Abstract
One of the main topics that generate the interest of experts nowadays involves the processes of organic matter chemical transformation during heavy and shale oil reservoirs’ development via thermally enhanced oil recovery. It is common knowledge that the host rock has a catalytic effect on the ongoing processes. In addition, oil transformation is mostly associated with destructive processes of resins and asphaltenes molecules. As a result, this would provide an increase in oil mobility as a result of kerogen destruction in shale oil. This ensures the formation of synthetic oil and an increase in the filtration characteristics of the rock. Besides, iron-containing compounds in the composition of the rock are catalytically active in the above processes. Moreover, clay minerals have high catalytic activity for many reactions of organic matter transformation. This review considers publications that study the role played by the rock and its individual components in the processes of in situ upgrading of heavy and shale oil.
1. Introduction
The sustainable development and production of oil and gas are significant for the hydrocarbon industry and its relationship to the world market. Therefore, heavy and shale oils deposits, which are more evenly distributed around the globe, are very attractive. However, the exploitation of such reservoirs requires application of thermally enhanced oil recovery methods. The heat energy is crucial for reducing heavy oil viscosity or supplying synthetic oil generation [1,2,3]. However, the in situ process is similar to the oil refinery process. If the application of catalysts is an essential part of oil refinery processes, then co-injection of catalysts is a built-in part of thermally enhanced oil recovery methods. The feasibility and economy of the thermal oil recovery techniques depend on the application of catalysts, which are injected into the reservoir formations as they influence the heat energy distribution [4,5,6,7]. There are several field tests of various catalysts for heavy oil development that were successfully completed. In China, the pilot tests of co-injecting catalysts with steam were assessed in two oil fields: Liaohe [8] and Xinjiang [9]. The industrial-scale application of catalysts in heavy oil carbonate deposit with the oil viscosity around 270 MPa·s was successfully implemented by our group [10].
It is widely considered that reservoir rock minerals have a great influence on oil production processes during the development of various hydrocarbons’ deposits. This is especially noticeable when using thermally enhanced oil recovery methods. Various mineral components have catalytic properties in relation to various reactions. A number of reactions, such as cracking, destructive hydrogenation, hydrolysis, etc., occur with resins and with asphaltenes in heavy oils composition or in kerogen of the shale composition. Minerals containing transition metals are especially active as catalysts for the above reactions. Increasing attention is being drawn to the possibility of injecting catalysts into the reservoir to convert oil or shale using thermally enhanced oil recovery methods. In this case, minerals can affect the action of the catalyst. During the adsorption of catalyst particles on the surface of the host rock, a catalytic complex can form. Consequently, the efficiency of such a complex can be higher than that of the catalyst itself or the rock separately. Therefore, the role of mineral components is actually generating considerable interest in terms of developing and exploiting heavy and shale oils.
2. Content
2.1. Minerals at the Stage of Formation
The mineral components of the reservoir system have a significant impact on the formation of hydrocarbon deposits. The composition of minerals changes with the migration of hydrocarbons during the further formation and destruction of the deposit. Authigenic mineral formation in the zones of hydrocarbon flows and in areas of their concentration is associated with a change in the physicochemical conditions at the contact of mobile hydrocarbons and water and occurs due to the dissolution of gases in water, variations in pH and Eh of the medium, the activity of anaerobic bacteria, etc. [10]. Epigenetic mineral formation in the areas of occurrence of oil and gas deposits has its own unique features and patterns.
Terrestrial heat from the bowels ensures the transformation of organic matter in clayey rocks. On the way to hydrocarbon flow, a “hydrocarbon footprint” is formed. In the zone of this “trace”, glauconite forms in the presence of hydrocarbons, in addition to the appearance of pyrolusite, pyrochlore, often uranium pyrochlore, plumbopirochlore and strontium pyrochlore; meanwhile, ferruginous minerals become converted. In zones of intense heat and hydrocarbon flow, new formations appear in the form of phenocrysts (impregnations), intergrowths of crystals.
At subsequent stages, biotite, ilmenite and calcite are formed in the hydrocarbon flow zone. In the central zone of the “hydrocarbon footprint”, ferruginous minerals hematite, pyrite and magnesioferrite are often found, and, in the far outer zones of the flow, pentahydrocalcite is sometimes formed.
In the zone where the concentration of hydrocarbons coming from the depths and from the oil source strata occurs (as a result of their compaction or dehydration of silty-clay deposits), a hydrocarbon deposit is slowly formed. In areas where hydrocarbons are collected, they are concentrated and converted. The concentration zone is characterized by lower temperatures (50–60 °C). Secondary processes include primarily the precipitation of clay minerals from fluid solution. Hydromicas and kaolinite precipitate. Kaolinite is probably formed as a result of the hydrolysis of CO2-containing silicate rocks with acidic solutions. Dickite, hydromica, illite and saussurite are found here. Kaolinite is the most representative in samples. Kaolinite is often fine-grained, granular. New formations of clay minerals are typical for peripheral zones of hydrocarbon deposits [11].
The formation of kaolinite is associated with periods of intensive growth of structures and mass flows of hydrocarbons up the section [12,13,14], and may be due, in the active tectonic phase, to the appearance of aggressive carbon dioxide solutions coming from the basement and lower horizons of the sedimentary cover. Kaolin formation occurs mainly due to the dissolution of detrital silicates and aluminosilicates of sandy–silty rocks. The formation of kaolinite is favored by high temperatures, pressure and the presence of protons in the solution (low pH values), while a carbonic environment, on the contrary, shifts the process in the opposite direction. The formation of kaolinite is known to be associated with the hydrolysis of silicate rocks:
2K[AlSi3Os] + 7H2O → Al2[Si2O5](OH)4 + 4H2SiO3 + 2КОН.
Clay minerals undergo hydrolysis under conditions of lowering the pH of formation waters, due to the release of CO2 and organic acids and during the decomposition of organic matter. According to previously published works [14,15,16], authigenic kaolinite is formed in a well-permeable medium mainly due to clay cement, consisting of hydromicas, hydromica–montmorillonite formations and chlorite. A more perfect structure, according to Sakhibgareev et al. [17], is possessed by kaolinite from aquifers, which is explained by the preservative effect of oil on authigenic mineral formation. Perfect aggregates of kaolinite are sometimes found in the contours of deposits [12]. Kaolinite formation is less common in oil-bearing reservoirs.
The maximum amount of kaolinite is observed in gas-saturated rocks. Minerals of the kaolinite and chlorite group are the most typical for unproductive sections. Hydromicas and montmorillonite formations are observed within oil deposits, often in the form of basal-pore cement, with predominantly random orientation of clay particles [12]. Organic matter adsorbed on clay material increases the stability of clay minerals [15,18]. When organic matter interacts with clay minerals, the fluid-resistant properties of screens increase significantly [12].
