Research Progress in High-Throughput Screening of CO2 Reduction Catalysts
Abstract
1. Introduction
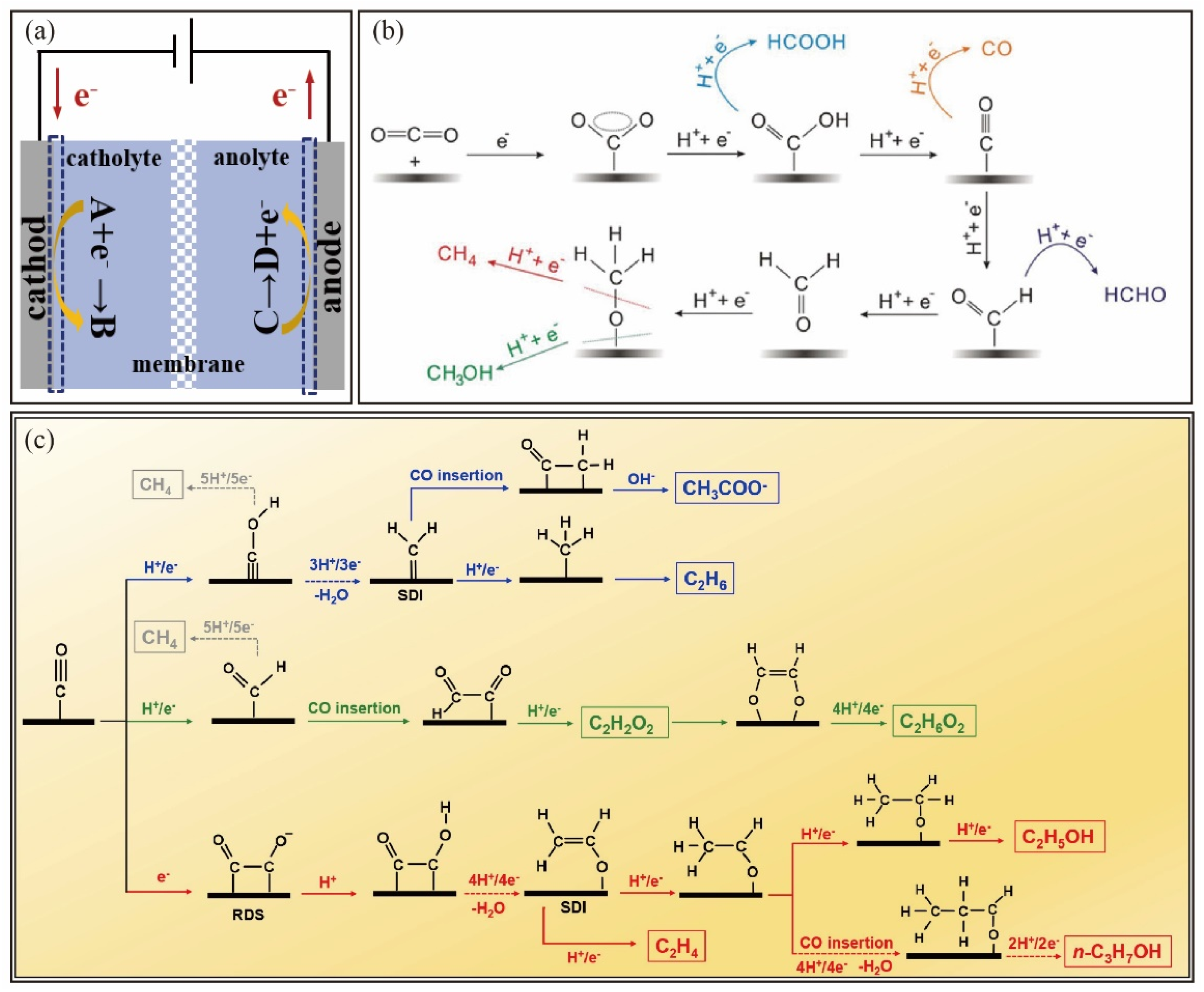
2. Mechanism of CO2 Electrocatalytic Reduction
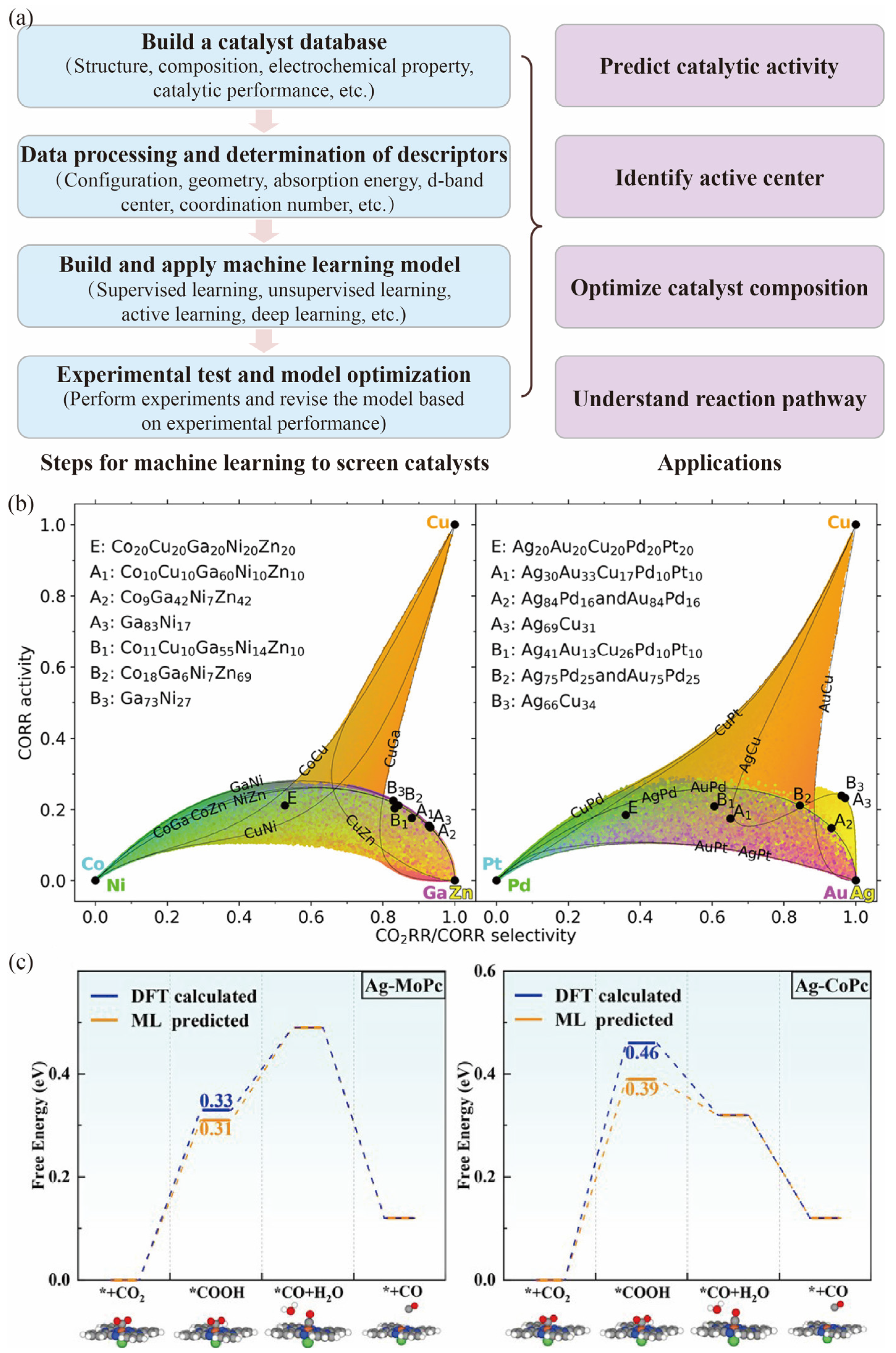
3. High-Throughput Computational Methods
4. High-Throughput Experimental Methods
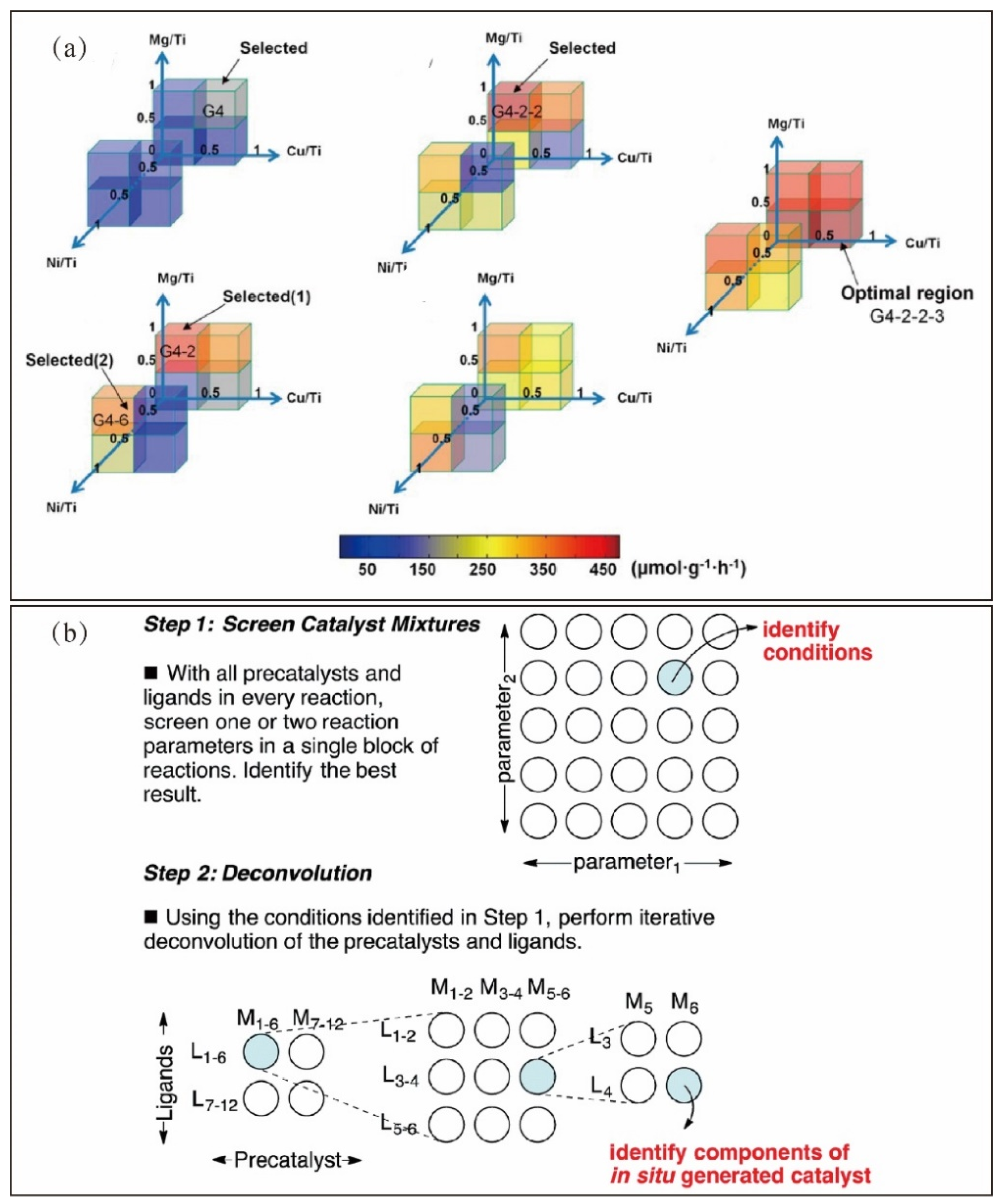
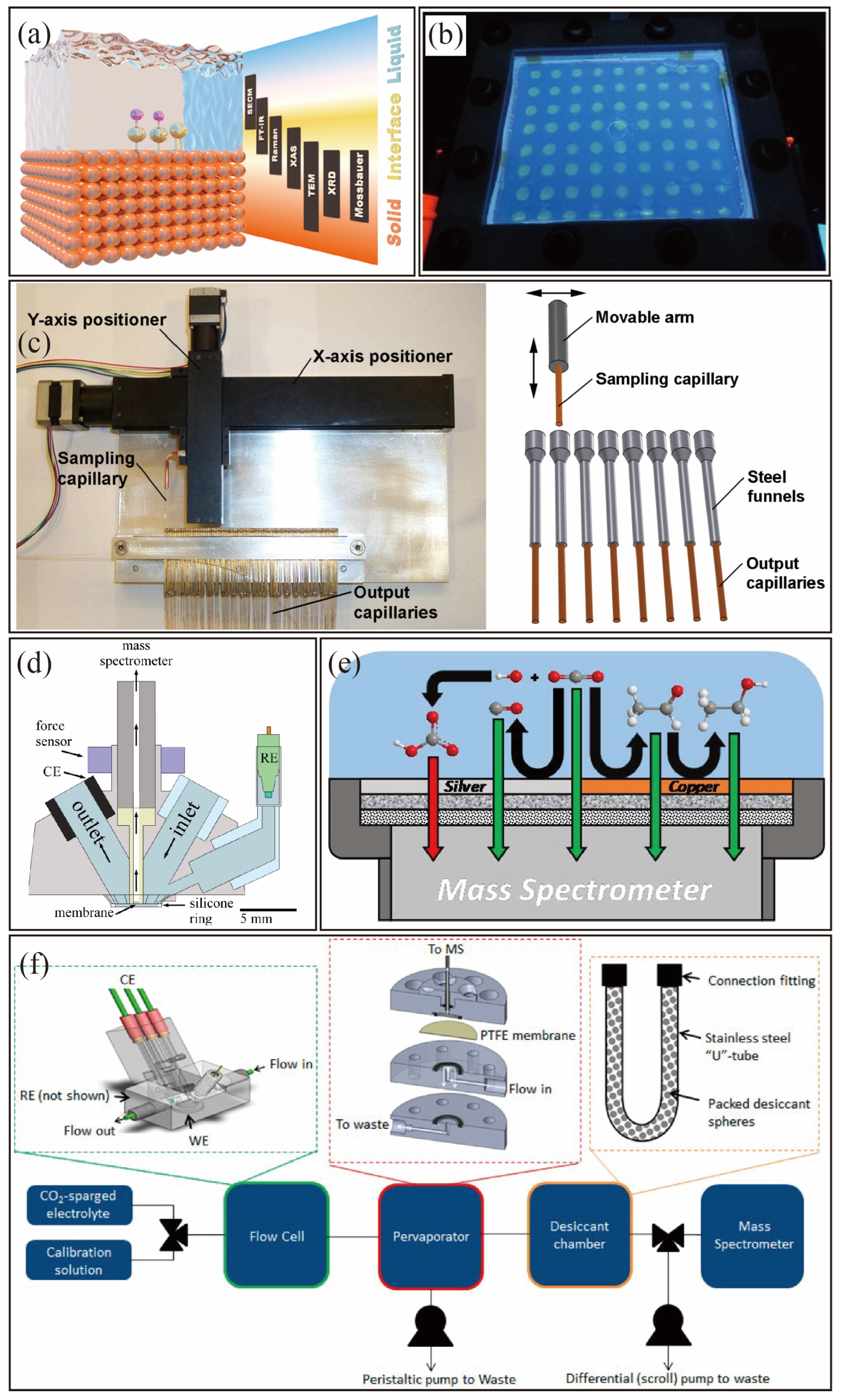
4.1. Experimental Design
4.2. High-Throughput Synthesis
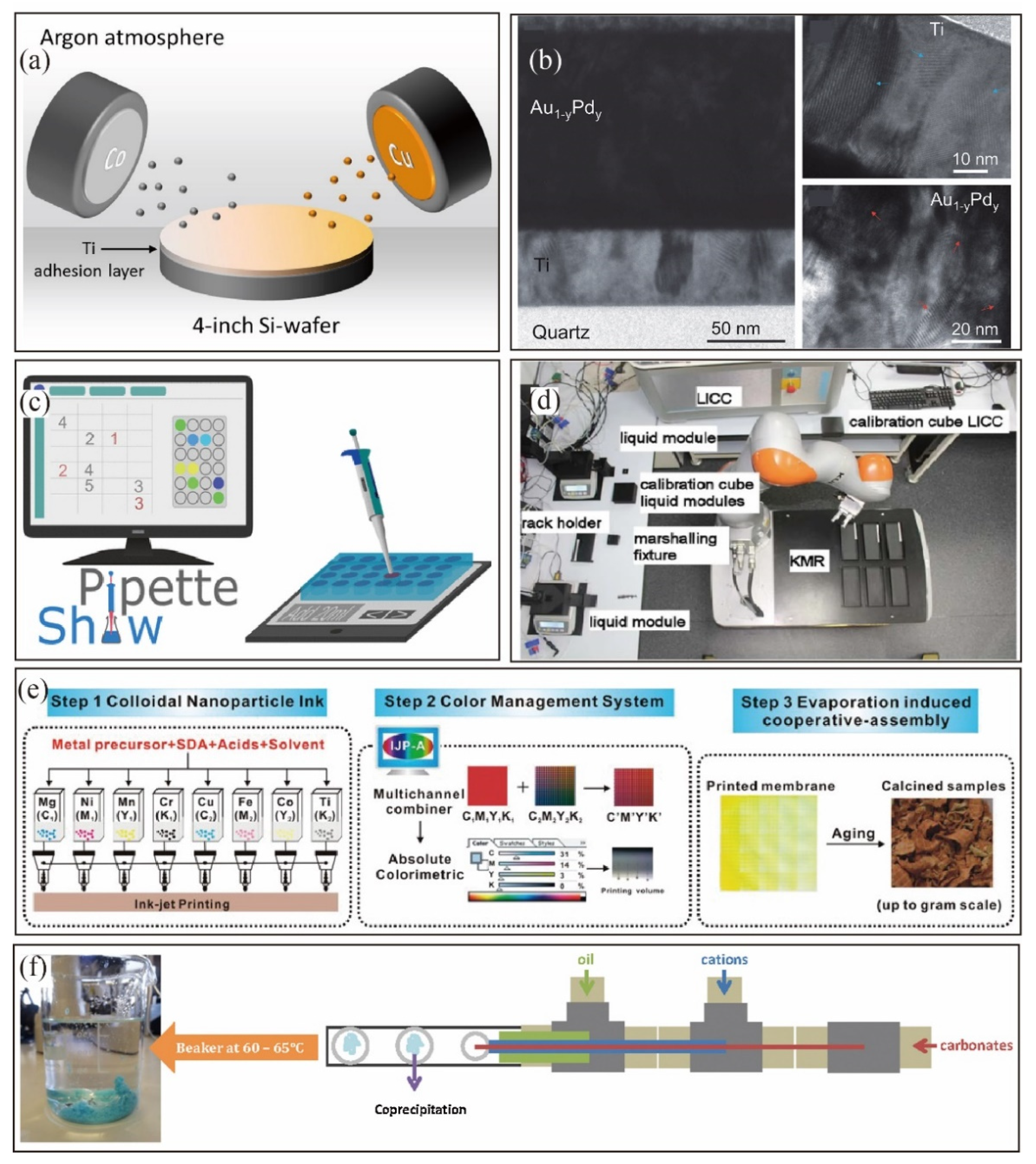
4.3. In Situ Characterization
4.4. High-Throughput Testing
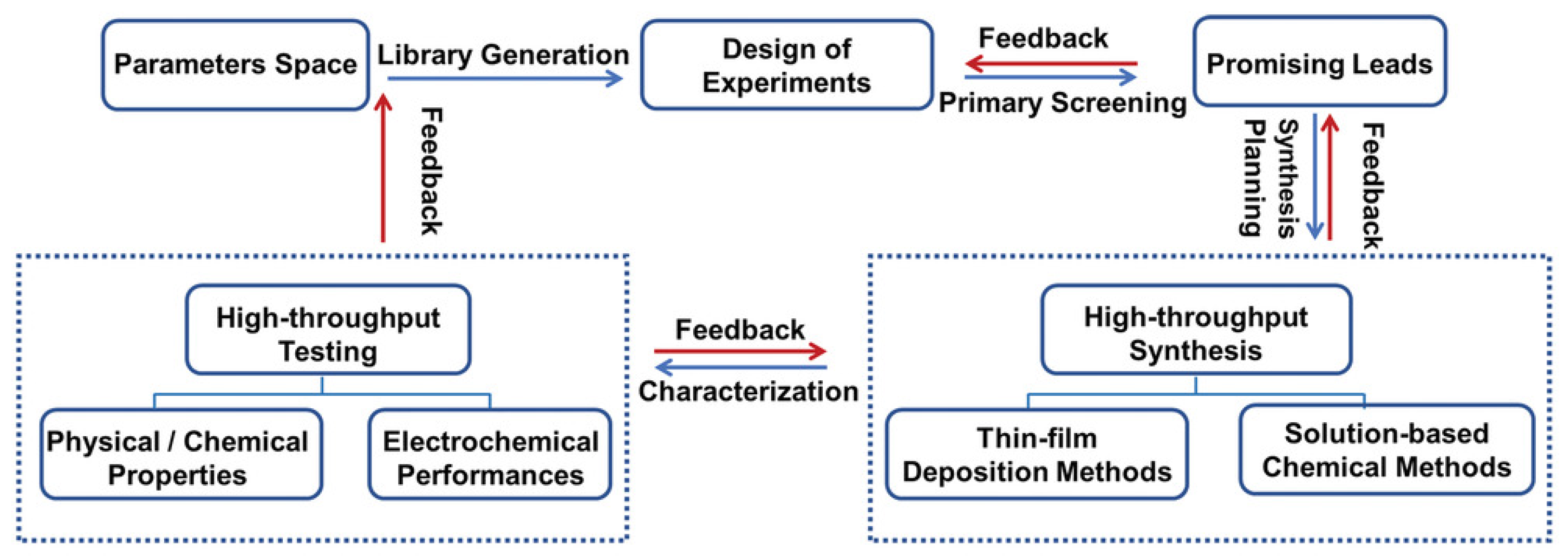
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.C. Reduction of CO2 to chemicals and fuels: A solution to global warming and energy crisis. ACS Energy Lett. 2018, 3, 1557–1561. [Google Scholar] [CrossRef]
- Zhong, H.; Ghorbani-Asl, M.; Ly, K.H.; Zhang, J.; Ge, J.; Wang, M.; Liao, Z.; Makarov, D.; Zschech, E.; Brunner, E. Synergistic electroreduction of carbon dioxide to carbon monoxide on bimetallic layered conjugated metal-organic frameworks. Nat. Commun. 2020, 11, 1–10. [Google Scholar]
- Kattel, S.; Ramírez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef]
- Arandiyan, H.; Kani, K.; Wang, Y.; Jiang, B.; Kim, J.; Yoshino, M.; Rezaei, M.; Rowan, A.E.; Dai, H.; Yamauchi, Y. Highly selective reduction of carbon dioxide to methane on novel mesoporous Rh catalysts. ACS Appl. Mater. Interfaces 2018, 10, 24963–24968. [Google Scholar] [CrossRef]
- Phillips, K.R.; Katayama, Y.; Hwang, J.; Shao-Horn, Y. Sulfide-Derived Copper for Electrochemical Conversion of CO2 to Formic Acid. J. Phys. Chem. Lett. 2018, 9, 4407–4412. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.S.; Aranha, B.S.; Ghosh, A.; Lobo, A.O.; da Silva, E.T.; Alves, D.C.; Viana, B.C. Production of oxalic acid by electrochemical reduction of CO2 using silver-carbon material from babassu coconut mesocarp. J. Phys. Chem. Solids 2020, 147, 109678. [Google Scholar] [CrossRef]
- Chan, F.L.; Altinkaya, G.; Fung, N.; Tanksale, A. Low temperature hydrogenation of carbon dioxide into formaldehyde in liquid media. Catal. Today 2018, 309, 242–247. [Google Scholar] [CrossRef]
- Kim, S.; Yang, Y.; Lippi, R.; Choi, H.; Kim, S.; Chun, D.; Im, H.; Lee, S.; Yoo, J. Low-Rank Coal Supported Ni Catalysts for CO2 Methanation. Energies 2021, 14, 2040. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S.p. Tailoring hybrid nonstoichiometric ceria redox cycle for combined solar methane reforming and thermochemical conversion of H2O/CO2. Energy Fuels 2016, 30, 6050–6058. [Google Scholar] [CrossRef]
- Hare, B.J.; Maiti, D.; Ramani, S.; Ramos, A.E.; Bhethanabotla, V.R.; Kuhn, J.N. Thermochemical conversion of carbon dioxide by reverse water-gas shift chemical looping using supported perovskite oxides. Catal. Today 2019, 323, 225–232. [Google Scholar] [CrossRef]
- Feng, K.; Wang, Y.; Guo, M.; Zhang, J.; Li, Z.; Deng, T.; Zhang, Z.; Yan, B. In-situ/operando techniques to identify active sites for thermochemical conversion of CO2 over heterogeneous catalysts. J. Energy Chem. 2021, 62, 153–171. [Google Scholar] [CrossRef]
- Suvarna, M.; Araújo, T.P.; Pérez-Ramírez, J. A generalized machine learning framework to predict the space-time yield of methanol from thermocatalytic CO2 hydrogenation. Appl. Catal. B 2022, 121530. [Google Scholar] [CrossRef]
