Research Progress in Metal-Organic Framework Based Nanomaterials Applied in Battery Cathodes
Abstract
:1. Introduction
2. Lithium Batteries
3. Metal Ion Batteries
4. Metal-Air Batteries
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mechili, M.; Vaitsis, C.; Argirusis, N.; Pandis, P.K.; Sourkouni, G.; Argirusis, C. Research progress in transition metal oxide based bifunctional electrocatalysts for aqueous electrically rechargeable zinc-air batteries. Renew. Sustain. Energy Rev. 2022, 156, 111970. [Google Scholar] [CrossRef]
- Kwak, W.-J.; Rosy; Sharon, D.; Xia, C.; Kim, H.; Johnson, L.R.; Bruce, P.G.; Nazar, L.F.; Sun, Y.-K.; Frimer, A.A.; et al. Lithium–Oxygen Batteries and Related Systems: Potential, Status, and Future. Chem. Rev. 2020, 120, 6626–6683. [Google Scholar] [CrossRef]
- Wang, H.-F.; Xu, Q. Materials Design for Rechargeable Metal-Air Batteries. Matter 2019, 1, 565–595. [Google Scholar] [CrossRef]
- Gaines, L.L.; Dunn, J.B. 21-Lithium-Ion Battery Environmental Impacts. In Lithium-Ion Batteries; Pistoia, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 483–508. [Google Scholar]
- Arinicheva, Y.; Wolff, M.; Lobe, S.; Dellen, C.; Fattakhova-Rohlfing, D.; Guillon, O.; Böhm, D.; Zoller, F.; Schmuch, R.; Li, J.; et al. 10-Ceramics for electrochemical storage. In Advanced Ceramics for Energy Conversion and Storage; Guillon, O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 549–709. [Google Scholar]
- Vaitsis, C.; Kanellou, E.; Angelara, C.; Pandis, P.K.; Argirusis, N.; Sourkouni, G.; Zorpas, A.A.; Karantonis, A.; Argirusis, C. Chapter 18-MOFs–metal oxides/sulfides/phosphides nanocomposites for supercapacitors. In Metal-Organic Framework-Based Nanomaterials for Energy Conversion and Storage; Gupta, R.K., Nguyen, T.A., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 393–412. [Google Scholar]
- Vaitsis, C.; Sourkouni, G.; Argirusis, C. Metal Organic Frameworks (MOFs) and ultrasound: A review. Ultrason. Sonochem. 2019, 52, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Vaitsis, C.; Mechili, M.; Argirusis, N.; Kanellou, E.; Pandis, P.K.; Sourkouni, G.; Zorpas, A.; Argirusis, C. Ultrasound-Assisted Preparation Methods of Nanoparticles for Energy-Related Applications. In Nanotechnology and the Environment; IntechOpen: Rijeka, Croatia, 2020; pp. 77–103. [Google Scholar]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; El Gabaly, F.; Yoon, H.P.; et al. Tunable electrical conductivity in metal-organic framework thin-film devices. Science 2014, 343, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Giménez, V.; Tatay, S.; Martí-Gastaldo, C. Electrical conductivity and magnetic bistability in metal–organic frameworks and coordination polymers: Charge transport and spin crossover at the nanoscale. Chem. Soc. Rev. 2020, 49, 5601–5638. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhu, W.; Zhang, Z.; Wu, W.; Chen, R.; Mu, S.; Lv, H.; Cheng, N. Electronic tuning of confined sub-nanometer cobalt oxide clusters boosting oxygen catalysis and rechargeable Zn–air batteries. Nano Energy 2021, 83, 105813. [Google Scholar] [CrossRef]
- Wild, M.; O’Neill, L.; Zhang, T.; Purkayastha, R.; Minton, G.; Marinescu, M.; Offer, G.J. Lithium sulfur batteries, a mechanistic review. Energy Environ Sci. 2015, 8, 3477–3494. [Google Scholar] [CrossRef]
- Ren, W.; Ma, W.; Zhang, S.; Tang, B. Recent advances in shuttle effect inhibition for lithium sulfur batteries. Energy Storage Mater. 2019, 23, 707–732. [Google Scholar] [CrossRef]
- Deng, W.; Phung, J.; Li, G.; Wang, X. Realizing high-performance lithium-sulfur batteries via rational design and engineering strategies. Nano Energy 2021, 82, 105761. [Google Scholar] [CrossRef]
- Liu, T.; Hu, H.; Ding, X.; Yuan, H.; Jin, C.; Nai, J.; Liu, Y.; Wang, Y.; Wan, Y.; Tao, X. 12 years roadmap of the sulfur cathode for lithium sulfur batteries (2009–2020). Energy Storage Mater. 2020, 30, 346–366. [Google Scholar] [CrossRef]
- Eftekhari, A.; Ramanujam, B. In pursuit of catalytic cathodes for lithium–oxygen batteries. J. Mater. Chem. A 2017, 5, 7710–7731. [Google Scholar] [CrossRef]
- Vaitsis, C.; Mechili, M.; Argirusis, N.; Pandis, P.K.; Sourkouni, G.; Argirusis, C. Chapter 10-MOF nanomaterials for battery cathodes. In Metal-Organic Framework-Based Nanomaterials for Energy Conversion and Storage; Gupta, R.K., Nguyen, T.A., Yasin, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 207–226. [Google Scholar]
- Demir-Cakan, R.; Morcrette, M.; Nouar, F.; Davoisne, C.; Devic, T.; Gonbeau, D.; Dominko, R.; Serre, C.; Férey, G.; Tarascon, J.-M. Cathode Composites for Li–S Batteries via the Use of Oxygenated Porous Architectures. J. Am. Chem. Soc. 2011, 133, 16154–16160. [Google Scholar] [CrossRef]
- Hou, Y.; Mao, H.; Xu, L. MIL-100(V) and MIL-100(V)/rGO with various valence states of vanadium ions as sulfur cathode hosts for lithium-sulfur batteries. Nano Res. 2017, 10, 344–353. [Google Scholar] [CrossRef]
- Baumann, A.E.; Aversa, G.E.; Roy, A.; Falk, M.L.; Bedford, N.M.; Thoi, V.S. Promoting sulfur adsorption using surface Cu sites in metal–organic frameworks for lithium sulfur batteries. J. Mater. Chem. A 2018, 6, 4811–4821. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, X.; Fan, X.; Wang, X.; Li, H.; Zhang, Y.; Li, W.; Zheng, J.; Wang, B.; Li, X. Impact of the particle size of a metal-organic framework for sulfur storage in Li-S batteries. J. Mater. Chem. A 2015, 3, 8272–8275. [Google Scholar] [CrossRef]
