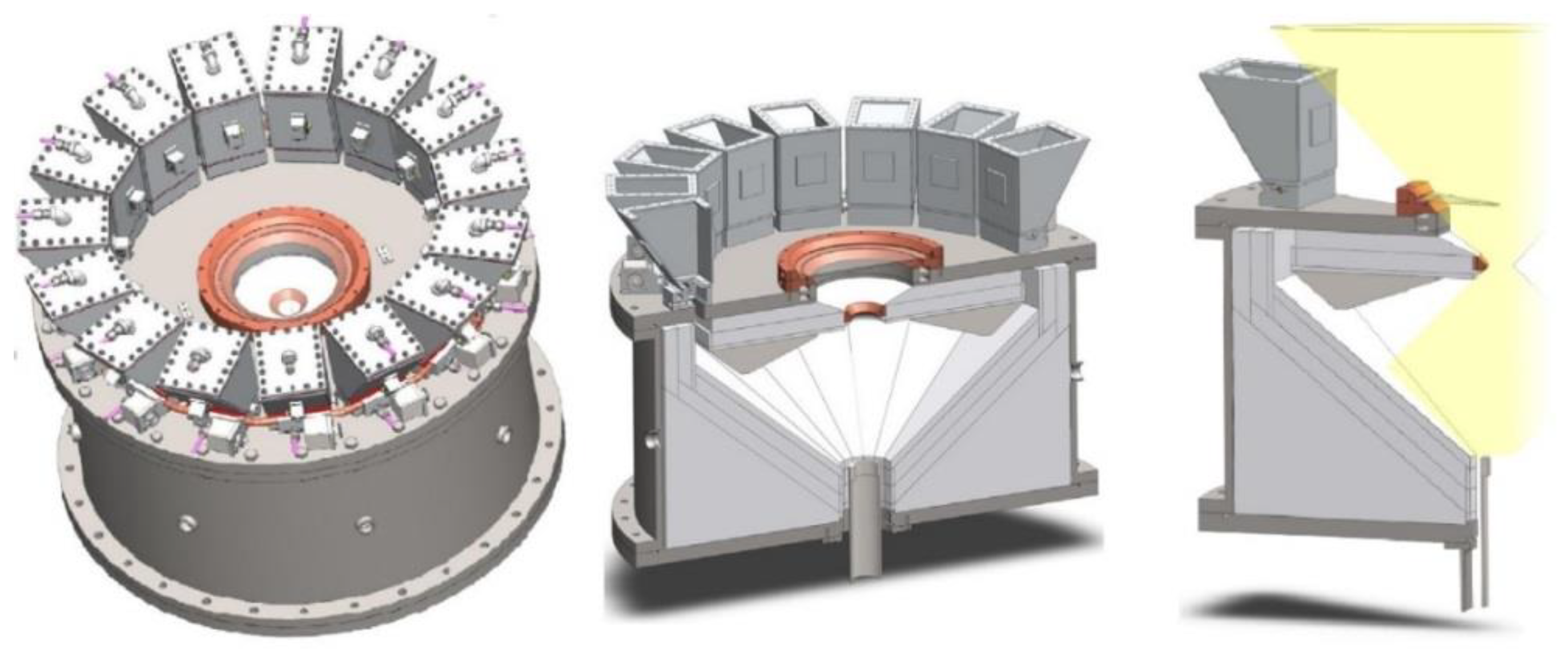
- Kodama, T.; Bellan, S.; Gokon, N.; Cho, H.S. Particle Reactors for Solar Thermochemical Processes. Sol. Energy 2017, 156, 113–132. [Google Scholar] [CrossRef]
- Abanades, S.; Rodat, S.; Boujjat, H. Solar Thermochemical Green Fuels Production: A Review of Biomass Pyro-Gasification, Solar Reactor Concepts and Modelling Methods. Energies 2021, 14, 1494. [Google Scholar] [CrossRef]
- Alonso, E.; Romero, M. Review of Experimental Investigation on Directly Irradiated Particles Solar Reactors. Renew. Sustain. Energy Rev. 2015, 41, 53–67. [Google Scholar] [CrossRef]
- Gokon, N.; Takahashi, S.; Yamamoto, H.; Kodama, T. Thermochemical Two-Step Water-Splitting Reactor with Internally Circulating Fluidized Bed for Thermal Reduction of Ferrite Particles. Int. J. Hydrogen Energy 2008, 33, 2189–2199. [Google Scholar] [CrossRef]
- Al-Shankiti, I.; Ehrhart, B.D.; Weimer, A.W. Isothermal Redox for H2O and CO2 Splitting—A Review and Perspective. Sol. Energy 2017, 156, 21–29. [Google Scholar] [CrossRef]
- Davenport, T.C.; Yang, C.-K.; Kucharczyk, C.J.; Ignatowich, M.J.; Haile, S.M. Maximizing Fuel Production Rates in Isothermal Solar Thermochemical Fuel Production. Appl. Energy 2016, 183, 1098–1111. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, C.-K.; Haile, S.M. High-Temperature Isothermal Chemical Cycling for Solar-Driven Fuel Production. Phys. Chem. Chem. Phys. 2013, 15, 17084. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, A.L.; Millican, S.L.; Czernik, C.E.; Alshankiti, I.; Netter, J.C.; Wendelin, T.J.; Musgrave, C.B.; Weimer, A.W. Continuous On-Sun Solar Thermochemical Hydrogen Production via an Isothermal Redox Cycle. Appl. Energy 2019, 249, 368–376. [Google Scholar] [CrossRef]
- Kong, H.; Hao, Y.; Jin, H. Isothermal versus Two-Temperature Solar Thermochemical Fuel Synthesis: A Comparative Study. Appl. Energy 2018, 228, 301–308. [Google Scholar] [CrossRef]
- Ma, T.; Wang, L.; Chang, C.; Akhatov, J.S.; Fu, M.; Li, X. A Comparative Thermodynamic Analysis of Isothermal and Non-Isothermal CeO2-Based Solar Thermochemical Cycle with Methane-Driven Reduction. Renew. Energy 2019, 143, 915–921. [Google Scholar] [CrossRef]
