Geological Controls on Geochemical Anomaly of the Carbonaceous Mudstones in Xian’an Coalfield, Guangxi Province, China
Abstract
1. Introduction
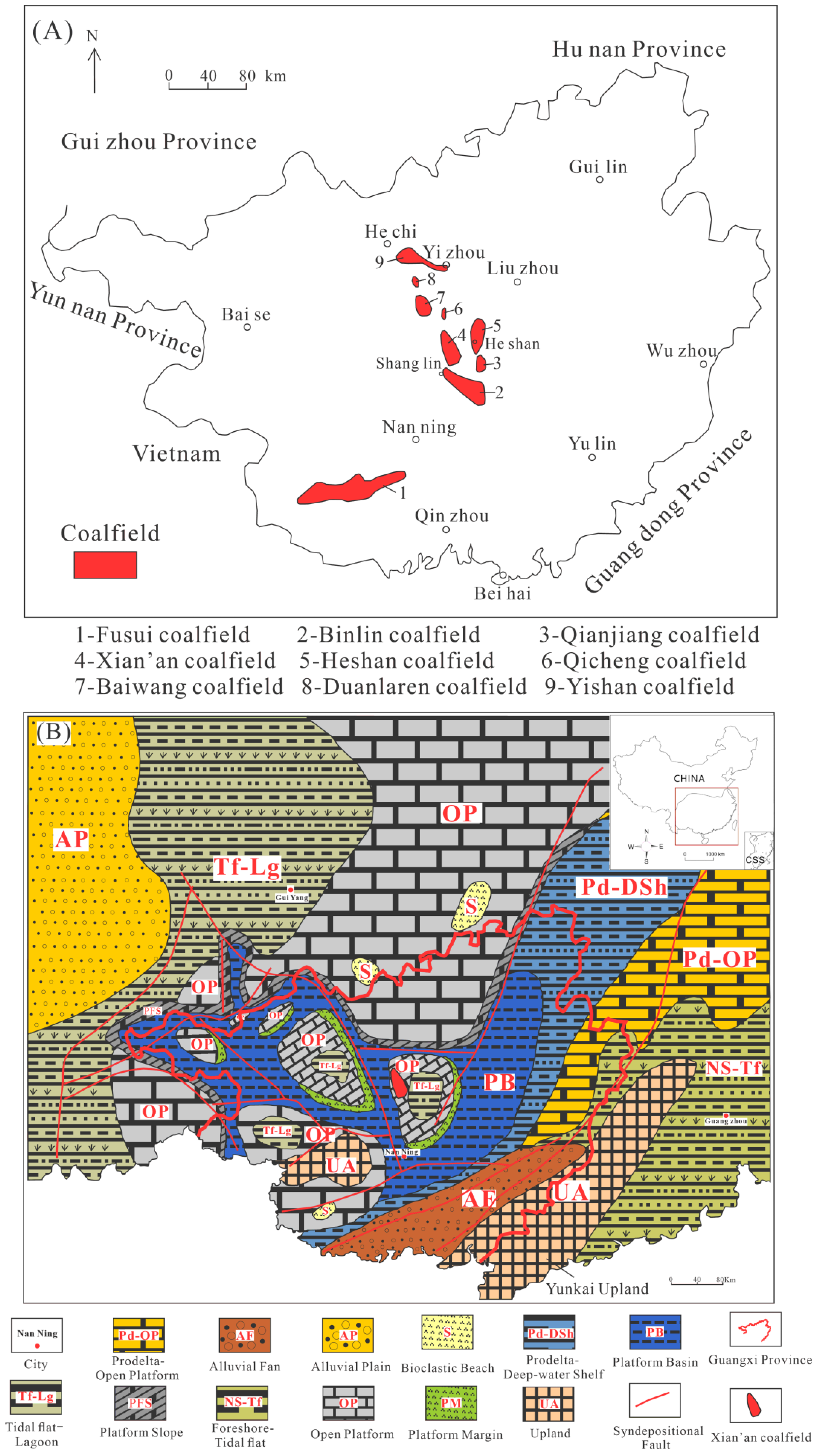
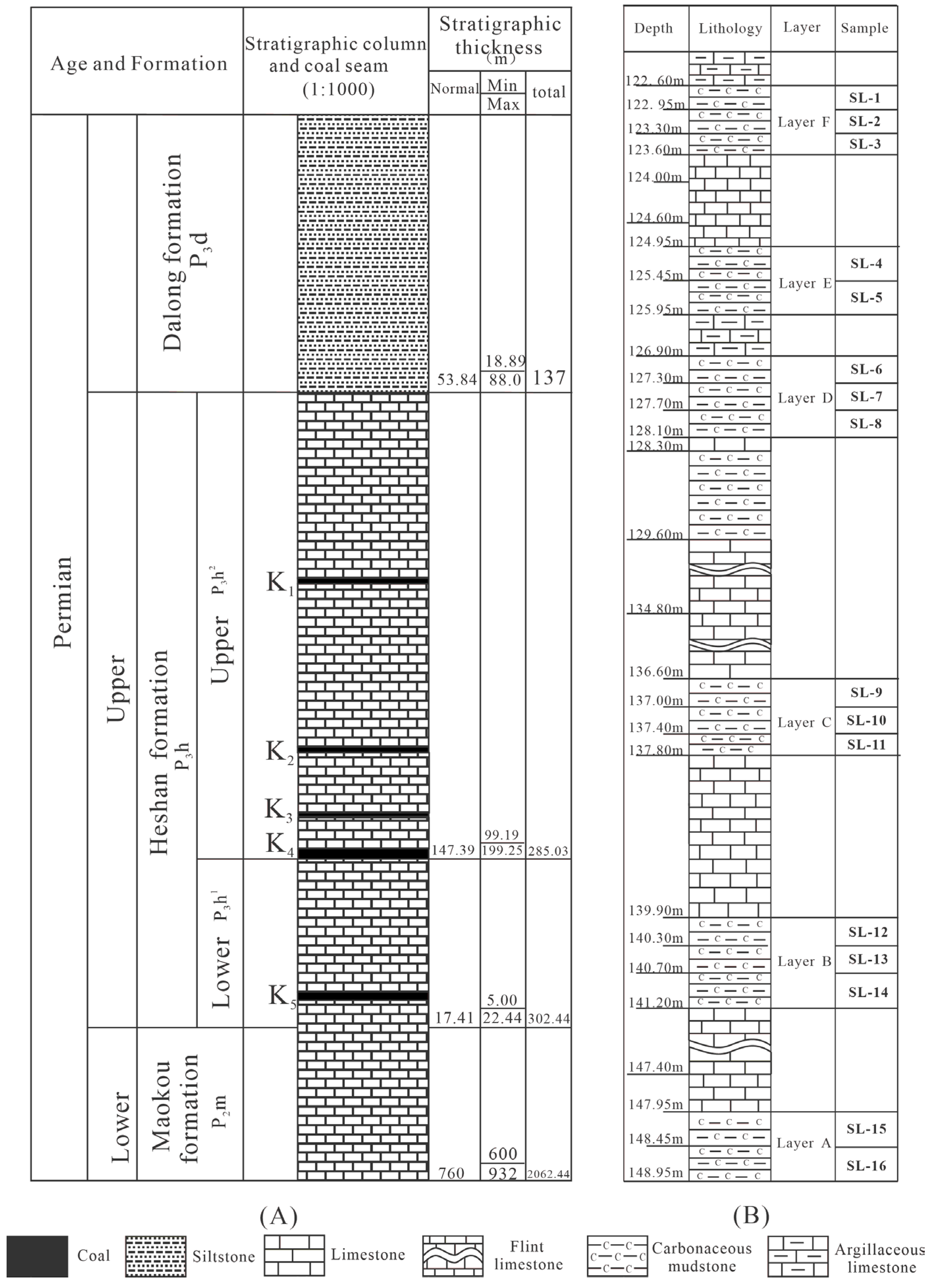
2. Geological Setting
3. Methodology
4. Results
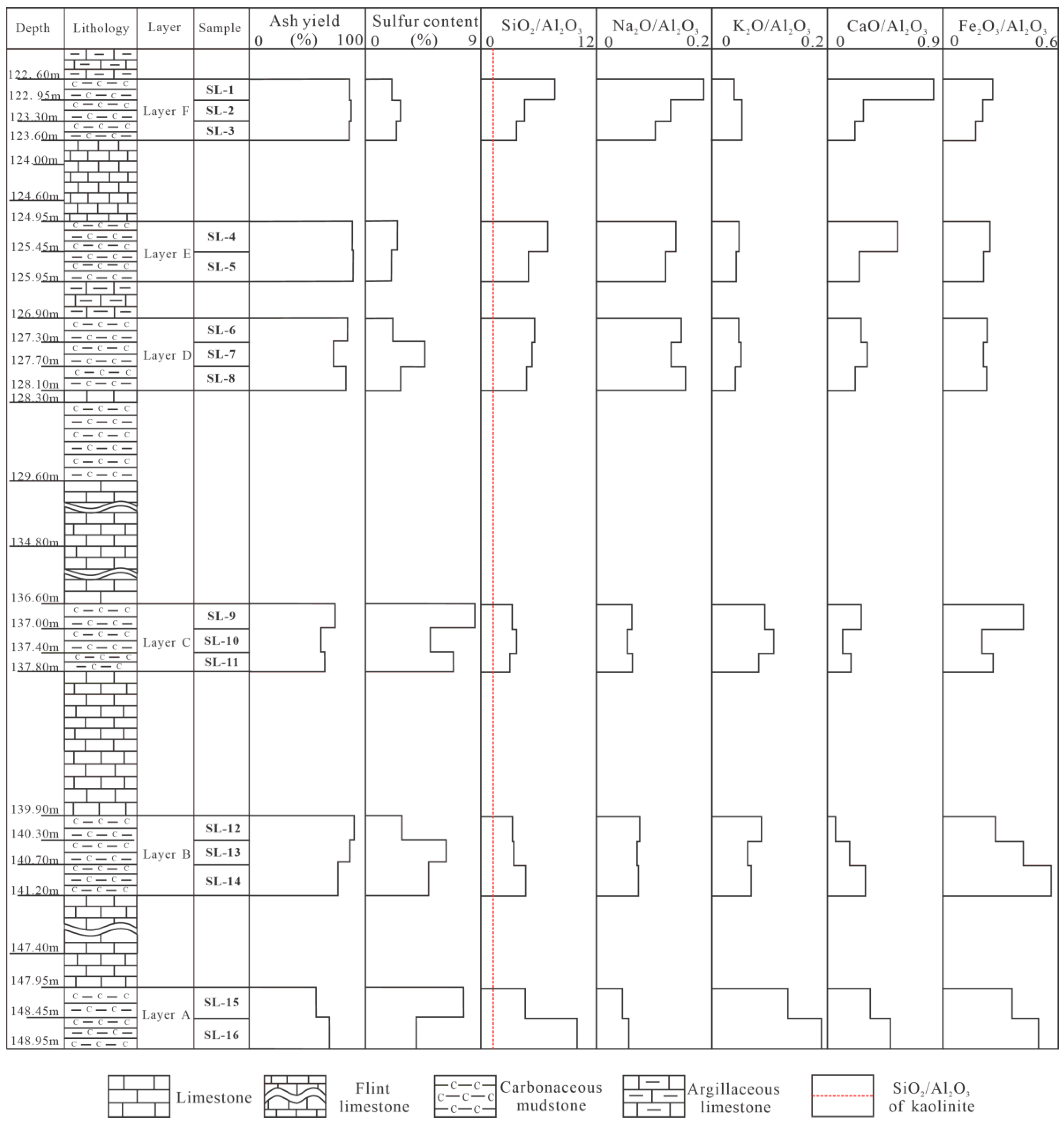
4.1. Coal Chemistry
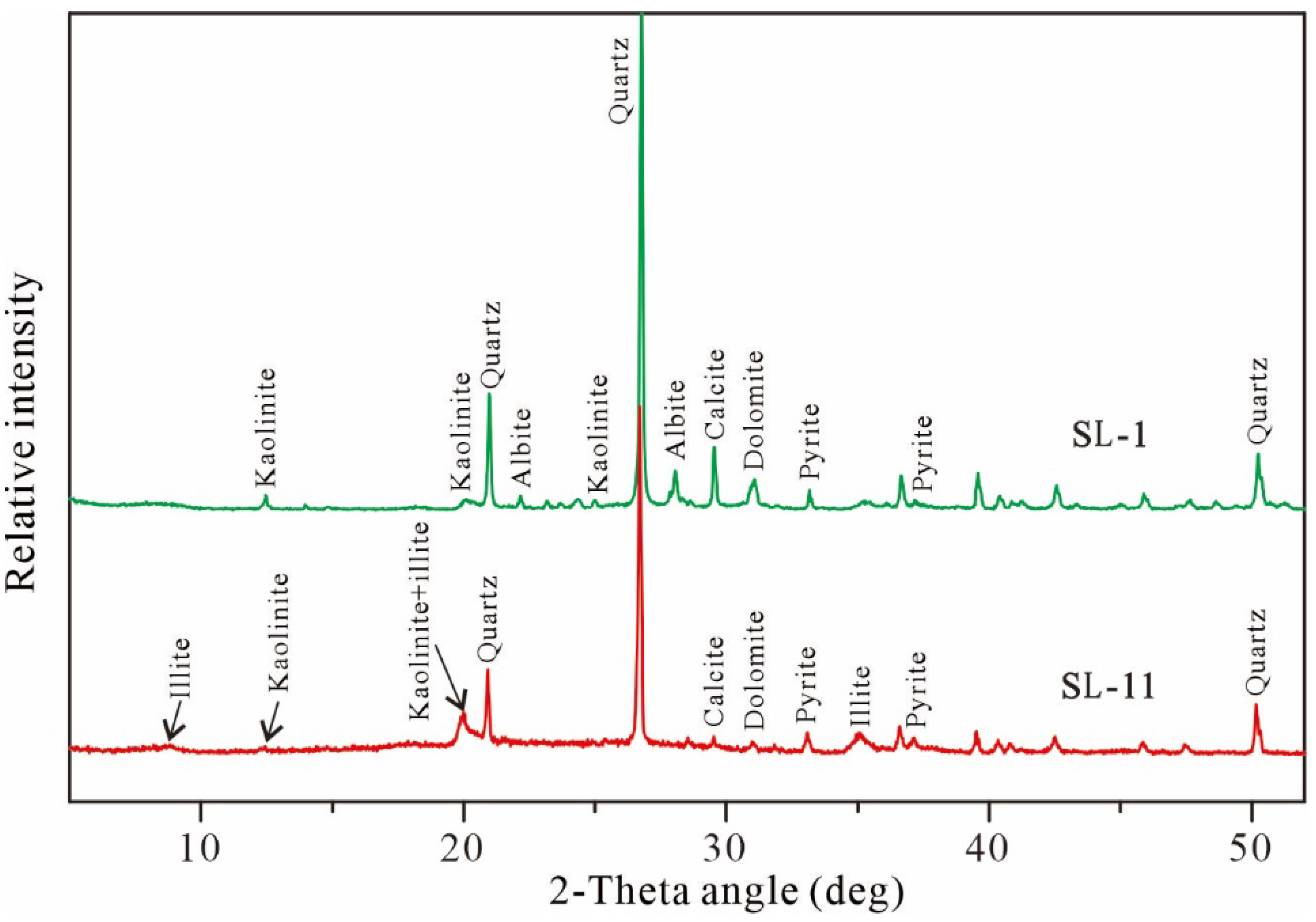
4.2. Mineralogy
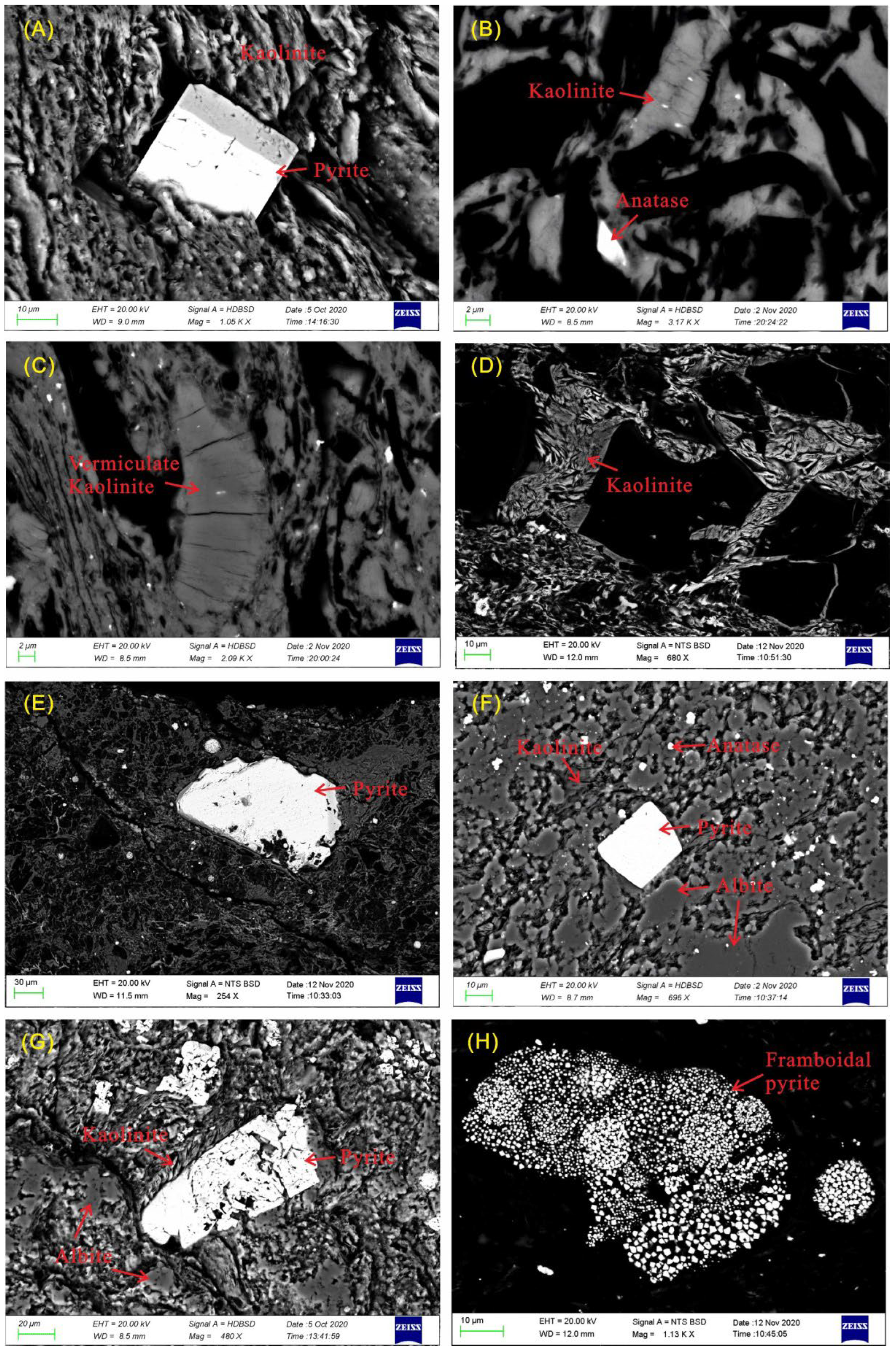
4.2.1. Mineral Phases
4.2.2. Mode of Occurrence of Minerals
4.3. Geochemistry
4.3.1. Major Elements
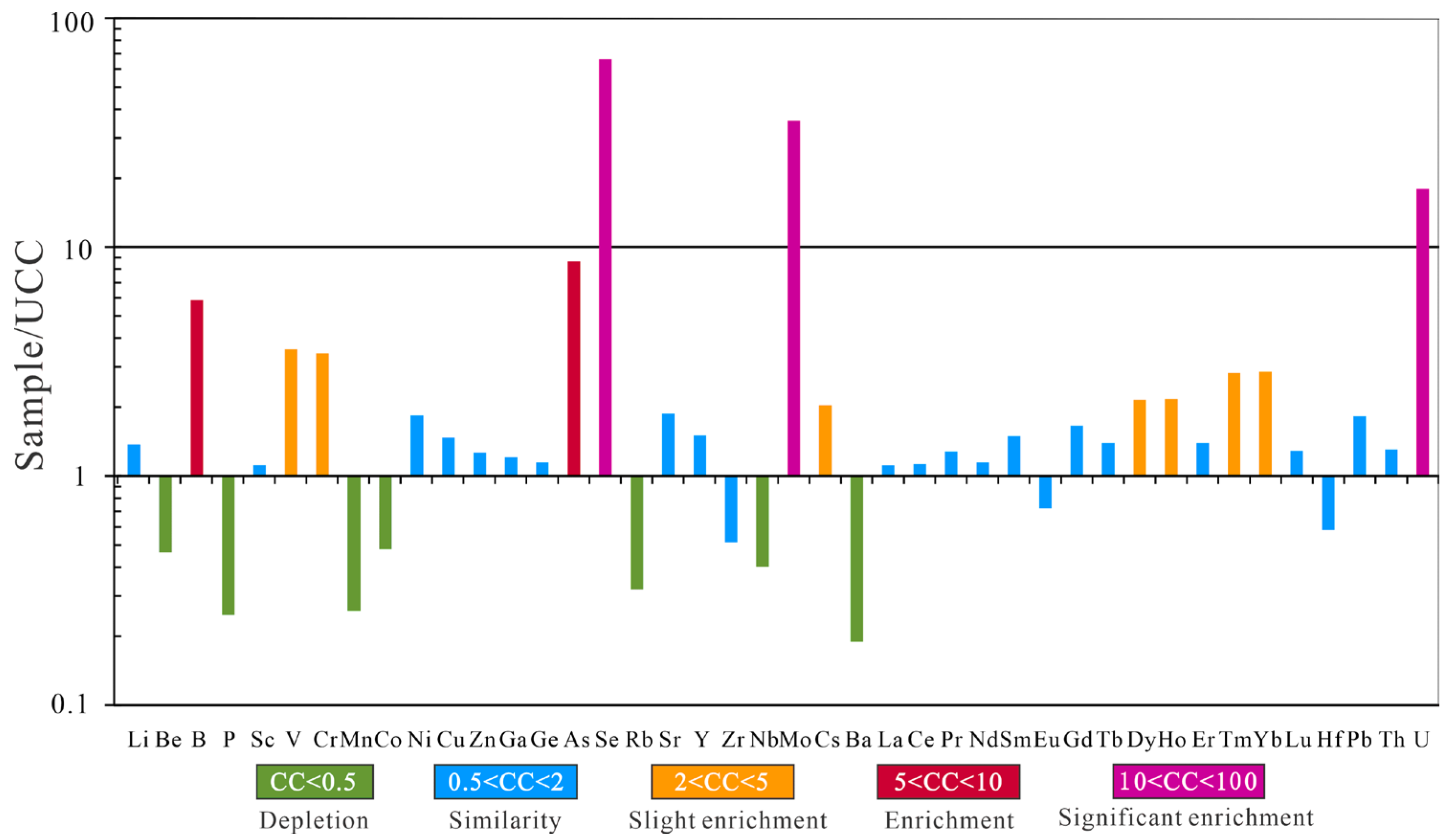
4.3.2. Trace Elements
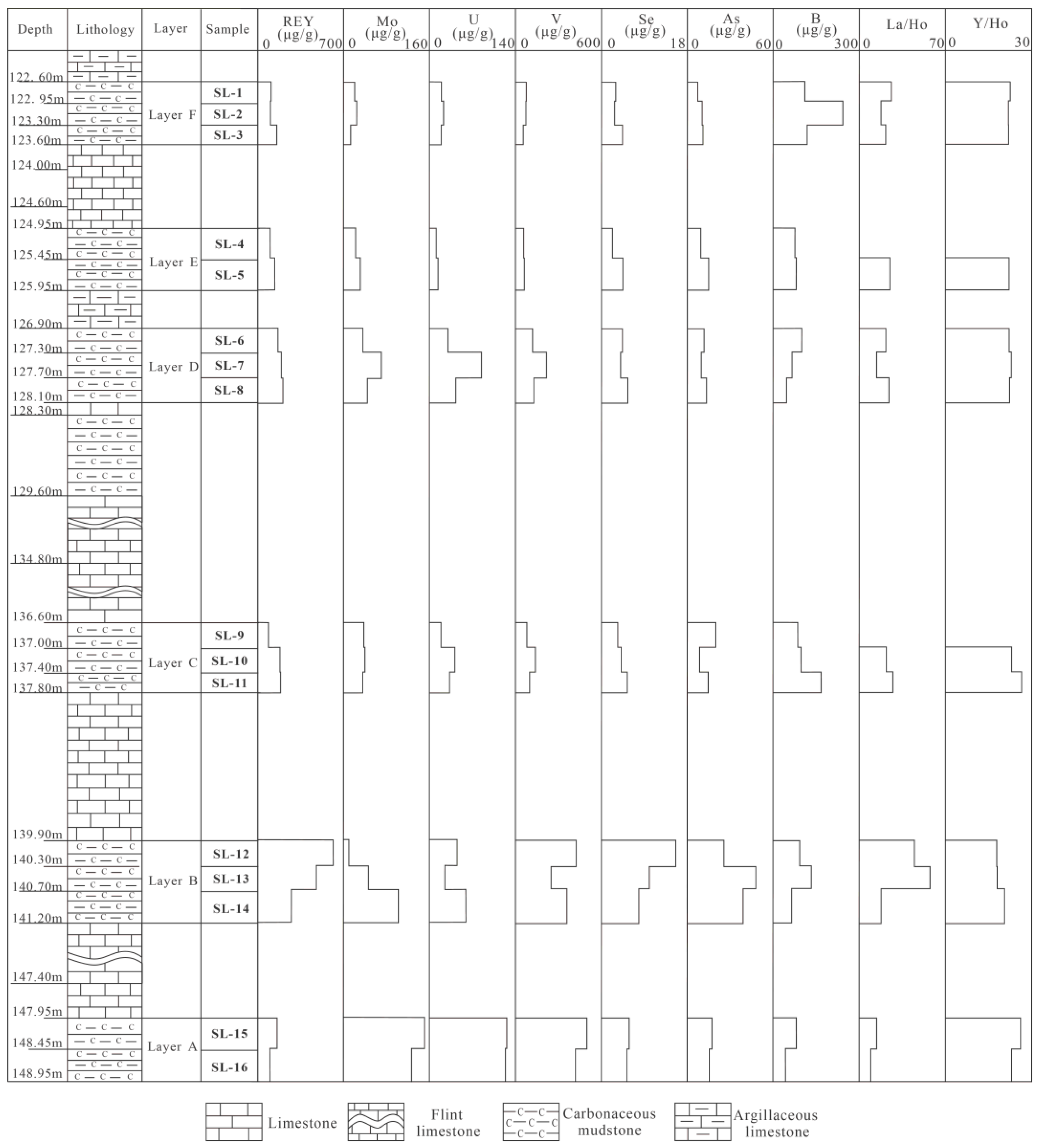
4.3.3. Rare Earth Element and Yttrium (REY)
5. Discussion
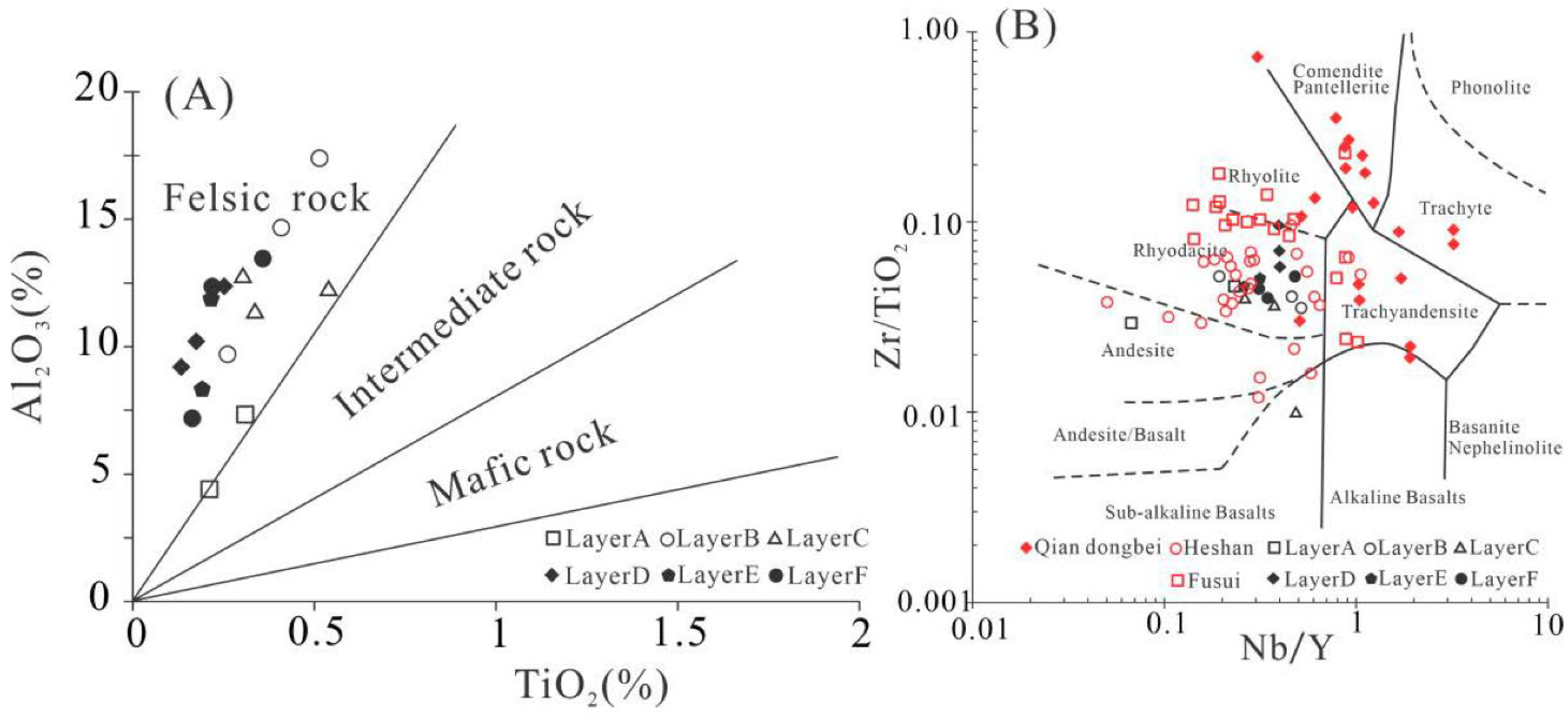
5.1. The Nature of Detrital Materials
5.2. Influence of Seawater Invasion
5.3. Mode of Occurrence of Elements
5.4. Elevated Concentrations of Trace Elements
5.4.1. Rare Earth Elements and Yttrium (REY)
5.4.2. Uranium, Mo, V, As, and Se
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shao, L.Y.; Jones, T.; Gayer, R.; Dai, S.F.; Li, S.S.; Jiang, Y.F.; Zhang, P.F. Petrology and geochemistry of the high-sulphur coals from the Upper Permian carbonate coal measures in the Heshan Coalfield, southern China. Int. J. Coal Geol. 2003, 55, 1–26. [Google Scholar] [CrossRef]
- Zeng, R.S.; Zhuang, X.G.; Koukouzas, N.; Xu, W.D. Characterization of trace elements in sulphur-rich Late Permian coals in the Heshan coal field, Guangxi, South China. Int. J. Coal Geol. 2005, 61, 87–95. [Google Scholar] [CrossRef]
- Dai, S.F.; Zhang, W.G.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Song, W.J.; Wang, X.B.; Li, X.; Zhao, L.X.; Kang, H.; et al. Factors controlling geochemical and mineralogical compositions of coals preserved within marine carbonate successions: A case study from the Heshan Coalfield, southern China. Int. J. Coal Geol. 2013, 109, 77–100. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Liang, H.Z.; Zeng, F.G.; Tang, Y.G.; Liang, L.T.; Takahashi, F. Origins and occurrences of Ti-nanominerals in a superhigh-organic-sulfur coal. Fuel 2020, 259, 116302. [Google Scholar] [CrossRef]
- Dai, S.F.; Zhang, W.G.; Ward, C.R.; Seredin, V.V.; Hower, J.C.; Li, X.; Song, W.J.; Wang, X.B.; Kang, H.; Zheng, L.C.; et al. Mineralogical and geochemical anomalies of late Permian coals from the Fusui Coalfield, Guangxi Province, southern China: Influences of terrigenous materials and hydrothermal fluids. Int. J. Coal Geol. 2013, 105, 60–84. [Google Scholar] [CrossRef]
