Model Based Optimal Control of the Photosynthetic Growth of Microalgae in a Batch Photobioreactor
Abstract
:1. Introduction
2. Mathematical Modeling of the Photosynthetic Growth Process
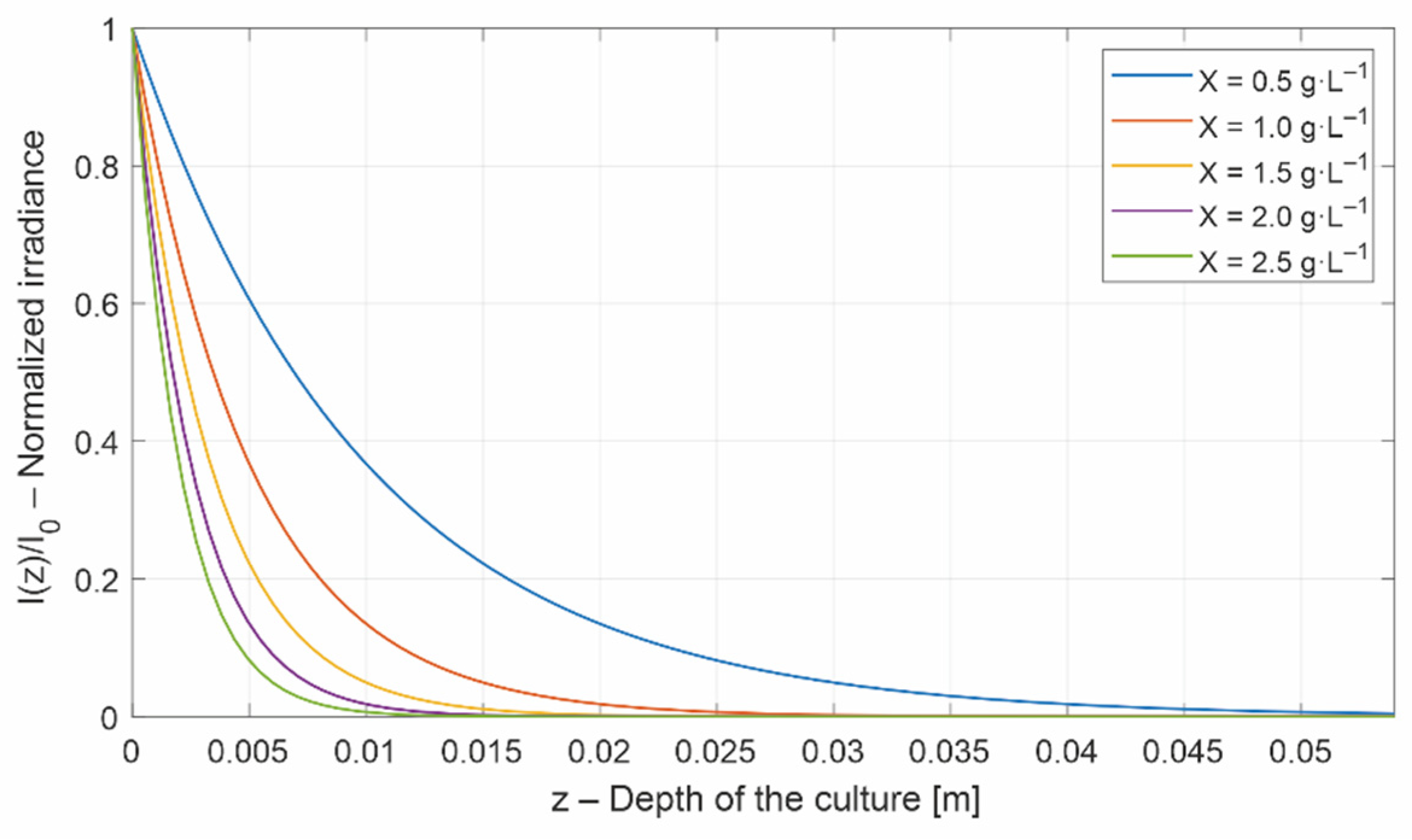
2.1. The Radiative Model
2.2. The Kinetic Growth Model
- -
- -
- local photosynthetic responses, , can be calculated for any depth of the culture. These local photosynthetic responses are simply averaged into an average photosynthetic response, (or average specific growth rate) [31]. The denote an averaged value.