- Geng, P.; Wang, L.; Du, M.; Bai, Y.; Li, W.; Liu, Y.; Chen, S.; Braunstein, P.; Xu, Q.; Pang, H. MIL-96-Al for Li–S Batteries: Shape or Size? Adv. Mater. 2022, 34, 2107836. [Google Scholar] [CrossRef]
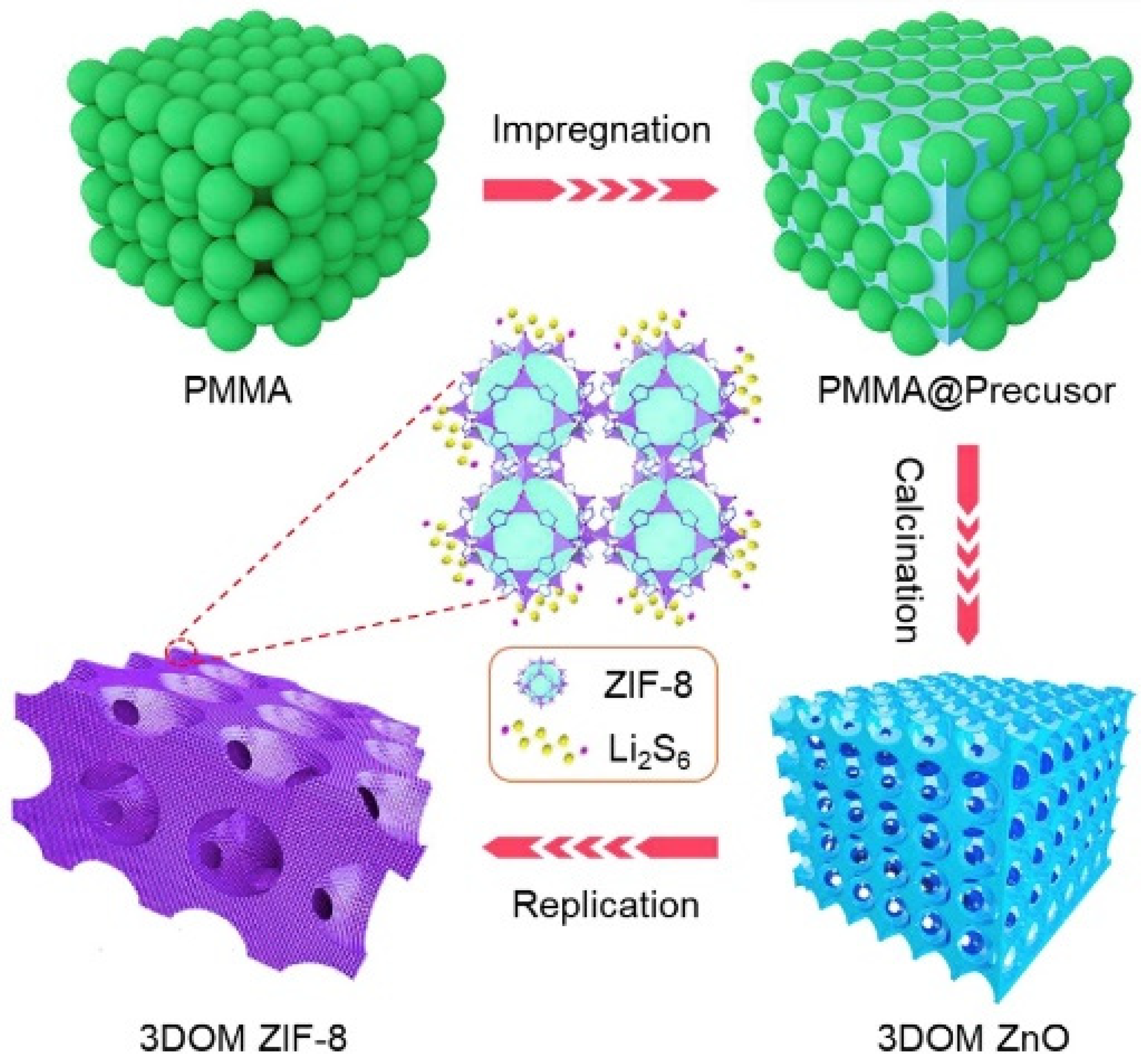
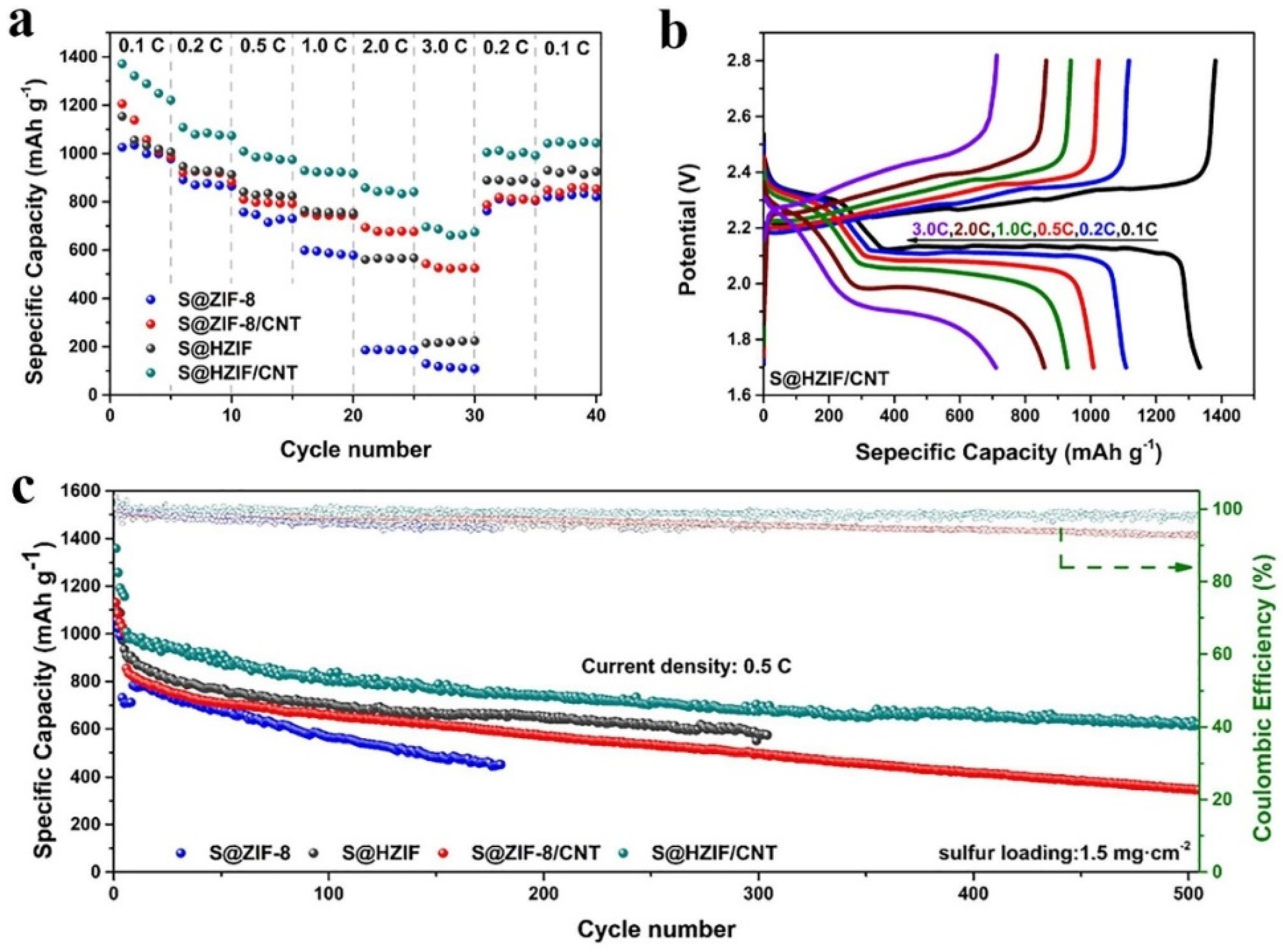
- Cui, G.; Li, G.; Luo, D.; Zhang, Y.; Zhao, Y.; Wang, D.; Wang, J.; Zhang, Z.; Wang, X.; Chen, Z. Three-dimensionally ordered macro-microporous metal organic frameworks with strong sulfur immobilization and catalyzation for high-performance lithium-sulfur batteries. Nano Energy 2020, 72, 104685. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Y.; Liu, X.; Cui, G.; Zhang, Y.; Wang, X. Three-dimensionally Ordered Macro-porous Metal-organic Framework for High-performance Lithium-sulfur Battery. ChemElectroChem 2022, 9, e202101099. [Google Scholar] [CrossRef]
- Liu, X.-W.; Sun, T.-J.; Hu, J.-L.; Wang, S.-D. Composites of metal–organic frameworks and carbon-based materials: Preparations, functionalities and applications. J. Mater. Chem. A 2016, 4, 3584–3616. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L.; Zhao, J.; Zheng, J.; Wang, J.; Huang, J. A facile in-situ synthesis of ZIF-8 nanoparticles anchored on reduced graphene oxide as a sulfur host for Li-S batteries. Mater. Res. Bull. 2021, 133, 111061. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, L.; Chen, S.; Zhu, X.; Deng, Q.; Wang, J.; Zeng, Z.; Deng, S. Facile and low-temperature strategy to prepare hollow ZIF-8/CNT polyhedrons as high-performance lithium-sulfur cathodes. Chem. Eng. J. 2021, 404, 126579. [Google Scholar] [CrossRef]
- Ge, X.; Li, C.; Li, Z.; Yin, L. Tannic acid tuned metal-organic framework as a high-efficiency chemical anchor of polysulfide for lithium-sulfur batteries. Electrochim. Acta 2018, 281, 700–709. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, Z.; Qu, Y.; Zhou, C.; Wang, X.; Li, J. Confine sulfur in mesoporous metal–organic framework @ reduced graphene oxide for lithium sulfur battery. J. Alloys Compd. 2014, 582, 334–340. [Google Scholar] [CrossRef]
- Xu, G.; Ding, B.; Shen, L.; Nie, P.; Han, J.; Zhang, X. Sulfur embedded in metal organic framework-derived hierarchically porous carbon nanoplates for high performance lithium–sulfur battery. J. Mater. Chem. A 2013, 1, 4490–4496. [Google Scholar] [CrossRef]
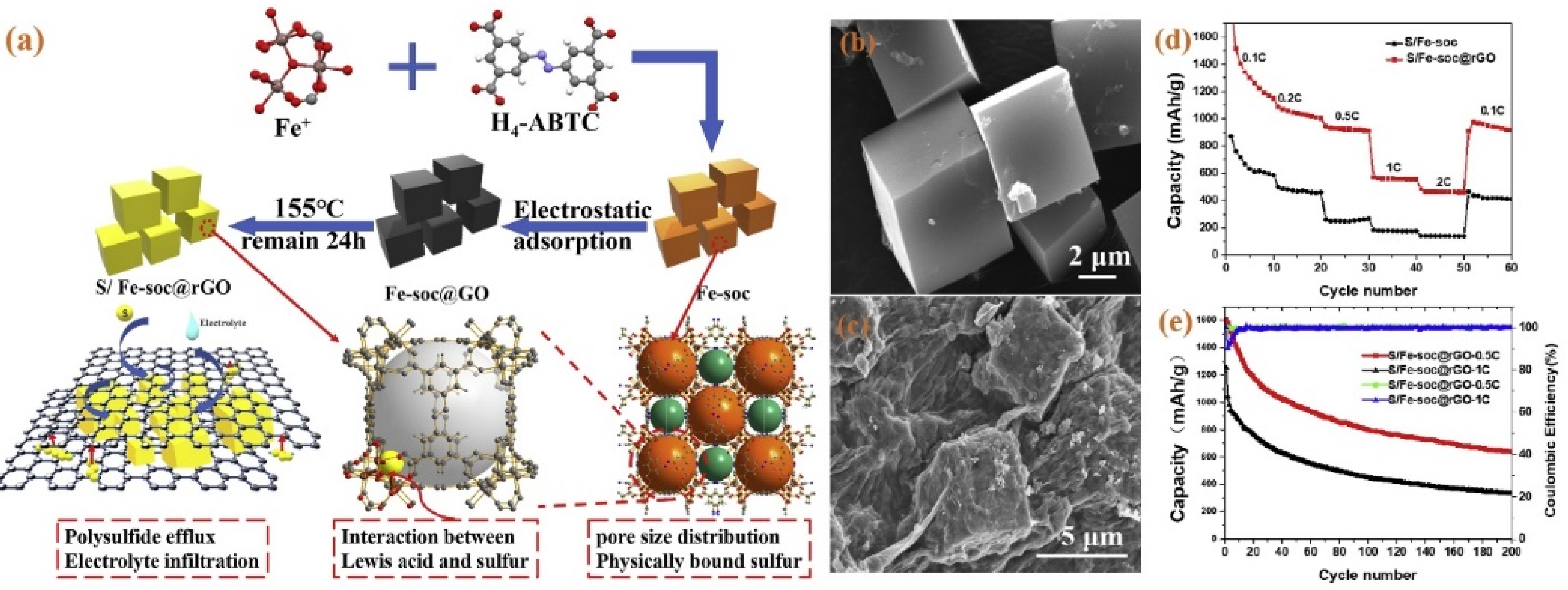
- Yao, J.; Zhang, M.; Han, G.; Wang, X.; Wang, Z.; Wang, J. Reduced graphene oxide coated Fe-soc as a cathode material for high-performance lithium-sulfur batteries. Ceram. Int. 2020, 46, 24155–24161. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Ge, X.; Ma, J.; Zhang, Z.; Li, Q.; Wang, C.; Yin, L. Reduced graphene oxide wrapped MOFs-derived cobalt-doped porous carbon polyhedrons as sulfur immobilizers as cathodes for high performance lithium sulfur batteries. Nano Energy 2016, 23, 15–26. [Google Scholar] [CrossRef]
- Xu, G.; Zuo, Y.; Huang, B. Metal-organic framework-74-Ni/carbon nanotube composite as sulfur host for high performance lithium-sulfur batteries. J. Electroanal. Chem. 2018, 830, 43–49. [Google Scholar] [CrossRef]
- Pu, Y.; Wu, W.; Liu, J.; Liu, T.; Ding, F.; Zhang, J.; Tang, Z. A defective MOF architecture threaded by interlaced carbon nanotubes for high-cycling lithium–sulfur batteries. RSC Adv. 2018, 8, 18604–18612. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.L.; Feng, D.; Liu, T.F.; Li, J.R.; Zhou, H.C. Pore surface engineering with controlled loadings of functional groups via click chemistry in highly stable metal-organic frameworks. J. Am. Chem. Soc. 2012, 134, 14690–14693. [Google Scholar] [CrossRef]
- Wu, H.; Chua, Y.S.; Krungleviciute, V.; Tyagi, M.; Chen, P.; Yildirim, T.; Zhou, W. Unusual and highly tunable missing-linker defects in zirconium metal-organic framework UiO-66 and their important effects on gas adsorption. J. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.; Slater, B. Dynamic acidity in defective UiO-66. Chem. Sci. 2016, 7, 4706–4712. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Li, Q.; Yu, H.; Hu, X.; Luo, Y.; Li, B. Three-dimensional ordered macroporous ZIF-8 nanoparticle-derived nitrogen-doped hierarchical porous carbons for high-performance lithium–sulfur batteries. RSC Adv. 2020, 10, 41983–41992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, M.; Xi, B.; Mi, K.; Yuan, A.; Xiong, S. Systematic Study of Effect on Enhancing Specific Capacity and Electrochemical Behaviors of Lithium-Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1701330. [Google Scholar] [CrossRef]
- Li, X.; Sun, Q.; Liu, J.; Xiao, B.; Li, R.; Sun, X. Tunable porous structure of metal organic framework derived carbon and the application in lithium–sulfur batteries. J. Power Sources 2016, 302, 174–179. [Google Scholar] [CrossRef]
- Li, Z.; Yin, L. Nitrogen-doped MOF-derived micropores carbon as immobilizer for small sulfur molecules as a cathode for lithium sulfur batteries with excellent electrochemical performance. ACS Appl. Mater. Interfaces 2015, 7, 4029–4038. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, H.; Tan, X.; Guo, L.; Zhang, J.; Liu, S.; Guo, Y.; Zhang, J.; Wang, H.; Chu, W. Monoclinic ZIF-8 Nanosheet-Derived 2D Carbon Nanosheets as Sulfur Immobilizer for High-Performance Lithium Sulfur Batteries. ACS Appl. Mater. Interfaces 2017, 9, 25239–25249. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.; Tian, T.; Cao, S.; Coxon, P.R.; Xi, K.; Fairen-Jimenez, D.; Vasant Kumar, R.; Cheetham, A.K. Graphene-wrapped sulfur/metal organic framework-derived microporous carbon composite for lithium sulfur batteries. APL Mater. 2014, 2, 124109. [Google Scholar] [CrossRef] [Green Version]
