Effect of Potassium Salts on Biochar Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biomass Preparation
2.3. Thermal Characterization
2.3.1. Design of Experiments
2.3.2. Kinetics Parameter
- α is conversion degree in the process (%) (dry basis)
- β is heating rate (K/min)
- T is temperature (K)
- A is frequency factor or pre-exponential factor (second−1)
- Ea is activation energy (kJ/mol)
- R is a gas constant (J/K·mol).
3. Results and Discussion
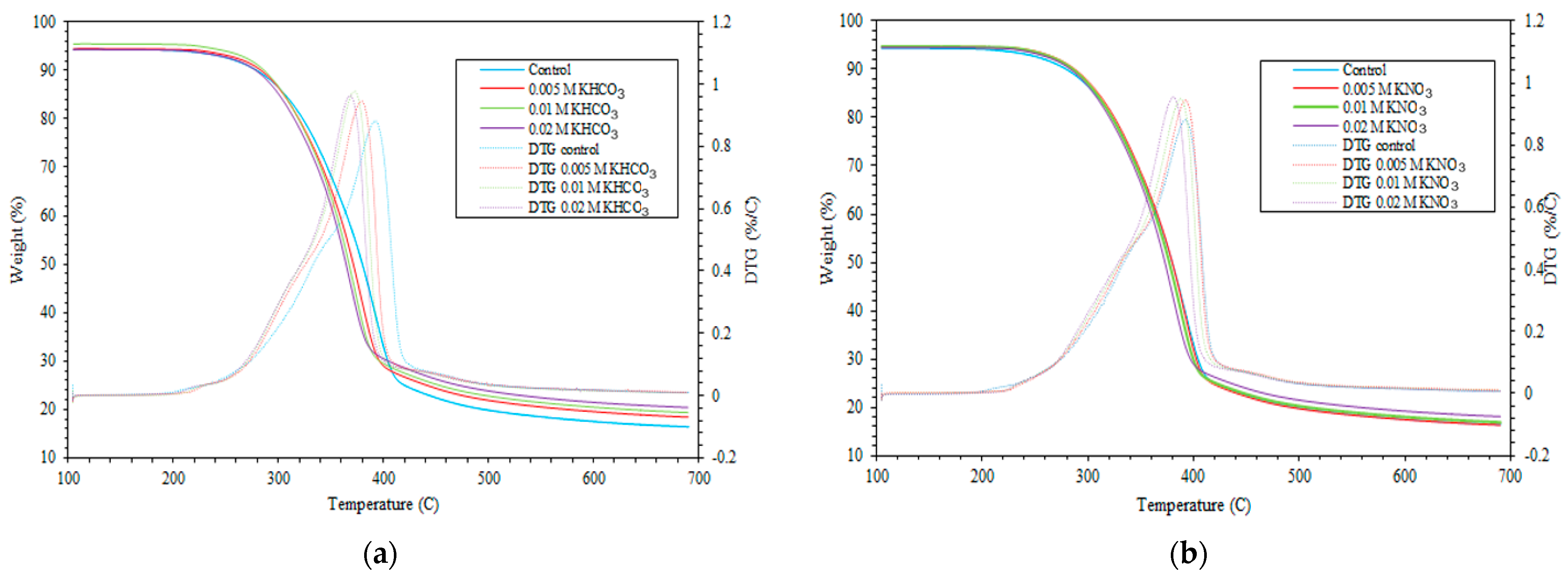
3.1. Char Production from Pine Wood
3.2. Char Production from Wheat Straw
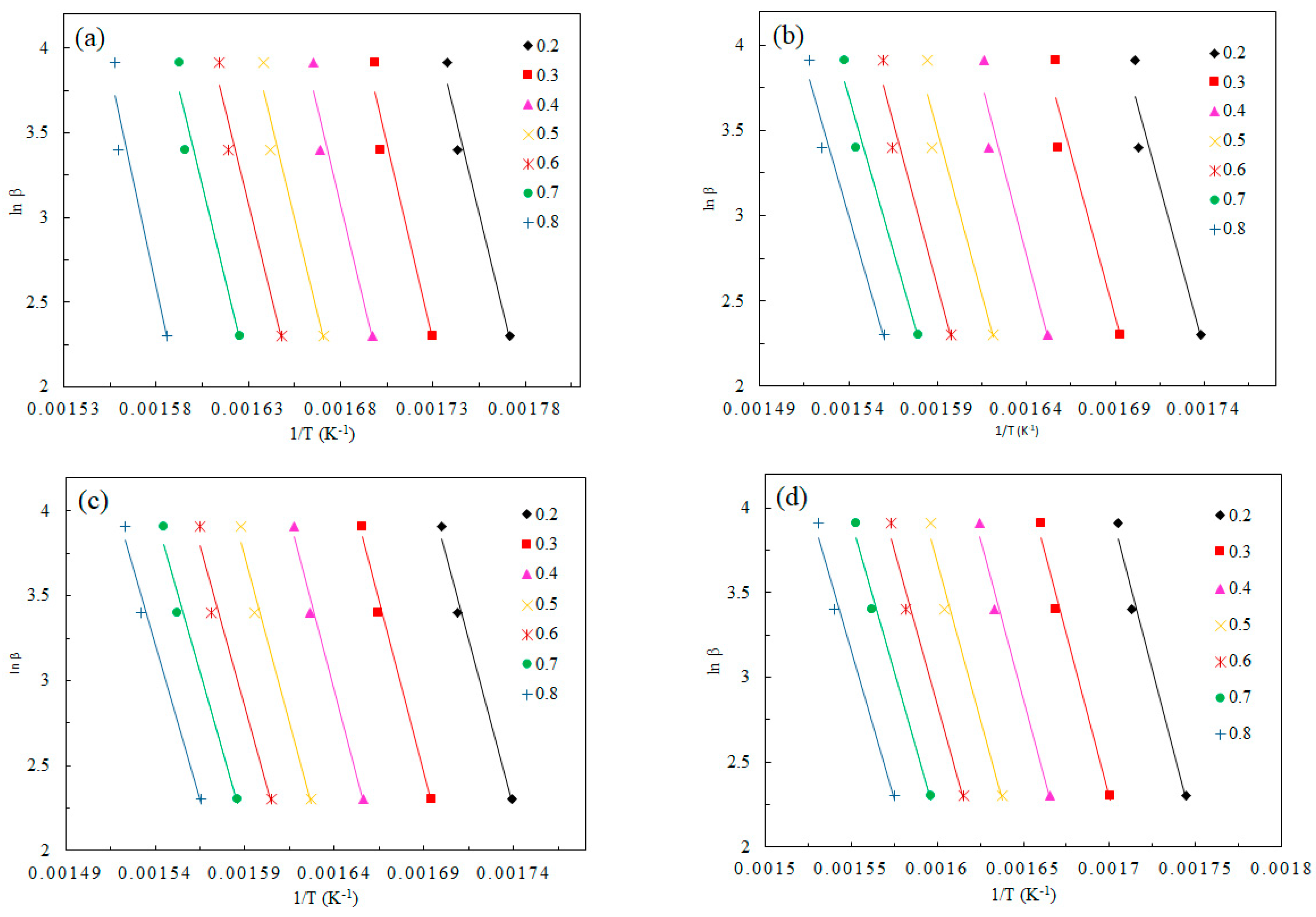
3.3. Kinetic Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Chen, M.Q.; Wang, J.; Zhang, M.X.; Chen, M.G.; Zhu, X.F.; Min, F.F.; Tan, Z.C. Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J. Anal. Appl. Pyrolysis 2008, 82, 145–150. [Google Scholar] [CrossRef]
- Misra, M.K.; Ragland, K.W.; Baker, A.J. Wood ash composition as a function of furnace temperature. Biomass Bioenergy 1993, 4, 103–116. [Google Scholar] [CrossRef]
- Dayton, D.C.; French, R.J.; Milne, T.A. Direct Observation of Alkali Vapor Release during Biomass Combustion and Gasification. 1. Application of Molecular Beam/Mass Spectrometry to Switchgrass Combustion. Energy Fuels 1995, 9, 855–865. [Google Scholar] [CrossRef]
- Davidsson, K.O.; Stojkova, B.J.; Pettersson, J.B.C. Alkali Emission from Birchwood Particles during Rapid Pyrolysis. Energy Fuels 2002, 16, 1033–1039. [Google Scholar] [CrossRef]
- Fagerström, J.; Steinvall, E.; Boström, D.; Boman, C. Alkali transformation during single pellet combustion of soft wood and wheat straw. Fuel Process. Technol. 2016, 143, 204–212. [Google Scholar] [CrossRef]