- Huang, H.; Lin, J.; Zhu, G.; Weng, Y.; Wang, X.; Fu, X.; Long, J. A long-lived mononuclear cyclopentadienyl ruthenium complex grafted onto anatase TiO2 for efficient CO2 photoreduction. Angew. Chem. Int. Ed. 2016, 55, 8314–8318. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Li, L.; Shen, J.; Zhang, Y.; Liang, J. Zn2SnxTi1–xO4 Continuous Solid-Solution Photocatalyst for Efficient Photocatalytic CO2 Conversion into Solar Fuels. ACS Appl. Energy Mater. 2022, 5, 3748–3756. [Google Scholar] [CrossRef]
- Cheng, S.; Sun, Z.; Lim, K.H.; Gani, T.Z.H.; Zhang, T.; Wang, Y.; Yin, H.; Liu, K.; Guo, H.; Du, T. Emerging Strategies for CO2 Photoreduction to CH4: From Experimental to Data-Driven Design. Adv. Energy Mater. 2022, 12, 2200389. [Google Scholar] [CrossRef]
- Yang, G.; Qiu, P.; Xiong, J.; Zhu, X.; Cheng, G. Facilely anchoring Cu2O nanoparticles on mesoporous TiO2 nanorods for enhanced photocatalytic CO2 reduction through efficient charge transfer. Chin. Chem. Lett. 2022, 33, 3709–3712. [Google Scholar] [CrossRef]
- Sen, S.; Liu, D.; Palmore, G.T.R. Electrochemical reduction of CO2 at copper nanofoams. ACS Catal. 2014, 4, 3091–3095. [Google Scholar] [CrossRef]
- Xie, H.; Wang, T.; Liang, J.; Li, Q.; Sun, S. Cu-based nanocatalysts for electrochemical reduction of CO2. Nano Today 2018, 21, 41–54. [Google Scholar] [CrossRef]
- Jiang, K.; Huang, Y.; Zeng, G.; Toma, F.M.; Goddard, W.A., III; Bell, A.T. Effects of surface roughness on the electrochemical reduction of CO2 over Cu. ACS Energy Lett. 2020, 5, 1206–1214. [Google Scholar] [CrossRef]
- Masana, J.J.; Peng, B.; Shuai, Z.; Qiu, M.; Yu, Y. Influence of Halide Ions on Electrochemical Reduction of Carbon dioxide over Copper Surface. J. Mater. Chem. A 2022, 10, 1086. [Google Scholar] [CrossRef]
- Liu, C.; Colón, B.C.; Ziesack, M.; Silver, P.A.; Nocera, D.G. Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 2016, 352, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Harris, D.F.; Dean, D.R.; Liu, T.L.; Yang, Z.-Y.; Seefeldt, L.C. Electrocatalytic CO2 reduction catalyzed by nitrogenase MoFe and FeFe proteins. Bioelectrochemistry 2018, 120, 104–109. [Google Scholar] [CrossRef]
- Shafaat, H.S.; Yang, J.Y. Uniting biological and chemical strategies for selective CO2 reduction. Nat. Catal. 2021, 4, 928–933. [Google Scholar] [CrossRef]
- Lopes, E.J.; Ribeiro, A.P.; Martins, L.M. New trends in the conversion of CO2 to cyclic carbonates. Catalysts 2020, 10, 479. [Google Scholar] [CrossRef]
- Schneider, J.; Jia, H.; Muckerman, J.T.; Fujita, E. Thermodynamics and kinetics of CO2, CO, and H+ binding to the metal centre of CO2 reduction catalysts. Chem. Soc. Rev. 2012, 41, 2036–2051. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhang, D.; Lin, C.Y.; Zhu, Y.; Shen, Y.; Zhang, J.; Han, X.; Zhang, L.; Xia, Z. Catalytic mechanisms and design principles for single-atom catalysts in highly efficient CO2 conversion. Adv. Energy Mater. 2019, 9, 1902625. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, S.-X.; Gandionco, K.A.; Bond, A.M.; Zhang, J. Electrocatalytic carbon dioxide reduction: From fundamental principles to catalyst design. Mater. Today 2020, 7, 100074. [Google Scholar] [CrossRef]
- Grote, J.-P.; Zeradjanin, A.R.; Cherevko, S.; Savan, A.; Breitbach, B.; Ludwig, A.; Mayrhofer, K.J. Screening of material libraries for electrochemical CO2 reduction catalysts—Improving selectivity of Cu by mixing with Co. J. Catal. 2016, 343, 248–256. [Google Scholar] [CrossRef]
- Li, W.; Nie, X.; Jiang, X.; Zhang, A.; Ding, F.; Liu, M.; Liu, Z.; Guo, X.; Song, C. ZrO2 support imparts superior activity and stability of Co catalysts for CO2 methanation. Appl. Catal. B. 2018, 220, 397–408. [Google Scholar] [CrossRef]