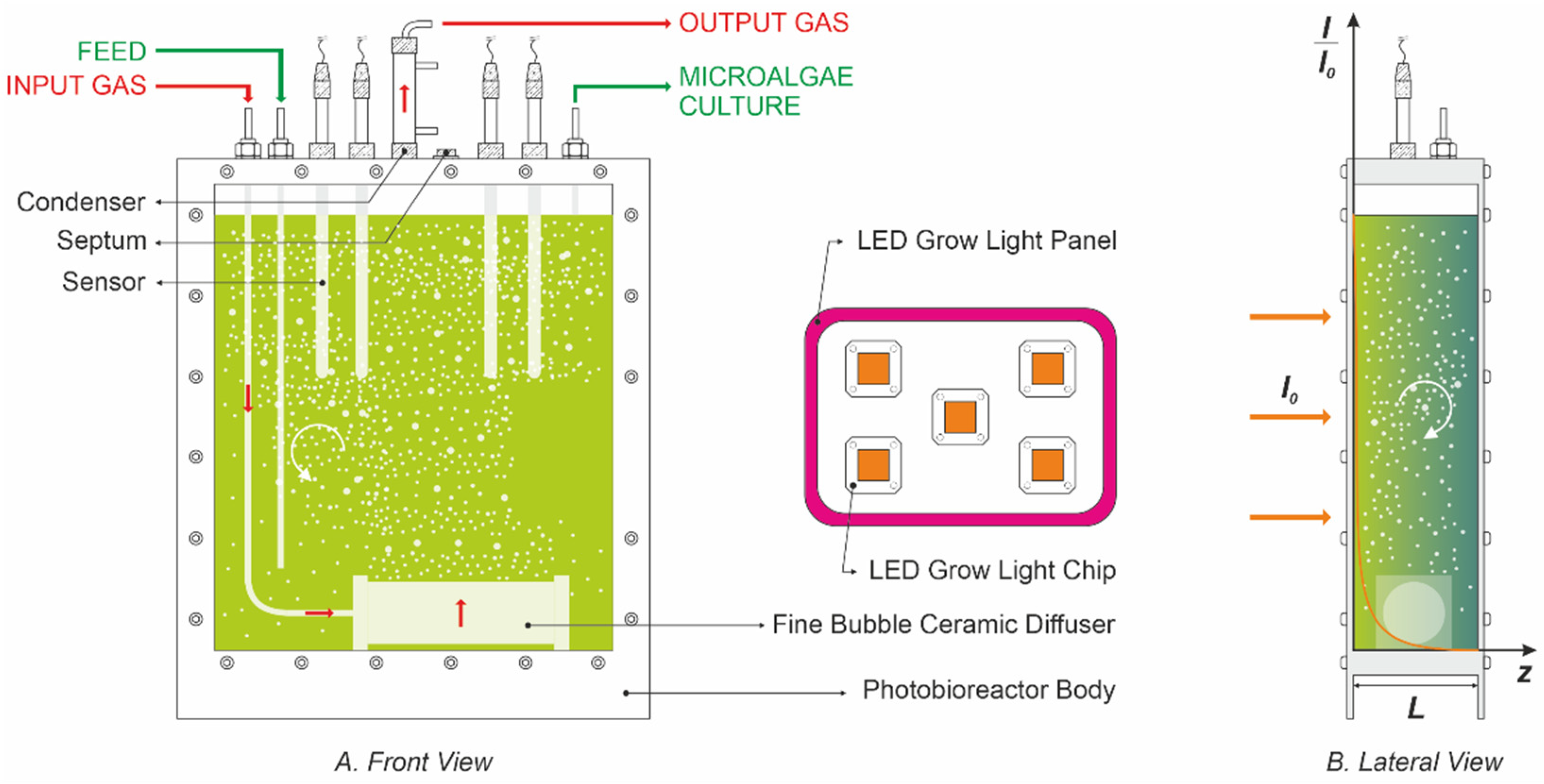
2.3. The Mass Balance Model
3. The Optimal Control Problem
- -
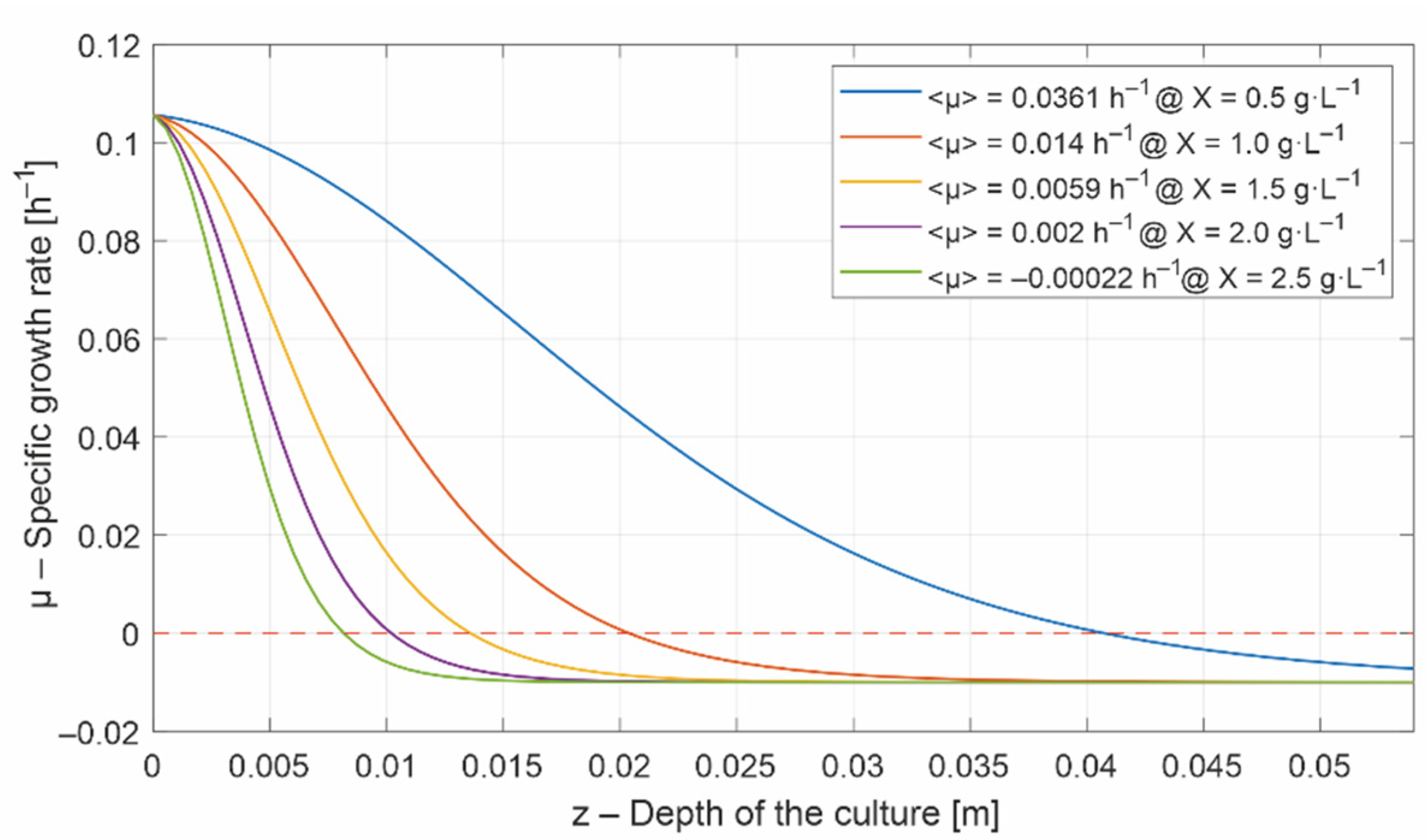
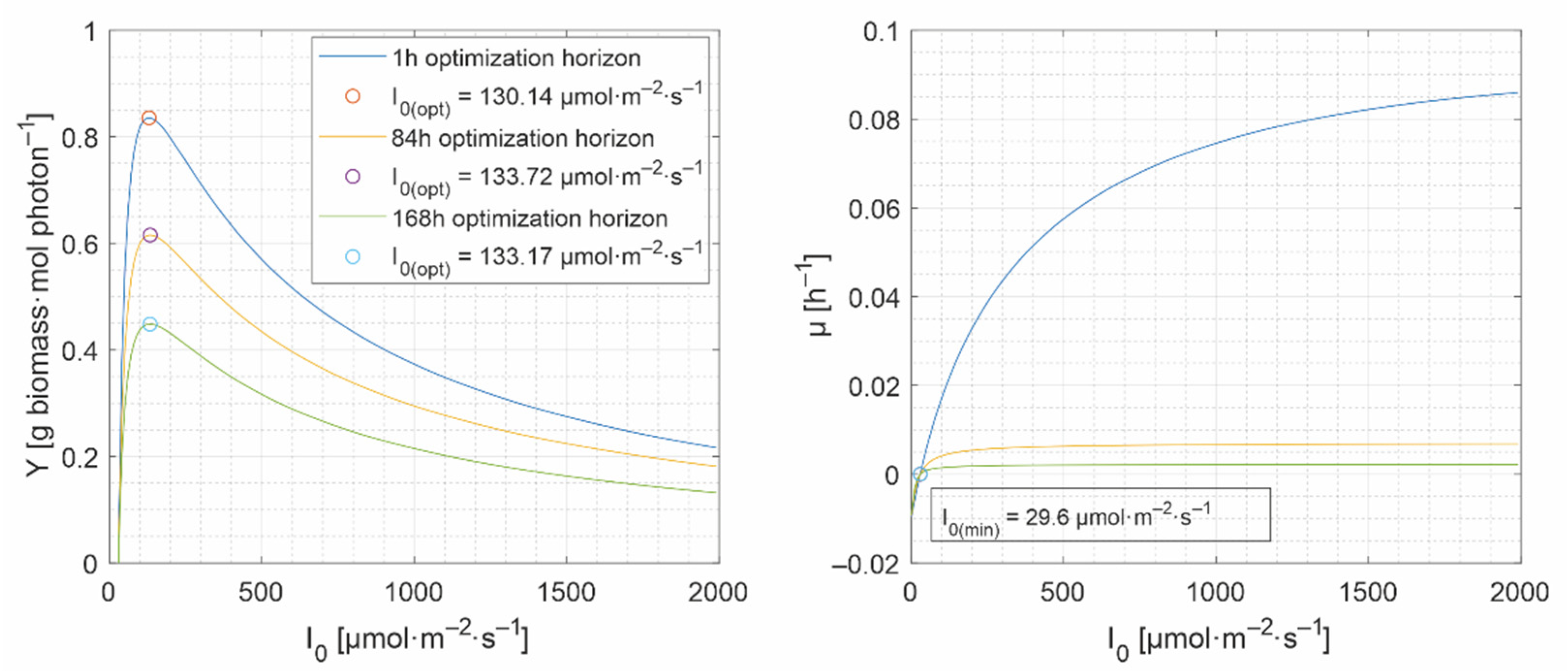
- a lower bound, , under which the biomass would decrease. Below the specific growth rate is negative (Figure 4), and
- -
- an upper bound, , which is set in Figure 4 at 80% of the maximum growth rate.
