Foam-Assisted Capillary Trapping in Saline Aquifers—An Initial–Residual Saturation Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
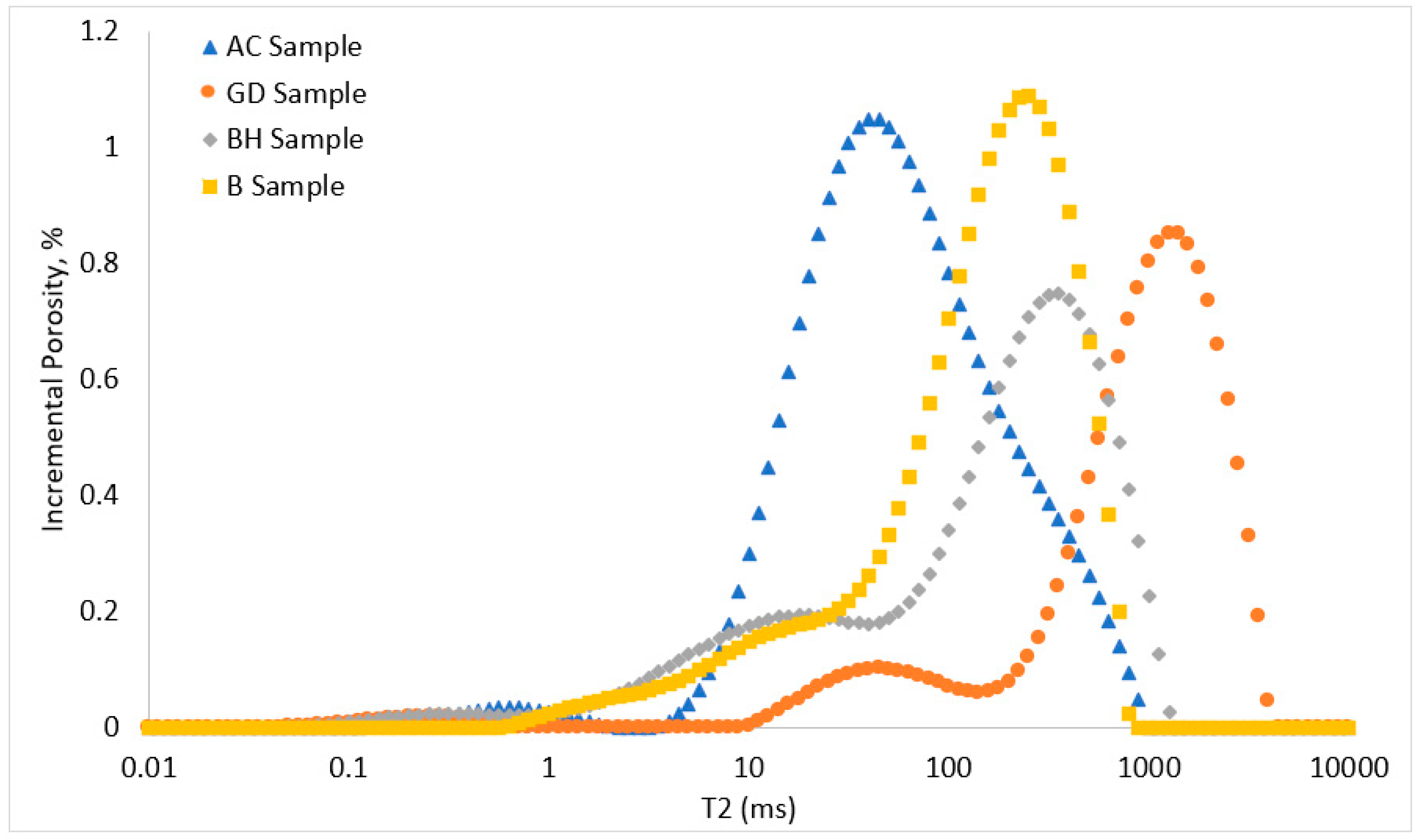
2.2. NMR Relaxometry
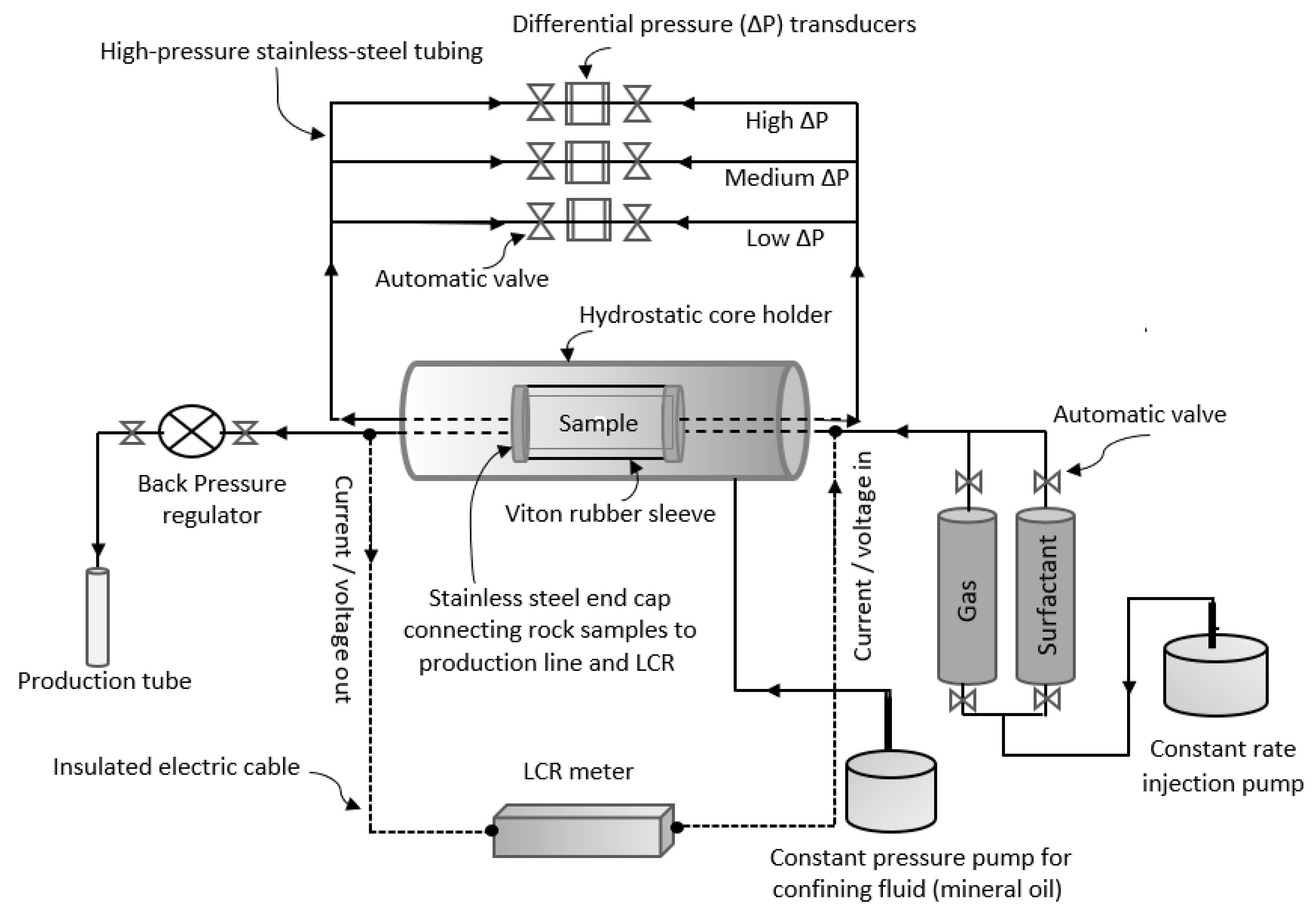
2.3. Core Flooding Setup
2.4. Experimental Procedure
2.5. Trapping Model
3. Results and Discussion
3.1. Core Flooding Results
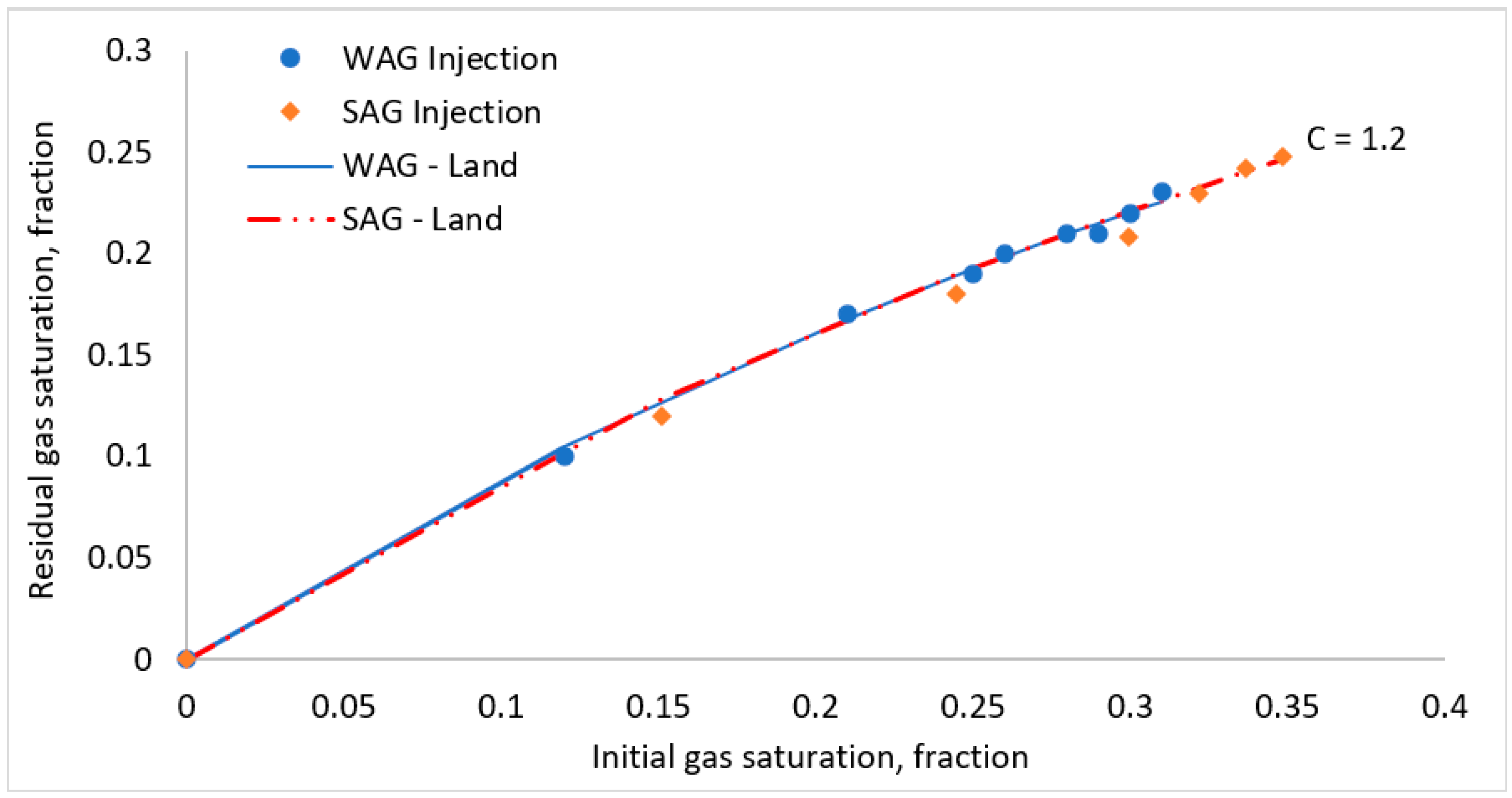
3.2. Initial–Residual (IR) Saturation Curves
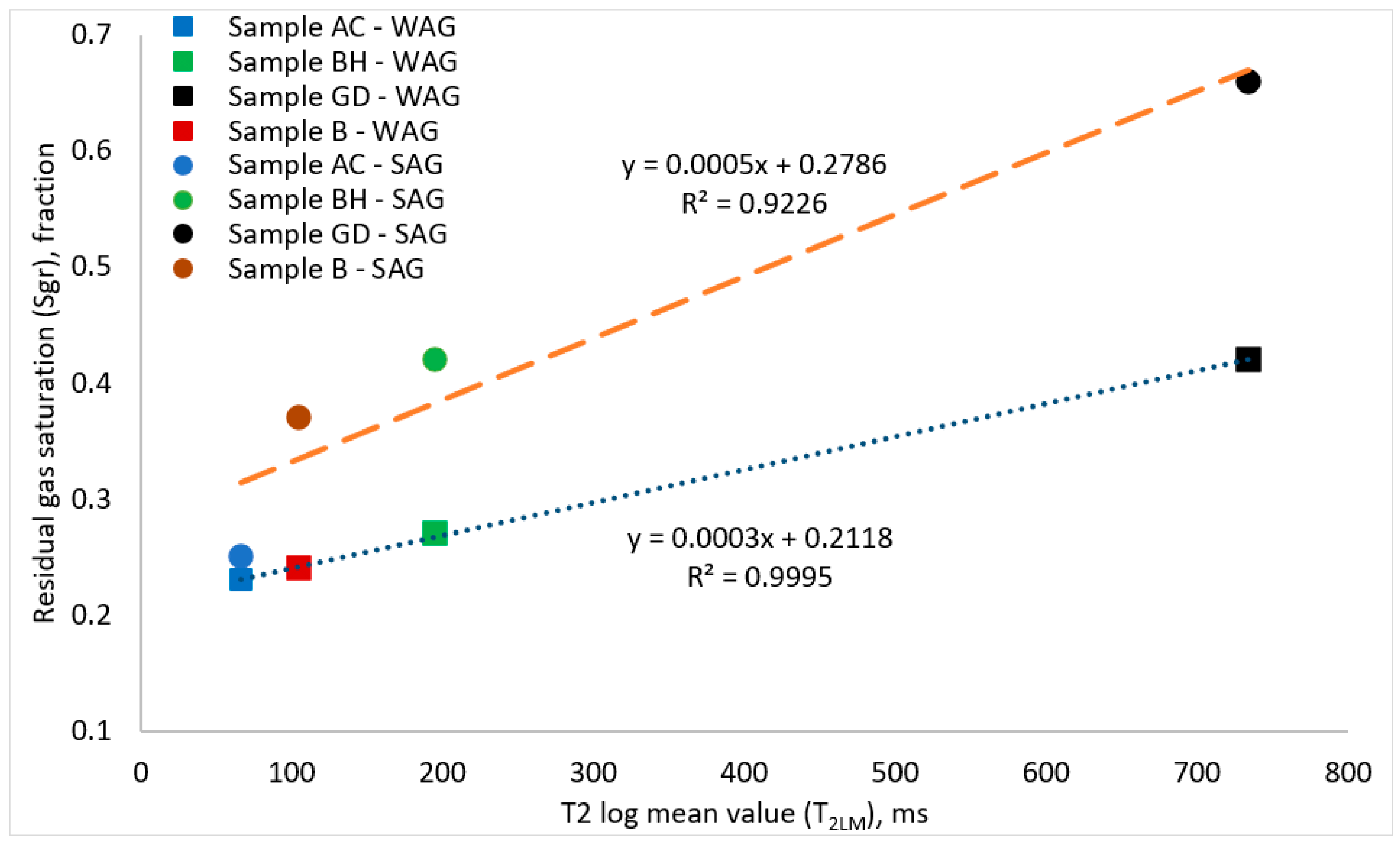
3.3. NMR T2 Relaxation Times and Residual Gas Saturations
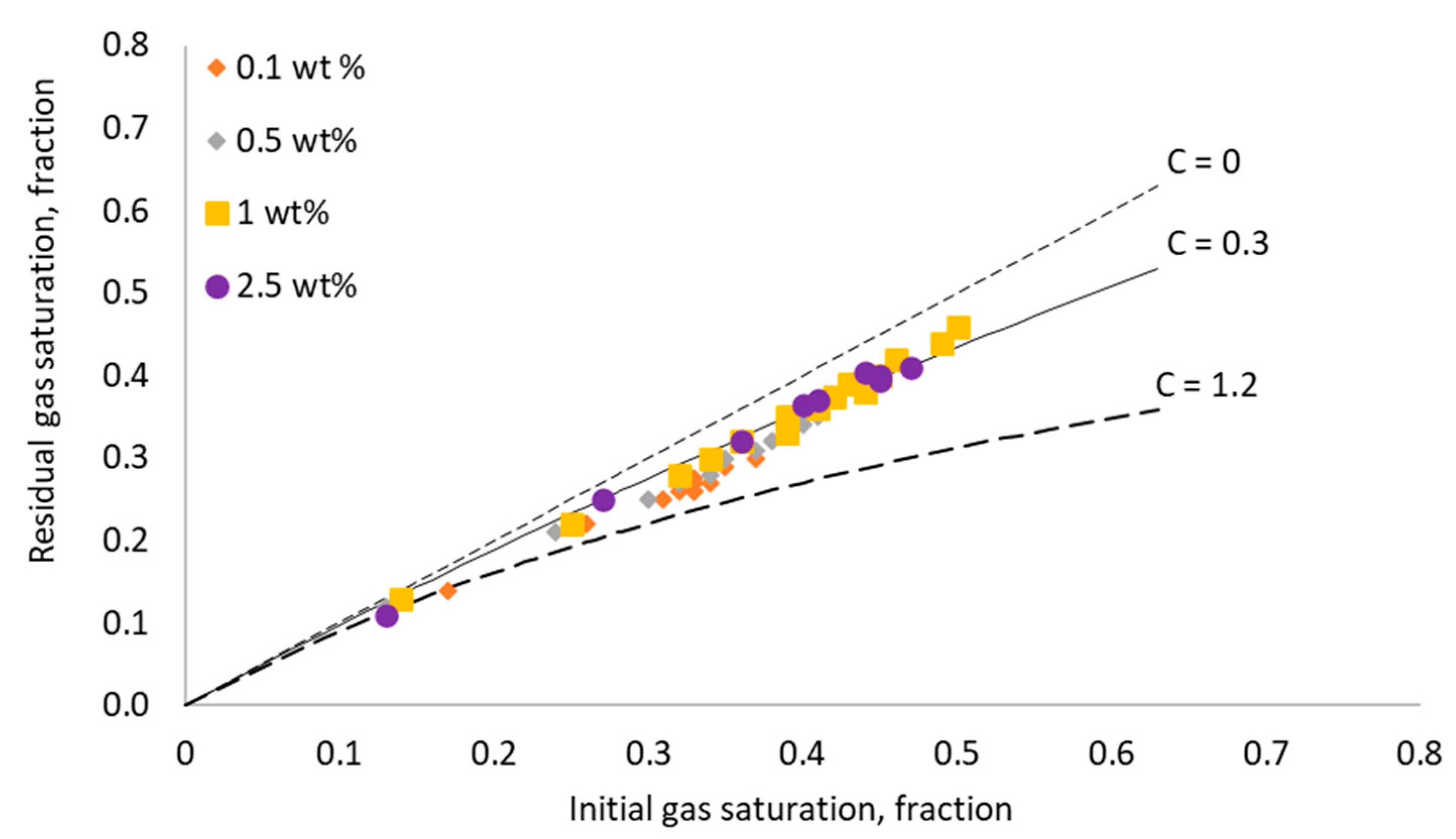
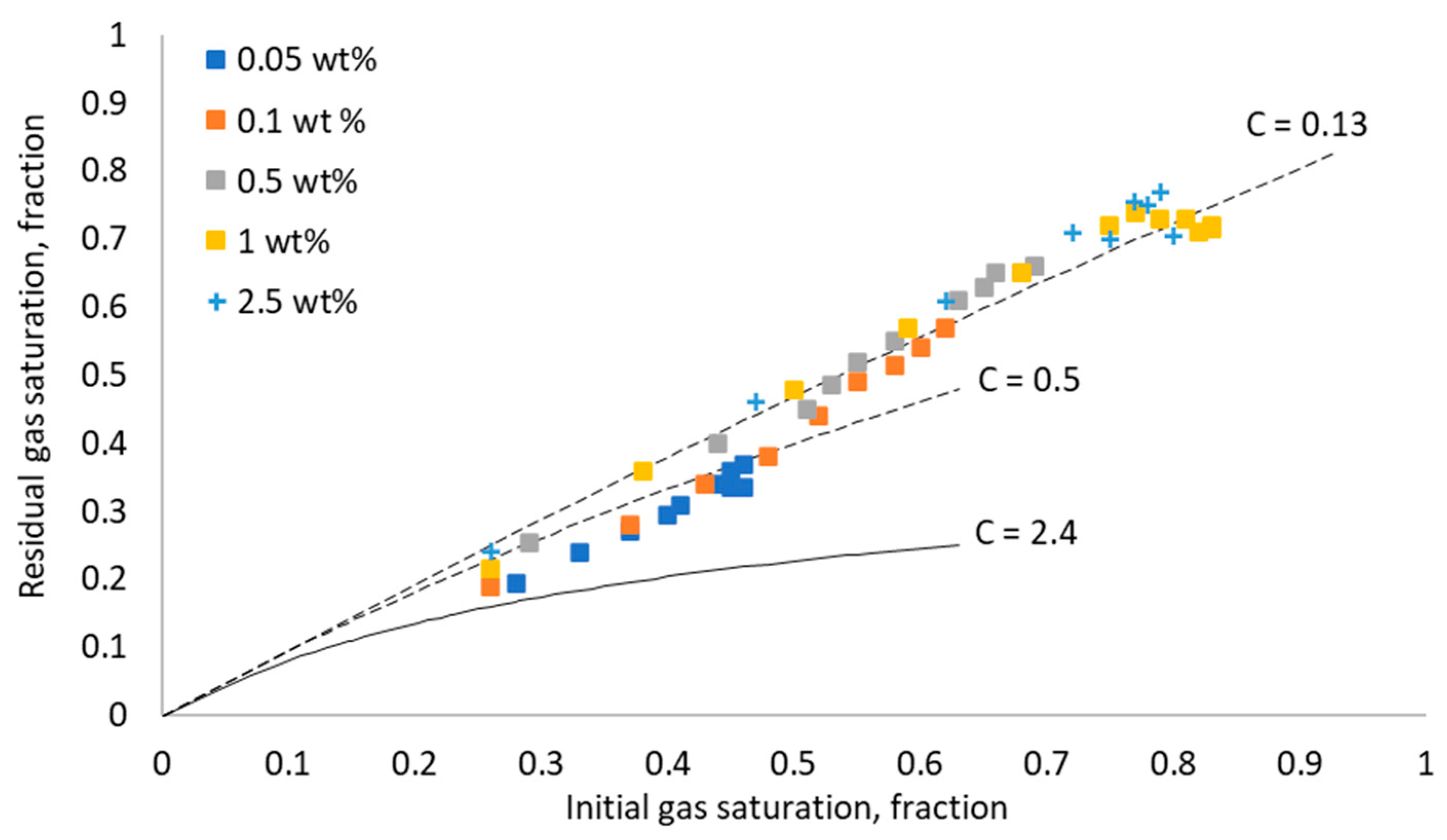
3.4. Effect of Surfactant Concentration on Gas Trapping Efficiency of Foam
4. Conclusions