- Venstrom, L.J.; De Smith, R.M.; Hao, Y.; Haile, S.M.; Davidson, J.H. Efficient Splitting of CO2 in an Isothermal Redox Cycle Based on Ceria. Energy Fuels 2014, 28, 2732–2742. [Google Scholar] [CrossRef]
- Haeussler, A.; Chuayboon, S.; Abanades, S. Solar Redox Cycling of Ceria in a Monolithic Reactor for Two-Step H2O/CO2 Splitting: Isothermal Methane-Induced Reduction versus Temperature-Swing Cycle. AIP Conf. Proc. 2020, 2303, 170009. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Abanades, S.; Salvado, I.M.M.; Ferreira, J.M.F.; Pullar, R.C. Robocasting of 3D Printed and Sintered Ceria Scaffold Structures with Hierarchical Porosity for Solar Thermochemical Fuel Production from the Splitting of CO2. Nanoscale 2022, 14, 4994–5001. [Google Scholar] [CrossRef]
- Haeussler, A.; Abanades, S.; Jouannaux, J.; Julbe, A. Demonstration of a Ceria Membrane Solar Reactor Promoted by Dual Perovskite Coatings for Continuous and Isothermal Redox Splitting of CO2 and H2O. J. Membr. Sci. 2021, 634, 119387. [Google Scholar] [CrossRef]
- Tou, M.; Michalsky, R.; Steinfeld, A. Solar-Driven Thermochemical Splitting of CO2 and In Situ Separation of CO and O2 across a Ceria Redox Membrane Reactor. Joule 2017, 1, 146–154. [Google Scholar] [CrossRef]
- Tou, M.; Jin, J.; Hao, Y.; Steinfeld, A.; Michalsky, R. Solar-Driven Co-Thermolysis of CO 2 and H 2 O Promoted by in Situ Oxygen Removal across a Non-Stoichiometric Ceria Membrane. React. Chem. Eng. 2019, 4, 1431–1438. [Google Scholar] [CrossRef]
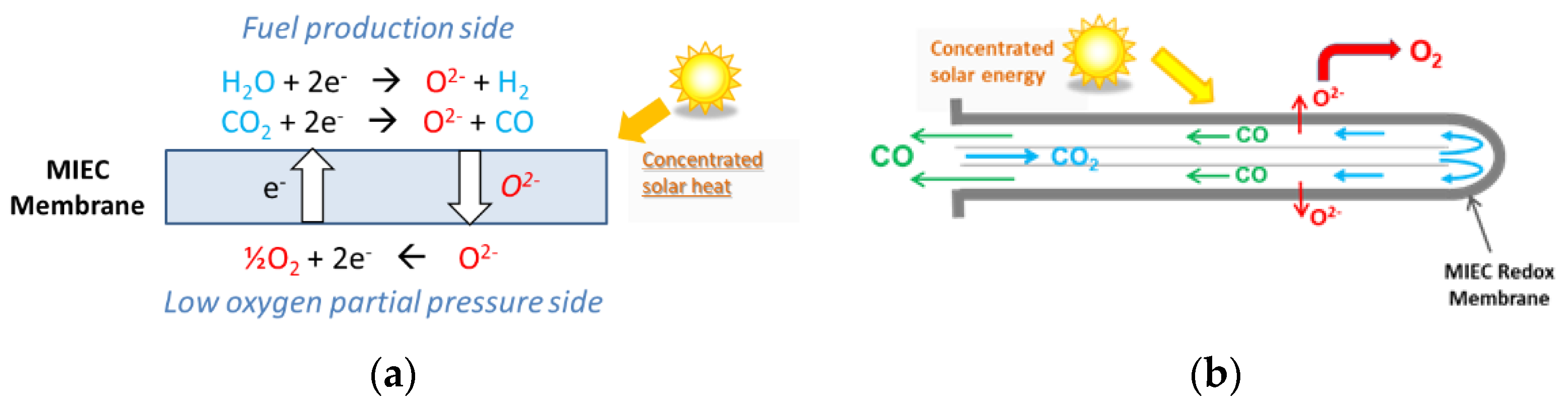
- Wu, X.-Y.; Ghoniem, A.F. Mixed Ionic-Electronic Conducting (MIEC) Membranes for Thermochemical Reduction of CO2: A Review. Prog. Energy Combust. Sci. 2019, 74, 1–30. [Google Scholar] [CrossRef]
- Charvin, P.; Abanades, S.; Neveu, P.; Lemont, F.; Flamant, G. Dynamic Modeling of a Volumetric Solar Reactor for Volatile Metal Oxide Reduction. Chem. Eng. Res. Des. 2008, 86, 1216–1222. [Google Scholar] [CrossRef]
- Koepf, E.; Villasmil, W.; Meier, A. Pilot-Scale Solar Reactor Operation and Characterization for Fuel Production via the Zn/ZnO Thermochemical Cycle. Appl. Energy 2016, 165, 1004–1023. [Google Scholar] [CrossRef]