- Zhang, F.Q.; Li, B.Q.; Zhuang, X.G.; Xavier, Q.; Natalia, M.; Shangguan, Y.F.; Zhou, J.M.; Liao, J.L. Geological Controls on Enrichment of Rare Earth Elements and Yttrium (REY) in Late Permian Coals and Non-Coal Rocks in the Xian’an Coalfield, Guangxi Province. Minerals 2021, 11, 301. [Google Scholar] [CrossRef]
- Dai, S.F.; Ji, D.P.; Ward, C.R.; French, D.; Hower, J.C.; Yan, X.Y.; Wei, Q. Mississippian anthracites in Guangxi Province, southern China: Petrological, mineralogical, and rare earth element evidence for high-temperature solutions. Int. J. Coal Geol. 2018, 197, 84–114. [Google Scholar] [CrossRef]
- Dai, S.F.; Seredin, V.V.; Ward, C.R.; Hower, J.C.; Xing, Y.W.; Zhang, W.G.; Song, W.J.; Wang, P.P. Enrichment of U-Se-Mo-Re-V in coals preserved within marine carbonate successions: Geochemical and mineralogical data from the Late Permian Guiding Coalfield, Guizhou, China. Min. Depos. 2015, 50, 159–186. [Google Scholar] [CrossRef]
- Dai, S.F.; Ren, D.Y.; Zhou, Y.P.; Chou, C.L.; Wang, X.B.; Zhao, L.; Zhu, X.W. Mineralogy and geochemistry of a superhigh-organic-sulfur coal, Yanshan Coalfield, Yunnan, China: Evidence for a volcanic ash component and influence by submarine exhalation. Chem. Geol. 2008, 255, 182–194. [Google Scholar] [CrossRef]
- Li, W.W.; Tang, Y.G. Sulfur isotopic composition of superhigh-organic-sulfur coals from the Chenxi coalfield, southern China. Int. J. Coal Geol. 2014, 127, 3–13. [Google Scholar] [CrossRef]
- Chou, C.L. Geologic factors affecting the abundance, distribution, and speciation of sulfur in coals. In Geology of Fossil Fuels—Coal; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Chou, C.L. Sulfur in coals: A review of geochemistry and origins. Int. J. Coal Geol. 2012, 100, 1–13. [Google Scholar] [CrossRef]
- Dai, S.F.; Xie, P.P.; Ward, C.R.; Yan, X.Y.; Guo, W.M.; French, D.; Graham, I.T. Anomalies of rare metals in Lopingian super-high-organic-sulfur coals from the Yishan Coalfield, Guangxi, China. Ore Geol. Rev. 2017, 88, 235–250. [Google Scholar] [CrossRef]
- Dai, S.F.; Ren, D.Y.; Chou, C.L.; Finkelman, R.B.; Seredin, V.V.; Zhou, Y.P. Geochemistry of trace elements in Chinese coals: A review of abundances, genetic types, impacts on human health, and industrial utilization. Int. J. Coal Geol. 2012, 94, 3–21. [Google Scholar] [CrossRef]
- Liao, J.L.; Zhang, F.Q.; Wei, S.M.; Liang, X.D. Lithium and gallium abundance and enrichment factors in typical Late Permian coal-accumulating basin in Guangxi. Coal Geol. Explor. 2020, 48, 1. [Google Scholar]
- Yang, H.Y. Devonian-Middle Triassic Tectono-Palaeogeographic Pattern and Its Evolution in Hunan-Guangxi Area; China University of Petroleum (East China): Beijingm, China, 2010. [Google Scholar]