- Tchoffor, P.A.; Davidsson, K.O.; Thunman, H.; Tcho, P.A. Transformation and Release of Potassium, Chlorine, and Sulfur from Wheat Straw under Conditions Relevant to Dual Fluidized Bed Gasification. Energy Fuels 2013, 27, 7510–7520. [Google Scholar] [CrossRef]
- Jensen, P.A.; Frandsen, F.J.; Dam-Johansen, K.; Sander, B. Experimental Investigation of the Transformation and Release to Gas Phase of Potassium and Chlorine during Straw Pyrolysis. Energy Fuels 2000, 14, 1280–1285. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Jensen, P.A.; Dam-Johansen, K. Transformation and Release to the Gas Phase of Cl, K, and S during Combustion of Annual Biomass. Energy Fuels 2004, 18, 1385–1399. [Google Scholar] [CrossRef]
- Van Lith, S.C.; Alonso-Ramírez, V.; Jensen, P.A.; Frandsen, F.J.; Glarborg, P. Release to the Gas Phase of Inorganic Elements during Wood Combustion. Part 1: Development and Evaluation of Quantification Methods. Energy Fuels 2006, 20, 964–978. [Google Scholar] [CrossRef]
- Van Lith, S.C.; Jensen, P.A.; Frandsen, F.J.; Glarborg, P. Release to the Gas Phase of Inorganic Elements during Wood Combustion. Part 2: Influence of Fuel Composition. Energy Fuels 2008, 22, 1598–1609. [Google Scholar] [CrossRef]
- Johansen, J.M.; Jakobsen, J.G.; Frandsen, F.J.; Glarborg, P. Release of K, Cl, and S during Pyrolysis and Combustion of High-Chlorine Biomass. Energy Fuels 2011, 25, 4961–4971. [Google Scholar] [CrossRef]