- Abanades, S.; Charvin, P.; Flamant, G. Design and Simulation of a Solar Chemical Reactor for the Thermal Reduction of Metal Oxides: Case Study of Zinc Oxide Dissociation. Chem. Eng. Sci. 2007, 62, 6323–6333. [Google Scholar] [CrossRef]
- Chambon, M.; Abanades, S.; Flamant, G. Design of a Lab-Scale Rotary Cavity-Type Solar Reactor for Continuous Thermal Dissociation of Volatile Oxides Under Reduced Pressure. J. Sol. Energy Eng. 2010, 132, 021006. [Google Scholar] [CrossRef]
- Koepf, E.; Advani, S.G.; Steinfeld, A.; Prasad, A.K. A Novel Beam-down, Gravity-Fed, Solar Thermochemical Receiver/Reactor for Direct Solid Particle Decomposition: Design, Modeling, and Experimentation. Int. J. Hydrogen Energy 2012, 37, 16871–16887. [Google Scholar] [CrossRef]
- Diver, R.B.; Miller, J.E.; Allendorf, M.D.; Siegel, N.P.; Hogan, R.E. Solar Thermochemical Water-Splitting Ferrite-Cycle Heat Engines. J. Sol. Energy Eng. 2008, 130, 041001. [Google Scholar] [CrossRef]
- Ermanoski, I.; Siegel, N.P.; Stechel, E.B. A New Reactor Concept for Efficient Solar-Thermochemical Fuel Production. J. Sol. Energy Eng. 2013, 135, 031002. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abanades, S. Redox Cycles, Active Materials, and Reactors Applied to Water and Carbon Dioxide Splitting for Solar Thermochemical Fuel Production: A Review. Energies 2022, 15, 7061. https://doi.org/10.3390/en15197061
Abanades S. Redox Cycles, Active Materials, and Reactors Applied to Water and Carbon Dioxide Splitting for Solar Thermochemical Fuel Production: A Review. Energies. 2022; 15(19):7061. https://doi.org/10.3390/en15197061
Chicago/Turabian StyleAbanades, Stéphane. 2022. "Redox Cycles, Active Materials, and Reactors Applied to Water and Carbon Dioxide Splitting for Solar Thermochemical Fuel Production: A Review" Energies 15, no. 19: 7061. https://doi.org/10.3390/en15197061
APA StyleAbanades, S. (2022). Redox Cycles, Active Materials, and Reactors Applied to Water and Carbon Dioxide Splitting for Solar Thermochemical Fuel Production: A Review. Energies, 15(19), 7061. https://doi.org/10.3390/en15197061