- Standard D3175-11; Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2011.
- Standard D3174-11; Annual Book of ASTM Standards. Test Method for Ash in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2011.
- Standard D3173-11; Standard Test Method for Moisture in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2011.
- Li, B.Q.; Zhuang, X.G.; Querol, X.; Moreno, N.; Cordoba, P.; Li, J.; Zhou, J.B.; Ma, X.P.; Liu, S.B.; Shangguan, Y.F. The mode of occurrence and origin of minerals in the Early Permian high-rank coals of the Jimunai depression, Xinjiang Uygur Autonomous Region, NW China. Int. J. Coal Geol. 2019, 205, 58–74. [Google Scholar] [CrossRef]
- Querol, X.; Whateley, M.K.G.; FernandezTuriel, J.L.; Tuncali, E. Geological controls on the mineralogy and geochemistry of the Beypazari lignite, central Anatolia, Turkey. Int. J. Coal Geol. 1997, 33, 255–271. [Google Scholar] [CrossRef]
- Taylor, S.R.; Mclennan, S.M. The continental crust: Its composition and evolution. J. Geol. 1985, 94, 57–72. [Google Scholar]
- Ketris, M.P.; Yudovich, Y.E. Estimations of Clarkes for Carbonaceous biolithes: World averages for trace element contents in black shales and coals. Int. J. Coal Geol. 2009, 78, 135–148. [Google Scholar] [CrossRef]
- Seredin, V.V.; Dai, S.F. Coal deposits as potential alternative sources for lanthanides and yttrium. Int. J. Coal Geol. 2012, 94, 67–93. [Google Scholar] [CrossRef]
- Huang, H.; Du, Y.S.; Yang, J.H.; Zhou, L.; Hu, L.S.; Huang, H.W.; Huang, Z.Q.J.L. Origin of Permian basalts and clastic rocks in Napo, Southwest China: Implications for the erosion and eruption of the Emeishan large igneous province. Lithos 2014, 208–209, 324–338. [Google Scholar] [CrossRef]
- Yang, J.; Cawood, P.A.; Du, Y.J.E.; Letters, P.S. Voluminous silicic eruptions during late Permian Emeishan igneous province and link to climate cooling. Earth Planet. Sci. Lett. 2015, 432, 166–175. [Google Scholar] [CrossRef]
- Deng, J.; Wang, Q.; Yang, S.; Liu, X.; Zhang, Q.; Yang, L.; Yang, Y. Genetic relationship between the Emeishan plume and the bauxite deposits in Western Guangxi, China: Constraints from U–Pb and Lu–Hf isotopes of the detrital zircons in bauxite ores—ScienceDirect. J. Asian Earth Sci. 2010, 37, 412–424. [Google Scholar] [CrossRef]
- Hayashi, T.; Hinoda, Y.; Takahashi, T.; Adachi, M.; Miura, S.; Izumi, T.; Kojima, H.; Yano, S.; Imai, K.J.I.M. Idiopathic CD4+ T-lymphocytopenia with Bowen’s disease. Intern. Med. 1997, 36, 822–824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hower, J.C.; Dai, S.F. Petrology and chemistry of sized Pennsylvania anthracite, with emphasis on the distribution of rare earth elements. Fuel 2016, 185, 305–315. [Google Scholar] [CrossRef]