The post-sedimentary formation of chlorite minerals is facilitated by the development of anaerobic bacterial activity, accompanied by iron reduction and an increase in the pH of pore solutions, the increased content of which is often accompanied by a deterioration in the reservoir properties of rocks. Minerals of the hydromica group, including illite, are rare in oil-saturated reservoirs. In pelitomorphic rocks, the process of illitization is more likely. Illite formation is assumed to be more alkaline than kaolinite formation [19]. Among the epigenetic carbonates, calcite is the most common; siderite, dolomite and ankerite are common [20].
In the sandy–silty reservoirs of Western Siberia, the most common cements are clayey and carbonate. In zones of secondary carbonatization, often associated with the migration of hydrocarbons, several generations of calcite stand out. It is believed that the bulk of calcite is formed at the stage of early epigenesis, before oil enters the trap. Calcite of late generations is formed in connection with the processes of sulfate reduction near hydrocarbon deposits [21]. The structures of diagenetic carbonates are most often pelitomorphic and granular with nodules. Among carbonates, there are calcite, manganocalcite, rhodochrosite, dolomite, siderite, ankerite, often manganosiderite, manganese calcite, magnesium-containing siderite, ferruginous magnesite and the Mg content in these carbonates in oil-saturated sandstones increases. In the oil-bearing contours, predominantly calcium-iron magnesite with a high content of Mg crystallizes [14].
For some deposits of the Ural-Volga region and Western Siberia, a multi-stage formation of deposits has been proven [22,23].
In a productive oil-saturated reservoir, where the preservative effect of hydrocarbons has a significant impact on the course of secondary processes, the leading process is silicification, as a result of which productive sandstones often contain up to 5–10% regeneration quartz cement.
As a result of the interaction of hydrocarbons with host rocks, subvertical zonal-ring-shaped geochemical, geophysical and biogeochemical fields are established above oil and gas deposits. Subvertical annular zones of secondary alteration of rocks arise as a result of the processes of chemical and biochemical destruction of hydrocarbons dissipating from deposits and the interaction of hydrocarbons with mineral components of rocks [19]. The migration of hydrocarbons causes a change in the parameters of the mineral environment in various geochemical zones.
In the hydrodynamic systems of the oil and gas section, in addition to temperature and pressure, the processes of secondary mineral formation are significantly affected by the mineralization of solutions and their pH and Eh. First of all, this affects the pH and Eh values of water-mineral media [24].
Carbon dioxide and hydrogen sulfide are of great importance in the successive occurrence and spatial variability of the concentration fields of various elements in the diffusion flow of hydrocarbons. They have a significant effect on the Eh and pH values of rocks and are involved in the processes of leaching and authigenesis. Carbon dioxide is formed during the oxidation of hydrocarbons, is contained in a dissolved state in waters, is present in the mineral environment, enhances the removal of such cations as Fe2+, Mn2+, Cu2+, Co2+ and alkaline earth metals: Mg2+, Ca2+, Sr2+ and Ba2+. Newly formed minerals are deposited in sediments located in the contour of hydrocarbon anomalies. Ions that have a greater affinity for sulfur: Fe2+, Cu2+, Co2+ and Mi2+ form sulfides, and those that have a greater affinity for oxygen: Mg2+, Ca2+, Sr2+, Ba2+ form carbonates.
Numerous studies have noted that, in overproductive deposits in the form of halos that outline hydrocarbon deposits, elevated concentrations of elements are established: Mi, Fe, Ti, Cu, Pb, Sn, V, Ni, Co, Si, Al, Ca, Ba, Sr, etc. Paragenetic associations of chemical elements, the nature of their relationship with neoformations, the sequence of deposition and selective confinement to different parts of productive sections are revealed. The multivalent and chalcophile elements Fe, Mi, Pb and Zn tend to precipitate in reducing media together with siderite and sulfides. Complexes of compounds with organic components form Cu, V, Ni and Co. In the presence of CO2, Ca, Ba and Sr, carbonates are intensively dissolved, turning into soluble hydrocarbons.
In other words, rocks containing hydrocarbons include a wide variety of compounds that actively influence the transformation of hydrocarbons at the stage of reservoir formation. During the development of deposits by thermal methods, many rock components can actively participate in chemical processes that provide an increase in the mobility of heavy oil or the destruction of kerogen in shale deposits.
2.2. At the Production Stage as Catalysts
The course of catalytic processes during thermal action on oil deposits has long been known. The term aquathermolysis itself provides for the important role of the minerals of the reservoir rock [25]. Aquathermolysis is considered as one of the methods of in situ upgrading of high-viscosity crude oil, along with in situ combustion, low-temperature oxidation and other methods, which are common for formation heating and injection of reagents and/or catalysts [25,26,27,28,29].
The catalytic participation of rock-forming minerals was studied on an oil sample from the Ashalcha field [30]. Physical modeling of the catalytic aquathermolysis of heavy oil with different chemical compositions has been performed. The processes were carried out at a temperature of 300 °C in the presence of kaolin (montmorillonite content 44%) and catalysts based on transition metal carboxylates (Fe, Co, Cu). The process medium was a mixture of carbon dioxide and water vapor. Distinctive features of hydrothermal–catalytic conversion of various types of oil were evaluated by fractional, structural-group, microelement composition and change in the H:C ratio. These variations are due to the initial properties of crude oil and the degree of activation of C-C, C-N, C-O and C-S bond destruction reactions, leading to different levels of increase in the content of saturated fractions and a decrease in the content of resins and asphaltenes. The thermal analysis method was used to evaluate the oil content on the rock before and after the experiments. The high molecular weight components of naphthenic aromatic oil type B2 showed a greater adsorption capacity for rocks compared to oil type A1. Therefore, the adsorption of catalyst components on rocks is also greater [31], as shown by the effect of montmorillonite, kaolinite, quartz, calcite, etc., on the change in the molecular weight of asphaltenes in heavy oils from various fields during aquathermolysis [32].
The process of aquathermolysis has been studied for the kerogen-containing Domanik rock. Fragments of kerogen fall into the fraction of aromatic hydrocarbons. Thermal action changes the ratio of ortho-, para- and meta-isomers of alkyltoluenes. The content of heavy aromatic hydrocarbons increases with increasing temperature, and the ratio of kerogen macroelements changes under the influence of thermal exposure, which is an indicator of the process of maturation of organic matter [33].