- Foam significantly enhanced sweep efficiency and capillary trapping during gas injection in a saline aquifer. Residual trapping from foam-assisted gas injection was higher than gas injection without foam. Additionally, the multi-cycle foam injection (SAG) was found to be more effective than the single-cycle foam injection.
- Although the initial gas saturation obtained from the single cycle and the multi-cycle SAG injection tests were similar, capillary trapping of the gas following water imbibition was higher in the multi-cycle foam injection (SAG) than in the single-cycle foam injection. This may be attributed to the multiple hysteresis effect associated with the cyclic injection.
- Rock porosity did not show a good-fitted correlation with the measured residual gas saturations in various samples tested. However, residual gas saturation showed an excellent linear relationship with NMR T2 log mean values measured in all the tested cores. The good fittings correlations between the residual gas saturations obtained from various injection scenarios, and the measured T2LM, showed that the T2LM measurements for various rock types can give a reliable prediction method for the residual gas saturations in the porous media.
- The trapping efficiency of the rock samples, indicated by Land’s coefficient, was significantly improved by increasing the surfactant concentration. This is due to the improvement in the in situ generated foam properties at high surfactant concentrations, such as foam texture, foam stability, and apparent foam viscosity, in addition to the limiting capillary pressure value. Additionally, the snap-off trapping mechanism was more dominant at high surfactant concentrations. Nevertheless, above a certain range of surfactant concentrations (0.5–1 wt% in this study), the improvement