- Li, B.Q.; Zhuang, X.G.; Li, J.; Querol, X.; Font, O.; Moreno, N. Geological controls on mineralogy and geochemistry of the Late Permian coals in the Liulong Mine of the Liuzhi Coalfield, Guizhou Province, Southwest China. Int. J. Coal Geol. 2016, 154, 1–15. [Google Scholar] [CrossRef]
- Li, B.Q.; Zhuang, X.G.; Querol, X.; Moreno, N.; Zhang, F. Geological controls on the distribution of REY-Zr (Hf)-Nb (Ta) enrichment horizons in late Permian coals from the Qiandongbei Coalfield, Guizhou Province, SW China. Int. J. Coal Geol. 2020, 231, 103604. [Google Scholar] [CrossRef]
- Dai, S.F.; Chekryzhov, I.Y.; Seredin, V.V.; Nechaev, V.P.; Graham, I.T.; Hower, J.C.; Ward, C.R.; Ren, D.Y.; Wang, X.B. Metalliferous coal deposits in East Asia (Primorye of Russia and South China): A review of geodynamic controls and styles of mineralization. Gondwana Res. 2016, 29, 60–82. [Google Scholar] [CrossRef]
- Dai, S.F.; Bechtel, A.; Eble, C.F.; Flores, R.M.; French, D.; Graham, I.T.; Hood, M.M.; Hower, J.C.; Korasidis, V.A.; Moore, T.A.; et al. Recognition of peat depositional environments in coal: A review. Int. J. Coal Geol. 2020, 219, 103383. [Google Scholar]
- Golab, A.N.; Carr, P.F. Changes in geochemistry and mineralogy of thermally altered coal, Upper Hunter Valley, Australia. Int. J. Coal Geol. 2004, 57, 197–210. [Google Scholar] [CrossRef]
- Rimmer, S.M.; Davis, A. Geologic Controls on the Inorganic Composition of Lower Kittanning Coal. In Geology of Fossil Fuels—Coal; ACS Publication: Washington, DC, USA, 1986; pp. 41–52. [Google Scholar]
- Bau, M.; Dulski, P. Petrology, Comparative study of yttrium and rare-earth element behaviours in fluorine-rich hydrothermal fluids. Contrib. Mineral. Petrol. 1995, 119, 213–223. [Google Scholar] [CrossRef]
- Bau, M.; Koschinsky, A.; Dulski, P.; Hein, J.R. Comparison of the partitioning behaviours of yttrium, rare earth elements, and titanium between hydrogenetic marine ferromanganese crusts and seawater. Geochim. Cosmochim. Acta 1996, 60, 1709–1725. [Google Scholar] [CrossRef]
- Byrne, R.H.; Kim, K.-H. Rare earth element scavenging in seawater. Geochim. Cosmochim. Acta 1990, 54, 2645–2656. [Google Scholar] [CrossRef]
- Xu, C.; Kynicky, J.I.; Smith, M.P.; Kopriva, A.; Brtnicky, M.; Urubek, T.; Yang, Y.; Zhao, Z.; He, C.; Song, W.J.N.C. Origin of heavy rare earth mineralization in South China. Nat. Commun. 2017, 8, 14598. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.J.; Riotte, J.; Battacharya, S.; Violette, A.; Oliva, P.; Prunier, J.; Subramanian, S. REY-Th-U Dynamics in the Critical Zone: Combined Influence of Reactive Bedrock Accessory Minerals, Authigenic Phases, and Hydrological Sorting (Mule Hole Watershed, South India). Geochem. Geophys. Geosyst. 2018, 19, 1611–1635. [Google Scholar] [CrossRef]