In situ combustion is generating a considerable interest in terms of enhancing heavy oil recovery. It is commonly known that promoting the combustion front is based on the formation and oxidation of coke. In their analysis of heavy oil combustion and pyrolysis from China in the presence of montmorillonite, a major type of clay, by means of thermogravimetric analyzer (TGA) and a fixed-bed reactor [34], the authors found that the characteristic temperatures of thermal conversion decreased with montmorillonite in the oxidizing atmosphere while remaining unaffected in the pyrolysis atmosphere. Moreover, the authors reported that the fuel deposition increased in both atmospheres because of montmorillonite’s strong adsorption. It has been found as well that the presence of montmorillonite obviously promoted the progress of coke formation and increased coke yield in the oxidizing atmosphere. In addition, the content of O was found more contrarily to the contents of C and H, which were found decreased in coke. The obtained results pointed towards the idea that the oxidation activity of coke was improved, while trailing occurred due to the blocking in the pore structures of the montmorillonite skeleton. However, in the pyrolysis atmosphere, the presence of montmorillonite did not influence the coke formation temperature but increased coke yield. What is more, the content of C was found increased, and the contents of H and O were found decreased in coke. On the other hand, the coke oxidation activity was reduced with a more serious trailing phenomenon. In fact, montmorillonite significantly affected coke formation through its strong adsorption to polar components in heavy oil and obvious catalysis on dehydrogenation as an acid catalyst in the pyrolysis atmosphere. It has enhanced the oxygenation due to its large surface area and catalyzed polycondensation in the air atmosphere.
In other works [35], authors have studied the influence of the rock matrix on oxidation kinetics by means of kinetic experiments for a Colombian heavy crude using various porous media, such as synthetic sand, drilling cuttings, reservoir core and outcrop. Authors have mainly aimed to evaluate the kinetic behavior of packing with porous media other than the original rock matrix to determine the feasibility of using them as a replacement for the original reservoir rock while maintaining the representativeness of the results. It was supposed that the importance resides in the fact that the observed kinetics can be used to assess the technical feasibility for the implementation of in situ combustion projects. The experimental design included kinetic cell tests varying the porous medium and keeping the same fluid and the same operating conditions. The obtained results showed the possibility of creating reliable laboratory studies for the air injection process using packing other than the reservoir core as a more convenient alternative [35].
Heavy oil extraction resolves economic and environmental challenges as a result of low prices and costly energy usage for their exploitation and transport. However, enhancing heavy oil recovery requires novel and well-engineered technologies. In situ combustion is a technique that increases heat generation by means of air injection assisted with nano catalysts. The application of such technology requires performance assessment and a kinetic database for each application and each type of reservoir with specific rock–oil in place characteristics. In their work [36], Durman et al. have studied the hydrocracking kinetics of dolomite core pack at a temperature range of 335 °C to 365 °C in a period of 12 h–72 h under constant pressure of 10 MPa, which are the typical ISUT’s conditions for carbonate reservoirs. The obtained results showed similar results with the previous kinetic model. The scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) techniques have showed that nanocatalysts of 13 nm to 1220 nm with two types, spherical and agglomerated-shaped, have deposited on the rock. The X-ray fluorescence spectroscopy (XRF) and the ISUT’s kinetic modeling studies have proposed the convenience of using the same model for the reservoir’s studies. This includes the effects of different conditions, such as temperature, pressure or residence time, used during ISUT’s process application.
The roles of various clay and non-clay minerals present in the steam distillation process in reservoir formation have been studied by Tavakkoli et al. [37]. Two heavy oil samples mixed with water and crushed rock mixed with different clay minerals were kept under steam pressure at 150, 200 and 250 °C in a batch autoclave reactor for 40 h. The obtained results were compared with respect to the changes in the density, viscosity and chemical composition of the remaining heavy oil. The authors added three different clay minerals (bentonite, kaolinite and sepiolite) to the crushed rock in order to study their effects. It has been found that kaolinite, among all the clay minerals, had the greatest effect on steam distillation. Moreover, the authors suggest that kaolinite has an inert surface compared to other clay minerals, which can be considered as a catalytic effect to make easier the evaporation of the volatile components of heavy oil during steam distillation. Moreover, the authors believe that bentonite may not allow the entrance of oil molecules because of its low permeability. It has been shown as well that the increase in density and viscosity of the remaining oil is the result of kaolinite addition. The results of this study have shown that the asphaltene content of the remaining oil after distillation is more than that of the original oil sample for all added clay minerals.
Nowadays, improving the efficiency of enhanced oil recovery methods presents a difficult task. Mukhammatdinov et al. have attempted to improve oil quality and reduce resins and asphaltenes content in heavy oil by applying hydrothermal treatment to a sample of Aschalcha oil. The authors simulated the process of heavy oil hydrothermal processing in the presence of iron tallates under reservoir conditions (200 °C, 30 bar). The composition and physicochemical characteristics of the obtained substances were characterized by elemental and SARA analysis, MALDI, GC–MS and FT-IR. The authors have extracted rocks and analyzed them by means of XRD and DSA (drop shape analyzer). The obtained data from this study showed that the introduction of a catalyst in combination with a hydrogen donor reduces the content of resins by 22 wt.% and increases saturated hydrocarbons by 27 wt.%. What is more, the destructive hydrogenation has been found to result in a decrease in the sulfur content in the upgrading products. The authors believe that this is crucial for the oil reservoirs of Tatarstan Republic because of their high sulfur content. In addition, DSA data showed that the hydrophilicity of the rock surface increases due to inhibition of the coke formation after the introduction of the catalytic complex, which means that the oil recovery factor can be increased as a result of reservoir rocks’ oil-wetting properties alteration [38].
In another work, Sitnov et al. studied the process of heavy oil aquathermolysis in the presence of dolomite and calcite. It has been shown that calcite reduces the amount of asphaltenes and resins in addition to their condensation and aromaticity as a result of C-S-C bonds cleavage, as shown by SARA analysis and IR spectroscopy. Moreover, the gas chromatography analysis in this study pointed towards the increment of hydrocarbon content with homologous series of С19-С30 as a result of heavy oil thermal conversion. However, the presence of dolomite leads to the isomerization of new formed alkanes (Figure 1). The authors have shown as well the high maturity of organic matters in thermally conversed heavy oil from a model system with dolomite by geochemical coefficients calculation [39].
