Investigation of Pyrolysis Behavior of Sewage Sludge by Thermogravimetric Analysis Coupled with Fourier Transform Infrared Spectrometry Using Different Heating Rates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Apparent Kinetic Parameters on the Basis of Arrhenius Equation Using First-Order Kinetic Approach
2.3. Kinetic Parameters on the Basis of Model-Free Methods
2.3.1. Flynn–Wall–Ozawa Model
2.3.2. Kissinger–Akira–Sunose Model
2.3.3. Friedman Model
3. Results and Discussion
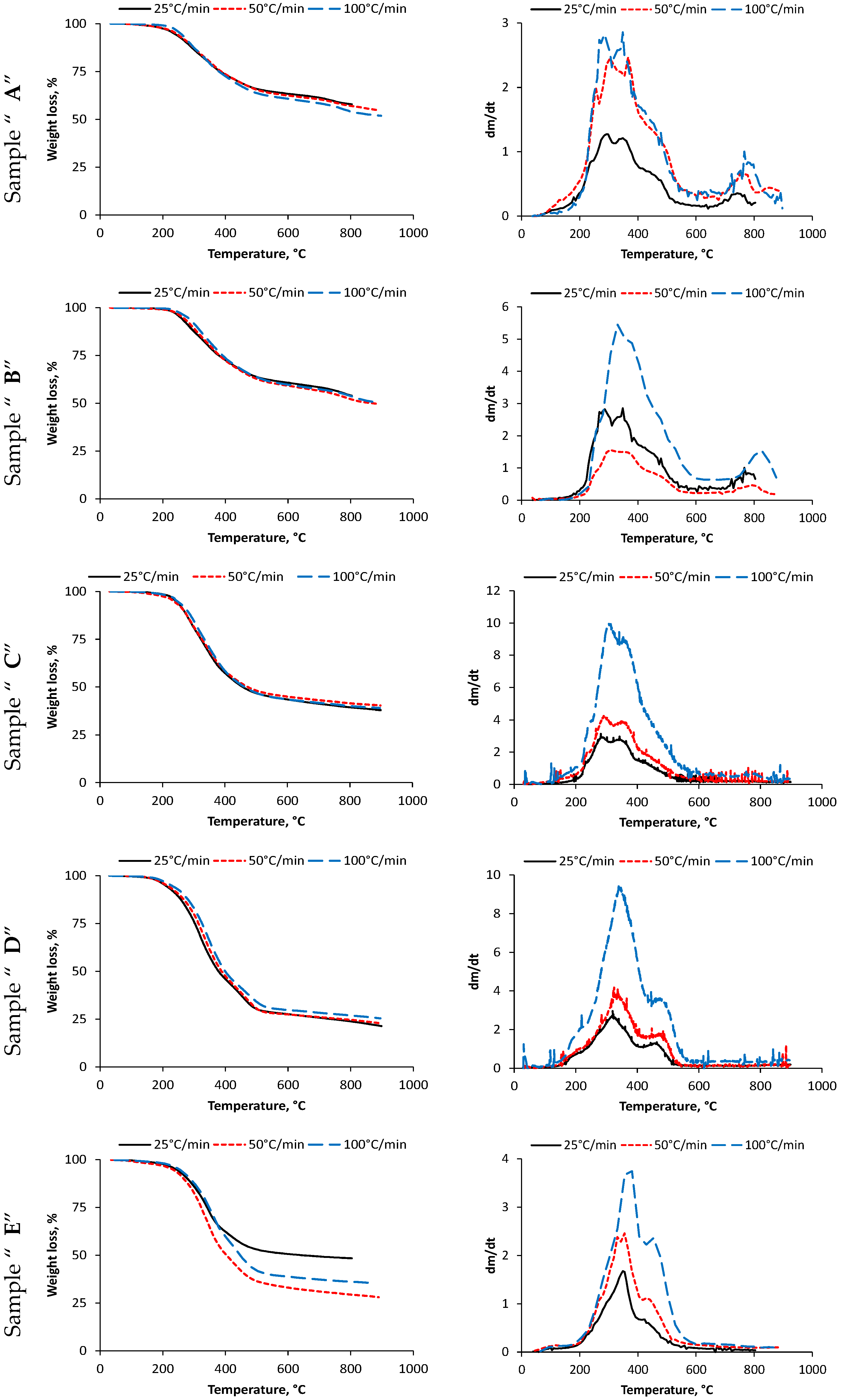
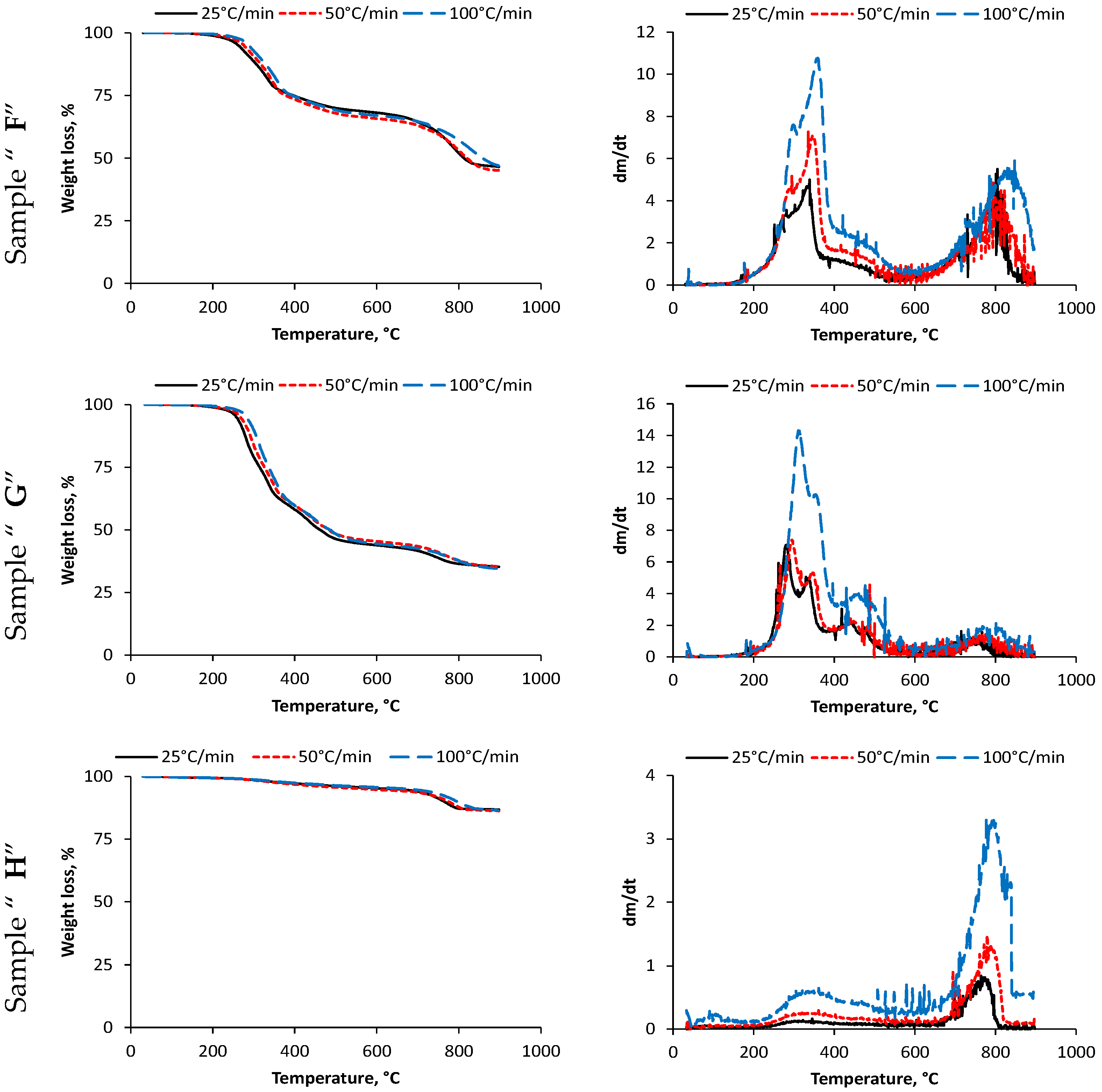