- Chesley, S.; Lumpkin, M.; Schatzki, A.; Galpern, W.R.; Greenblatt, D.J.; Shader, R.I.; Miller, L.G. Prenatal exposure to benzodiazepine—I. Prenatal exposure to lorazepam in mice alters open-field activity and GABAA receptor function. Neuropharmacology 1991, 30, 53–58. [Google Scholar] [CrossRef]
- Winchester, J.A.; Floyd, P.A. Geochemical discrimination of different magma series and their differentiation products using immobile elements. Chem. Geol. 1977, 20, 325–343. [Google Scholar] [CrossRef]
- Dai, S.F.; Ward, C.R.; Graham, I.T.; French, D.; Hower, J.C.; Zhao, L.; Wang, X.B. Altered volcanic ashes in coal and coal-bearing sequences: A review of their nature and significance. Earth-Sci. Rev. 2017, 175, 44–74. [Google Scholar] [CrossRef]
- Dai, S.F.; Graham, I.T.; Ward, C.R. A review of anomalous rare earth elements and yttrium in coal. Int. J. Coal Geol. 2016, 159, 82–95. [Google Scholar] [CrossRef]
- Ichimura, K.; Sanematsu, K.; Kon, Y.; Takagi, T.; Murakami, T.J.A.M. REE redistributions during granite weathering: Implications for Ce anomaly as a proxy for paleoredox states. Am. Mineral. 2020, 105, 848–859. [Google Scholar] [CrossRef]
- Dai, S.; Finkelman, R.B. Coal geology in China: An overview. Int. Geol. Rev. 2018, 60, 531–534. [Google Scholar] [CrossRef]
- Li, B.Q.; Zhuang, X.G.; Li, J.; Querol, X.; Font, O.; Moreno, N. Enrichment and distribution of elements in the Late Permian coals from the Zhina Coalfield, Guizhou Province, Southwest China. Int. J. Coal Geol. 2017, 171, 111–129. [Google Scholar] [CrossRef]



| Layer | Sample | LTA (d, wt%) | Moisture (ad, wt%) | Ash Yield (d, wt%) | Volatile Matter Yield (daf, wt%) | Total Sulfur Content (d, wt%) |
|---|---|---|---|---|---|---|
| Layer F | SL-1 | 93.2 | 0.39 | 84.8 | 12.0 | 2.1 |
| SL-2 | 93.0 | 0.73 | 84.1 | 10.0 | 2.7 | |
| SL-3 | 96.3 | 1.17 | 83.2 | 10.7 | 2.4 | |
| Layer E | SL-4 | 96.1 | 0.58 | 86.3 | 10.2 | 2.5 |
| SL-5 | 88.9 | 0.80 | 86.9 | 10.6 | 2.0 | |
| Layer D | SL-6 | 89.7 | 0.59 | 82.5 | 9.6 | 2.1 |
| SL-7 | 77.6 | 0.37 | 69.0 | 10.1 | 4.6 | |
| SL-8 | 88.7 | 0.79 | 80.0 | 9.8 | 2.7 | |
| Layer C | SL-9 | 85.6 | 1.32 | 70.0 | 13.2 | 8.6 |
| SL-10 | 72.0 | 1.33 | 59.2 | 10.8 | 5.1 | |
| SL-11 | 83.6 | 1.59 | 63.3 | 14.3 | 6.9 | |
| Layer B | SL-12 | 93.8 | 2.14 | 86.2 | 12.2 | 2.8 |
| SL-13 | 97.5 | 2.00 | 83.2 | 16.1 | 6.3 | |
| SL-14 | 89.7 | 1.18 | 72.3 | 15.4 | 4.9 | |