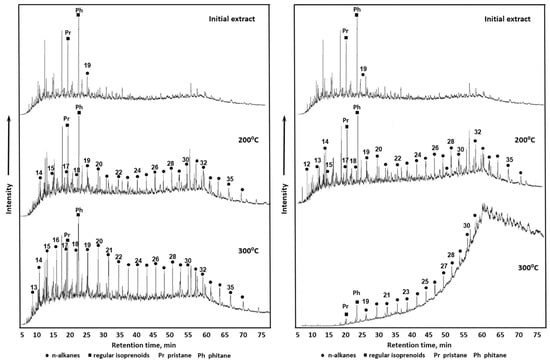
Figure 1.
GC–MS chromatograms (TIC = total ion chromatography) of saturated hydrocarbon fraction of initial oil and after thermal treatment in the presence of calcite (CaCO3)—left and in the presence of calcite (CaCO3)—right.
2.3. As Substrates for Embedded Catalysts
Reservoir rocks have an important influence on catalysts’ functioning. In the work about catalytic redox transformations in rock matrices by Dobrynkin et al. [40], the authors provided the results of studying the properties of catalytic systems based on iron oxide and inorganic matrices of rocks (basalt, clay, sandstone) in relation to the reactions of ammonium nitrate decomposition, methane oxidation and asphaltene hydrocracking. The used catalytic systems were iron oxide fixed on matrices during the joint hydrolysis of carbamide and iron chloride under hydrothermal conditions at T = 433–473 K and pressures of 0.6–1.6 MPa. In the reaction of asphaltene hydrocracking to maltenes, the activity of catalytic systems decreases in the order Fe2O3/basalt > Fe2O3/clay > Fe2O3/sandstone, while clay-based iron oxide catalysts turned out to be the most selective.
In another study, Chen et al. have taken reservoir minerals from Daqing and Liaohe oilfields oil sand. The experiments were carried out with the addition of the same oil. Reservoir minerals included quartz, potash feldspar, plagioclase, some calcite and dolomite (about 1%). The total content of clay minerals was about 10%. In contrast to simple aquathermolysis, the addition of formation minerals resulted in a decrease in the average molecular weight of higher saturated and aromatic content, lower resins and asphaltenes content. The coefficient of viscosity of the two oil samples has been found to increase from 7.41% and 12.95% up to 16.05% and 25.29%, respectively. With the addition of NiSO4, the average molecular weight of the reaction products and the contents of resin and asphalt formation are further reduced, saturated and aromatic; with a further increase in the content of hydrocarbons, the viscosity is significantly reduced, with a recovery factor of up to 84.39% [41].
In the work performed by Tavakkoli et al. [37], the effect of carbonate and sandstone rock, temperature and clay and non-clay minerals was evaluated during the steam distillation of Chamurlu and Bati Raman heavy oil samples. The density and viscosity of the remaining oil were measured, and SARA analysis was performed. It has been found that kaolinite particles allow the volatile components to evaporate, creating an inert surface, which can be considered as a kind of catalytic effect. As for other minerals, especially bentonite, the behavior of clay particles seems to be different. The swelling of bentonite in water due to special mineralogical composition prevents the evaporation of volatile components of oil. Consequently, the density of the remaining oil decreases. The behavior of sepiolite was similar to that of kaolinite as the sepiolite sample contains kaolinite as a clay mineral. The content of asphaltenes in the remaining oil after steam distillation was found to be higher than that of the original crude oil. It increases somewhat in the presence of minerals. On the other hand, the addition of clay minerals leads to a significant increase in the content of asphaltenes due to a decrease in the amount of volatile components.
Zhang et al. investigated the effect of minerals together with nickel sulfate used as a catalyst and added it to the reaction system. The viscosity reduction rate was found to reach 50%. The authors have shown that, if formic acid is used as a hydrogen donor, the rate of viscosity reduction can increase even more to a total amount of up to 71.8%. The reaction of heavy oil aquathermolysis under steam injection conditions was affected by the reaction temperature, reaction time, amount of added minerals, catalyst density and hydrogen donor. Moreover, the authors have reported that minerals, a catalyst and a hydrogen donor can jointly enhance the aquathermolysis reaction of heavy oil in the presence of high-temperature steam. In fact, the mineral composition included quartz, kaolinite, montmorillonite, illite and others. The applied value of the study lay in the possibility of regulating the process of chemical transformation of oil. In the process, minerals can electrify metal ions—nickel, vanadium, iron and molybdenum. This process speeds up the breaking of the C-S bond. In this case, acid cents are formed, accelerating the reactions of aquathermolysis. For example, when exposed to vapor, Al3+ in the composition of clay can adhere to the surface of H4SiO4. It is formed in the process of exposure to steam. In this case, highly active hydroxyl groups are formed. The H+ ion from water can combine with oxygen bound to Si4+. This results in the formation of a highly active hydroxyl group, where hydroxyl groups can release the H+ ion by analogy with Bronsted acids; meanwhile, visbreaking and hydrogen transfer occur in the presence of Brønsted acids [42].
Similar works have been provided by Hyne et al., who studied the catalytic effect of minerals on the hydrocarbon groups, viscosity and element distribution of heavy oil during thermally enhanced oil recovery. It has been found that minerals have a catalytic effect in heavy oil aquathermolysis. The authors showed that, in the presence of 10 wt.% mineral, the saturate and aromatic content increased, while the resins and asphaltenes components decreased. Moreover, the obtained data found that the average molecular weight of heavy oil and asphaltene decreased after thermal treatment in addition to sulfur content. This study has reported a decrease in heavy oil viscosity by 23.4–25.6% in the reaction system after aquathermolysis. In fact, the term “aquathermolysis”, as described by the authors, is the process of chemical interaction of high-temperature, high-pressure water and heavy oil and bitumen reactive components, which differs from “hydrothermolysis”, which has come to be associated with the interaction with hydrogen at elevated temperature and pressure [43]. Model compounds studying showed that mineral components, in particular, trace metals such as vanadium and nickel, are able to accelerate heavy oil organ sulfur components cracking, which leads to producing carbon monoxide and other gases. What is more, aquathermolysis may lead in an increase in saturates and aromatics content and a decrease in resins and asphaltenes content. This may lower the average molecular weight and viscosity of oil and improve the its properties. This has attracted the interest of many researchers to study how to apply reservoir minerals to accelerate the aquathermolysis of heavy oil. Belgrave et al. studied heavy oil aquathermolysis kinetics with different model compounds and found that the mineralogy plays an important role in the generation of CO2 and H2S [44]. In a similar work, Clark et al. investigated the steam–oil chemical reaction and found that some metal ions, minerals and reservoir sands can change the heavy oil composition drastically [45]. Monin et al. studied heavy oil/mineral matrix systems’ thermal cracking at temperatures and pressures encountered during thermal recovery. They found that the chemical reactions, which include oil, possibly water, and a mineral matrix, may change the heavy oil composition significantly. The authors have used four thermal crude oils with different geochemical compositions. They showed that a large number of light hydrocarbons, CO2 and H2S could be generated in the presence of minerals in the reaction system [46,47,48].