- Song, R.-S.; Wang, B.; Ruan, T.-T.; Wang, L.; Luo, H.; Wang, F.; Gao, T.-T.; Wang, D.-L. A three-dimensional cathode matrix with bi-confinement effect of polysulfide for lithium-sulfur battery. Appl. Surf. Sci. 2018, 427, 396–404. [Google Scholar] [CrossRef]
- Tan, Y.; Jia, Z.; Lou, P.; Cui, Z.; Guo, X. Self-assembly sandwiches of reduced graphene oxide layers with zeolitic-imidazolate-frameworks-derived mesoporous carbons as polysulfides reservoirs for lithium-sulfur batteries. J. Power Sources 2017, 341, 68–74. [Google Scholar] [CrossRef]
- Chen, K.; Sun, Z.; Fang, R.; Shi, Y.; Cheng, H.-M.; Li, F. Metal-Organic Frameworks (MOFs)-Derived Nitrogen-Doped Porous Carbon Anchored on Graphene with Multifunctional Effects for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1707592. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, P.; Zhang, Q.; Zeng, S.; Chen, S.; Zou, G.; Zou, J.; Zeng, X.; Li, X. Nitrogen-doped micropores binder-free carbon-sulphur composites as the cathode for long-life lithium-sulphur batteries. Mater. Lett. 2018, 231, 159–162. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.; Su, D.; Zhang, W.; Guo, X.; Wang, G. 3D Metal Carbide@Mesoporous Carbon Hybrid Architecture as a New Polysulfide Reservoir for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2016, 26, 8746–8756. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, Z.; Chen, W.; Zhou, C.; Lai, Y.; Li, J. Facile synthesis of graphene oxide @ mesoporous carbon hybrid nanocomposites for lithium sulfur battery. Electrochim. Acta 2014, 127, 342–348. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Xie, J.; Ge, H.; Hu, X. Multi-walled carbon nanotubes and metal-organic framework nanocomposites as novel hybrid electrode materials for the determination of nano-molar levels of lead in a lab-on-valve format. Analyst 2013, 138, 5113–5120. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Lin, N.; long Cai, W.; Guo, C.; Zhang, K.; Zhou, J.; Zhu, Y.; Qian, Y. Synthesis of S/CoS2 Nanoparticles-Embedded N-doped Carbon Polyhedrons from Polyhedrons ZIF-67 and their Properties in Lithium-Sulfur Batteries. Electrochim. Acta 2016, 218, 243–251. [Google Scholar] [CrossRef]
- Luo, S.; Zheng, C.; Sun, W.; Wang, Y.; Ke, J.; Guo, Q.; Liu, S.; Hong, X.; Li, Y.; Xie, W. Multi-functional CoS2-N-C porous carbon composite derived from metal-organic frameworks for high performance lithium-sulfur batteries. Electrochim. Acta 2018, 289, 94–103. [Google Scholar] [CrossRef]
- Gao, H.; Ning, S.; Zhou, Y.; Men, S.; Kang, X. Polyacrylonitrile-induced formation of core-shell carbon nanocages: Enhanced redox kinetics towards polysulfides by confined catalysis in Li-S batteries. Chem. Eng. J. 2021, 408, 127323. [Google Scholar] [CrossRef]
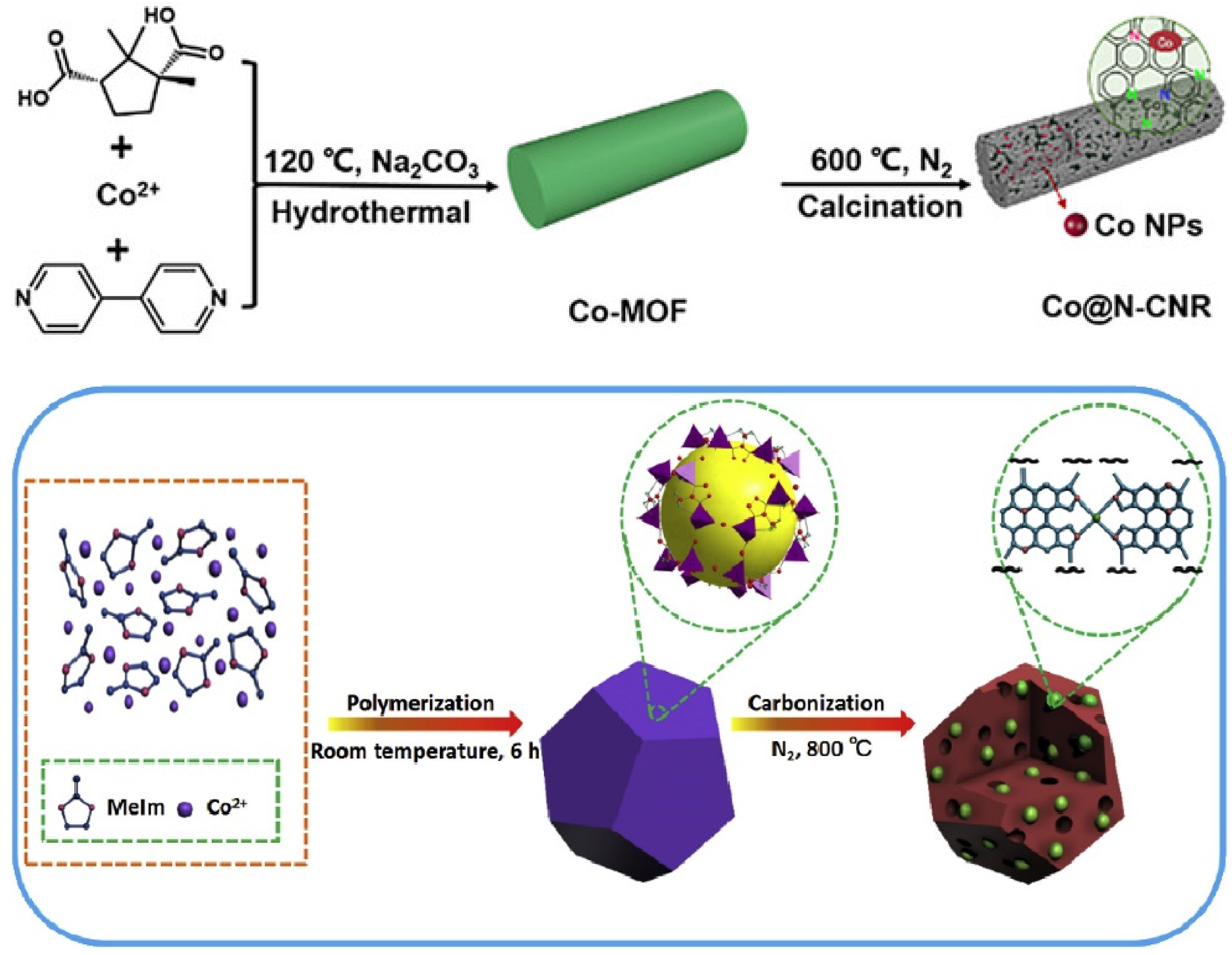
- He, J.; Chen, Y.; Lv, W.; Wen, K.; Xu, C.; Zhang, W.; Li, Y.; Qin, W.; He, W. From Metal-Organic Framework to Li2S@C-Co-N Nanoporous Architecture: A High-Capacity Cathode for Lithium-Sulfur Batteries. ACS Nano 2016, 10, 10981–10987. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Lu, X.; Li, X.; Jiang, N.; Huo, Y.; Zheng, Q.; Lin, D. Tailored multifunctional hybrid cathode substrate configured with carbon nanotube-modified polar Co(PO3)2/CoP nanoparticles embedded nitrogen-doped porous-shell carbon polyhedron for high-performance lithium-sulfur batteries. J. Colloid Interface Sci. 2020, 575, 220–230. [Google Scholar] [CrossRef]
- Li, M.; Feng, W.; Wang, X. The dual-play of carbon nanotube embedded with CoNi N codoped porous polyhedra toward superior Lithium–Sulfur batteries. J. Alloys Compd. 2021, 853, 157194. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, C.; Zhang, Y.; Huang, Y.; Liu, M.; Liu, T. Simultaneous growth of carbon nanotubes on inner/outer surfaces of porous polyhedra: Advanced sulfur hosts for lithium-sulfur batteries. Nano Res. 2018, 11, 6155–6166. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, H.; Cen, J.; Ali, A.; Chen, X.; Shen, P.K. High performance lithium-sulfur batteries based on CoP nanoparticle-embedded nitrogen-doped carbon nanotube hollow polyhedra. J. Electroanal. Chem. 2021, 885, 114996. [Google Scholar] [CrossRef]
- Xu, F.; Dong, C.; Jin, B.; Li, H.; Wen, Z.; Jiang, Q. MOF-derived LDH wrapped with rGO as an efficient sulfur host for lithium-sulfur batteries. J. Electroanal. Chem. 2020, 876, 114545. [Google Scholar] [CrossRef]
- Wan, T.; Liu, S.; Wu, C.; Tan, Z.; Lin, S.; Zhang, X.; Zhang, Z.; Liu, G. Rational design of Co nano-dots embedded three-dimensional graphene gel as multifunctional sulfur cathode for fast sulfur conversion kinetics. J. Energy Chem. 2021, 56, 132–140. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, S.-H.; Wu, Y.-J.; Su, J.-T.; Cheng, C.-C.; Cheng, P.-Y.; Ting, Y.-C.; Lu, S.-Y. MOF-derived cobalt Disulfide/Nitrogen-doped carbon composite polyhedrons linked with Multi-walled carbon nanotubes as sulfur hosts for Lithium-Sulfur batteries. Chem. Eng. J. 2022, 431, 133924. [Google Scholar] [CrossRef]
- Fan, L.; Wu, H.; Wu, X.; Wang, M.; Cheng, J.; Zhang, N.; Feng, Y.; Sun, K. Fe-MOF derived jujube pit like Fe3O4/C composite as sulfur host for lithium-sulfur battery. Electrochim. Acta 2019, 295, 444–451. [Google Scholar] [CrossRef]