- Karmodak, N.; Vijay, S.; Kastlunger, G.; Chan, K. Computational Screening of Single and Di-Atom Catalysts for Electrochemical CO2 Reduction. ACS Catal. 2022, 12, 4818–4824. [Google Scholar] [CrossRef]
- Liu, K.; Wang, J.; Shi, M.; Yan, J.; Jiang, Q. Simultaneous achieving of high faradaic efficiency and CO partial current density for CO2 reduction via robust, noble-metal-free Zn nanosheets with favorable adsorption energy. Adv. Energy Mater. 2019, 9, 1900276. [Google Scholar] [CrossRef]
- Mayer, F.D.; Hosseini-Benhangi, P.; Sánchez-Sánchez, C.M.; Asselin, E.; Gyenge, E.L. Scanning electrochemical microscopy screening of CO2 electroreduction activities and product selectivities of catalyst arrays. Commun. Chem. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- De Gregorio, G.L.; Burdyny, T.; Loiudice, A.; Iyengar, P.; Smith, W.A.; Buonsanti, R. Facet-dependent selectivity of Cu catalysts in electrochemical CO2 reduction at commercially viable current densities. ACS Catal. 2020, 10, 4854–4862. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Katiyar, N.K.; Kumar, R.; Parui, A.; Malviya, K.D.; Pradeep, K.; Singh, A.K.; Sharma, S.; Tiwary, C.S.; Biswas, K. High-entropy alloys as catalysts for the CO2 and CO reduction reactions: Experimental realization. ACS Catal. 2020, 10, 3658–3663. [Google Scholar] [CrossRef]
- Batchelor, T.A.; Löffler, T.; Xiao, B.; Krysiak, O.A.; Strotkötter, V.; Pedersen, J.K.; Clausen, C.M.; Savan, A.; Li, Y.; Schuhmann, W. Complex-Solid-Solution Electrocatalyst Discovery by Computational Prediction and High-Throughput Experimentation. Angew. Chem. Int. Ed. 2021, 60, 6932–6937. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef] [PubMed]
- Kibria, M.G.; Edwards, J.P.; Gabardo, C.M.; Dinh, C.T.; Seifitokaldani, A.; Sinton, D.; Sargent, E.H. Electrochemical CO2 reduction into chemical feedstocks: From mechanistic electrocatalysis models to system design. Adv. Mater. 2019, 31, 1807166. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, Z.J.; Gong, J. Nanostructured materials for heterogeneous electrocatalytic CO2 reduction and their related reaction mechanisms. Angew. Chem. Int. Ed. 2017, 56, 11326–11353. [Google Scholar] [CrossRef]
- Zheng, Y.; Vasileff, A.; Zhou, X.; Jiao, Y.; Jaroniec, M.; Qiao, S.-Z. Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts. J. Am. Chem. Soc. 2019, 141, 7646–7659. [Google Scholar] [CrossRef]
- Handoko, A.D.; Chen, H.; Lum, Y.; Zhang, Q.; Anasori, B.; Seh, Z.W. Two-dimensional titanium and molybdenum carbide MXenes as electrocatalysts for CO2 reduction. IScience 2020, 23, 101181. [Google Scholar] [CrossRef] [PubMed]
- Hooe, S.L.; Dressel, J.M.; Dickie, D.A.; Machan, C.W. Highly efficient electrocatalytic reduction of CO2 to CO by a molecular chromium complex. ACS Catal. 2019, 10, 1146–1151. [Google Scholar] [CrossRef]
- He, J.; Dettelbach, K.E.; Huang, A.; Berlinguette, C.P. Brass and bronze as effective CO2 reduction electrocatalysts. Angew. Chem. Int. Ed. 2017, 129, 16806–16809. [Google Scholar] [CrossRef]
- Li, Z.; He, D.; Yan, X.; Dai, S.; Younan, S.; Ke, Z.; Pan, X.; Xiao, X.; Wu, H.; Gu, J. Size-dependent nickel-based electrocatalysts for selective CO2 reduction. Angew. Chem. Int. Ed. 2020, 132, 18731–18736. [Google Scholar] [CrossRef]
- Umeda, M.; Niitsuma, Y.; Horikawa, T.; Matsuda, S.; Osawa, M. Electrochemical reduction of CO2 to methane on platinum catalysts without overpotentials: Strategies for improving conversion efficiency. ACS Appl. Energy Mater. 2019, 3, 1119–1127. [Google Scholar] [CrossRef]