in the trapping efficiencies became insignificant. This highlights the importance of selecting an optimum surfactant concentration that leads to a high sweep efficiency during foam injection for sequestration purposes.
- The experimental results have shown that the apparent foam viscosity increased with increasing the surfactant concentration to a certain range of the surfactant concentration, above which no further improvement in the foam apparent viscosity was observed. Additionally, the changes in the apparent foam viscosity with different surfactant concentrations were directly reflected in the measured residual gas saturation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fink, J. Chapter 16—Enhanced oil recovery. In Petroleum Engineer’s Guide to Oil Field Chemicals and Fluids, 3rd ed.; Fink, J., Ed.; Gulf Professional Publishing: Houston, TX, USA, 2021; pp. 643–731. [Google Scholar]
- Rezk, M.G.; Foroozesh, J.; Zivar, D.; Mumtaz, M. CO2 storage potential during CO2 enhanced oil recovery in sandstone reservoirs. J. Nat. Gas Sci. Eng. 2019, 66, 233–243. [Google Scholar] [CrossRef]
- Rezk, M.G.; Foroozesh, J. Uncertainty effect of CO2 molecular diffusion on oil recovery and gas storage in underground formations. Fuel 2022, 324, 124770. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L. Carbon Dioxide Capture and Storage: Intergovernmental Panel on Climate Change Special Report; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- International Energy Agency (IEA). Global Energy and CO2 Status Report—2017; IEA: Paris, France, 2018; Available online: https://www.iea.org/publications/freepublications/publication/GECO2017.pdf (accessed on 1 April 2022).