- Liu, G.; Feng, K.; Cui, H.; Li, J.; Liu, Y.; Wang, M. MOF derived in-situ carbon-encapsulated Fe3O4@C to mediate polysulfides redox for ultrastable Lithium-sulfur batteries. Chem. Eng. J. 2020, 381, 122652. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Z.; Zhong, B.; Wang, H.; Xia, L.; Zhang, T.; Duan, X.; Jia, D.; Zhou, Y.; Huang, X. O-, N-Coordinated single Mn atoms accelerating polysulfides transformation in lithium-sulfur batteries. Energy Storage Mater. 2021, 35, 12–18. [Google Scholar] [CrossRef]
- Tian, H.; Tian, H.; Wang, S.; Chen, S.; Zhang, F.; Song, L.; Liu, H.; Liu, J.; Wang, G. High-power lithium–selenium batteries enabled by atomic cobalt electrocatalyst in hollow carbon cathode. Nat Commun 2020, 11, 5025. [Google Scholar] [CrossRef]
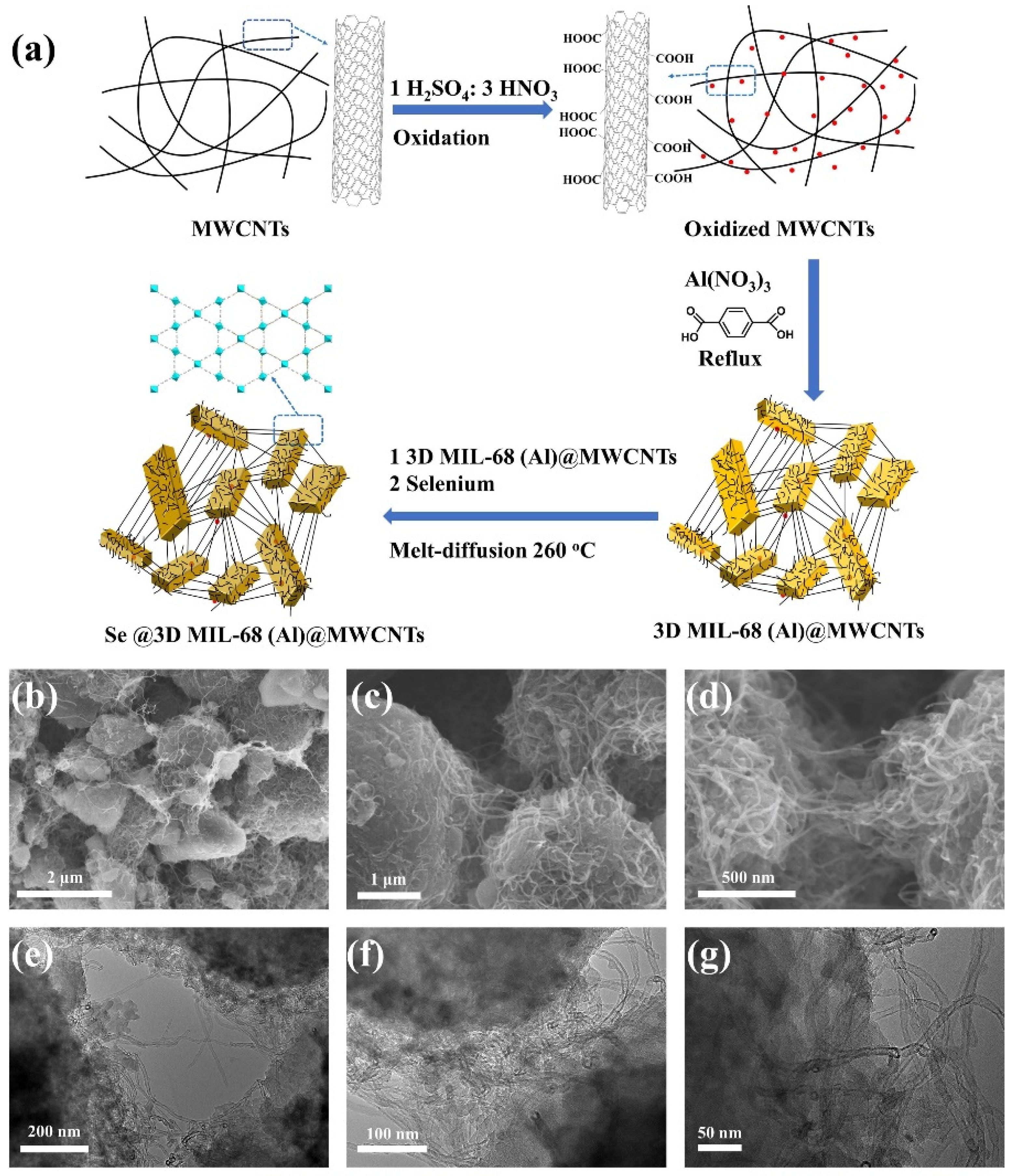
- Li, C.; Wang, Y.; Li, H.; Liu, J.; Song, J.; Fusaro, L.; Hu, Z.-Y.; Chen, Y.; Li, Y.; Su, B.-L. Weaving 3D highly conductive hierarchically interconnected nanoporous web by threading MOF crystals onto multi walled carbon nanotubes for high performance Li–Se battery. J. Energy Chem. 2021, 59, 396–404. [Google Scholar] [CrossRef]
- Liu, Y.; Si, L.; Zhou, X.; Liu, X.; Xu, Y.; Bao, J.; Dai, Z. A selenium-confined microporous carbon cathode for ultrastable lithium–selenium batteries. J. Mater. Chem. A 2014, 2, 17735–17739. [Google Scholar] [CrossRef]
- Lai, Y.; Yongqing, G.; Zhang, Z.; Chen, W.; Li, J. Metal-organic frameworks-derived mesoporous carbon for high performance lithium–selenium battery. Electrochim. Acta 2014, 146, 134–141. [Google Scholar] [CrossRef]
- Jin, W.-W.; Li, H.-J.; Zou, J.-Z.; Inguva, S.; Zhang, Q.; Zeng, S.-Z.; Xu, G.-Z.; Zeng, X.-R. Metal organic framework-derived carbon nanosheets with fish-scale surface morphology as cathode materials for lithium–selenium batteries. J. Alloys Compd. 2020, 820, 153084. [Google Scholar] [CrossRef]
- Park, S.-K.; Park, J.-S.; Kang, Y.C. Selenium-infiltrated metal–organic framework-derived porous carbon nanofibers comprising interconnected bimodal pores for Li–Se batteries with high capacity and rate performance. J. Mater. Chem. A 2018, 6, 1028–1036. [Google Scholar] [CrossRef]
- Park, S.K.; Park, J.S.; Kang, Y.C. Metal-Organic-Framework-Derived N-Doped Hierarchically Porous Carbon Polyhedrons Anchored on Crumpled Graphene Balls as Efficient Selenium Hosts for High-Performance Lithium-Selenium Batteries. ACS Appl. Mater. Interfaces 2018, 10, 16531–16540. [Google Scholar] [CrossRef]
- Liu, T.; Jia, M.; Zhang, Y.; Han, J.; Li, Y.; Bao, S.; Liu, D.; Jiang, J.; Xu, M. Confined selenium within metal-organic frameworks derived porous carbon microcubes as cathode for rechargeable lithium–selenium batteries. J. Power Sources 2017, 341, 53–59. [Google Scholar] [CrossRef]
- He, J.; Lv, W.; Chen, Y.; Xiong, J.; Wen, K.; Xu, C.; Zhang, W.; Li, Y.; Qin, W.; He, W. Three-dimensional hierarchical C-Co-N/Se derived from metal-organic framework as superior cathode for Li-Se batteries. J. Power Sources 2017, 363, 103–109. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Wang, J.; Song, H.; Yang, Y.; Liu, Y.; Li, J.; Wang, L.; Yu, C. Hollow Mesoporous Carbon Nanocubes: Rigid-Interface-Induced Outward Contraction of Metal-Organic Frameworks. Adv. Funct. Mater. 2017, 28, 1705253. [Google Scholar] [CrossRef]
- He, J.; Lv, W.; Chen, Y.; Xiong, J.; Wen, K.; Xu, C.; Zhang, W.; Li, Y.; Qin, W.; He, W. Direct impregnation of SeS2 into a MOF-derived 3D nanoporous Co–N–C architecture towards superior rechargeable lithium batteries. J. Mater. Chem. A 2018, 6, 10466–10473. [Google Scholar] [CrossRef]
- Cong, J.; Zheng, Z.; Hao, J.; You, H.; Liu, X.; Yang, H. Hierarchical cathode from hollow CoP embedded in graphene nanosheets to synergistically confine SeS2 for advanced Li/SeS2 batteries. J. Alloys Compd. 2021, 867, 159089. [Google Scholar] [CrossRef]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Liu, Q.; Su, X.; Lei, D.; Qin, Y.; Wen, J.; Guo, F.; Wu, Y.A.; Rong, Y.; Kou, R.; Xiao, X.; et al. Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping. Nat. Energy 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Lung-Hao Hu, B.; Wu, F.-Y.; Lin, C.-T.; Khlobystov, A.N.; Li, L.-J. Graphene-modified LiFePO4 cathode for lithium ion battery beyond theoretical capacity. Nat. Commun. 2013, 4, 1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Cheng, F.; Zhang, S.; Chen, J. Shape-controlled synthesis and lithium-storage study of metal-organic frameworks Zn4O(1,3,5-benzenetribenzoate)2. J. Power Sources 2006, 160, 542–547. [Google Scholar] [CrossRef]
- Ferey, G.; Millange, F.; Morcrette, M.; Serre, C.; Doublet, M.L.; Greneche, J.M.; Tarascon, J.M. Mixed-valence li/fe-based metal-organic frameworks with both reversible redox and sorption properties. Angew. Chem. 2007, 46, 3259–3263. [Google Scholar] [CrossRef]
- de Combarieu, G.; Morcrette, M.; Millange, F.; Guillou, N.; Cabana, J.; Grey, C.P.; Margiolaki, I.; Férey, G.; Tarascon, J.M. Influence of the Benzoquinone Sorption on the Structure and Electrochemical Performance of the MIL-53(Fe) Hybrid Porous Material in a Lithium-Ion Battery. Chem. Mater. 2009, 21, 1602–1611. [Google Scholar] [CrossRef]
- Fateeva, A.; Horcajada, P.; Devic, T.; Serre, C.; Marrot, J.; Grenèche, J.-M.; Morcrette, M.; Tarascon, J.-M.; Maurin, G.; Férey, G. Synthesis, Structure, Characterization, and Redox Properties of the Porous MIL-68(Fe) Solid. Eur. J. Inorg. Chem. 2010, 2010, 3789–3794. [Google Scholar] [CrossRef]