- Ma, M.; Liu, K.; Shen, J.; Kas, R.; Smith, W.A. In Situ fabrication and reactivation of highly selective and stable Ag catalysts for electrochemical CO2 conversion. ACS Energy Lett. 2018, 3, 1301–1306. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Sethuraman, V.; Michalsky, R.; Peterson, A.A. Competition between CO2 reduction and H2 evolution on transition-metal electrocatalysts. ACS Catal. 2014, 4, 3742–3748. [Google Scholar] [CrossRef]
- Wang, Z.; She, X.; Yu, Q.; Zhu, X.; Li, H.; Xu, H. Minireview on the Commonly Applied Copper-Based Electrocatalysts for Electrochemical CO2 Reduction. Energy Fuels 2021, 35, 8585–8601. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Schouten, K.J.P.; Gallent, E.P.; Koper, M.T. The influence of pH on the reduction of CO and CO2 to hydrocarbons on copper electrodes. J. Electroanal. Chem. 2014, 716, 53–57. [Google Scholar] [CrossRef]
- Wang, L.; Nitopi, S.A.; Bertheussen, E.; Orazov, M.; Morales-Guio, C.G.; Liu, X.; Higgins, D.C.; Chan, K.; Nørskov, J.K.; Hahn, C. Electrochemical carbon monoxide reduction on polycrystalline copper: Effects of potential, pressure, and pH on selectivity toward multicarbon and oxygenated products. ACS Catal. 2018, 8, 7445–7454. [Google Scholar] [CrossRef]
- Wu, J.; Sharifi, T.; Gao, Y.; Zhang, T.; Ajayan, P.M. Emerging carbon-based heterogeneous catalysts for electrochemical reduction of carbon dioxide into value-added chemicals. Adv. Mater. 2019, 31, 1804257. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Quan, X. Carbon-based materials for electrochemical reduction of CO2 to C2+ oxygenates: Recent progress and remaining challenges. ACS Catal. 2021, 11, 2076–2097. [Google Scholar] [CrossRef]
- Martínez-Hincapié, R.; Čolić, V. Electrocatalysts for the Oxygen Reduction Reaction: From Bimetallic Platinum Alloys to Complex Solid Solutions. ChemEng. 2022, 6, 19. [Google Scholar] [CrossRef]
- Chen, S.; Liu, T.; Olanrele, S.O.; Lian, Z.; Si, C.; Chen, Z.; Li, B. Boosting electrocatalytic activity for CO2 reduction on nitrogen-doped carbon catalysts by co-doping with phosphorus. J. Energy Chem. 2021, 54, 143–150. [Google Scholar] [CrossRef]
- Löffler, T.; Ludwig, A.; Rossmeisl, J.; Schuhmann, W. What Makes High-Entropy Alloys Exceptional Electrocatalysts? Angew. Chem. Int. Ed. 2021, 60, 26894–26903. [Google Scholar] [CrossRef]
- Liu, X.; Liu, B.; Ding, J.; Deng, Y.; Han, X.; Zhong, C.; Hu, W. Building a Library for Catalysts Research Using High-Throughput Approaches. Adv. Funct. Mater. 2022, 2107862. [Google Scholar] [CrossRef]
- Steinmann, S.N.; Hermawan, A.; Jassar, M.B.; Seh, Z.W. Autonomous high-throughput computations in catalysis. Chem Catal. 2022, 2, 917–1240. [Google Scholar] [CrossRef]
- Qin, X.; Zhu, S.; Xiao, F.; Zhang, L.; Shao, M. Active sites on heterogeneous single-iron-atom electrocatalysts in CO2 reduction reaction. ACS Energy Lett. 2019, 4, 1778–1783. [Google Scholar] [CrossRef]
- Tian, D.; Denny, S.R.; Li, K.; Wang, H.; Kattel, S.; Chen, J.G. Density functional theory studies of transition metal carbides and nitrides as electrocatalysts. Chem. Soc. Rev. 2021, 50, 12338. [Google Scholar] [CrossRef]
- Zhong, M.; Tran, K.; Min, Y.; Wang, C.; Wang, Z.; Dinh, C.-T.; De Luna, P.; Yu, Z.; Rasouli, A.S.; Brodersen, P. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature. 2020, 581, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, W.; Jiang, Q. A machine learning scheme for the catalytic activity of alloys with intrinsic descriptors. J. Mater. Chem. A. 2020, 8, 17507–17515. [Google Scholar] [CrossRef]