| Layer A | SL-15 | 78.7 | 0.60 | 54.4 | 9.8 | 7.7 |
| SL-16 | 83.0 | 0.60 | 65.4 | 8.4 | 4.0 |
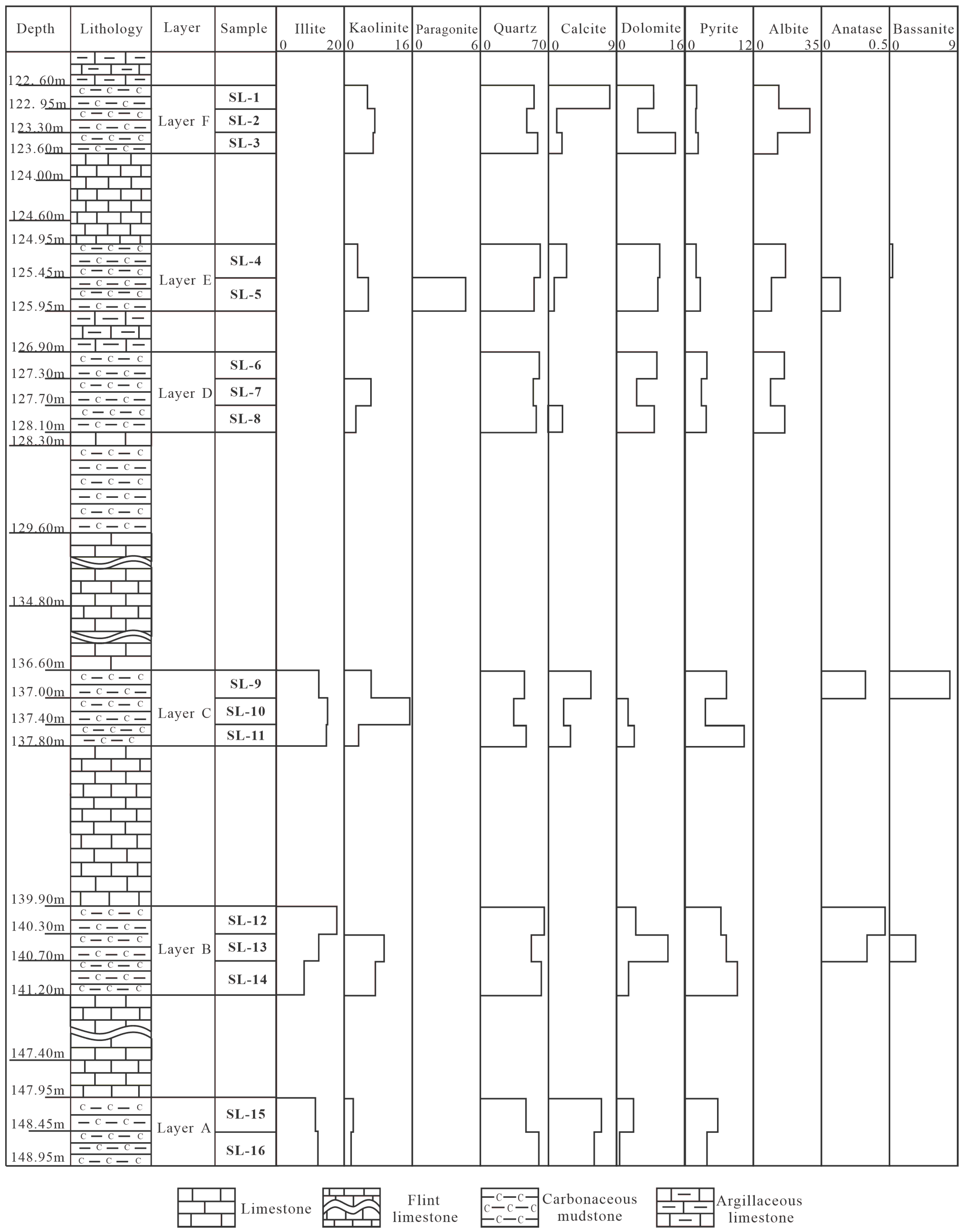
| Sample | LTA | Illite | Kaolinite | Paragonite | Quartz | Calcite | Dolomite | Pyrite | Albite | Anatase | Bassanite |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SL-1 | 93.2 | <dL | 5.6 | <dL | 55.2 | 8.4 | 8.6 | 2.1 | 13.4 | <dl | <dL |
| SL-2 | 93.0 | <dL | 7.1 | <dL | 48.3 | 1.1 | 4.9 | 2.0 | 29.6 | <dl | <dL |
| SL-3 | 96.3 | <dL | 6.9 | <dL | 58.8 | 1.6 | 14.0 | 2.2 | 12.9 | <dl | <dL |
| SL-4 | 96.1 | <dL | 3.2 | <dL | 60.6 | 2.5 | 10.2 | 2.0 | 17.1 | <dl | 0.4 |
| SL-5 | 88.9 | <dL | 5.7 | 5.3 | 55.1 | 0.7 | 9.7 | 2.7 | 9.6 | 0.1 | <dL |
| SL-6 | 89.7 | <dL | <dl | <dL | 59.8 | <dL | 9.5 | 4.1 | 16.3 | <dl | <dL |
| SL-7 | 77.6 | <dL | 6.2 | <dL | 54.2 | <dL | 4.6 | 2.9 | 9.7 | <dl | <dL |
| SL-8 | 88.7 | <dL | 2.8 | <dL | 55.0 | 1.8 | 8.9 | 3.8 | 16.5 | <dl | <dL |
| SL-9 | 85.6 | 12.3 | 6.4 | <dL | 45.3 | 5.8 | <dL | 7.5 | <dL | 0.3 | 8.0 |
| SL-10 | 72.0 | 15.0 | 15.1 | <dL | 33.7 | 2.0 | 2.5 | 3.7 | <dL | <dl | <dL |
| SL-11 | 83.6 | 14.8 | 3.7 | <dL | 47.6 | 3.0 | 4.0 | 10.5 | <dL | <dl | <dL |
| SL-12 | 93.8 | 17.6 | <dl | <dL | 64.8 | <dL | 4.4 | 6.5 | <dL | 0.4 | <dL |
| SL-13 | 97.5 | 12.5 | 9.4 | <dL | 52.3 | <dL | 12.2 | 7.4 | <dL | 0.3 | 3.4 |
| SL-14 | 89.7 | 7.8 | 7.5 | <dL | 62.4 | <dL | 2.7 | 9.3 | <dL | <dL | <dL |
| SL-15 | 78.7 | 11.5 | 2.1 | <dL | 48.1 | 7.3 | 3.9 | 5.9 | <dL | <dL | <dL |
| SL-16 | 83.0 | 11.8 | 1.3 | <dL | 59.3 | 6.2 | 0.5 | 3.9 | <dL | <dL | <dL |
| SL-1 | SL-2 | SL-3 | SL-4 | SL-5 | SL-6 | SL-7 | SL-8 | SL-9 | SL-10 | SL-11 | SL-12 | SL-13 | SL-14 | SL-15 | SL-16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 66 | 70 | 69 | 72 | 67 | 69 | 62 | 66 | 55 | 48 | 57 | 73 | 63 | 70 | 55 | 66 |
| TiO2 | 0.17 | 0.23 | 0.36 | 0.20 | 0.22 | 0.18 | 0.13 | 0.25 | 0.54 | 0.34 | 0.30 | 0.51 | 0.41 | 0.26 | 0.31 | 0.21 |
| Al2O3 | 7.2 | 12 | 13 | 8.3 | 12 | 10 | 9.1 | 12 | 12 | 11 | 13 | 17 | 15 | 9.7 | 7.3 | 4.4 |
| Fe2O3 | 1.8 | 2.5 | 2.3 | 1.9 | 2.4 | 2.2 | 1.8 | 2.7 | 5.1 | 2.3 | 3.5 | 5.1 | 6.2 | 5.6 | 2.6 | 2.1 |
| MgO | 1.8 | 1.8 | 2.0 | 2.6 | 2.6 | 2.3 | 2.0 | 2.1 | 0.67 | 0.63 | 0.75 | 0.76 | 2.0 | 1.9 | 0.87 | 0.32 |
| CaO | 6.0 | 3.5 | 3.0 | 4.6 | 3.2 | 2.7 | 2.8 | 2.7 | 3.3 | 1.4 | 2.5 | 1.1 | 2.9 | 3.0 | 2.5 | 2.2 |
| K2O | 0.29 | 0.62 | 0.74 | 0.38 | 0.51 | 0.47 | 0.45 | 0.51 | 1.1 | 1.2 | 1.0 | 1.4 | 0.91 | 0.62 | 0.95 | 0.85 |
| Na2O | 1.4 | 1.6 | 1.5 | 1.2 | 1.5 | 1.5 | 1.2 | 1.9 | 0.73 | 0.60 | 0.84 | 1.3 | 1.1 | 0.71 | 0.31 | 0.26 |
| Li | 16 | 22 | 23 | 28 | 55 | 35 | 32 | 45 | 40 | 35 | 55 | 27 | 25 | 23 | 14 | 8.0 |