- Shin, J.; Kim, M.; Cirera, J.; Chen, S.; Halder, G.J.; Yersak, T.A.; Paesani, F.; Cohen, S.M.; Meng, Y.S. MIL-101(Fe) as a lithium-ion battery electrode material: A relaxation and intercalation mechanism during lithium insertion. J. Mater. Chem. A 2015, 3, 4738–4744. [Google Scholar] [CrossRef]
- Yamada, T.; Shiraishi, K.; Kitagawa, H.; Kimizuka, N. Applicability of MIL-101(Fe) as a cathode of lithium ion batteries. Chem. Commun. 2017, 53, 8215–8218. [Google Scholar] [CrossRef]
- Li, C.; Zhang, C.; Xie, J.; Wang, K.; Li, J.; Zhang, Q. Ferrocene-based metal-organic framework as a promising cathode in lithium-ion battery. Chem. Eng. J. 2021, 404, 126463. [Google Scholar] [CrossRef]
- Peng, Z.; Yi, X.; Liu, Z.; Shang, J.; Wang, D. Triphenylamine-Based Metal-Organic Frameworks as Cathode Materials in Lithium-Ion Batteries with Coexistence of Redox Active Sites, High Working Voltage, and High Rate Stability. ACS Appl. Mater. Interfaces 2016, 8, 14578–14585. [Google Scholar] [CrossRef] [PubMed]
- Nagatomi, H.; Yanai, N.; Yamada, T.; Shiraishi, K.; Kimizuka, N. Synthesis and Electric Properties of a Two-Dimensional Metal-Organic Framework Based on Phthalocyanine. Chemistry 2018, 24, 1806–1810. [Google Scholar] [CrossRef] [PubMed]
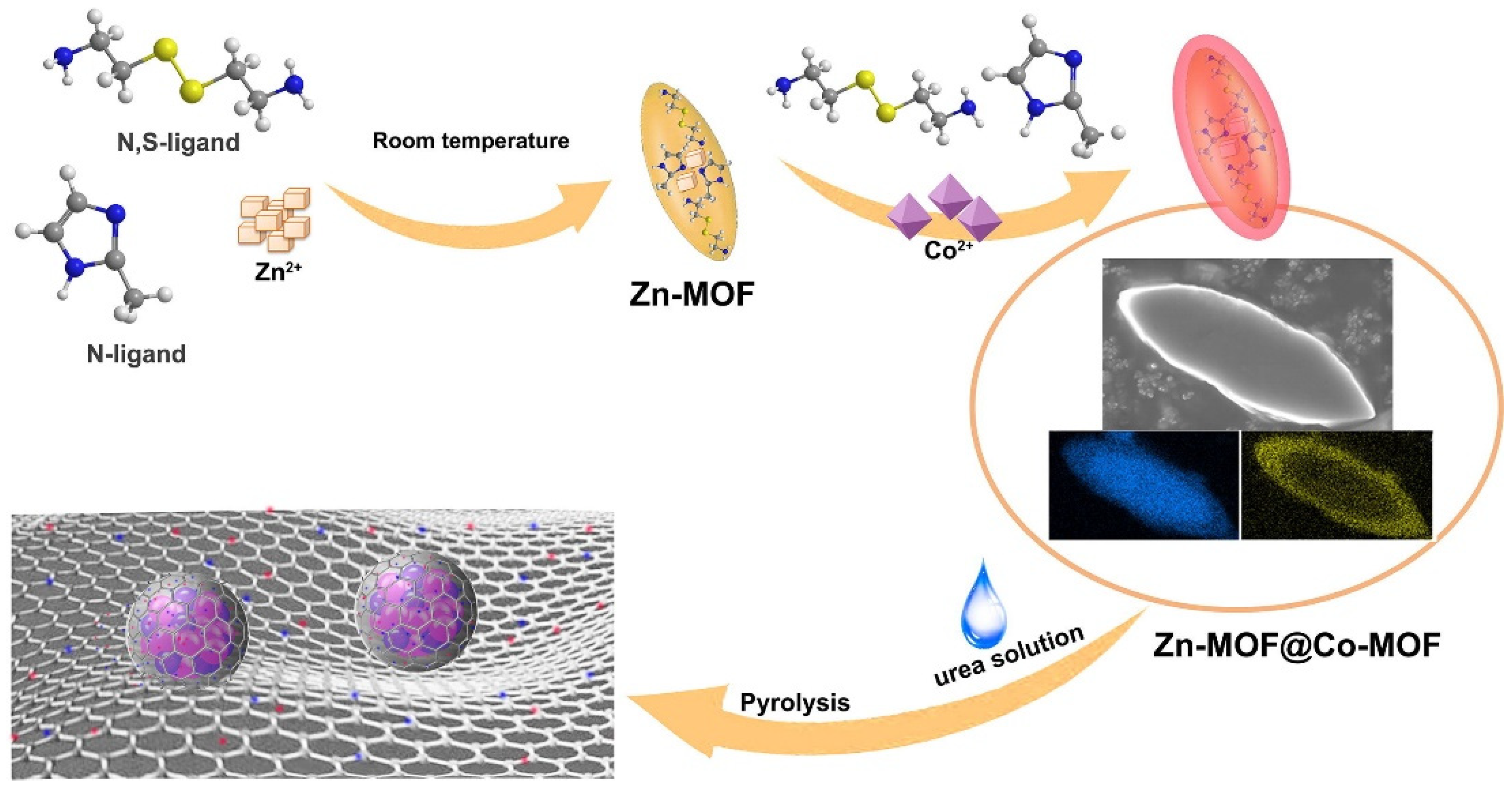
- Sun, P.-P.; Zhang, Y.-H.; Shi, H.; Shi, F.-N. Study on the properties of Cu powder modified 3-D Co-MOF in electrode materials of lithium ion batteries. J. Solid State Chem. 2022, 307, 122740. [Google Scholar] [CrossRef]
- Zheng, W.; Hu, T.; Fang, Y.; Li, L.; Yuan, W. High capacity of microspheric manganese and cobalt trimesic dual-metal organic framework for Li-ion battery. J. Solid State Chem. 2022, 306, 122719. [Google Scholar] [CrossRef]
- He, B.; Zhang, Q.; Man, P.; Zhou, Z.; Li, C.; Li, Q.; Xie, L.; Wang, X.; Pang, H.; Yao, Y. Self-sacrificed synthesis of conductive vanadium-based Metal–Organic framework nanowire-bundle arrays as binder-free cathodes for high-rate and high-energy-density wearable Zn-Ion batteries. Nano Energy 2019, 64, 103935. [Google Scholar] [CrossRef]
- Xu, J.; Lin, F.; Doeff, M.M.; Tong, W. A review of Ni-based layered oxides for rechargeable Li-ion batteries. J. Mater. Chem. A 2017, 5, 874–901. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Li, G.; Zhang, D.; Fan, J.; Chen, D.; Liu, X.; Feng, T.; Li, L. Zeolitic imidazolate framework-8 modified LiNi1/3Co1/3Mn1/3O2: A durable cathode showing excellent electrochemical performances in Li-ion batteries. Electrochim. Acta 2020, 336, 135724. [Google Scholar] [CrossRef]
- Liang, S.; Hu, J.; Zhang, Y.; Wang, Y.; Cao, X.; Pan, A. Facile synthesis of sandwich-structured Li3V2(PO4)3/carbon composite as cathodes for high performance lithium-ion batteries. J. Alloys Compd. 2016, 683, 178–185. [Google Scholar] [CrossRef]
- Liao, Y.; Li, C.; Lou, X.; Hu, X.; Ning, Y.; Yuan, F.; Chen, B.; Shen, M.; Hu, B. Carbon-coated Li3V2(PO4)3 derived from metal-organic framework as cathode for lithium-ion batteries with high stability. Electrochim. Acta 2018, 271, 608–616. [Google Scholar] [CrossRef]
- Wang, Z.; He, W.; Zhang, X.; Yue, Y.; Liu, J.; Zhang, C.; Fang, L. Multilevel structures of Li3V2(PO4)3/phosphorus-doped carbon nanocomposites derived from hybrid V-MOFs for long-life and cheap lithium ion battery cathodes. J. Power Sources 2017, 366, 9–17. [Google Scholar] [CrossRef]
- Kim, T.; Kim, H.; You, T.-S.; Kim, J. Carbon-coated V2O5 nanoparticles derived from metal-organic frameworks as a cathode material for rechargeable lithium-ion batteries. J. Alloys Compd. 2017, 727, 522–530. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, A.; Wang, Y.; Wei, W.; Su, Y.; Hu, J.; Cao, G.; Liang, S. Dodecahedron-Shaped Porous Vanadium Oxide and Carbon Composite for High-Rate Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 17303–17311. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, S.; Lin, Z.; Yang, W.; Zou, H.; Sun, R.W.-Y. Enhanced electrochemical performance of Li-rich layered oxide, Li1.2Mn0.54Co0.13Ni0.13O2, by surface modification derived from a MOF-assisted treatment. Electrochem. Commun. 2019, 99, 65–70. [Google Scholar] [CrossRef]
- Xiao, Z.; Meng, J.; Li, Q.; Wang, X.; Huang, M.; Liu, Z.; Han, C.; Mai, L. Novel MOF shell-derived surface modification of Li-rich layered oxide cathode for enhanced lithium storage. Sci. Bull. 2018, 63, 46–53. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Yu, J.; Xing, C.; Ullah, Z.; Yu, C.; Zhu, S.; Chen, M.; Li, W.; Li, Q.; Liu, L. Nanopore confined anthraquinone in MOF-derived N-doped microporous carbon as stable organic cathode for lithium-ion battery. Energy Storage Mater. 2019, 22, 433–440. [Google Scholar] [CrossRef]
- Zhao, L.; Guan, Z.; Ullah, Z.; Yu, C.; Song, H.; Chu, R.; Zhang, Y.; Li, W.; Li, Q.; Liu, L. Significantly stable organic cathode for lithium-ion battery based on nanoconfined poly(anthraquinonyl sulfide)@MOF-derived microporous carbon. Electrochim. Acta 2020, 335, 135681. [Google Scholar] [CrossRef]
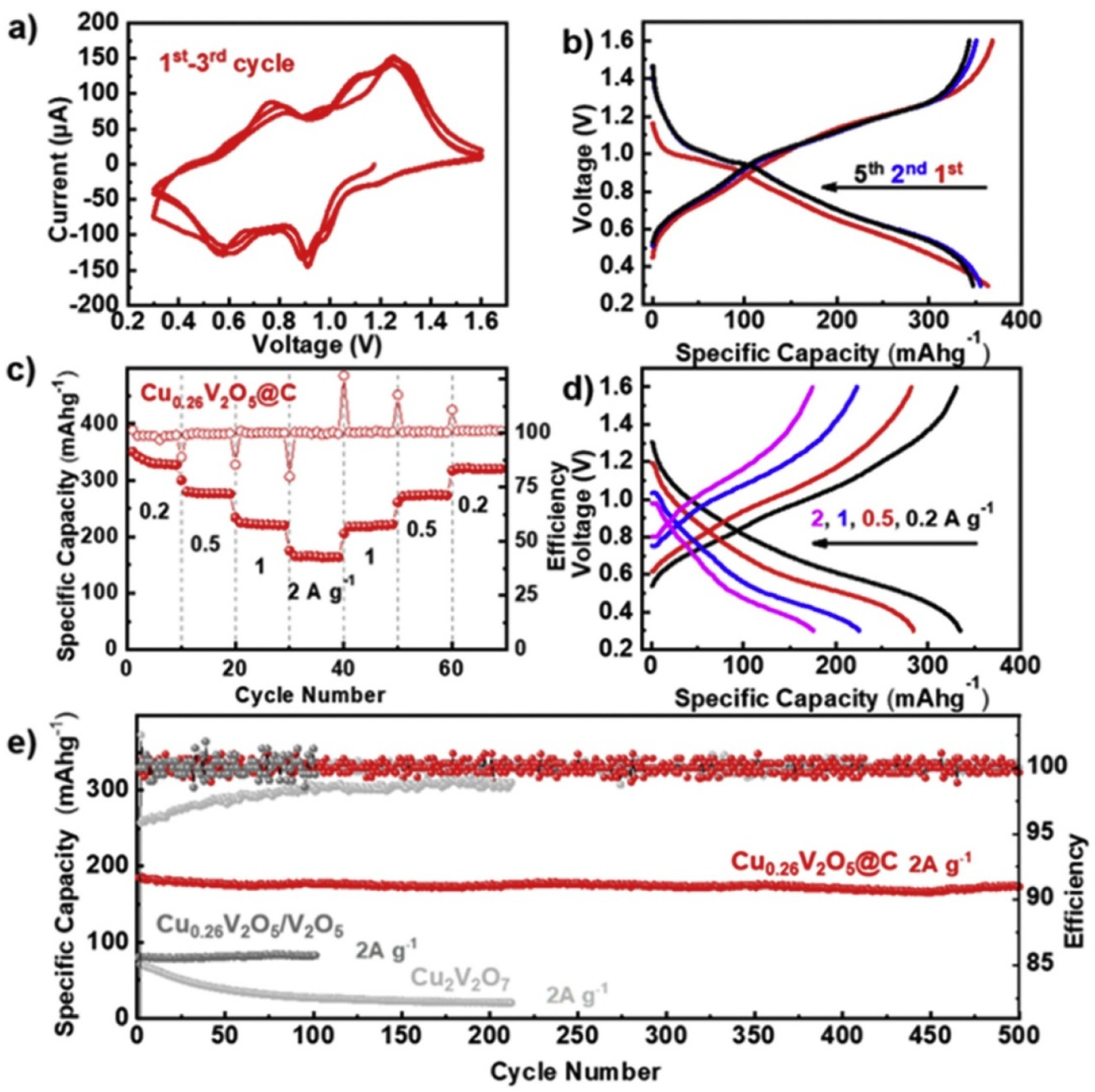
- Wang, X.; Zhang, B.; Feng, J.; Wang, L.; Wu, B.; Zhang, J.; Ou, X.; Hou, F.; Liang, J. Cu-MOF-derived and porous Cu0.26V2O5@C composite cathode for aqueous zinc-ion batteries. Sustain. Mater. Technol. 2020, 26, e00236. [Google Scholar] [CrossRef]