- Ting, K.W.; Kamakura, H.; Poly, S.S.; Takao, M.; Siddiki, S.H.; Maeno, Z.; Matsushita, K.; Shimizu, K.-i.; Toyao, T. Catalytic Methylation of m-Xylene, Toluene, and Benzene Using CO2 and H2 over TiO2-Supported Re and Zeolite Catalysts: Machine-Learning-Assisted Catalyst Optimization. ACS Catal. 2021, 11, 5829–5838. [Google Scholar] [CrossRef]
- Zhi, X.; Jiao, Y.; Zheng, Y.; Qiao, S.Z. Impact of interfacial electron transfer on electrochemical CO2 reduction on graphitic carbon nitride/doped graphene. Small 2019, 15, 1804224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, X.; Kang, Y.; Ye, C.; Jin, R.; Yan, H.; Lin, R.; Yang, J.; Xu, Q.; Wang, Y. A supported Pd2 dual-atom site catalyst for efficient electrochemical CO2 reduction. Angew. Chem. Int. Ed. 2021, 133, 13500–13505. [Google Scholar] [CrossRef]
- McCullough, K.; Williams, T.; Mingle, K.; Jamshidi, P.; Lauterbach, J. High-throughput experimentation meets artificial intelligence: A new pathway to catalyst discovery. Phys. Chem. Chem. Phys. 2020, 22, 11174–11196. [Google Scholar] [CrossRef]
- Ribeiro, M.T.; Singh, S.; Guestrin, C. “Why should I trust you”? Explaining the predictions of any classifier. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, San Francisco, CA, USA, 13–17 August 2016; pp. 1135–1144. [Google Scholar]
- Sun, Z.; Yin, H.; Liu, K.; Cheng, S.; Li, G.K.; Kawi, S.; Zhao, H.; Jia, G.; Yin, Z. Machine learning accelerated calculation and design of electrocatalysts for CO2 reduction. SmartMat 2022, 3, 68–83. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, Y.; Ran, M.; Zhang, X.; Liu, S.; Yang, Y.; Hu, W.; Zheng, C.; Gao, X. Accelerated identification of high-performance catalysts for low-temperature NH3-SCR by machine learning. J. Mater. Chem. A 2021, 9, 23850–23859. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, B.; Liu, K.; Li, H.; Chen, G.; Qiu, X.; Li, W.; Hu, J.; Fu, J.; Jiang, Y. Machine Learning in Screening High Performance Electrocatalysts for CO2 Reduction. Small Methods 2021, 5, 2100987. [Google Scholar] [CrossRef]
- Roy, D.; Mandal, S.C.; Pathak, B. Machine Learning-Driven High-Throughput Screening of Alloy-Based Catalysts for Selective CO2 Hydrogenation to Methanol. ACS Appl. Mater. Interfaces 2021, 13, 56151–56163. [Google Scholar] [CrossRef]
- Pedersen, J.K.; Batchelor, T.A.; Bagger, A.; Rossmeisl, J. High-entropy alloys as catalysts for the CO2 and CO reduction reactions. ACS Catal. 2020, 10, 2169–2176. [Google Scholar] [CrossRef]
- Daiyan, R.; Saputera, W.H.; Masood, H.; Leverett, J.; Lu, X.; Amal, R. A Disquisition on the Active Sites of Heterogeneous Catalysts for Electrochemical Reduction of CO2 to Value-Added Chemicals and Fuel. Adv. Energy Mater. 2020, 10, 1902106. [Google Scholar] [CrossRef]
- Tran, K.; Ulissi, Z.W. Active learning across intermetallics to guide discovery of electrocatalysts for CO2 reduction and H2 evolution. Nat. Catal. 2018, 1, 696–703. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Cheng, T.; Goddard, W.A., III. Identifying active sites for CO2 reduction on dealloyed gold surfaces by combining machine learning with multiscale simulations. J. Am. Chem. Soc. 2019, 141, 11651–11657. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Yang, H.; Fu, X.; Cheng, X.; Guo, J.; Fu, L. A DFT study of two-dimensional P2Si monolayer modified by single transition metal (Sc-Cu) atoms for efficient electrocatalytic CO2 reduction. Chin. Chem. Lett. 2022. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, Z.; Niu, H.; Yin, Y.; Kuai, C.; Wang, J.; Shao, C.; Guo, Y. Machine-Learning-Accelerated Catalytic Activity Predictions of Transition Metal Phthalocyanine Dual-Metal-Site Catalysts for CO2 Reduction. J. Phys. Chem. Lett. 2021, 12, 6111–6118. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, W.; Wang, L.; Zhang, J.; Fu, X.Z.; Luo, J.L. Toward Excellence of Electrocatalyst Design by Emerging Descriptor-Oriented Machine Learning. Adv. Funct. Mater. 2022, 32, 2110748. [Google Scholar] [CrossRef]