- -
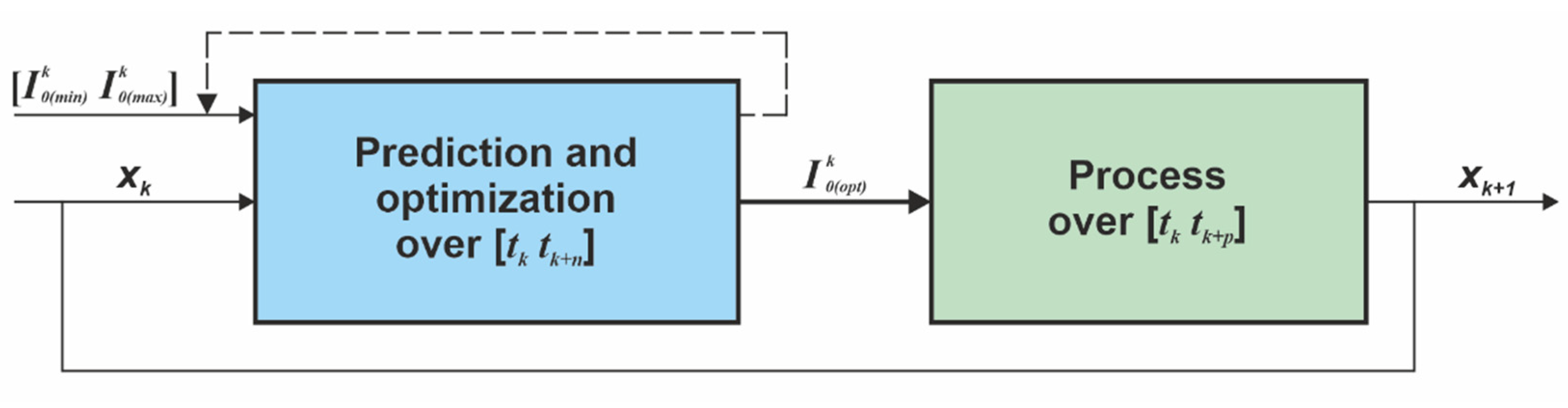
- the control horizon: , where and are the initial and the final time of the control horizon (the batch period),
- -
- the initial conditions: ,
- -
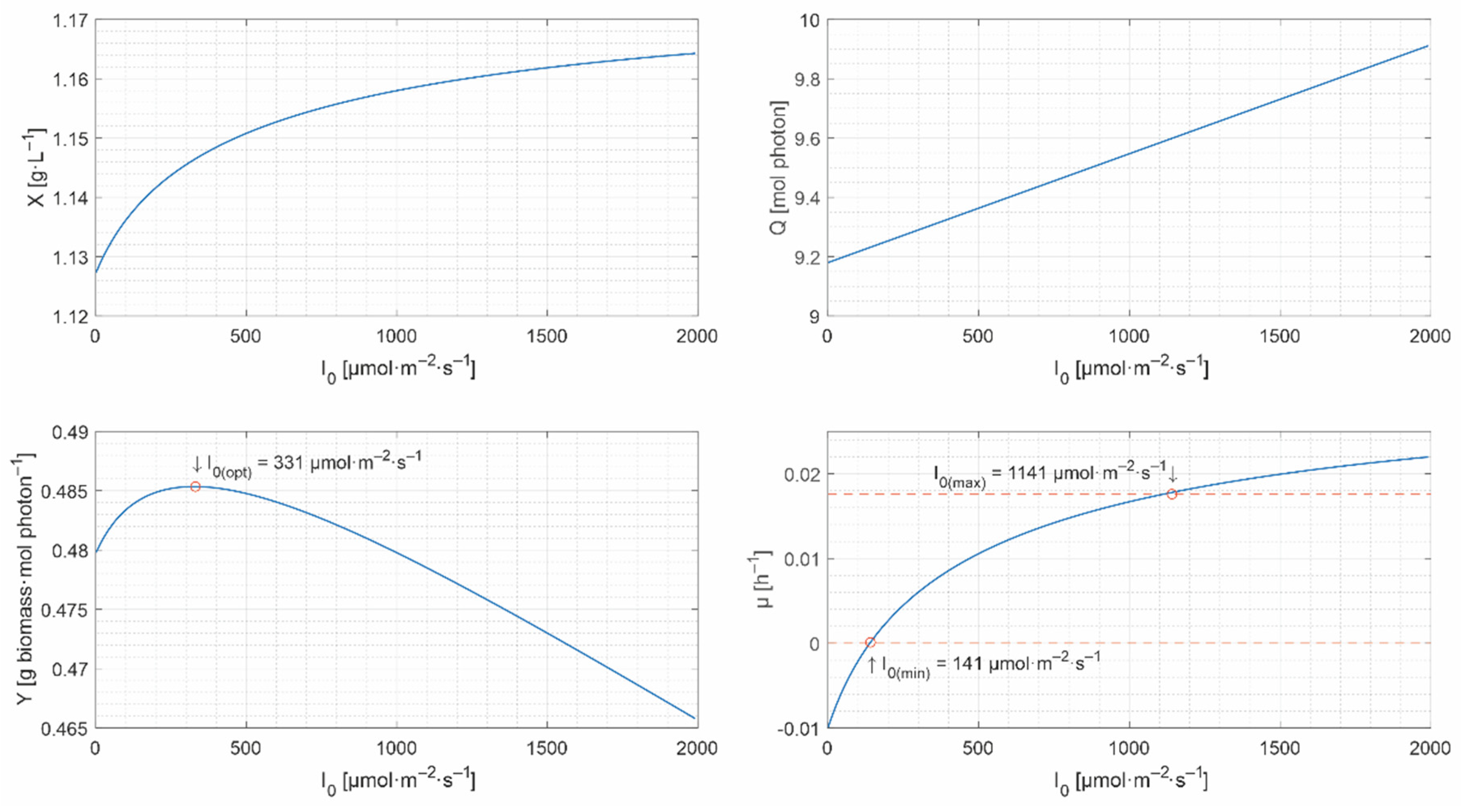
- the set of lower and upper bounds: , with . The lower bound, , is critical because the biomass decreases under this value, while the upper bound is not, and can remain constant (e.g., 2000 μmol·m−2·s−1). Even though can inhibit the microalgae growth, it is attenuated inside the culture. The lower and the upper bounds are required by most of the unidimensional optimization methods [41], e.g., fminbnd function in Matlab.