| Be | <dl | 1.7 | 1.4 | 1.6 | 1.8 | 0.98 | 2.1 | 0.85 | 1.4 | 1.2 | 1.6 | 3.6 | 3.1 | 0.84 | <dl | 1.0 |
| B | 108 | 241 | 115 | 77 | 81 | 98 | 65 | 46 | 82 | 96 | 166 | 98 | 134 | 66 | 79 | 45 |
| P | 98 | 97 | 219 | 82 | 100 | 113 | 97 | 222 | 168 | 69 | 81 | 372 | 139 | 77 | 62 | 635 |
| Sc | 4.1 | 5.4 | 9.7 | 5.3 | 7.6 | 6.6 | 5.9 | 7.8 | 6.9 | 11 | 8.5 | 14 | 12 | 7.7 | 8.2 | 3.7 |
| V | 71 | 70 | 54 | 56 | 57 | 120 | 216 | 126 | 75 | 137 | 100 | 424 | 248 | 359 | 504 | 424 |
| Cr | 28 | 14 | 15 | 87 | 32 | 73 | 100 | 75 | 33 | 51 | 28 | 298 | 121 | 444 | 357 | 165 |
| Mn | 95 | 102 | 138 | 129 | 136 | 160 | 132 | 168 | 405 | 122 | 208 | 49 | 83 | 93 | 85 | 75 |
| Co | 5.6 | 4.5 | 3.4 | 2.8 | 3.7 | 3.0 | 2.6 | 3.5 | 9.0 | 3.7 | 4.5 | 11 | 12 | 8.0 | 6.2 | 5.3 |
| Ni | 18 | 11 | 6.8 | 31 | 14 | 22 | 22 | 23 | 19 | 21 | 13 | 44 | 49 | 77 | 84 | 92 |
| Cu | 20 | 10 | 27 | 17 | 10 | 32 | 16 | 23 | 23 | 21 | 15 | 27 | 23 | 26 | 23 | 23 |
| Zn | 62 | 41 | 59 | 45 | 50 | 71 | 62 | 66 | 95 | 83 | 74 | 80 | 64 | 62 | 66 | 72 |
| Ga | 9.0 | 14 | 18 | 11 | 17 | 15 | 15 | 18 | 19 | 22 | 20 | 36 | 27 | 15 | 11 | 4.0 |
| Ge | 1.2 | 1.3 | 1.4 | 1.1 | 1.5 | 1.7 | 1.6 | 1.8 | 1.5 | 1.8 | 1.8 | 3.4 | 2.5 | 1.7 | 1.1 | <dl |
| As | 7.6 | 10 | 11 | 9.1 | 15 | 12 | 9.4 | 13 | 21 | 9.2 | 15 | 26 | 48 | 40 | 17 | 14 |
| Se | 3.0 | 2.7 | 4.4 | 2.3 | 4.4 | 4.3 | 4.0 | 5.5 | 3.4 | 4.0 | 5.4 | 15 | 10.0 | 7.8 | 5.8 | 5.2 |
| Rb | 16 | 35 | 44 | 22 | 30 | 27 | 24 | 26 | 42 | 49 | 42 | 71 | 46 | 30 | 39 | 20 |
| Sr | 495 | 664 | 650 | 525 | 567 | 554 | 514 | 567 | 677 | 501 | 771 | 798 | 825 | 539 | 508 | 337 |
| Y | 19 | 23 | 28 | 19 | 23 | 30 | 49 | 35 | 11 | 33 | 33 | 38 | 33 | 53 | 43 | 29 |
| Zr | 67 | 103 | 186 | 89 | 108 | 124 | 132 | 148 | 54 | 124 | 122 | 184 | 167 | 137 | 146 | 63 |
| Nb | 6.5 | 7.0 | 14 | 4.8 | 7.3 | 12 | 19 | 14 | 5.3 | 12 | 8.6 | 19 | 15 | 10 | 9.9 | 1.9 |
| Mo | 20 | 25 | 13 | 22 | 31 | 36 | 69 | 43 | 38 | 39 | 35 | 8.7 | 45 | 101 | 149 | 126 |
| Cs | 4.3 | 9.7 | 12 | 6.2 | 8.9 | 9.7 | 7.7 | 8.5 | 15 | 17 | 16 | 27 | 19 | 13 | 9.4 | 6.7 |
| Ba | 84 | 221 | 250 | 132 | 178 | 170 | 140 | 239 | 74 | 91 | 81 | 107 | 81 | 51 | 74 | 45 |
| La | 22 | 18 | 28 | 21 | 26 | 29 | 30 | 38 | 17 | 32 | 34 | 99 | 105 | 44 | 21 | 11 |
| Ce | 36 | 32 | 49 | 35 | 45 | 50 | 51 | 66 | 32 | 59 | 62 | 313 | 208 | 86 | 41 | 23 |
| Pr | 4.6 | 4.1 | 6.2 | 4.3 | 5.8 | 6.4 | 6.4 | 8.2 | 4.3 | 7.4 | 7.8 | 24 | 21 | 9.8 | 5.5 | 3.7 |
| Nd | 18 | 15 | 23 | 16 | 22 | 24 | 23 | 30 | 17 | 27 | 30 | 88 | 70 | 36 | 22 | 15 |
| Sm | 4.1 | 4.1 | 6.0 | 3.9 | 6.4 | 5.6 | 5.4 | 7.3 | 3.8 | 6.5 | 6.5 | 16 | 14 | 8.7 | 5.3 | 4.3 |