- Dupont, C.; Jacob, S.; Marrakchy, K.O.; Hognon, C.; Grateau, M.; Labalette, F.; Da Silva Perez, D. How inorganic elements of biomass influence char steam gasification kinetics. Energy 2016, 109, 430–435. [Google Scholar] [CrossRef]
- Pereira, S.; Martins, P.C.R.; Costa, M. Kinetics of Poplar Short Rotation Coppice Obtained from Thermogravimetric and Drop Tube Furnace Experiments. Energy Fuels 2016, 30, 6525–6536. [Google Scholar] [CrossRef]
- Wagner, D.R.; Broström, M. Time-dependent variations of activation energy during rapid devolatilization of biomass. J. Anal. Appl. Pyrolysis 2016, 118, 98–104. [Google Scholar] [CrossRef]
- Mani, T.; Murugan, P.; Abedi, J.; Mahinpey, N. Pyrolysis of wheat straw in a thermogravimetric analyzer: Effect of particle size and heating rate on devolatilization and estimation of global kinetics. Chem. Eng. Res. Design 2010, 88, 952–958. [Google Scholar] [CrossRef]
- Safar, M.; Lin, B.J.; Chen, W.H.; Langauer, D.; Chang, J.S.; Raclavska, H.; Pétrissans, M. Catalytic effects of potassium on biomass pyrolysis, combustion and torrefaction. Appl. Energy 2019, 235, 346–355. [Google Scholar] [CrossRef]
- Mishra, G.; Kumar, J.; Bhaskar, T. Kinetic studies on the pyrolysis of pinewood. Bioresour. Technol. 2015, 182, 282–288. [Google Scholar] [CrossRef]
- Cotana, F.; Barbanera, M.; Foschini, D.; Lascaro, E.; Buratti, C. Preliminary optimization of alkaline pretreatment for ethanol production from vineyard pruning. Energy Procedia 2015, 82, 389–394. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Wang, X.; Qin, G.; Chen, M.; Wang, J. Kinetic Study of Pine Wood Pyrolysis Using Thermogravimetric Analysis. J. Biobased Mater. Bioenergy 2018, 12, 97–101. [Google Scholar] [CrossRef]