- Huo, Y.; Teng, Y.; Cai, K.; Chen, H. Honeycomb ZnO/N/C obtained from cornsilk and ZIF-8 dual induced method for long-life aqueous zinc-ion batteries. J. Alloys Compd. 2021, 855, 157398. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Zhang, G.; Wang, X.; Zhang, J.; Hu, Y.; Wang, D.; Zuin, L.; Zhou, T.; Wu, Y.; et al. High-Performance Reversible Aqueous Zn-Ion Battery Based on Porous MnOxNanorods Coated by MOF-Derived N-Doped Carbon. Adv. Energy Mater. 2018, 8, 1801445. [Google Scholar] [CrossRef]
- Jia, D.; Yang, Z.; Gu, W.; Liu, X. Manganese-based metal-organic-framework derived hydrophilic cathode with carbon nanotubes introduced for long-life and high-performance aqueous zinc-ion battery. J. Alloys Compd. 2022, 910, 164876. [Google Scholar] [CrossRef]
- Chu, R.; Song, H.; Ullah, Z.; Guan, Z.; Zhang, Y.; Zhao, L.; Chen, M.; Li, W.; Li, Q.; Liu, L. ZIF-8 derived nitrogen-doped carbon composites boost the rate performance of organic cathodes for sodium ion batteries. Electrochim. Acta 2020, 362, 137115. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, N.; Zhang, Y.; Xue, P.; Guo, M.; Tang, B.; Xu, X.; Wang, W.; Bai, Z.; Dou, S. Metal–Organic Framework-Derived Sea-Cucumber-like FeS2@C Nanorods with Outstanding Pseudocapacitive Na-Ion Storage Properties. ACS Appl. Energy Mater. 2018, 1, 6234–6241. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-S.; Tai Kim, S.; Cao, R.; Choi, N.-S.; Liu, M.; Lee, K.T.; Cho, J. Metal–Air Batteries with High Energy Density: Li–Air versus Zn–Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Yan, X.; Ha, Y.; Wu, R. Binder-Free Air Electrodes for Rechargeable Zinc-Air Batteries: Recent Progress and Future Perspectives. Small Methods 2021, 5, 2000827. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, Z.; Hu, N.; Xu, C.; Shen, Z.; Liu, J. Nanocarbon-Based Electrocatalysts for Rechargeable Aqueous Li/Zn-Air Batteries. ChemElectroChem 2018, 5, 1745–1763. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, J.; Cheng, C.; Tan, P.; Ni, M. Mini-review of perovskite oxides as oxygen electrocatalysts for rechargeable zinc–air batteries. Chem. Eng. J. 2020, 397, 125516. [Google Scholar] [CrossRef]
- Xu, L.; Wu, S.; Deng, D.; Wang, C.; Qian, J.; Lu, G.; Li, H. Fabricating highly active and stable tungsten carbide electrocatalyst for rechargeable zinc–air batteries: An approach of dual metal Co-adjusted the electronic structure. J. Alloys Compd. 2021, 868, 159236. [Google Scholar] [CrossRef]
- Li, J.; Wan, T.; Li, J.; Zhang, Z.; Wang, Y.; Liu, G. Three-dimensionally ordered mesoporous trimetal sulfide as efficient electrocatalyst for rechargeable zinc-air batteries. Appl. Surf. Sci. 2022, 575, 151728. [Google Scholar] [CrossRef]
- Vayenas, M.; Vaitsis, C.; Sourkouni, G.; Pandis, P.K.; Argirusis, C. Investigation of alternative materials as bifunctional catalysts for electrochemical applications. Chim. Techno Acta 2019, 6, 120–129. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Guo, Z.; Yin, X.; Pang, Q.; Tu, B.; Zhang, L.; Wang, Y.G.; Li, Q. Metal-organic frameworks as cathode materials for Li-O2 batteries. Adv. Mater. 2014, 26, 3258–3262. [Google Scholar] [CrossRef]
- Yan, W.; Guo, Z.; Xu, H.; Lou, Y.; Chen, J.; Li, Q. Downsizing metal–organic frameworks with distinct morphologies as cathode materials for high-capacity Li–O2 batteries. Mater. Chem. Front. 2017, 1, 1324–1330. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, Y.J.; Kim, D.H.; Lee, Y.J. Bimetallic Metal-Organic Frameworks as Efficient Cathode Catalysts for Li-O2 Batteries. ACS Appl. Mater. Interfaces 2018, 10, 660–667. [Google Scholar] [CrossRef]
- Shin, S.; Yoon, Y.; Shin, M.W. Co/Zn-based bimetallic MOF-derived hierarchical porous Co/C composite as cathode material for high-performance lithium-air batteries. Int. J. Energy Res. 2022, 46, 9900–9910. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, P.; Lee, J.-I.; Gray, J.T.; Cha, Y.-H.; Ha, S.; Song, M.-K. Enhanced cycling performance of rechargeable Li–O2 batteries via LiOH formation and decomposition using high-performance MOF-74@CNTs hybrid catalysts. Energy Storage Mater. 2019, 17, 167–177. [Google Scholar] [CrossRef]
- Shin, S.; Yoon, H.; Yoon, Y.; Park, S.; Shin, M.W. Porosity tailoring of the Zn-MOF-5 derived carbon materials and its effects on the performance as a cathode for lithium-air batteries. Microporous Mesoporous Mater. 2021, 311, 110726. [Google Scholar] [CrossRef]
- Ge, Q.; Chen, M.; Lou, X.; Zhang, W.; Shen, M.; Yang, Q.; Hu, B. Centrifugal Field Guided Dual Templating Synthesis of Functional Macro-Microporous Carbon. Part. Part. Syst. Charact. 2018, 35, 1800262. [Google Scholar] [CrossRef]
- Chatterjee, A.; Or, S.W. Metal–organic framework-derived MnO/CoMn2O4@N–C nanorods with nanoparticle interstitial decoration in core@shell structure as improved bifunctional electrocatalytic cathodes for Li–O2 batteries. Electrochim. Acta 2020, 338, 135809. [Google Scholar] [CrossRef]
- Zhang, P.; Li, J.; Feng, J.; Wang, Y.; Xu, A.; Chen, T.; Zhao, L.; Dang, F.; Zhang, X.; Wang, H. MOF-template derived hollow CeO2/Co3O4 polyhedrons with efficient cathode catalytic capability in Li-O2 batteries. Chin. Chem. Lett. 2021, in press. [CrossRef]
- Tan, G.; Chong, L.; Amine, R.; Lu, J.; Liu, C.; Yuan, Y.; Wen, J.; He, K.; Bi, X.; Guo, Y.; et al. Toward Highly Efficient Electrocatalyst for Li-O2 Batteries Using Biphasic N-Doping Cobalt@Graphene Multiple-Capsule Heterostructures. Nano Lett. 2017, 17, 2959–2966. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wu, S.; Wang, T.; Gong, H.; Zhang, H.; Alshehri, S.M.; Ahamad, T.; Zhou, H.; Yamauchi, Y. Cage-Type Highly Graphitic Porous Carbon-Co3O4 Polyhedron as the Cathode of Lithium-Oxygen Batteries. ACS Appl. Mater. Interfaces 2016, 8, 2796–2804. [Google Scholar] [CrossRef]