3.1. Weight Loss of Samples
3.2. FTIR Results of Sample Decompositions
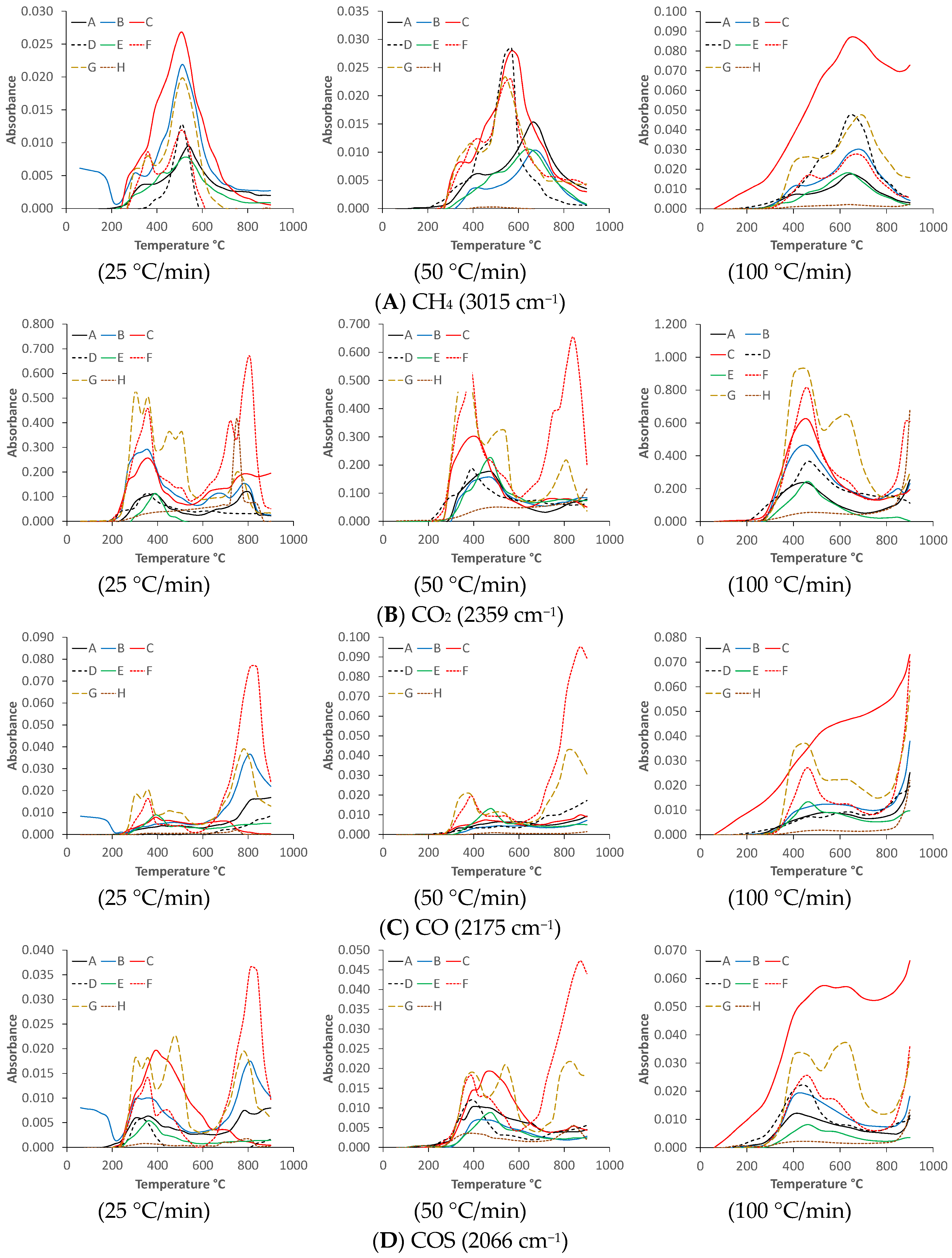
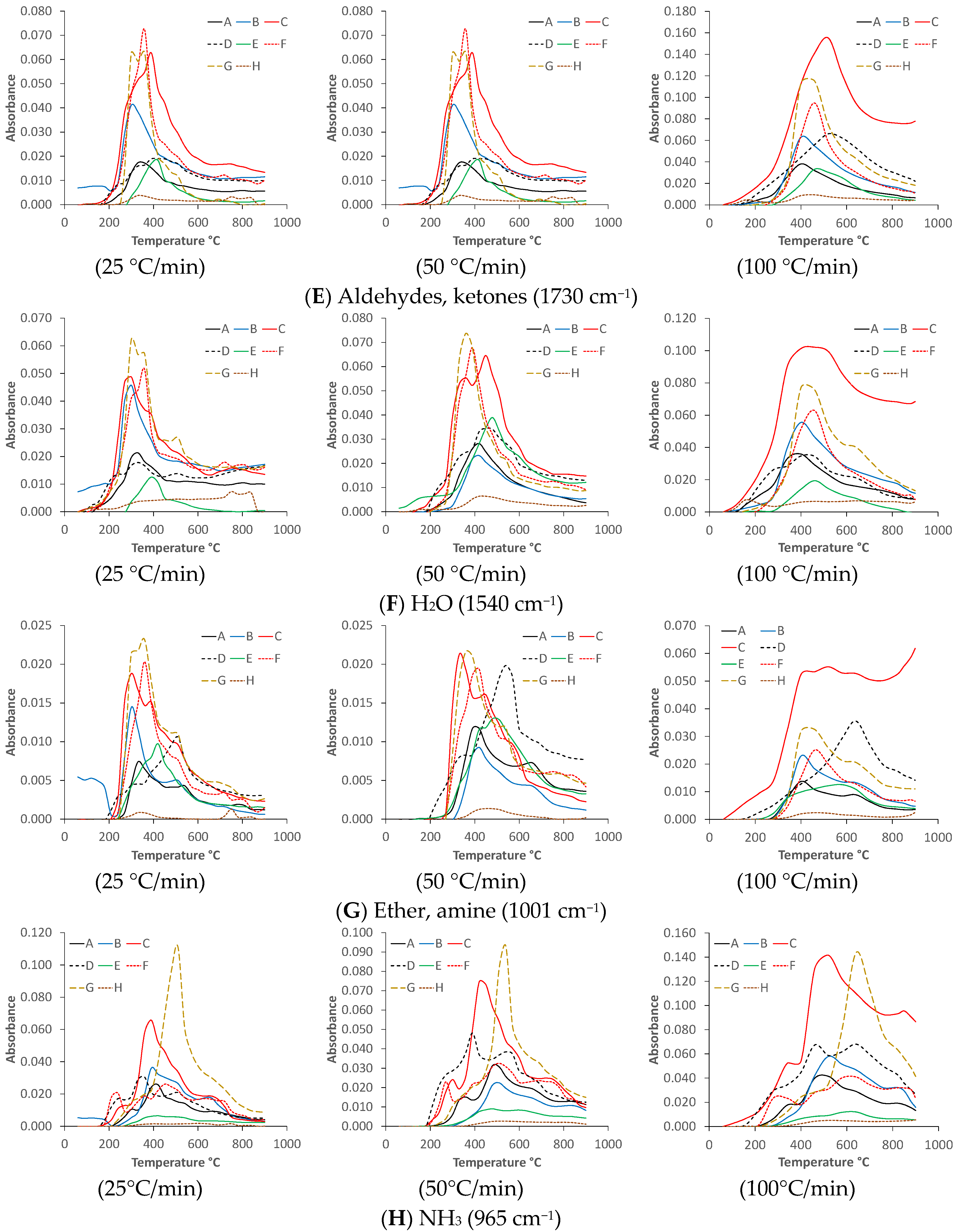