Several studies have studied the influence of rock-forming minerals on the physical and chemical properties of heavy crude oil under steam-stimulation [49]. For example, Petrov et al. performed an analysis of minerals’ effect on heavy oil conversion in the presence of rock-forming additives among which calcites, dolomite, kaolin clay and manganese oxide were present in the studied medium. The experiments considered different temperatures and pressures. The obtained results showed that the temperature and pressure have a significant influence on the occurred processes. In addition, the obtained samples after the aquathermolysis experiments were characterized by a lower structural and Newtonian flow behavior and by greater output of fuel and oil fractions than the heavy oil. On the other hand, resins were found to be converted into lighter components during the destruction. The authors have reported as well that additives partially structure a monomolecular surface layer with a decrease in entropy of the observed molecules on the surface. This leads to a shift in the equilibrium towards a thermal decomposition unimolecular reaction of -C-C- bonds by radical chain mechanism. Therefore, there are two competing mechanisms. Besides, it has been found that a temperature increase raises the processes of cracking macromolecular compounds; meanwhile, a growing temperature background in the absence of high pressure has been found to reduce the adsorption probability on the additive surface [50].
Kayukova et al. have studied the specific features of Ashal’cha heavy oil conversion at hydrothermal conditions in a CO2 atmosphere in the presence of different oil-soluble carboxylate metals based on Fe, Co and Cu at 300 °C. The obtained results have shown that the catalyst and reservoir rocks minerals impact significantly the transformation behavior of hydrocarbon compositions and the SARA fractions. Moreover, it has been found that the most significant changes in the composition of heavy oil were established after hydrothermal treatment in the presence of a rock-forming additive, clay mineral (kaolin), which are reflected in a significant increase in the content of saturated and aromatic hydrocarbons. Additionally, this was fixed by resins and asphaltenes’ amount decrease. What is more, asphaltenes’ structure changes have been manifested in an increase in aromaticity and oxidation degrees because the catalyst metals mainly concentrate on the asphaltene structures and on the rock-forming reservoir minerals (Figure 2). It has been found as well that the Fe concentration increases to 5.84% after the catalytic treatment of oil with kaolin, and the concentration of Cu increases as well from 0 to 0.25%.
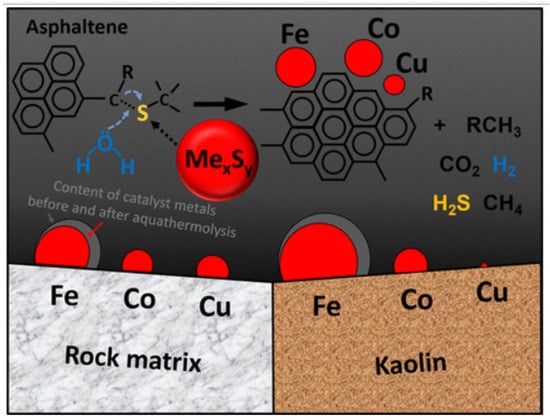
Figure 2.
The amount of adsorbed metals from the catalyst on the components of the reservoir system [51].
A similar change in asphaltene’s content was observed in rock extracts where iron and copper concentrations reached 1.38 and 0.31%, respectively. Meanwhile, cobalt was absent in asphaltenes content. In addition, active metals, such as Ti, Fe, Cr, Mn, Co, Ni, Cu, Zr and Bawere found in the rock samples after organic matter extraction. Besides, the Fe concentration has been associated with a slight decrease from 2.27 to 1.93% in the catalytic complex. On the other hand, the Co and Cu concentrations have been found to increase to 0.036 and 0.038% compared to their content in the original rock in which they were practically absent. This study has shown as well that the studied metal complexes (Fe, Co and Cu) with carboxylate ligands can penetrate not only into the rock pore space but also into asphaltenes. This may provide a possible catalytic effect on the heavy oil upgrading process under hydrothermal conditions [51].
Experimental studies have shown that the mineral matrix is actively involved in the process of kerogen cracking under hydrothermal conditions. An increase in contacts with process activators during the crushing of samples leads to an increase in the released synthetic oil. In this case, the amount of hydrocarbons obtained primarily depends directly on the mineral composition of the original rock and the contact of organic matter with process activators and inhibitors. Silica (primarily quartz) and clay minerals have a positive effect on the synthesis of oil from kerogen: an increase in their concentration in the rock leads to an increase in the yield of synthetic oil [10,52].
Even such an inert component as calcium carbonate can contribute to the conversion of kerogen because it reacts with the resulting sulfur and leads to the formation of calcium sulfates [53,54].
More recent evidence [55] suggests that mineral matrices’ intrinsic catalytic properties of various kinds (basalts, clays, sandstones) are of interest for in situ heavy oil upgrading. In fact, this may result in advanced technologies for enhanced oil recovery. In Ref. [55], the authors studied the elemental, surface and phase composition in addition to matrix particle morphology, surface and acidic properties using elemental analysis, X-ray diffraction, adsorption and desorption of nitrogen and ammonia. The obtained results have fixed for the first time the inorganic matrices’ catalytic activity of ammonium nitrate decomposition, oxidation of hydrocarbons and carbon monoxide and hydrocracking of asphaltenes into maltenes. In order to check their applicability for the asphaltenes’ hydrocracking catalytic systems development, basalt and clay matrices were used as supports for iron/basalt, nickel/basalt and iron/clay catalysts, and the obtained results confirmed the previously catalytic effect of the matrices in the reactions of ammonium nitrate, oxidation of hydrocarbons and carbon monoxide and hydrocracking of asphaltenes decomposition.
A significant effect of clay minerals on the efficiency of nickel-based aquathermolysis catalyst was demonstrated in our previous works [56,57,58]. Significant changes in the composition of aromatic fractions have been established. Resins cracking products have been found to transform into aromatic hydrocarbons. This has increased the relative content of monoaromatic compounds in the aromatic fractions’ composition, which were studied in the range of m/z = 132.5–133.5. These results are shown in Figure 3 as confirmed by the results of oil viscosity measurement after hydrothermal exposure.