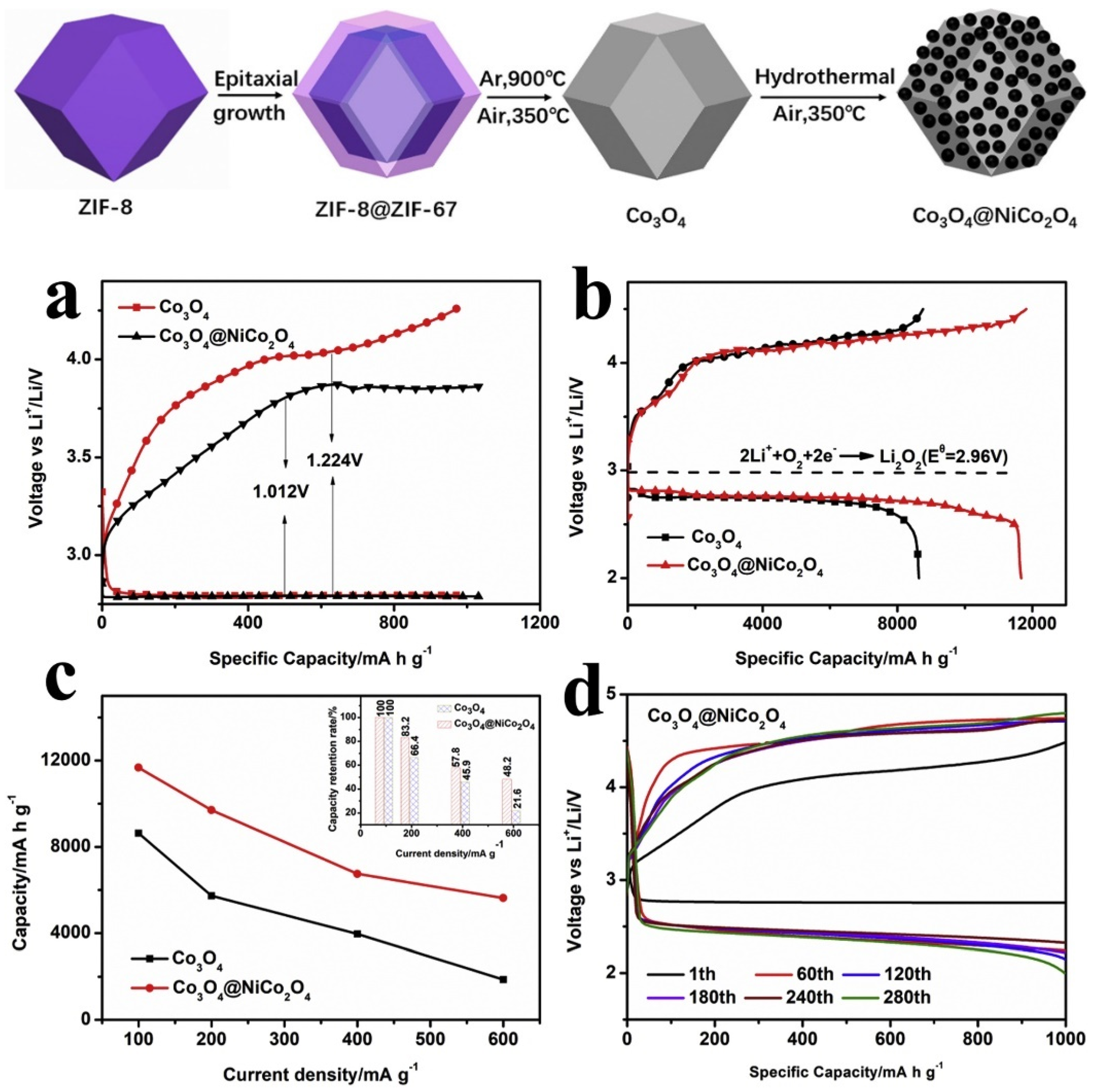
- Zhao, Y.; Ding, L.; Wang, X.; Yang, X.; He, J.; Yang, B.; Wang, B.; Zhang, D.; Li, Z. Yolk-shell ZIF-8@ZIF-67 derived Co3O4@NiCo2O4 catalysts with effective electrochemical properties for Li-O2 batteries. J. Alloys Compd. 2021, 861, 157945. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Z.; Bao, W.; Lai, Y.; Li, J.; Gan, Y.; Wang, J. Hierarchical mesoporous γ-Fe2O3/carbon nanocomposites derived from metal organic frameworks as a cathode electrocatalyst for rechargeable Li-O2 batteries. Electrochim. Acta 2014, 134, 293–301. [Google Scholar] [CrossRef]
- Gan, Y.; Lai, Y.; Zhang, Z.; Chen, W.; Du, K.; Li, J. Hierarchical Cr2O3@OPC composites with octahedral shape for rechargeable nonaqueous lithium-oxygen batteries. J. Alloys Compd. 2016, 665, 365–372. [Google Scholar] [CrossRef]
- Chen, K.; Yang, D.-Y.; Huang, G.; Zhang, X.-B. Lithium–Air Batteries: Air-Electrochemistry and Anode Stabilization. Acc. Chem. Res. 2021, 54, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, G.; Zhu, Y.; Liu, B.; Zhou, W.; Shao, Z. B-Site Cation Ordered Double Perovskites as Efficient and Stable Electrocatalysts for Oxygen Evolution Reaction. Chemistry 2017, 23, 5722–5728. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.; Sun, Y.-F.; Li, M.; Yan, N.; Chen, J.; Zhang, Y.-Q.; Zeng, Y.; Shalchi Amirkhiz, B.; Luo, J.-L. Stabilizing Double Perovskite for Effective Bifunctional Oxygen Electrocatalysis in Alkaline Conditions. Chem. Mater. 2017, 29, 6228–6237. [Google Scholar] [CrossRef] [Green Version]
- Mai, Z.; Duan, W.; Wang, K.; Tang, Z.; Chen, S. Integrating ZnCo2O4 submicro/nanospheres with CoxSey nanosheets for the oxygen evolution reaction and zinc–air batteries. Sustain. Energy Fuels 2020, 4, 2184–2191. [Google Scholar] [CrossRef]
- Gao, Z.W.; Liu, J.Y.; Chen, X.M.; Zheng, X.L.; Mao, J.; Liu, H.; Ma, T.; Li, L.; Wang, W.C.; Du, X.W. Engineering NiO/NiFe LDH Intersection to Bypass Scaling Relationship for Oxygen Evolution Reaction via Dynamic Tridimensional Adsorption of Intermediates. Adv. Mater. 2019, 31, e1804769. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Guo, S.; Wang, R.; Lin, S.; Hussain, N.; Wei, H.; Deng, B.; Long, Y.; Lei, M.; Tang, H.; et al. Two-dimensional MOF/MOF derivative arrays on nickel foam as efficient bifunctional coupled oxygen electrodes. Chin. J. Catal. 2020, 41, 1754–1760. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, L.; Xiao, Z.; Chen, R.; Jiang, Z.; Wang, S. 3D Carbon Electrocatalysts In Situ Constructed by Defect-Rich Nanosheets and Polyhedrons from NaCl-Sealed Zeolitic Imidazolate Frameworks. Adv. Funct. Mater. 2018, 28, 1705356. [Google Scholar] [CrossRef]
- Yang, M.; Hu, X.; Fang, Z.; Sun, L.; Yuan, Z.; Wang, S.; Hong, W.; Chen, X.; Yu, D. Bifunctional MOF-Derived Carbon Photonic Crystal Architectures for Advanced Zn–Air and Li–S Batteries: Highly Exposed Graphitic Nitrogen Matters. Adv. Funct. Mater. 2017, 27, 1701971. [Google Scholar] [CrossRef]
- Yan, J.; Zheng, X.; Wei, C.; Sun, Z.; Zeng, K.; Shen, L.; Sun, J.; Rümmeli, M.H.; Yang, R. Nitrogen-doped hollow carbon polyhedron derived from salt-encapsulated ZIF-8 for efficient oxygen reduction reaction. Carbon 2021, 171, 320–328. [Google Scholar] [CrossRef]
- Xu, K.; Bao, H.; Tang, C.; Maliutina, K.; Li, F.; Fan, L. Engineering hierarchical MOFs-derived Fe–N–C nanostructure with improved oxygen reduction activity for zinc-air battery: The role of iron oxide. Mater. Today Energy 2020, 18, 100500. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, H.; Zhu, J.; Li, W.; Zhang, C.; Zhao, J.; Luo, F.; Sun, Z.; Mu, S. 3D flower-like ZnFe-ZIF derived hierarchical Fe, N-Codoped carbon architecture for enhanced oxygen reduction in both alkaline and acidic media, and zinc-air battery performance. Carbon 2020, 161, 502–509. [Google Scholar] [CrossRef]
- Jin, H.; Zhou, H.; Li, W.; Wang, Z.; Yang, J.; Xiong, Y.; He, D.; Chen, L.; Mu, S. In situ derived Fe/N/S-codoped carbon nanotubes from ZIF-8 crystals as efficient electrocatalysts for the oxygen reduction reaction and zinc–air batteries. J. Mater. Chem. A 2018, 6, 20093–20099. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Wu, H.B.; Shen, G.; Le, Z.; Chen, G.; Lu, Y. Iron-decorated nitrogen-rich carbons as efficient oxygen reduction electrocatalysts for Zn-air batteries. Nanoscale 2018, 10, 16996–17001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, X.; Jiang, Z.; Xu, J.; Chen, L.; Xue, Y.; Nie, A.; Xie, Z.; Kuang, Q.; Zheng, L. Atomically dispersed hierarchically ordered porous Fe–N–C electrocatalyst for high performance electrocatalytic oxygen reduction in Zn-Air battery. Nano Energy 2020, 71, 104547. [Google Scholar] [CrossRef]
- Chen, K.; Ci, S.; Xu, Q.; Cai, P.; Li, M.; Xiang, L.; Hu, X.; Wen, Z. Iron-incorporated nitrogen-doped carbon materials as oxygen reduction electrocatalysts for zinc-air batteries. Chin. J. Catal. 2020, 41, 858–867. [Google Scholar] [CrossRef]
- Han, H.; Chao, S.; Bai, Z.; Wang, X.; Yang, X.; Qiao, J.; Chen, Z.; Yang, L. Metal-Organic-Framework-Derived Co Nanoparticles Deposited on N-Doped Bimodal Mesoporous Carbon Nanorods as Efficient Bifunctional Catalysts for Rechargeable Zinc−Air Batteries. ChemElectroChem 2018, 5, 1868–1873. [Google Scholar] [CrossRef]