- Su, Y.; Luo, Y.; Wu, W.; Zhang, Y.; Zhao, S. Characteristics of pine wood oxidative pyrolysis: Degradation behavior, carbon oxide production and heat properties. J. Anal. Appl. Pyrolysis 2012, 98, 137–143. [Google Scholar] [CrossRef]
- Wu, K.; Liu, J.; Wu, Y.; Chen, Y.; Li, Q.; Xiao, X.; Yang, M. Pyrolysis characteristics and kinetics of aquatic biomass using thermogravimetric analyzer. Bioresour. Technol. 2014, 163, 18–25. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Zhou, L.; Jia, Y.; Nguyen, T.H.; Adesina, A.A.; Liu, Z. Hydropyrolysis characteristics and kinetics of potassium-impregnated pine wood. Fuel Processing Technol. 2013, 116, 149–157. [Google Scholar] [CrossRef]
- Jensen, A.; Dam-Johansen, K.; Wójtowicz, M.A.; Serio, M.A. TG-FTIR study of the influence of potassium chloride on wheat straw pyrolysis. Energy Fuels 1998, 12, 929–938. [Google Scholar] [CrossRef]
- Sun, Z.; Xu, B.; Rony, A.H.; Toan, S.; Chen, S.; Gasem, K.A.; Xiang, W. Thermogravimetric and kinetics investigation of pine wood pyrolysis catalyzed with alkali-treated CaO/ZSM-5. Energy Convers. Manag. 2017, 146, 182–194. [Google Scholar] [CrossRef]
- Müller-Hagedorn, M.; Bockhorn, H.; Krebs, L.; Müller, U. A comparative kinetic study on the pyrolysis of three different wood species. J. Anal. Appl. Pyrolysis 2003, 68, 231–249. [Google Scholar] [CrossRef]
- Carrier, M.; Auret, L.; Bridgwater, A.; Knoetze, J.H. Using apparent activation energy as a reactivity criterion for biomass pyrolysis. Energy Fuels 2016, 30, 7834–7841. [Google Scholar] [CrossRef]