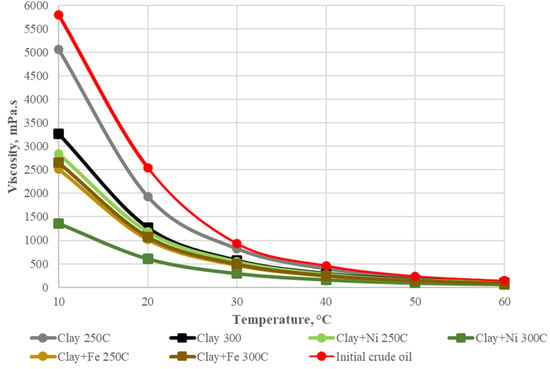
Figure 3.
Dynamic viscosity of initial crude oil before and after various catalytic hydrothermal treatments.
3. Conclusions
A significant part of unconventional hydrocarbon resources requires the use of thermally enhanced oil recovery technologies during the development stages. In the case of heavy oils development, a decrease in viscosity is required to facilitate their extraction, which can be achieved by chemical transformation of resins and asphaltenes. It is well known that oil shale development is found to be realized by the destruction of kerogen, which ensures the generation of shale oil.
Minerals actively participate in the formation of hydrocarbon deposits. They can also influence the transformation processes in the application of thermally enhanced oil recovery methods. A number of cracking, destructive hydrogenation, hydrolysis, etc., reactions occur with resins and asphaltenes in the composition of heavy oils or kerogen contained in oil shales. Minerals containing transition metals are especially active as catalysts for the above reactions. Increasing attention is being drawn to the possibility of injecting catalysts into the reservoir to convert oil or shale using thermally enhanced oil recovery methods. Moreover, minerals can affect the action of the catalyst. During the adsorption of catalyst particles on the surface of the host rock, a catalytic complex can form. The efficiency of such a complex can be higher than that of the catalyst itself and the rock separately. However, this process is less studied. In order to further in situ catalysis processes during development of unconventional hydrocarbons, it is necessary to study in detail the behavior of catalytic complex formation between catalyst particles and reservoir rock minerals. It is highly important to focus on the adsorption process of injected catalytic particles on the surface of minerals. For these purposes, it is probably required to develop special techniques as the processes in porous media will be different from the downstream processes with heterogeneous catalysts. Scientists and scholars have to consider the influence of reservoir rock minerals when they investigate the application of catalysts in enhanced heavy oil recovery methods or in situ upgrading technologies. Finally, it is worthy to note that the role of mineral components is important in the development of heavy and shale oils and should attract the interest of researchers and scholars of different areas in order to meet energy demands around the world.
Funding
This work was supported by the Russian Science Foundation (grant no. 21-73-30023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Muraza, O.; Galadima, A. Aquathermolysis of heavy oil: A review and perspective on catalyst development. Fuel 2015, 157, 219–231. [Google Scholar] [CrossRef]
- Maity, S.K.; Ancheyta, J.; Marroquın, G. Catalytic Aquathermolysis Used for Viscosity Reduction of Heavy Crude Oils: A Review. Energy Fuels 2010, 24, 2809–2816. [Google Scholar] [CrossRef]
- Tumanyan, B.P.; Petrukhina, N.N.; Kayukova, G.P.; Nurgaliev, D.K.; Foss, L.E.; Romanov, G.V. Aquathermolysis of crude oils and natural bitumen: Chemistry, catalysts and prospects for industrial implementation. Russ. Chem. Rev. 2015, 84, 1145–1175. [Google Scholar] [CrossRef]
- Li, C.; Huang, W.; Zhou, C.; Chen, Y. Advances on the transition-metal based catalysts for aquathermolysis upgrading of heavy crude oil. Fuel 2019, 257, 115779. [Google Scholar] [CrossRef]
- Qu, X.; Zhou, G.; Lu, Y.; Li, S.; Zhang, L.; Wang, J.; Xu, H. Catalytic aquathermolysis of Mackay River bitumen with different types of Mo-based catalysts. Fuel 2022, 326, 125134. [Google Scholar] [CrossRef]
- Tirado, A.; Yuan, C.; Varfolomeev, M.A.; Ancheyta, J. Kinetic modeling of aquathermolysis for upgrading of heavy oils. Fuel 2022, 310, 122286. [Google Scholar] [CrossRef]
- Wen, S.; Zhao, Y.; Liu, Y.; Hu, S. A Study on Catalytic Aquathermolysis of Heavy Crude Oil During Steam Stimulation. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 28 February–2 March 2007; pp. 417–421. [Google Scholar]
- Chao, K.; Chen, Y.; Liu, H.; Zhang, X.; Li, J. Laboratory Experiments and Field Test of a Difunctional Catalyst for Catalytic Aquathermolysis of Heavy Oil. Energy Fuels 2012, 26, 1152–1159. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Mukhamatdinov, I.I.; Aliev, F.A.; Feoktistov, D.F.; Sitnov, S.A.; Gafurov, M.R.; Minkhanov, I.F.; Varfolomeev, M.A.; Nurgaliev, D.K.; Simakov, I.O. Industrial application of nickel tallate catalyst during cyclic steam stimulation in Boca De Jaruco Reservoir. In Proceedings of the SPE Russian Petroleum Technology Conference, Virtual, 12–15 October 2021. [Google Scholar]
- Justinova, V.N.; Wiltsan, I.A.; Djilina, Е.N.; Mishenina, L.N. The Formation of Secondary Minerals in Oil- Gas-Bearing Sections and in Oil-Containing Thechnogenic Objects. 2000. Available online: https://www.elibrary.ru/item.asp?id=26460519 (accessed on 15 August 2022).