- Jha, N.K.; Al-Yaseri, A.; Ghasemi, M.; Al-Bayati, D.; Lebedev, M.; Sarmadivaleh, M. Pore scale investigation of hydrogen injection in sandstone via X-ray micro-tomography. Int. J. Hydrog. Energy 2021, 46, 34822–34829. [Google Scholar] [CrossRef]
- Iglauer, S. Optimum geological storage depths for structural H2 geo-storage. J. Pet. Sci. Eng. 2021, 212, 109498. [Google Scholar] [CrossRef]
- Foroozesh, J.; Dier, M.A.; Rezk, M.G. A simulation study on CO2 sequestration in saline aquifers: Trapping mechanisms and risk of CO2 leakage. MATEC Web Conf. 2018, 225, 03004. [Google Scholar] [CrossRef]
- Iglauer, S. Dissolution trapping of carbon dioxide in reservoir formation brine—A carbon storage mechanism. In Mass Transfer-Advanced Aspects; InTech: London, UK, 2011. [Google Scholar]
- Rezk, M.G.; Foroozesh, J. Study of convective-diffusive flow during CO2 sequestration in fractured heterogeneous saline aquifers. J. Nat. Gas Sci. Eng. 2019, 69, 102926. [Google Scholar] [CrossRef]
- Rezk, M.G.; Foroozesh, J.; Abdulrahman, A.; Gholinezhad, J. CO2 Diffusion and Dispersion in Porous Media: Review of Advances in Experimental Measurements and Mathematical Models. Energy Fuels 2022, 36, 133–155. [Google Scholar] [CrossRef]
- Saadatpoor, E.; Bryant, S.L.; Sepehrnoori, K. Effect of Heterogeneous Capillary Pressure on Buoyancy-Driven CO2 Migration. In Proceedings of the SPE Symp. On Improved Oil Recovery, Tulsa, AK, USA, 20–23 April 2008. [Google Scholar]
- Nghiem, L.; Yang, C.; Shrivastava, V.; Kohse, B.; Hassam, M.; Card, C. Risk mitigation through the optimization of residual gas and solubility trapping for CO2 storage in saline aquifers. Energy Procedia 2009, 1, 3015–3022. [Google Scholar] [CrossRef]
- Harris, C.; Jackson, S.J.; Benham, G.P.; Krevor, S.; Muggeridge, A.H. The impact of heterogeneity on the capillary trapping of CO2 in the Captain Sandstone. Int. J. Greenh. Gas Control 2021, 112, 103511. [Google Scholar] [CrossRef]
- Krevor, S.; Blunt, M.J.; Benson, S.M.; Pentland, C.H.; Reynolds, C.; Al-Menhali, A.; Niu, B. Capillary trapping for geologic carbon dioxide storage – From pore scale physics to field scale implications. Int. J. Greenh. Gas Control 2015, 40, 221–237. [Google Scholar] [CrossRef] [Green Version]
- Iglauer, S.; Wulling, W.; Pentland, C.H.; al Mansoori, S.K.; Blunt, M.J. Capillary Trapping Capacity of Rocks and Sandpacks. In Proceedings of the EUROPEC/EAGE Conference and Exhibition, Amsterdam, The Netherlands, 8–11 June 2009. [Google Scholar]
- Al-Menhali, A.S.; Krevor, S. Capillary Trapping of CO2 in Oil Reservoirs: Observations in a Mixed-Wet Carbonate Rock. Environ. Sci. Technol. 2016, 50, 2727–2734. [Google Scholar] [CrossRef]
- Adebayo, A.R. Foam Flow in Different Pore Systems—Part 1: The Roles of Pore Attributes and Their Variation on Trapping and Apparent Viscosity of Foam. SPE J. 2021, 26, 3908–3925. [Google Scholar] [CrossRef]
- Suekane, T.; Nguyen, H.T. Relation between the Initial and Residual Gas Saturations of Gases Trapped by Capillarity in Natural Sandstones. J. Fluid Sci. Technol. 2013, 8, 322–336. [Google Scholar] [CrossRef]
- Bull, Ø.; Bratteli, F.; Ringen, J.K.; Melhuus, K.; Bye, A.L.; Iversen, J.E. The Quest for the True Residual Gas Saturation—An Experimental Approach. In Proceedings of the International Symposium of the Society of Core Analysts, Austin, TX, USA, 18–21 September 2011; pp. 1–12. [Google Scholar]
- Pentland, C.H.; Al-Mansoori, S.; Iglauer, s.; Bijeljic, B.; Blunt, M.J. Measurement of Non-Wetting Phase Trapping in Sand Packs. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008. [Google Scholar]
- Adebayo, A.R. Foam Flow in Different Pore Systems—Part 2: The Roles of Pore Attributes on the Limiting Capillary Pressure, Trapping Coefficient, and Relative Permeability of Foamed Gas. SPE J. 2021, 26, 3926–3948. [Google Scholar] [CrossRef]
- Singh, K.; Menke, H.; Andrew, M.; Lin, Q.; Rau, C.; Blunt, M.J.; Bijeljic, B. Dynamics of snap-off and pore-filling events during two-phase fluid flow in permeable media. Sci. Rep. 2017, 7, 5192. [Google Scholar] [CrossRef]