- Rani, P.; Bansal, M.; Pathak, V.V. Experimental and kinetic studies for improvement of biogas production from KOH pretreated wheat straw. Curr. Res. Green Sustain. Chem. 2022, 5, 100283. [Google Scholar] [CrossRef]
- Mittal, V.; Sinha, S. Effect of alkali treatment on the thermal properties of wheat straw fiber reinforced epoxy composites. J. Compos. Mater. 2017, 51, 323–331. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, M.; Zhu, X.; Guo, D.; Liu, S.; Hu, Z.; Laghari, M. Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour. Technol. 2015, 192, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, P.; Narayanan, K.S.; Arockiam, L. Study on kinetic parameters of different biomass samples using thermo-gravimetric analysis. Biomass Bioenergy 2013, 58, 58–66. [Google Scholar] [CrossRef]
- Guo, F.; Liu, Y.; Wang, Y.; Li, X.; Li, T.; Guo, C. Pyrolysis kinetics and behavior of potassium-impregnated pine wood in TGA and a fixed-bed reactor. Energy Convers. Manag. 2016, 130, 184–191. [Google Scholar] [CrossRef]
- Hu, B.; Gu, Z.; Su, J.; Li, Z. Pyrolytic characteristics and kinetics of Guanzhong wheat straw and its components for high-value products. BioResources 2021, 16, 1958. [Google Scholar] [CrossRef]
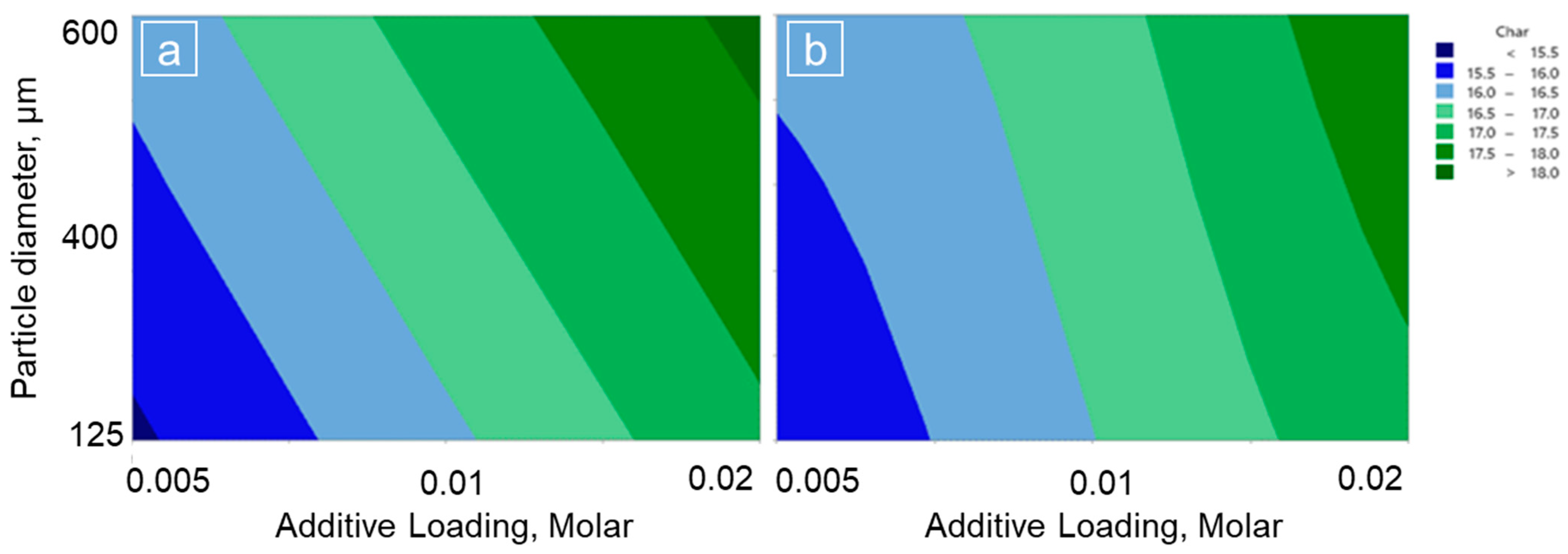
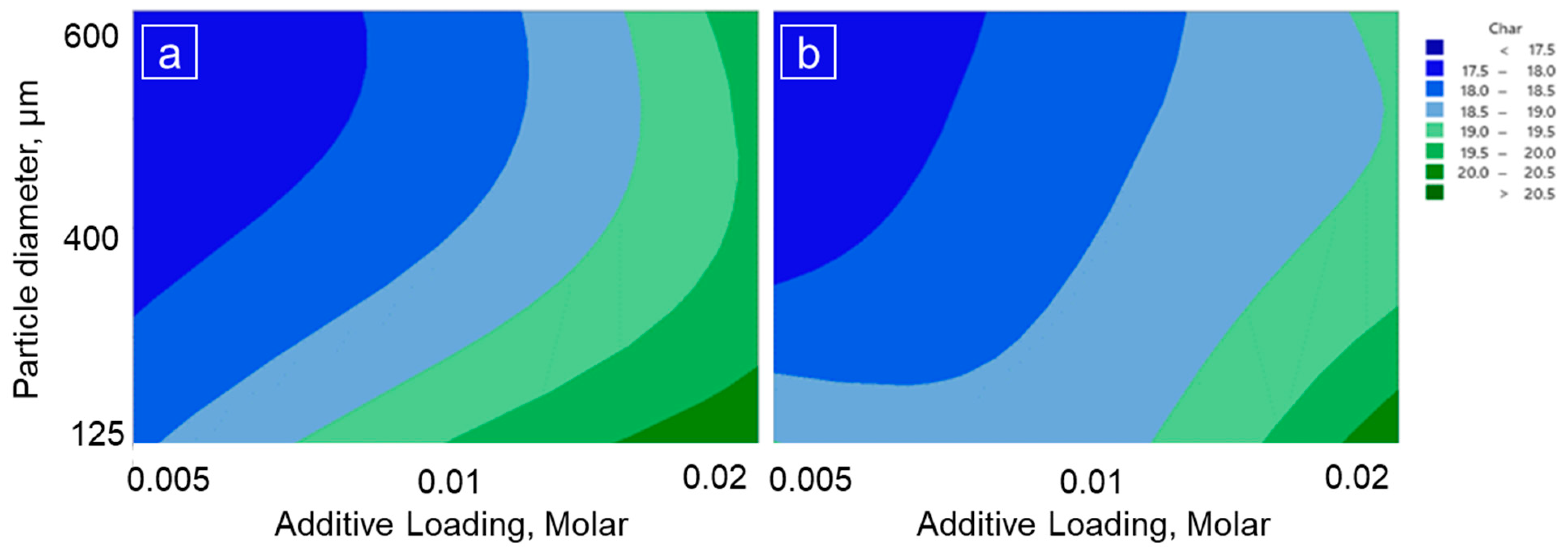






| Factor | Level 1 | Level 2 | Level 3 |
|---|---|---|---|
| Particle size ranges (μm) | 125–150 * | 400–425 | 600–630 |
| Heating rate (°C/min) | 10 | 30 | 50 |
| Additive concentration (M) | 0.005 | 0.01 | 0.02 |
| α | Control Pine | 0.005 K-Pine | 0.01 K-Pine | 0.02 K-Pine | ||||