- Gushchin, V.A.; Ryl’kov, A.V. Geochemical features of two-phase hydrocarbon-pool formation in northern west Siberia. Int. Geol. Rev. 1987, 29, 1113–1116. [Google Scholar] [CrossRef]
- Frolov, S.V.; Akhmanov, G.G.; Bakay, E.A.; Lubnina, N.V.; Korobova, N.I.; Karnyushina, E.E.; Kozlova, E.V. Meso-Neoproterozoic petroleum systems of the Eastern Siberian sedimentary basins. Precambrian Res. 2015, 259, 95–113. [Google Scholar] [CrossRef]
- Dmitrievsky, A.N.; Balanyuk, I.E.; Karakin, A.V.; Lodzhevskaya, M.I.; Dongaryan, L.S. New fluid dynamic model for formation of giant hydrocarbon deposits. J. Geochem. Explor. 2003, 78–79, 345–347. [Google Scholar] [CrossRef]
- Sakhibgareev, R.S.; Vinogradov, A.D. Ancient water-oil contacts as indicators of the history of the formation of destroyed deposits. Transactions (Doklady) of the USSR Academy of Sciences. Earth Sci. Sections 1981, 257, 445. [Google Scholar]
- Yapaskurt, O.V. A New typification scheme of postsedimentation transformations of terrigenous deposits of continents and their margins with allowance for geodynamic factors. Mosc. Univ. Geol. Bull. 2014, 69, 229–235. [Google Scholar] [CrossRef]
- Logvinenko, N.V.; Berger, M.G. Modelling of lithogenetic processes. Izvestiya—Akad. Nauk. SSSR Seriya Geol. 1989, 12, 115–126. [Google Scholar]
- Volokitin, Y.; Sakhibgareev, R.; Shaymardanov, M.; Nurieva, O. Chemical and analytical work in support of West Salym field enhanced oil recovery project (ASP). In Proceedings of the SPE Russian Oil and Gas Exploration and Production Technical Conference and Exhibition 2012, Moscow, Russia, 16–18 October 2012; Volume 4, pp. 2527–2542. [Google Scholar]
- Stolbova, N.F.; Shaldybin, M.V. The nature of the clay content of industrial rocks in reservoirs of oil fields in the southeastern part of Western Siberia. In Topical Issues of Geology and Geography of Siberia; Tomsk Publishing House TGU: Tomsk, Russia, 1998; pp. 146–148. [Google Scholar]
- Rudmin, M.; Wilson, M.J.; Wilson, L.; Ibraeva, K.; Mazurov, A. Geochemical and mineralogical features of the substrates of the Vasyugan Mire, Western Siberia, Russia. Catena 2020, 194, 104781. [Google Scholar] [CrossRef]
- Ilyina, V.I. Subdivision and correlation of the marine and non-marine Jurassic sediments in Siberia based on palynological evidence. Rev. Palaeobot. Palynol. 1986, 48, 357–364. [Google Scholar] [CrossRef]
- Shvartsev, S.L.; Silkina, T.N.; Zhukovskaya, E.A.; Trushkin, V.V. Underground waters of petroliferous deposits of the Nyurol’ka sedimentary basin (Tomsk Region). Geol. Geofiz. 2003, 44, 451–464. [Google Scholar]
- Stoupakova, A.V.; Khvedchuk, I.I.; Sautkin, R.S.; Korobova, N.I.; Sivkova, E.D. Reforming of deposits in ancient oil and gas basins (On the example of deposits of the baikit anteclise eastern slope of the siberian platform). Georesursy 2019, 21, 31–41. [Google Scholar] [CrossRef]
- Kuznetsov, O.L. Lithogeochemical Studies in the Search for Oil and Gas Fields; Nedra: Waltham, MA, USA, 1987. [Google Scholar]
- Hyne, J.B.; Clark, P.D.; Clarke, R.A.; Koo, J.; Greidanus, J.W. Aquathermolysis of heavy oils. Rev. Tec. INTEVEP (Venezuela) 1982, 2, 87–94. [Google Scholar]
- Weissman, J.G.; Kessler, R.V. Downhole heavy crude oil hydroprocessing. Appl. Catal. A Gen. 1996, 140, 1–16. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Eskin, A.A.; Nurgaliev, D.K.; Vakhin, A.V. Thermal Study on Stabilizing the Combustion Front via Bimetallic Mn@ Cu Tallates during Heavy Oil Oxidation. Energy Fuels 2019, 34, 5121–5127. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Salih, I.S.S.; Khelkhal, M.A.; Vakhin, A.V. Application of Aromatic and Industrial Solvents for Enhancing Heavy Oil Recovery from the Ashalcha Field. Energy Fuels 2020, 35, 374–385. [Google Scholar]
- Sitnov, S.; Mukhamatdinov, I.; Aliev, F.; Khelkhal, M.A.; Slavkina, O.; Bugaev, K. Heavy oil aquathermolysis in the presence of rock-forming minerals and iron oxide (II, III) nanoparticles. Pet. Sci. Technol. 2020, 38, 574–579. [Google Scholar] [CrossRef]
- Farhadian, A.; Khelkhal, M.A.; Tajik, A.; Lapuk, S.E.; Rezaeisadat, M.; Eskin, A.A.; Rodionov, N.O.; Vakhin, A.V. Effect of Ligand Structure on the Kinetics of Heavy Oil Oxidation: Toward Biobased Oil-Soluble Catalytic Systems for Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2021, 60, 14713–14727. [Google Scholar] [CrossRef]
- Petrov, S.M.; Abdelsalam, Y.I.; Vakhin, A.V.; Baibekova, L.R.; Kayukova, G.P.; Karalin, A.E. Study of the rheological properties of heat-treatment products of asphaltic oils in the presence of rock-forming minerals. Chem. Technol. Fuels Oils 2015, 51, 133–139. [Google Scholar]
- Kayukova, G.P.; Mikhailova, A.N.; Kosachev, I.P.; Feoktistov, D.A.; Vakhin, A.V. Conversion of heavy oil with different chemical compositions under catalytic aquathermolysis with an amphiphilic Fe-Co-Cu catalyst and kaolin. Energy Fuels 2018, 32, 6488–6497. [Google Scholar]
- Fan, H.-F.; Liu, Y.-J.; Zhong, L.-G. Studies on the synergetic effects of mineral and steam on the composition changes of heavy oils. Energy Fuels 2001, 15, 1475–1479. [Google Scholar]
- Vakhin, A.V.; Onishchenko, Y.V.; Chemodanov, A.E.; Sitnov, S.A.; Mukhamatdinov, I.I.; Nazimov, N.A.; Sharifullin, A.V. The composition of aromatic destruction products of Domanic shale kerogen after aquathermolysis. Pet. Sci. Technol. 2019, 37, 390–395. [Google Scholar] [CrossRef]
- Zheng, R.; Pan, J.; Chen, L.; Tang, J.; Liu, D.; Song, Q.; Chen, L.; Yao, Q. Catalytic effects of montmorillonite on coke formation during thermal conversion of heavy oil. Energy Fuels 2018, 32, 6737–6745. [Google Scholar]