- Holtz, M.H. Residual Gas Saturation to Aquifer Influx: A Calculation Method for 3-D Computer Reservoir Model Construction. In Proceedings of the SPE Gas Technology Symposium, Calgary, AB, Canada, 30 April–2 May 2002. [Google Scholar]
- Land, C.S. Calculation of Imbibition Relative Permeability for Two- and Three-Phase Flow From Rock Properties. Soc. Pet. Eng. J. 1968, 8, 149–156. [Google Scholar] [CrossRef]
- Jerauld, G.R. Prudhoe Bay Gas/Oil Relative Permeability. SPE Reserv. Eng. 1997, 12, 66–73. [Google Scholar] [CrossRef]
- Kleppe, J.; Delaplace, P.; Lenormand, R.; Hamon, G.; Chaput, E. Representation of Capillary Pressure Hysteresis in Reservoir Simulation. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 5–8 October 1997. [Google Scholar]
- Aissaoui, A. Etude Théorique et Expérimentale de L’hystérésis des Pressions Capillaires et des Perméabilités Relatives en Vue du Stockage Souterrain de Gaz; Ecole des Mines de Paris: Paris, France, 1983. [Google Scholar]
- Spiteri, E.J.; Juanes, R.; Blunt, M.J.; Orr, F.M. A New Model of Trapping and Relative Permeability Hysteresis for All Wettability Characteristics. SPE J. 2008, 13, 277–288. [Google Scholar] [CrossRef]
- Dance, T.; Paterson, L. Observations of carbon dioxide saturation distribution and residual trapping using core analysis and repeat pulsed-neutron logging at the CO2CRC Otway site. Int. J. Greenh. Gas Control 2016, 47, 210–220. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Lotfollahi, M.; Eftekhari, A.A.; Rossen, W.R.; Hirasaki, G.J.H. Effect of Permeability on Implicit-Texture Foam Model Parameters and the Limiting Capillary Pressure. Energy Fuels 2015, 29, 3011–3018. [Google Scholar] [CrossRef] [Green Version]
- Eftekhari, A.A.; Farajzadeh, R. Effect of Foam on Liquid Phase Mobility in Porous Media. Sci. Rep. 2017, 7, 43870. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Andrianov, A.; Bruining, H.; Zitha, P.L.J. Comparative Study of CO2 and N2 Foams in Porous Media at Low and High Pressure−Temperatures. Ind. Eng. Chem. Res. 2009, 48, 4542–4552. [Google Scholar] [CrossRef]
- Blaker, T.; Aarra, M.G.; Skauge, A.; Rasmussen, L.; Celius, H.K.; Martinsen, H.A.; Vassenden, F. Foam for Gas Mobility Control in the Snorre Field: The FAWAG Project. SPE Reserv. Eval. Eng. 2002, 5, 317–323. [Google Scholar] [CrossRef]
- Kamyab, M.; Simjoo, M.; Dejam, M.; Alamatsaz, A. Numerical Study of Immiscible Foam Propagation in Porous Media in the Presence of Oil Using an Implicit-Texture Foam Model. Energy Fuels 2021, 35, 6553–6565. [Google Scholar] [CrossRef]
- Heller, J.P. CO2 Foams in enhanced oil recovery. In Foams: Fundamentals and Applications in the Petroleum Industry; AMC: Washington, DC, USA, 1994; Volume 242, pp. 201–234. [Google Scholar]
- Meng, B.; Li, Z.; Lu, T.; Du, L.; Wang, Y.; He, X.; Zhang, Y. Experimental Study on the Mechanism of Nitrogen Foam to Improve the Recovery of Bottom-Water Heavy Oil Reservoir. Energy Fuels 2022, 36, 3457–3467. [Google Scholar] [CrossRef]
- Kahrobaei, S.; Farajzadeh, R. Insights into Effects of Surfactant Concentration on Foam Behavior in Porous Media. Energy Fuels 2019, 33, 822–829. [Google Scholar] [CrossRef]
- Janssen, M.T.G.; Mutawa, A.S.; Pilus, R.M.; Zitha, P.L.J. Foam-Assisted Chemical Flooding for Enhanced Oil Recovery: Effects of Slug Salinity and Drive Foam Strength. Energy Fuels 2019, 33, 4951–4963. [Google Scholar] [CrossRef]
- Apaydin, O.G.; Kovscek, A.R. Surfactant Concentration and End Effects on Foam Flow in Porous Media. Transp. Porous Media 2001, 43, 511–536. [Google Scholar] [CrossRef]
- Bernard, G.G.; Jacobs, W.L. Effect of Foam on Trapped Gas Saturation and on Permeability of Porous Media to Water. Soc. Pet. Eng. J. 1965, 5, 295–300. [Google Scholar] [CrossRef]
- Jones, S.A.; Getrouw, N.; Vincent-Bonnieu, S. Foam flow in a model porous medium: II. The effect of trapped gas. Soft Matter. 2018, 14, 3497–3503. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Q.; Kovscek, A.R. Trapped Gas Fraction During Steady-State Foam Flow. Transp. Porous Media 2006, 65, 287–307. [Google Scholar] [CrossRef]