|---|---|---|---|---|---|---|---|---|
| Ea | R2 | Ea | R2 | Ea | R2 | Ea | R2 | |
| Pine stem wood with KHCO3 | ||||||||
| 0.2 | 288.3 | 0.9775 | 307.9 | 0.9945 | 286.9 | 0.9815 | 282.5 | 0.9837 |
| 0.3 | 288.9 | 0.9649 | 294.8 | 0.9956 | 278.2 | 0.9713 | 288.9 | 0.9794 |
| 0.4 | 291.0 | 0.9729 | 281.8 | 0.9996 | 275.2 | 0.9828 | 274.9 | 0.9874 |
| 0.5 | 278.7 | 0.9821 | 273.0 | 0.9976 | 267.8 | 0.9720 | 265.9 | 0.9844 |
| 0.6 | 267.9 | 0.9831 | 264.9 | 0.9991 | 259.5 | 0.9858 | 261.4 | 0.9865 |
| 0.7 | 268.3 | 0.9798 | 255.8 | 0.9990 | 257.9 | 0.9845 | 262.8 | 0.9910 |
| 0.8 | 257.9 | 0.9880 | 259.5 | 0.9982 | 258.8 | 0.9853 | 271.5 | 0.9926 |
| Pine stem wood with KNO3 | ||||||||
| 0.2 | 288.3 | 0.9775 | 288.4 | 0.9618 | 293.0 | 0.9634 | 284.4 | 0.9611 |
| 0.3 | 288.9 | 0.9649 | 285.7 | 0.9697 | 288.1 | 0.9850 | 279.8 | 0.9579 |
| 0.4 | 291.0 | 0.9729 | 285.3 | 0.9606 | 281.7 | 0.9765 | 276.8 | 0.9693 |
| 0.5 | 278.7 | 0.9821 | 276.4 | 0.9673 | 267.8 | 0.9864 | 260.6 | 0.9759 |
| 0.6 | 267.9 | 0.9831 | 259.4 | 0.9762 | 265.8 | 0.9867 | 252.2 | 0.9822 |
| 0.7 | 268.3 | 0.9798 | 255.9 | 0.9789 | 261.8 | 0.9884 | 252.4 | 0.9745 |
| 0.8 | 257.9 | 0.9880 | 252.0 | 0.9748 | 253.6 | 0.9919 | 249.8 | 0.9814 |
| α | Control Wheat | 0.005 K-Wheat | 0.01 K-Wheat | 0.02 K-Wheat | ||||
|---|---|---|---|---|---|---|---|---|
| Ea | R2 | Ea | R2 | Ea | R2 | Ea | R2 | |
| Wheat straw with KHCO3 | ||||||||
| 0.2 | 350.3 | 0.9725 | 295.8 | 0.9404 | 262.4 | 0.9644 | 401.8 | 0.9312 |
| 0.3 | 365.8 | 0.9516 | 311.2 | 0.9234 | 272.8 | 0.9638 | 389.2 | 0.9139 |