4. The Closed-Loop Control System
4.1. The Control Structure
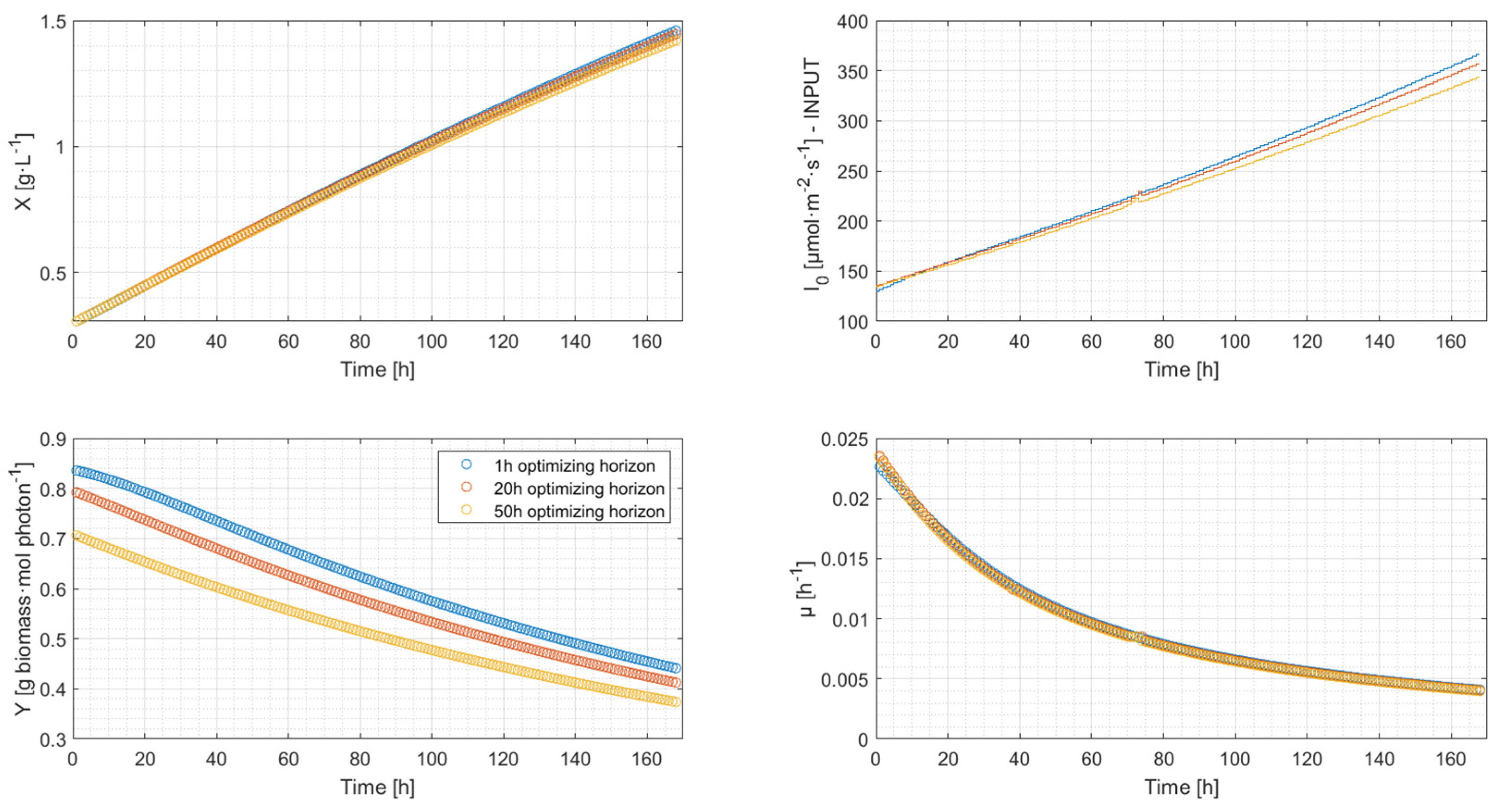
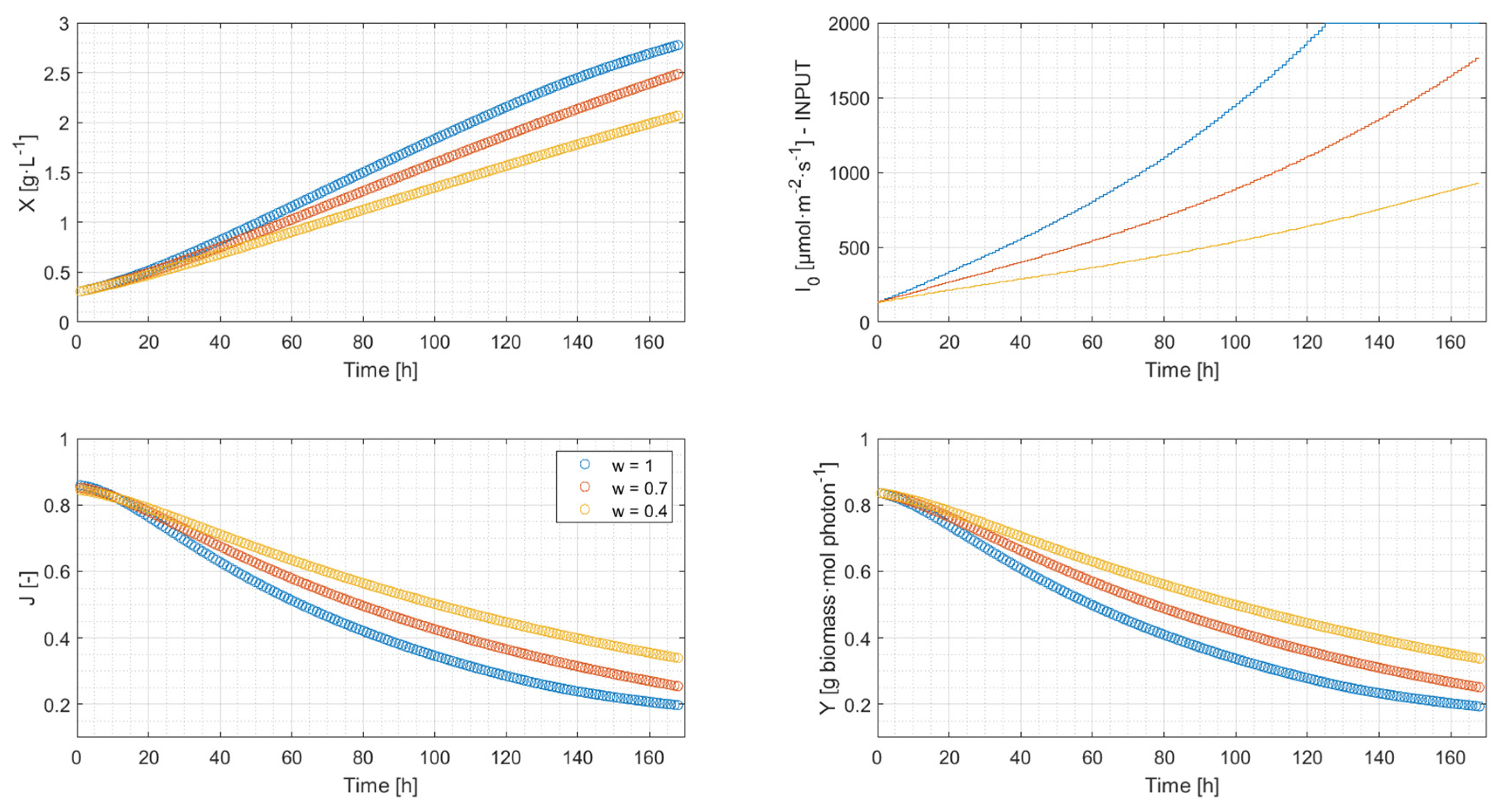
4.2. Simulation Results
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, J.; Chandra Saxena, R. An Introduction to Microalgae: Diversity and Significance. In Handbook of Marine Microalgae; Kim, S.-K., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 11–24. [Google Scholar] [CrossRef]
- Pagels, F.; Salvaterra, D.; Amaro, H.M.; Guedes, A.C. Pigments from microalgae. In Handbook of Microalgae-Based Processes and Products; Jacob-Lopes, E., Maroneze, M.M., Queiroz, M.I., Zepka, L.Q., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 465–492. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; Coimbra, J.S.R.; Leite, M.O.; Albino, L.F.T.; Martins, M.A. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef] [PubMed]
- Mahata, C.; Das, P.; Khan, S.; Thaher, M.I.A.; Abdul Quadir, M.; Annamalai, S.N.; Al Jabri, H. The Potential of Marine Microalgae for the Production of Food, Feed, and Fuel (3F). Fermentation 2022, 8, 316. [Google Scholar] [CrossRef]
- Borowiak, D.; Krzywonos, M. Bioenergy, Biofuels, Lipids and Pigments—Research Trends in the Use of Microalgae Grown in Photobioreactors. Energies 2022, 15, 5357. [Google Scholar] [CrossRef]
- Lupette, J.; Benning, C. Human health benefits of very-long-chain polyunsaturated fatty acids from microalgae. Biochimie 2020, 178, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Santin, A.; Balzano, S.; Russo, M.T.; Palma Esposito, F.; Ferrante, M.I.; Blasio, M.; Cavalletti, E.; Sardo, A. Microalgae-Based PUFAs for Food and Feed: Current Applications, Future Possibilities, and Constraints. J. Mar. Sci. Eng. 2022, 10, 844. [Google Scholar] [CrossRef]
- Jansen, M.; Wijffels, R.H.; Barbosa, M.J. Microalgae based production of single-cell protein. Curr. Opin. Biotechnol. 2022, 75, 102705. [Google Scholar] [CrossRef]
- Lucakova, S.; Branyikova, I.; Hayes, M. Microalgal Proteins and Bioactives for Food, Feed, and Other Applications. Appl. Sci. 2022, 12, 4402. [Google Scholar] [CrossRef]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell. Fact. 2019, 18, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnamoorthy, A.; Rodriguez, C.; Durrant, A. Sustainable Approaches to Microalgal Pre-Treatment Techniques for Biodiesel Production: A Review. Sustainability 2022, 14, 9953. [Google Scholar] [CrossRef]