- Li, J.-C.; Wu, X.-T.; Chen, L.-J.; Li, N.; Liu, Z.-Q. Bifunctional MOF-derived Co-N-doped carbon electrocatalysts for high-performance zinc-air batteries and MFCs. Energy 2018, 156, 95–102. [Google Scholar] [CrossRef]
- Amiinu, I.S.; Liu, X.; Pu, Z.; Li, W.; Li, Q.; Zhang, J.; Tang, H.; Zhang, H.; Mu, S. From 3D ZIF Nanocrystals to Co-Nx/C Nanorod Array Electrocatalysts for ORR, OER, and Zn-Air Batteries. Adv. Funct. Mater. 2018, 28, 1704638. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, Q.; Zheng, H.; Chen, M.; Dai, L. Novel MOF-Derived Co@N-C Bifunctional Catalysts for Highly Efficient Zn-Air Batteries and Water Splitting. Adv. Mater. 2018, 30, 1705431. [Google Scholar] [CrossRef] [PubMed]
- Zang, W.; Sumboja, A.; Ma, Y.; Zhang, H.; Wu, Y.; Wu, S.; Wu, H.; Liu, Z.; Guan, C.; Wang, J.; et al. Single Co Atoms Anchored in Porous N-Doped Carbon for Efficient Zinc−Air Battery Cathodes. ACS Catal. 2018, 8, 8961–8969. [Google Scholar] [CrossRef]
- Chen, S.; Shu, X.; Wang, H.; Zhang, J. Thermally driven phase transition of manganese oxide on carbon cloth for enhancing the performance of flexible all-solid-state zinc–air batteries. J. Mater. Chem. A 2019, 7, 19719–19727. [Google Scholar] [CrossRef]
- Chen, D.; Yu, J.; Cui, Z.; Zhang, Q.; Chen, X.; Sui, J.; Dong, H.; Yu, L.; Dong, L. Hierarchical architecture derived from two-dimensional zeolitic imidazolate frameworks as an efficient metal-based bifunctional oxygen electrocatalyst for rechargeable Zn–air batteries. Electrochim. Acta 2020, 331, 135394. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, P.; Zhang, Y.; Liu, B.; Gao, P.; Guo, S. 3D star-like atypical hybrid MOF derived single-atom catalyst boosts oxygen reduction catalysis. J. Energy Chem. 2021, 55, 355–360. [Google Scholar] [CrossRef]
- Zhou, D.; Fu, H.; Long, J.; Shen, K.; Gou, X. Novel fusiform core-shell-MOF derived intact metal@carbon composite: An efficient cathode catalyst for aqueous and solid-state Zn-air batteries. J. Energy Chem. 2022, 64, 385–394. [Google Scholar] [CrossRef]
- Huo, M.; Wang, B.; Zhang, C.; Ding, S.; Yuan, H.; Liang, Z.; Qi, J.; Chen, M.; Xu, Y.; Zhang, W.; et al. 2D Metal-Organic Framework Derived CuCo Alloy Nanoparticles Encapsulated by Nitrogen-Doped Carbonaceous Nanoleaves for Efficient Bifunctional Oxygen Electrocatalyst and Zinc-Air Batteries. Chemistry 2019, 25, 12780–12788. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.-M.; Zhang, Y.; Zhang, M.; Yu, J.; Liu, H.; Yu, Z.; Yang, B.; Zhu, C.; Xu, J. Leaf-like 2D nanosheet as efficient oxygen reduction reaction catalyst for Zn-air battery. J. Power Sources 2019, 434, 226717. [Google Scholar] [CrossRef]
- Zhong, Y.; Pan, Z.; Wang, X.; Yang, J.; Qiu, Y.; Xu, S.; Lu, Y.; Huang, Q.; Li, W. Hierarchical Co3O4 Nano-Micro Arrays Featuring Superior Activity as Cathode in a Flexible and Rechargeable Zinc-Air Battery. Adv. Sci. 2019, 6, 1802243. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Shi, X.; Gao, P.; Wang, S.; Hu, W.; Zhao, X.; Ni, Y.; Xu, X.; Xu, Y.; Yan, W.; et al. Well-elaborated, mechanochemically synthesized Fe-TPP⊂ZIF precursors (Fe-TPP = tetraphenylporphine iron) to atomically dispersed iron–nitrogen species for oxygen reduction reaction and Zn-air batteries. Nano Energy 2018, 52, 29–37. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, J.; Ju, J.; Zhang, W.; He, L.; Zhang, J.; Fu, C.; Sun, B. Space-confined synthesis of CoNi nanoalloy in N-doped porous carbon frameworks as efficient oxygen reduction catalyst for neutral and alkaline aluminum-air batteries. Energy Storage Mater. 2020, 27, 96–108. [Google Scholar] [CrossRef]
- Li, D.; Sun, L.; Hu, L.; Zhu, J.; Shi, J.; Guo, D. Rare earth insitu-doped ZIF-67 derived N doped C encapsulated Sm2O3/Co nanoparticles as excellent oxygen reduction reaction catalyst for Al-air batteries. J. Power Sources 2021, 482, 229052. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Hao, J.; Liu, Y.; Shen, H.; Li, W.; Li, J. Metal-Organic Framework-Derived Reduced Graphene Oxide-Supported ZnO/ZnCo2O4/C Hollow Nanocages as Cathode Catalysts for Aluminum-O2 Batteries. ACS Appl. Mater. Interfaces 2017, 9, 31841–31852. [Google Scholar] [CrossRef]
- Li, J.; Zhou, N.; Song, J.; Fu, L.; Yan, J.; Tang, Y.; Wang, H. Cu–MOF-Derived Cu/Cu2O Nanoparticles and CuNxCy Species to Boost Oxygen Reduction Activity of Ketjenblack Carbon in Al–Air Battery. ACS Sustain. Chem. Eng. 2017, 6, 413–421. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mechili, M.; Vaitsis, C.; Argirusis, N.; Pandis, P.K.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Research Progress in Metal-Organic Framework Based Nanomaterials Applied in Battery Cathodes. Energies 2022, 15, 5460. https://doi.org/10.3390/en15155460
Mechili M, Vaitsis C, Argirusis N, Pandis PK, Sourkouni G, Zorpas AA, Argirusis C. Research Progress in Metal-Organic Framework Based Nanomaterials Applied in Battery Cathodes. Energies. 2022; 15(15):5460. https://doi.org/10.3390/en15155460
Chicago/Turabian StyleMechili, Maria, Christos Vaitsis, Nikolaos Argirusis, Pavlos K. Pandis, Georgia Sourkouni, Antonis A. Zorpas, and Christos Argirusis. 2022. "Research Progress in Metal-Organic Framework Based Nanomaterials Applied in Battery Cathodes" Energies 15, no. 15: 5460. https://doi.org/10.3390/en15155460
APA StyleMechili, M., Vaitsis, C., Argirusis, N., Pandis, P. K., Sourkouni, G., Zorpas, A. A., & Argirusis, C. (2022). Research Progress in Metal-Organic Framework Based Nanomaterials Applied in Battery Cathodes. Energies, 15(15), 5460. https://doi.org/10.3390/en15155460