- Lai, Y.; Jones, R.J.; Wang, Y.; Zhou, L.; Richter, M.H.; Gregoire, J. The sensitivity of Cu for electrochemical carbon dioxide reduction to hydrocarbons as revealed by high throughput experiments. J. Mater. Chem. A. 2019, 7, 26785–26790. [Google Scholar] [CrossRef]
- Hitt, J.L.; Li, Y.C.; Tao, S.; Yan, Z.; Gao, Y.; Billinge, S.J.; Mallouk, T.E. A high throughput optical method for studying compositional effects in electrocatalysts for CO2 reduction. Nat. Commun. 2021, 12, 1–10. [Google Scholar]
- Lai, Y.; Watkins, N.B.; Rosas-Hernández, A.; Thevenon, A.; Heim, G.P.; Zhou, L.; Wu, Y.; Peters, J.C.; Gregoire, J.M.; Agapie, T. Breaking Scaling Relationships in CO2 Reduction on Copper Alloys with Organic Additives. ACS Cent. Sci. 2021, 7, 1756–1762. [Google Scholar] [CrossRef]
- Liu, X.; Shen, Y.; Yang, R.; Zou, S.; Ji, X.; Shi, L.; Zhang, Y.; Liu, D.; Xiao, L.; Zheng, X. Inkjet printing assisted synthesis of multicomponent mesoporous metal oxides for ultrafast catalyst exploration. Nano Lett. 2012, 12, 5733–5739. [Google Scholar] [CrossRef] [PubMed]
- Renom-Carrasco, M.; Lefort, L. Ligand libraries for high throughput screening of homogeneous catalysts. Chem. Soc. Rev. 2018, 47, 5038–5060. [Google Scholar] [CrossRef] [PubMed]
- Wolf, E.; Richmond, E.; Moran, J. Identifying lead hits in catalyst discovery by screening and deconvoluting complex mixtures of catalyst components. Chem. Sci. 2015, 6, 2501–2505. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, D.K.; Maier, W.F. Combinatorial discovery of new autoreduction catalysts for the CO2 reforming of methane. J. Catal. 2006, 238, 142–152. [Google Scholar] [CrossRef]
- Goryachev, A.; Pustovarenko, A.; Shterk, G.; Alhajri, N.S.; Jamal, A.; Albuali, M.; van Koppen, L.; Khan, I.S.; Russkikh, A.; Ramirez, A. A Multi-Parametric Catalyst Screening for CO2 Hydrogenation to Ethanol. ChemCatChem 2021, 13, 3324–3332. [Google Scholar] [CrossRef]
- Jeng, E.; Qi, Z.; Kashi, A.R.; Hunegnaw, S.; Huo, Z.; Miller, J.S.; Bayu Aji, L.B.; Ko, B.H.; Shin, H.; Ma, S. Scalable gas diffusion electrode fabrication for electrochemical CO2 reduction using physical vapor deposition methods. ACS Appl. Mater. Interfaces 2022, 14, 7731–7740. [Google Scholar] [CrossRef]
- Kortlever, R.; Peters, I.; Balemans, C.; Kas, R.; Kwon, Y.; Mul, G.; Koper, M. Palladium–gold catalyst for the electrochemical reduction of CO2 to C1–C5 hydrocarbons. Chem. Commun. 2016, 52, 10229–10232. [Google Scholar] [CrossRef]
- Zanellato, G.; Schiavi, P.G.; Zanoni, R.; Rubino, A.; Altimari, P.; Pagnanelli, F. Electrodeposited Copper Nanocatalysts for CO2 Electroreduction: Effect of Electrodeposition Conditions on Catalysts’ Morphology and Selectivity. Energies 2021, 14, 5012. [Google Scholar] [CrossRef]
- Hahn, C.; Abram, D.N.; Hansen, H.A.; Hatsukade, T.; Jackson, A.; Johnson, N.C.; Hellstern, T.R.; Kuhl, K.P.; Cave, E.R.; Feaster, J.T. Synthesis of thin film AuPd alloys and their investigation for electrocatalytic CO2 reduction. J. Mater. Chem. A 2015, 3, 20185–20194. [Google Scholar] [CrossRef]