- Cheng, D.; Li, D.; Yuan, Y.; Zhou, L.; Li, X.; Wu, T.; Wang, L.; Zhao, Q.; Wei, W.; Sun, Y. Improving carbohydrate and starch accumulation in Chlorella sp. AE10 by a novel two-stage process with cell dilution. Biotechnol. Biofuels 2017, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Olguín, E.J.; Sánchez-Galván, G.; Arias-Olguín, I.I.; Melo, F.J.; González-Portela, R.E.; Cruz, L.; De Philippis, R.; Adessi, A. Microalgae-Based Biorefineries: Challenges and Future Trends to Produce Carbohydrate Enriched Biomass, High-Added Value Products and Bioactive Compounds. Biology 2022, 11, 1146. [Google Scholar] [CrossRef] [PubMed]
- Al-Jabri, H.; Das, P.; Khan, S.; Thaher, M.; AbdulQuadir, M. Treatment of Wastewaters by Microalgae and the Potential Applications of the Produced Biomass—A Review. Water 2021, 13, 27. [Google Scholar] [CrossRef]
- Ruiz, J.; Olivieri, G.; de Vree, J.; Bosma, R.; Willems, P.; Reith, J.H.; Eppink, M.H.M.; Kleinegris, D.M.M.; Wijffels, R.H.; Barbosa, M.J. Towards industrial products from microalgae. Energy Environ. Sci. 2016, 10, 3036–3043. [Google Scholar] [CrossRef]
- Severo, I.A.; Siqueira, S.F.; Deprá, M.C.; Maroneze, M.M.; Zepka, L.Q.; Jacob-Lopes, E. Biodiesel facilities: What can we address to make biorefineries commercially competitive? Renew. Sustain. Energy Rev. 2019, 112, 686–705. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae biotechnology for industrial wastewater treatment, bioenergy production, and high-value bioproducts. Sci. Total Environ. 2022, 806, 150585. [Google Scholar] [CrossRef] [PubMed]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The Use of Microalgae for Coupling Wastewater Treatment with CO2 Biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Acién Fernández, F.G.; Fernández Sevilla, J.M.; Molina Grima, E. Photobioreactors for the production of microalgae. Rev. Environ. Sci. Biotechnol. 2013, 12, 131–151. [Google Scholar] [CrossRef]
- Legrand, J.; Arnaud, A.; Pruvost, J. A review on photobioreactor design and modelling for microalgae production. React. Chem. Eng. 2021, 6, 1134–1151. [Google Scholar] [CrossRef]
- Pottier, L.; Pruvost, J.; Deremetz, J.; Cornet, J.F.; Legrand, J.; Dussap, C.G. A fully predictive model for one-dimensional light attenuation by Chlamydomonas reinhardtii in a torus photobioreactor. Biotechnol. Bioeng. 2005, 91, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Ifrim, G.A.; Titica, M.; Barbu, M.; Boillereaux, L.; Cogne, G.; Caraman, S.; Legrand, J. Multivariable Feedback Linearizing Control of Chlamydomonas reinhardtii Photoautotrophic Growth Process in a Torus Photobioreactor. Chem. Eng. J. 2013, 218, 191–203. [Google Scholar] [CrossRef]
- Ifrim, G.A.; Titica, M.; Cogne, G.; Boillereaux, L.; Legrand, J.; Caraman, S. Dynamic pH Model for Autotrophic Growth of Microalgae in Photobioreactor: A Tool for Monitoring and Control Purposes. AIChe J. 2014, 60, 585–599. [Google Scholar] [CrossRef]