| 0.4 | 365.7 | 0.9556 | 311.8 | 0.9282 | 272.2 | 0.9684 | 389.0 | 0.9419 |
| 0.5 | 355.0 | 0.9530 | 299.7 | 0.9543 | 262.3 | 0.9619 | 365.2 | 0.9388 |
| 0.6 | 345.8 | 0.9689 | 289.0 | 0.9723 | 249.7 | 0.9752 | 349.6 | 0.9699 |
| 0.7 | 349.1 | 0.9502 | 276.2 | 0.9861 | 241.9 | 0.9771 | 339.9 | 0.9745 |
| 0.8 | 398.1 | 0.9425 | 276.5 | 0.9829 | 239.6 | 0.9834 | 347.5 | 0.9775 |
| Wheat straw with KNO3 | ||||||||
| 0.2 | 350.3 | 0.9725 | 300.2 | 0.9303 | 314.7 | 0.9877 | 307.3 | 0.9832 |
| 0.3 | 365.8 | 0.9516 | 305.2 | 0.9237 | 322.1 | 0.9911 | 302.5 | 0.9855 |
| 0.4 | 365.7 | 0.9556 | 316.3 | 0.9398 | 321.5 | 0.9913 | 299.8 | 0.9879 |
| 0.5 | 355.0 | 0.9530 | 304.9 | 0.9374 | 310.2 | 0.9833 | 294.3 | 0.9831 |
| 0.6 | 345.8 | 0.9689 | 305.4 | 0.9619 | 301.0 | 0.9749 | 287.5 | 0.9831 |
| 0.7 | 349.1 | 0.9502 | 287.1 | 0.9708 | 294.9 | 0.9783 | 282.0 | 0.9854 |
| 0.8 | 398.1 | 0.9425 | 288.3 | 0.9755 | 289.7 | 0.9856 | 276.6 | 0.9853 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wongmat, Y.; Wagner, D.R. Effect of Potassium Salts on Biochar Pyrolysis. Energies 2022, 15, 5779. https://doi.org/10.3390/en15165779
Wongmat Y, Wagner DR. Effect of Potassium Salts on Biochar Pyrolysis. Energies. 2022; 15(16):5779. https://doi.org/10.3390/en15165779
Chicago/Turabian StyleWongmat, Yuthapong, and David R. Wagner. 2022. "Effect of Potassium Salts on Biochar Pyrolysis" Energies 15, no. 16: 5779. https://doi.org/10.3390/en15165779
APA StyleWongmat, Y., & Wagner, D. R. (2022). Effect of Potassium Salts on Biochar Pyrolysis. Energies, 15(16), 5779. https://doi.org/10.3390/en15165779