- Zhang, M.; Lee, J.; Wang, L.; Duan, Q.; Zhang, J.; Qi, H. A Novel High-Throughput Screening of Multicomponent Photocatalysts for Decomposition of Organic Pollutants Based on Fluorescence Imaging. ChemCatChem 2015, 7, 3978–3984. [Google Scholar] [CrossRef]
- Falk, J.; Mendler, M.; Kabisch, J. Pipette Show: An Open Source Web Application to Support Pipetting into Microplates. ACS Synth. Biol. 2022, 11, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Burger, B.; Maffettone, P.M.; Gusev, V.V.; Aitchison, C.M.; Bai, Y.; Wang, X.; Li, X.; Alston, B.M.; Li, B.; Clowes, R. A mobile robotic chemist. Nature 2020, 583, 237–241. [Google Scholar] [CrossRef]
- Maleki, H.; Bertola, V. Recent advances and prospects of inkjet printing in heterogeneous catalysis. Catal. Sci. Technol. 2020, 10, 3140–3159. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Torres-Martínez, L.M. Ink-jet printing films of molybdates of alkaline earth metals with scheelite structure applied in the photocatalytic CO2 reduction. J. Photochem. Photobiol. A 2019, 368, 15–22. [Google Scholar] [CrossRef]
- Chen, L.; Yang, C.; Xiao, Y.; Yan, X.; Hu, L.; Eggersdorfer, M.; Chen, D.; Weitz, D.; Ye, F. Millifluidics, microfluidics, and nanofluidics: Manipulating fluids at varying length scales. MT Nano 2021, 16, 100136. [Google Scholar] [CrossRef]
- Jun, M.; Kwak, C.; Lee, S.Y.; Joo, J.; Kim, J.M.; Im, D.J.; Cho, M.K.; Baik, H.; Hwang, Y.J.; Kim, H. Microfluidics-Assisted Synthesis of Hierarchical Cu2O Nanocrystal as C2-Selective CO2 Reduction Electrocatalyst. Small Methods 2022, 6, 2200074. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, B.; Ren, Y.; Wang, Z.; Zhao, C.X.; Hai, M.; Weitz, D.A.; Chen, D. Diverse Particle Carriers Prepared by Co-Precipitation and Phase Separation: Formation and Applications. ChemPlusChem 2021, 86, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Angelo, L.; Girleanu, M.; Ersen, O.; Serra, C.; Parkhomenko, K.; Roger, A.-C. Catalyst synthesis by continuous coprecipitation under micro-fluidic conditions: Application to the preparation of catalysts for methanol synthesis from CO2/H2. Catal. Today 2016, 270, 59–67. [Google Scholar] [CrossRef]
- Handoko, A.D.; Wei, F.; Yeo, B.S.; Seh, Z.W. Understanding heterogeneous electrocatalytic carbon dioxide reduction through operando techniques. Nat. Catal. 2018, 1, 922–934. [Google Scholar] [CrossRef]
- Cao, X.; Tan, D.; Wulan, B.; Hui, K.; Hui, K.; Zhang, J. In situ characterization for boosting electrocatalytic carbon dioxide reduction. Small Methods 2021, 5, 2100700. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Shi, J.; Tan, D.; Liu, L.; Zhang, F.; Lu, C.; Su, Z.; Tan, X.; Cheng, X. Manganese acting as a high-performance heterogeneous electrocatalyst in carbon dioxide reduction. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, W.; Zhang, B. A review of electrocatalyst characterization by transmission electron microscopy. J. Energy Chem. 2017, 26, 1117–1135. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, J.; Chu, H.; Chu, Y.-C.; Chen, H.M. In situ/operando studies for designing next-generation electrocatalysts. ACS Energy Lett. 2020, 5, 1281–1291. [Google Scholar] [CrossRef]
- Vavra, J.; Shen, T.H.; Stoian, D.; Tileli, V.; Buonsanti, R. Real-time monitoring reveals dissolution/redeposition mechanism in copper nanocatalysts during the initial stages of the CO2 reduction reaction. Angew. Chem. Int. Ed. 2021, 133, 1367–1374. [Google Scholar] [CrossRef]