- Grognard, F.; Akhmetzhanov, A.R.; Masci, P.; Bernard, O. Optimization of a photobioreactor biomass production using natural light. In Proceedings of the 49th IEEE Conference on Decision and Control (CDC), Atlanta, GA, USA, 15–17 December 2010; pp. 4691–4696. [Google Scholar] [CrossRef]
- Grognard, F.; Akhmetzhanov, A.R.; Bernard, O. Optimal strategies for biomass productivity maximization in a photobioreactor using natural light. Automatica 2014, 50, 359–368. [Google Scholar] [CrossRef]
- de Andrade, G.A.; Berenguel, M.; Guzmán, J.L.; Pagano, D.J.; Acién, F.G. Optimization of biomass production in outdoor tubular photobioreactors. J. Process Control. 2016, 37, 58–69. [Google Scholar] [CrossRef]
- Yoo, S.J.; Jeong, D.H.; Kim, J.H.; Lee, J.M. Optimization of microalgal photobioreactor system using model predictive control with experimental validation. Bioprocess Biosyst. Eng. 2016, 39, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Carreño-Zagarra, J.J.; Guzmán, J.L.; Moreno, J.C.; Villamizar, R. Linear active disturbance rejection control for a raceway photobioreactor. Control. Eng. Pract. 2019, 85, 271–279. [Google Scholar] [CrossRef]
- Cornet, J.P.; Dussap, C.G. A Simple and Reliable Formula for Assessment of Maximum Volumetric Productivities in Photobioreactors. Biotechnol. Prog. 2009, 25, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, S.K.; Rhinehart, R.R. Modeling and Optimization of Algae Growth. Ind. Eng. Chem. Res. 2015, 54, 8063–8071. [Google Scholar] [CrossRef]
- Degrenne, B.; Pruvost, J.; Titica, M.; Takache, H.; Legrand, J. Kinetic modeling of light limitation and sulfur deprivation effects in the induction of hydrogen production with Chlamydomonas reinhardtii. Part II: Definition of model-based protocols and experimental validation. Biotechnol. Bioeng. 2011, 108, 2288–2299. [Google Scholar] [CrossRef] [PubMed]
- Ifrim, G.A.; Titica, M.; Boillereaux, L.; Caraman, S. Feedback linearizing control of light-to-microalgae ratio in artificially lighted photobioreactors. In Proceedings of the 12th IFAC Symposium on Computer Applications in Biotechnology (CAB), Mumbai, India, 16–18 December 2013; pp. 60–65. [Google Scholar] [CrossRef]
- Eriksen, N.T.; Geest, T.; Iversen, J.J.L. Phototrophic growth in the lumostat: A photo-bioreactor with on-line optimization of light intensity. J. Appl. Phycol. 1996, 8, 345–352. [Google Scholar] [CrossRef]
- Tebbani, S.; Titica, M.; Ifrim, G.; Barbu, M.; Caraman, S. Optimal Operation of a Lumostatic Microalgae Cultivation Process. In Developments in Model-Based Optimization and Control: Distributed Control and Industrial Applications; Olaru, S., Grancharova, A., Lobo Pereira, F., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 209–235. [Google Scholar] [CrossRef]