- Almajid, M.M.; Kovscek, A.R. Pore Network Investigation of Trapped Gas and Foam Generation Mechanisms. Transp. Porous Media 2020, 131, 289–313. [Google Scholar] [CrossRef]
- Adebayo, A.R. A Graphical Interpretation Technique to Evaluate Strength and Stability of Foam in Porous Media Based on Mobile-Trapped Foam Ratio. Transp. Porous Media 2021, 139, 327–355. [Google Scholar] [CrossRef]
- Archie, G.E. Classification of carbonate reservoir rocks and petrophysical considerations. AAPG Bull. 1952, 36, 278–298. [Google Scholar]
- Adebayo, A.R.; Isah, A.; Mahmoud, M.; Al-Shehri, D. Effects of Foam Microbubbles on Electrical Resistivity and Capillary Pressure of Partially Saturated Porous Media. Molecules 2020, 25, 3385. [Google Scholar] [CrossRef]
- Kovscek, A.R.; Tang, G.Q.; Radke, C.J. Verification of Roof snap off as a foam-generation mechanism in porous media at steady state. Colloids Surf. A: Physicochem. Eng. Aspects. 2007, 302, 251–260. [Google Scholar] [CrossRef]
- He, L.; Luo, Z.; Bai, B. Breakup of pancake droplets flowing through a microfluidic constriction. Chem. Eng. Sci. 2020, 220, 115649. [Google Scholar] [CrossRef]
- Blunt, M.J. Multiphase Flow in Permeable Media: A Pore-Scale Perspective; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Singh, K.; Bultreys, T.; Raeini, A.Q.; Shams, M.; Blunt, M.J. New type of pore-snap-off and displacement correlations in imbibition. J. Colloid Interface Sci. 2022, 609, 384–392. [Google Scholar] [CrossRef]
- Cohen, M.H.; Mendelson, K.S. Nuclear magnetic relaxation and the internal geometry of sedimentary rocks. J. Appl. Phys. 1982, 53, 1127–1135. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Krastev, R.; Zitha, P.L.J. Gas Permeability of Foam Films Stabilized by an α-Olefin Sulfonate Surfactant. Langmuir 2009, 25, 2881–2886. [Google Scholar] [CrossRef]
- Jones, S.A.; Laskaris, G.; Vincent-Bonnieu, S.; Farajzadeh, R.; Rossen, W.R. Effect of surfactant concentration on foam: From coreflood experiments to implicit-texture foam-model parameters. J. Ind. Eng. Chem. 2016, 37, 268–276. [Google Scholar] [CrossRef] [Green Version]







| Sample ID | Name of Outcrop | Rock Lithology | Permeability k (mD) | Porosity ϕ (%) | Length L (cm) | Diameter D (cm) |
|---|---|---|---|---|---|---|
| BH | Briarhill | Sandstone | 1100 | 18.92 | 10.248 | 3.747 |
| B | Berea sand | Sandstone | 278 | 16.01 | 9.351 | 3.783 |
| AC | Austin Chalk | Carbonate (Calcite) | 26 | 26.89 | 10.441 | 3.784 |
| GD | Guelph | Carbonate (Dolomite) | 637 | 14.83 | 10.243 | 3.787 |
| Salt Composition | Mass (g/L) |
|---|---|
| Sodium Chloride (NaCl) | 41.17 |
| Magnesium Chloride (MgCl2·6H2O) | 17.42 |
| Sodium sulphate (Na2SO4·10H2O) | 7.93 |
| Calcium Chloride (CaCl2·4H2O) | 2.97 |
| Sodium Bicarbonate (NaHCO3) | 0.17 |
| Total dissolved solids (TDS) | 69.66 |
| Injection Strategy | Sgr–T2LM Correlation | Goodness-of-Fit, R2 |
|---|---|---|
| WAG injection | Sgr = 0.0003 (T2LM) + 0.2118 | 0.9995 |
| SAG injection | Sgr = 0.0005 (T2LM) + 0.2789 | 0.9226 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezk, M.G.; Babu, R.S.; Badmus, S.O.; Adebayo, A.R. Foam-Assisted Capillary Trapping in Saline Aquifers—An Initial–Residual Saturation Analysis. Energies 2022, 15, 6305. https://doi.org/10.3390/en15176305
Rezk MG, Babu RS, Badmus SO, Adebayo AR. Foam-Assisted Capillary Trapping in Saline Aquifers—An Initial–Residual Saturation Analysis. Energies. 2022; 15(17):6305. https://doi.org/10.3390/en15176305
Chicago/Turabian StyleRezk, Mohamed Gamal, Rahul S. Babu, Suaibu O. Badmus, and Abdulrauf R. Adebayo. 2022. "Foam-Assisted Capillary Trapping in Saline Aquifers—An Initial–Residual Saturation Analysis" Energies 15, no. 17: 6305. https://doi.org/10.3390/en15176305
APA StyleRezk, M. G., Babu, R. S., Badmus, S. O., & Adebayo, A. R. (2022). Foam-Assisted Capillary Trapping in Saline Aquifers—An Initial–Residual Saturation Analysis. Energies, 15(17), 6305. https://doi.org/10.3390/en15176305