- Sakamoto, N.; Nishimura, Y.F.; Nonaka, T.; Ohashi, M.; Ishida, N.; Kitazumi, K.; Kato, Y.; Sekizawa, K.; Morikawa, T.; Arai, T. Self-assembled cuprous coordination polymer as a catalyst for CO2 electrochemical reduction into C2 products. ACS Catal. 2020, 10, 10412–10419. [Google Scholar] [CrossRef]
- Baruch, M.F.; Pander, J.E., III; White, J.L.; Bocarsly, A.B. Mechanistic insights into the reduction of CO2 on tin electrodes using in situ ATR-IR spectroscopy. ACS Catal. 2015, 5, 3148–3156. [Google Scholar] [CrossRef]
- Rosser, T.E.; Windle, C.D.; Reisner, E. Electrocatalytic and Solar-Driven CO2 Reduction to CO with a Molecular Manganese Catalyst Immobilized on Mesoporous TiO2. Angew. Chem. Int. Ed. 2016, 128, 7514–7518. [Google Scholar] [CrossRef]
- Guo, Y.; He, X.; Su, Y.; Dai, Y.; Xie, M.; Yang, S.; Chen, J.; Wang, K.; Zhou, D.; Wang, C. Machine-learning-guided discovery and optimization of additives in preparing Cu catalysts for CO2 reduction. J. Am. Chem. Soc. 2021, 143, 5755–5762. [Google Scholar] [CrossRef]
- Hossain, M.N.; Chen, S.; Chen, A. Thermal-assisted synthesis of unique Cu nanodendrites for the efficient electrochemical reduction of CO2. Appl. Catal. B 2019, 259, 118096. [Google Scholar] [CrossRef]
- Clark, E.L.; Bell, A.T. Direct observation of the local reaction environment during the electrochemical reduction of CO2. J. Am. Chem. Soc. 2018, 140, 7012–7020. [Google Scholar] [CrossRef]
- Zeng, L.; Shi, J.; Chen, H.; Lin, C. Ag Nanowires/C as a Selective and Efficient Catalyst for CO2 Electroreduction. Energies 2021, 14, 2840. [Google Scholar] [CrossRef]
- Frey, M.; Romero, T.; Roger, A.-C.; Edouard, D. Open cell foam catalysts for CO2 methanation: Presentation of coating procedures and in situ exothermicity reaction study by infrared thermography. Catal. Today 2016, 273, 83–90. [Google Scholar] [CrossRef]
- Kondratyuk, P.; Gumuslu, G.; Shukla, S.; Miller, J.B.; Morreale, B.D.; Gellman, A.J. A microreactor array for spatially resolved measurement of catalytic activity for high-throughput catalysis science. J. Catal. 2013, 300, 55–62. [Google Scholar] [CrossRef]
- Lai, Y.; Jones, R.J.; Wang, Y.; Zhou, L.; Gregoire, J.M. Scanning electrochemical flow cell with online mass spectroscopy for accelerated screening of carbon dioxide reduction electrocatalysts. ACS Comb Sci. 2019, 21, 692–704. [Google Scholar] [CrossRef] [PubMed]
| Group | Metal Element | Main Product |
|---|---|---|
| I | Ni, Fe, Pt, Ti, Ga, Co | H2 |
| II | Au, Ag, Zn | CO |
| III | Pd, Hg, In, Sn, Cd, Tl, Bi | HCOOH |
| IV | Cu | Hydrocarbons/Oxygenates |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Pan, M.; Zhang, S.; Sun, D.; Yang, Y.; Chen, D.; Weitz, D.A.; Gao, X. Research Progress in High-Throughput Screening of CO2 Reduction Catalysts. Energies 2022, 15, 6666. https://doi.org/10.3390/en15186666
Wu Q, Pan M, Zhang S, Sun D, Yang Y, Chen D, Weitz DA, Gao X. Research Progress in High-Throughput Screening of CO2 Reduction Catalysts. Energies. 2022; 15(18):6666. https://doi.org/10.3390/en15186666
Chicago/Turabian StyleWu, Qinglin, Meidie Pan, Shikai Zhang, Dongpeng Sun, Yang Yang, Dong Chen, David A. Weitz, and Xiang Gao. 2022. "Research Progress in High-Throughput Screening of CO2 Reduction Catalysts" Energies 15, no. 18: 6666. https://doi.org/10.3390/en15186666
APA StyleWu, Q., Pan, M., Zhang, S., Sun, D., Yang, Y., Chen, D., Weitz, D. A., & Gao, X. (2022). Research Progress in High-Throughput Screening of CO2 Reduction Catalysts. Energies, 15(18), 6666. https://doi.org/10.3390/en15186666