- Kandilian, R.; Tsao, T.C.; Pilon, L. Control of incident irradiance on a batch operated flat-plate photobioreactor. Chem. Eng. Sci. 2014, 119, 99–108. [Google Scholar] [CrossRef]
- Minzu, V.; Ifrim, G.; Arama, I. Control of Microalgae Growth in Artificially Lighted Photobioreactors Using Metaheuristic-Based Predictions. Sensors 2021, 21, 8065. [Google Scholar] [CrossRef] [PubMed]
- Ifrim, G.A.; Titica, M.; Deppe, S.; Frahm, B.; Barbu, M.; Caraman, S. Multivariable control strategy for the photosynthetic cultures of microalgae. In Proceedings of the 23rd International Conference on System Theory, Control and Computing (ICSTCC), Sinaia, Romania, 9–11 October 2019; pp. 218–223. [Google Scholar] [CrossRef]
- Gassenmeier, V.; Deppe, S.; Hernández Rodríguez, T.; Kuhfuß, F.; Moser, A.; Hass, V.C.; Kuchemüller, K.B.; Pörtner, R.; Möller, J.; Ifrim, G.; et al. Model-assisted DoE applied to microalgae processes. Curr. Res. Biotechnol. 2022, 4, 102–118. [Google Scholar] [CrossRef]
- Shampine, L.F.; Reichelt, M.W.; Kierzenka, J.A. Solving Index-1 DAEs in MATLAB and Simulink. SIAM Rev. 1999, 41, 538–552. [Google Scholar] [CrossRef]
- Yan, P.; Gai, M.; Wang, Y.; Gao, X. Review of Soft Sensors in Anaerobic Digestion Process. Processes 2021, 9, 1434. [Google Scholar] [CrossRef]
- Antoniu, A.; Lu, W.S. Practical Optimization. In Algorithms and Engineering Applications; Springer: New York, NY, USA, 2007. [Google Scholar] [CrossRef] [Green Version]
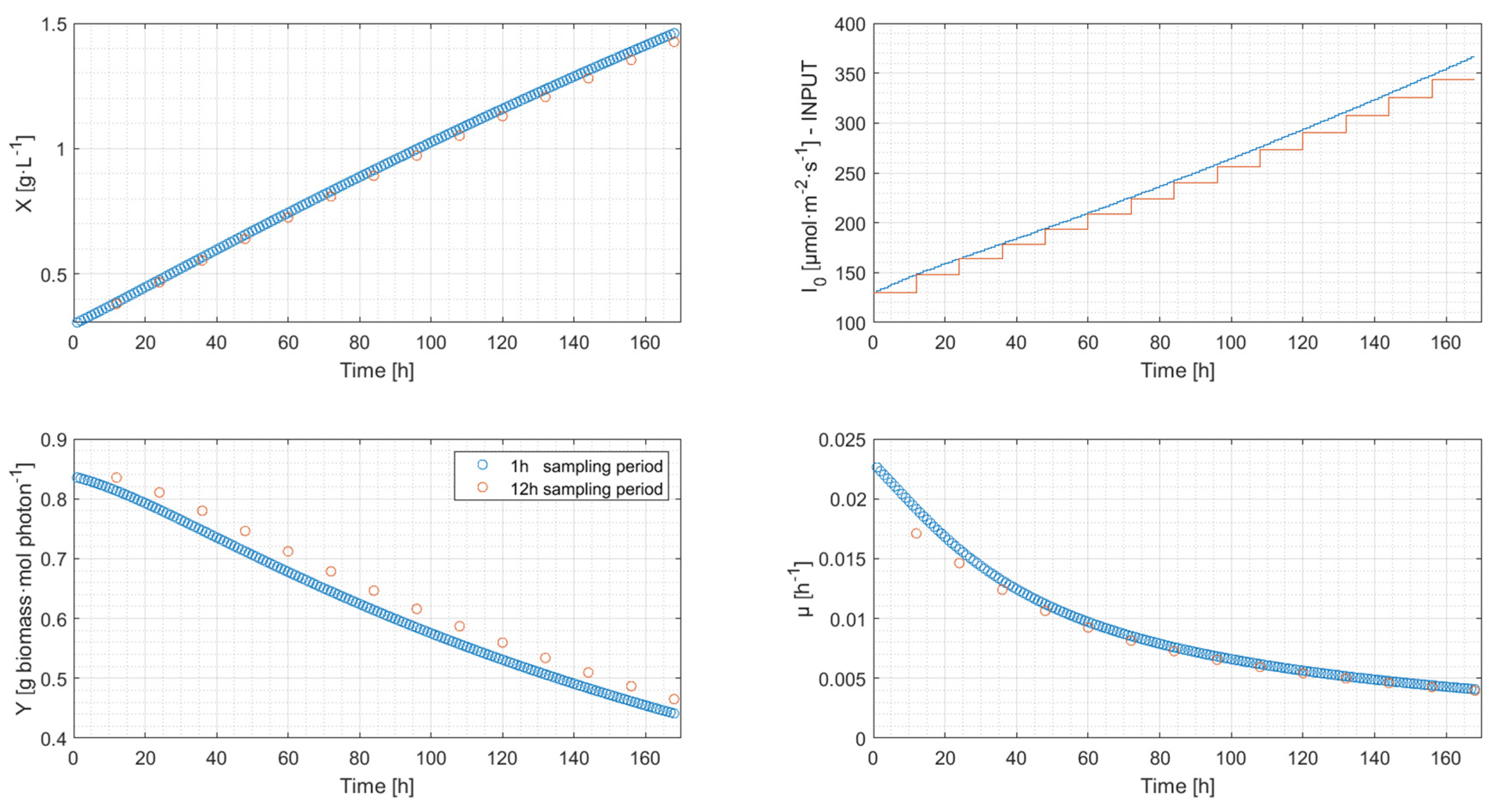
| Length of the Sampling Period | Biomass Concentration [g·L−1] | Decrease [%] |
|---|---|---|
| 1-h | 1.460 | |
| 12-h | 1.418 | −2.88 |
| 24-h | 1.374 | −5.89 |
| 168-h | 0.942 | −35.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ifrim, G.A.; Titica, M.; Horincar, G.; Antache, A.; Baicu, L.; Barbu, M.; Guzmán, J.L. Model Based Optimal Control of the Photosynthetic Growth of Microalgae in a Batch Photobioreactor. Energies 2022, 15, 6535. https://doi.org/10.3390/en15186535
Ifrim GA, Titica M, Horincar G, Antache A, Baicu L, Barbu M, Guzmán JL. Model Based Optimal Control of the Photosynthetic Growth of Microalgae in a Batch Photobioreactor. Energies. 2022; 15(18):6535. https://doi.org/10.3390/en15186535
Chicago/Turabian StyleIfrim, George Adrian, Mariana Titica, Georgiana Horincar, Alina Antache, Laurențiu Baicu, Marian Barbu, and José Luis Guzmán. 2022. "Model Based Optimal Control of the Photosynthetic Growth of Microalgae in a Batch Photobioreactor" Energies 15, no. 18: 6535. https://doi.org/10.3390/en15186535
APA StyleIfrim, G. A., Titica, M., Horincar, G., Antache, A., Baicu, L., Barbu, M., & Guzmán, J. L. (2022). Model Based Optimal Control of the Photosynthetic Growth of Microalgae in a Batch Photobioreactor. Energies, 15(18), 6535. https://doi.org/10.3390/en15186535