- Trujillo Portillo, M.L. Influence of rock matrix in kinetics oxidation for heavy oils. CT&F-Ciencia Tecnol. Futuro 2017, 7, 43–58. [Google Scholar]
- Armas, J.D.; Garcia-Vila, A.; Ortega, L.C.; Scott, C.E.; Maini, B.; Pereira-Almao, P. In-situ upgrading in a dolomite porous medium: A kinetic model comparison and nanocatalyst deposition study. J. Pet. Sci. Eng. 2021, 205, 108799. [Google Scholar]
- Tavakkoli Osgouei, Y.; Parlaktuna, M. Effects of minerals on steam distillation during thermal heavy-oil recovery: An experimental investigation. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 662–672. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Mukhamatdinov, I.I.; Feoktistov, D.A.; Onishchenko, Y.V.; Sudakov, V.A.; Amerkhanov, M.I.; Vakhin, A.V. Underground Upgrading of the Heavy Crude Oil in Content-Saturated Sandstone with Aquathermolysis in the Presence of an Iron Based Catalyst. Catalysts 2021, 11, 1255. [Google Scholar] [CrossRef]
- Sitnov, S.A.; Vakhin, A.V.; Mukhamatdinov, I.I.; Onishchenko, Y.V.; Feoktistov, D.A. Effects of calcite and dolomite on conversion of heavy oil under subcritical condition. Pet. Sci. Technol. 2019, 37, 687–693. [Google Scholar] [CrossRef]
- Dobrynkin, N.M.; Batygina, M.V.; Noskov, A.S. Catalytic Redox Transformations in Rock Matrices. Catal. Ind. 2018, 10, 91–96. [Google Scholar]
- Chen, Q.Y.; Liu, Y.J.; Zhao, J. Intensified viscosity reduction of heavy oil by using reservoir minerals and chemical agents in aquathermolysis. In Proceedings of the Advanced Materials Research, Nancy, France, 7–8 November 2011; Trans Tech Publications Ltd.: Freienbach, Switzerland, 2011; Volume 236, pp. 839–843. [Google Scholar]
- Zhang, X.; Ying-Cai, F.A.N.; Yong-Jian, L.I.U.; Hong-Chang, C.H.E. Effects of reservoir minerals and chemical agents on aquathermolysis of heavy oil during steam injection. China Pet. Process. Petrochem. Technol. 2010, 12, 25. [Google Scholar]
- Hyne, J.B.; Greidanus, J.W.; Tyrer, J.D.; Verona, D.; Clark, P.D.; Clarke, R.A.; Koo, J.H.F. The future of heavy crude and tar sands. In Proceedings of the Aquathermolysis of heavy oils, 2nd International Conference, Caracas, Venezuela, 7–17 February 1982; pp. 7–17. [Google Scholar]
- Belgrave, J.D.M.; Moore, R.G.; Ursenbach, M.G. Comprehensive kinetic models for the aquathermolysis of heavy oils. J. Can. Pet. Technol. 1997, 36, PETSOC-97-04-03. [Google Scholar] [CrossRef]
- Clark, P.D.; Hyne, J.B. Steam-oil chemical reactions: Mechanisms for the aquathermolysis of heavy oils. Aostra J. Res. 1984, 1, 15–20. [Google Scholar]
- Monin, J.C.; Audibert, A. Thermal cracking of heavy oil/mineral matrix systems. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 4 February 1987. [Google Scholar]
- Pahlavan, H.; Rafiqul, I. Laboratory simulation of geochemical changes of heavy crude oils during thermal oil recovery. J. Pet. Sci. Eng. 1995, 12, 219–231. [Google Scholar]
- Fan, H. The effects of reservoir minerals on the composition changes of heavy oil during steam stimulation. J. Can. Pet. Technol. 2003, 42, PETSOC-03-03-TN1. [Google Scholar] [CrossRef]
- Zhou, Z.; Slaný, M.; Kuzielová, E.; Zhanga, W.; Maa, L.; Donga, S.; Zhang, J.; Chen, G. Influence of reservoir minerals and ethanol on catalytic aquathermolysis of heavy oil. Fuel 2022, 307, 121871. [Google Scholar]
- Petrov, S.M.; Ibragimova, D.A.; Vakhin, A.V.; Zaidullin, I.M.; Valieva, G.R.; Zakirova, Z.R. Investigating the structure and composition of heavy oil under thermal-catalytic treatment in presence of carbonaceous minerals (Russian). Neftyanoe Khozyaystvo-Oil Ind. 2018, 2018, 44–47. [Google Scholar] [CrossRef]
- Kayukova, G.P.; Mikhailova, A.N.; Kosachev, I.P.; Nasyrova, Z.R.; Gareev, B.I.; Vakhin, A.V. Catalytic hydrothermal conversion of heavy oil in the porous media. Energy Fuels 2021, 35, 1297–1307. [Google Scholar] [CrossRef]
- Stennikov, A.V.; Bugaev, I.A.; Kalmykov, A.G.; Bychkov, A.Y.; Kozlova, E.V.; Kalmykov, G.A. An Experimental Study of the Oil Production from Domanik Formation Rocks Under Hydrothermal Conditions. Mosc. Univ. Geol. Bull. 2018, 73, 60–65. [Google Scholar] [CrossRef]
- Al-Otoom, A.Y.; Shawabkeh, R.A.; Al-Harahsheh, A.M.; Shawaqfeh, A.T. The chemistry of minerals obtained from the combustion of Jordanian oil shale. Energy 2005, 30, 611–619. [Google Scholar] [CrossRef]
- Kazakov, M.O.; Klimov, O.V.; Dik, P.P.; Shaverina, A.V.; Pereyma, V.Y.; Noskov, A.S. Hydroconversion of Oil Shale on Natural Mineral Matrices. Pet. Chem. 2017, 57, 1169–1172. [Google Scholar] [CrossRef]
- Dobrynkin, N.M.; Batygina, M.V.; Noskov, A.S. Studies of Catalytic Properties of Inorganic Rock Matrices in Redox Reactions. J. Sustain. Dev. Energy Water Environ. Syst. 2017, 5, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Vakhin, A.V.; Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Kudryashov, S.I.; Afanasiev, I.S.; Petrashov, O.V.; Nurgaliev, D.K. Extra-Heavy Oil Aquathermolysis Using Nickel-Based Catalyst: Some Aspects of In-Situ Transformation of Catalyst Precursor. Catalysts 2021, 11, 189. [Google Scholar] [CrossRef]
- Aliev, F.A.; Mukhamatdinov, I.I.; Sitnov, S.A.; Ziganshina, M.R.; Onishchenko, Y.V.; Sharifullin, A.V.; Vakhin, A.V. In-situ heavy oil aquathermolysis in the presence of nanodispersed catalysts based on transition metals. Processes 2021, 9, 127. [Google Scholar] [CrossRef]
- Aliev, F.A.; Kiekbaev, A.A.; Andreev, D.V.; Mitroshin, A.V.; Akkuzhin, A.A.; Sharifullin, A.V.; Vakhin, A.V. The Effect of Clay Minerals on Conversion of Yarega Heavy Oil During Catalytic Aquathermolysis Processes. SOCAR Proc. 2021, 2, 41–47. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).