| Eu | <dl | <dl | 1.0 | <dl | 0.90 | 0.83 | <dl | 1.0 | <dl | <dl | 1.1 | 2.5 | 1.6 | 1.2 | 0.85 | <dl |
| Gd | 2.8 | 3.0 | 4.2 | 2.8 | 4.3 | 4.4 | 4.2 | 4.7 | 2.0 | 4.5 | 3.8 | 12 | 8.0 | 6.5 | 4.0 | 3.2 |
| Tb | <dl | <dl | 0.82 | <dl | 0.83 | 0.88 | 0.99 | 0.95 | <dl | 0.92 | 0.72 | 2.0 | 1.4 | 1.5 | 0.92 | <dl |
| Dy | 3.7 | 4.5 | 5.7 | 3.6 | 5.0 | 6.1 | 8.3 | 6.8 | 2.3 | 6.3 | 5.3 | 11 | 8.7 | 11 | 6.4 | 5.2 |
| Ho | 0.83 | 1.0 | 1.3 | <dl | 1.1 | 1.3 | 2.1 | 1.6 | <dl | 1.4 | 1.3 | 2.2 | 1.8 | 2.6 | 1.6 | 1.3 |
| Er | 1.9 | 2.4 | 3.0 | 1.8 | 2.1 | 2.9 | 5.5 | 3.8 | 1.1 | 3.1 | 2.7 | 4.5 | 4.0 | 6.0 | 3.7 | 2.8 |
| Tm | <dl | 0.80 | 0.49 | <dl | 0.36 | 0.51 | 2.0 | 0.62 | <dl | 1.0 | 0.42 | 0.75 | 0.68 | 1.0 | 0.58 | 0.95 |
| Yb | 2.4 | 3.2 | 4.2 | 2.1 | 2.6 | 4.0 | 8.3 | 5.2 | 1.3 | 4.0 | 3.2 | 6.3 | 5.5 | 8.1 | 4.5 | 3.8 |
| Lu | <dl | <dl | 0.69 | <dl | 0.44 | 0.66 | 1.4 | 0.87 | <dl | <dl | 0.54 | 1.0 | 0.92 | 1.3 | 0.76 | <dl |
| Hf | 1.8 | 3.0 | 4.7 | 2.6 | 3.6 | 3.4 | 3.1 | 3.9 | 1.8 | 3.8 | 3.6 | 5.4 | 4.8 | 3.5 | 3.4 | 1.4 |
| Ta | <dl | <dl | <dl | <dl | <dl | <dl | <dl | <dl | <dl | <dl | <dl | <dl | <dl | <dl | <dl | <dl |
| Pb | 19 | 32 | 29 | 17 | 26 | 26 | 26 | 29 | 25 | 34 | 32 | 66 | 38 | 35 | 31 | 32 |
| Th | 7.3 | 17 | 15 | 9.6 | 16 | 15 | 15 | 20 | 7.8 | 15 | 11 | 21 | 19 | 13 | 7.8 | 5.2 |
| U | 20 | 23 | 19 | 11 | 14 | 31 | 85 | 43 | 19 | 42 | 33 | 44 | 25 | 60 | 126 | 125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Zhang, F.; Liao, J.; Li, B.; Zhuang, X.; Querol, X.; Moreno, N.; Shangguan, Y. Geological Controls on Geochemical Anomaly of the Carbonaceous Mudstones in Xian’an Coalfield, Guangxi Province, China. Energies 2022, 15, 5196. https://doi.org/10.3390/en15145196
Li B, Zhang F, Liao J, Li B, Zhuang X, Querol X, Moreno N, Shangguan Y. Geological Controls on Geochemical Anomaly of the Carbonaceous Mudstones in Xian’an Coalfield, Guangxi Province, China. Energies. 2022; 15(14):5196. https://doi.org/10.3390/en15145196
Chicago/Turabian StyleLi, Bo, Fuqiang Zhang, Jialong Liao, Baoqing Li, Xinguo Zhuang, Xavier Querol, Natalia Moreno, and Yunfei Shangguan. 2022. "Geological Controls on Geochemical Anomaly of the Carbonaceous Mudstones in Xian’an Coalfield, Guangxi Province, China" Energies 15, no. 14: 5196. https://doi.org/10.3390/en15145196
APA StyleLi, B., Zhang, F., Liao, J., Li, B., Zhuang, X., Querol, X., Moreno, N., & Shangguan, Y. (2022). Geological Controls on Geochemical Anomaly of the Carbonaceous Mudstones in Xian’an Coalfield, Guangxi Province, China. Energies, 15(14), 5196. https://doi.org/10.3390/en15145196