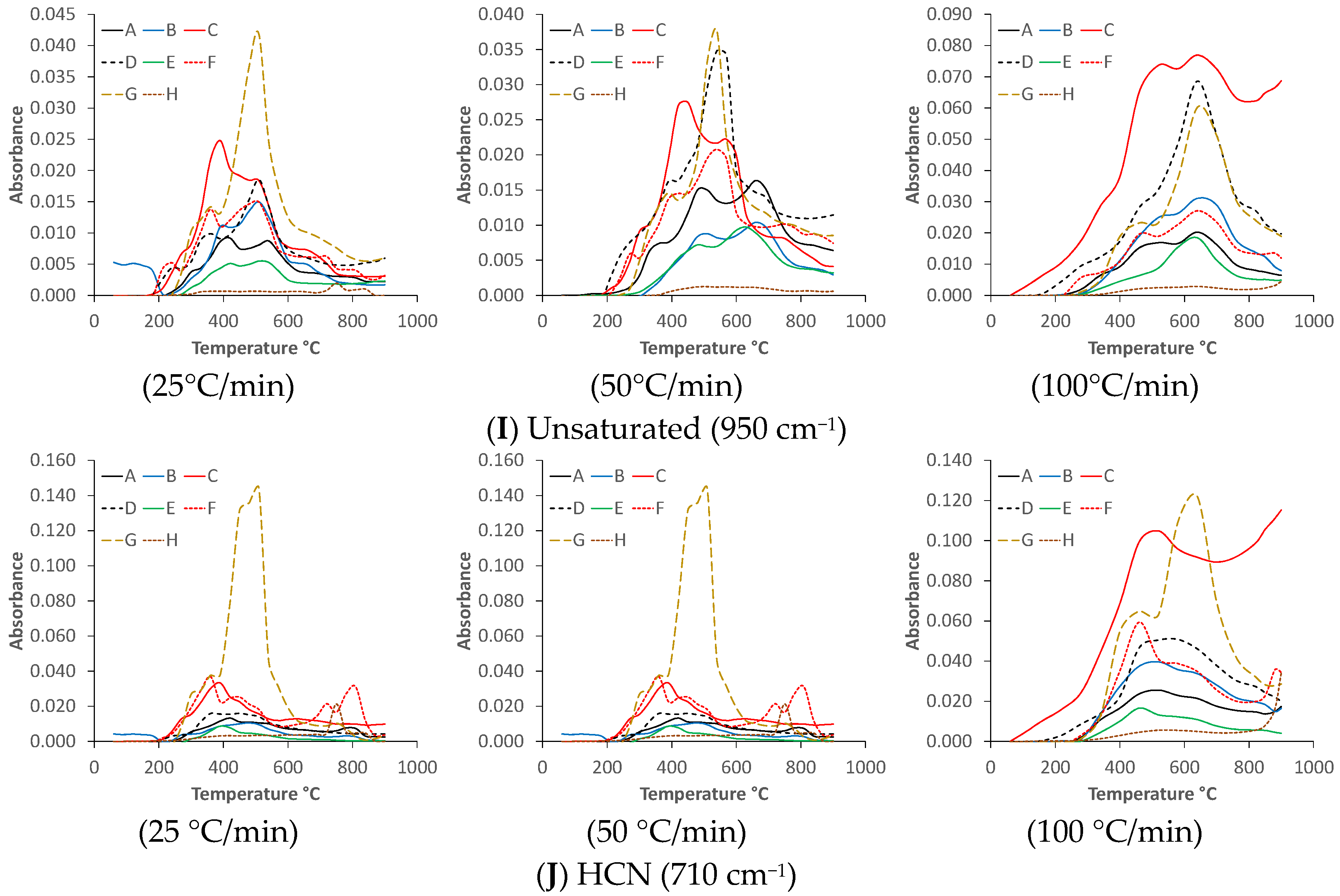
3.3. Reaction Kinetic Calculations
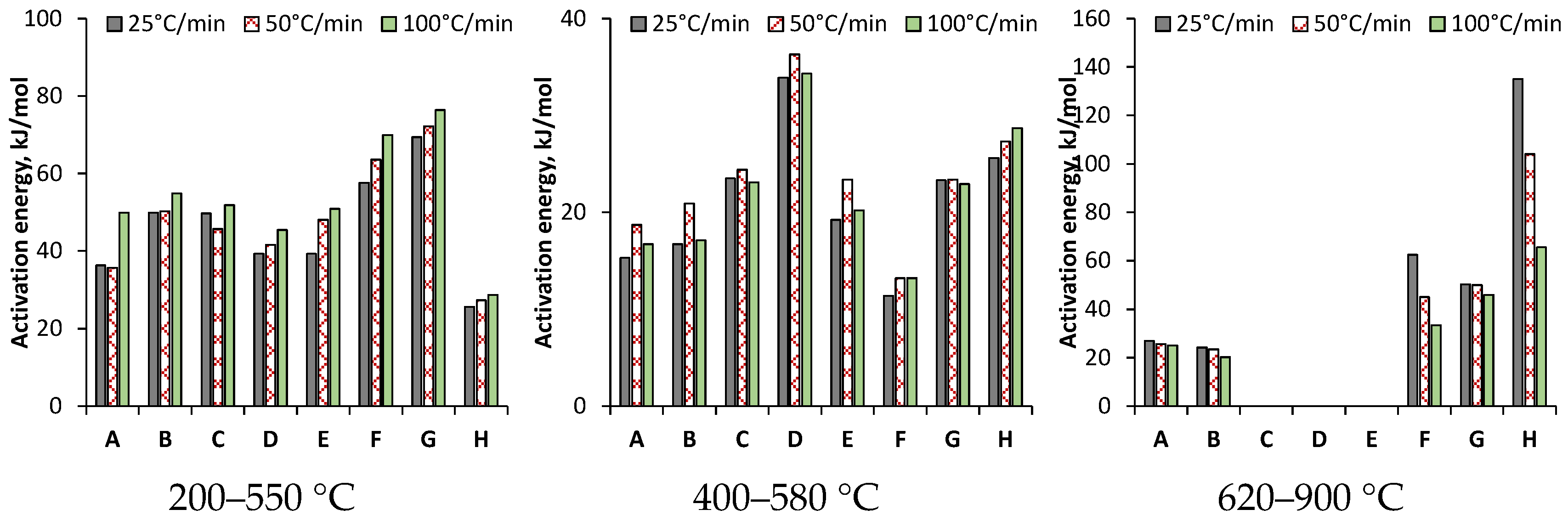
3.3.1. On the Basis of Arrhenius Equation Using First-Order Kinetic Approach
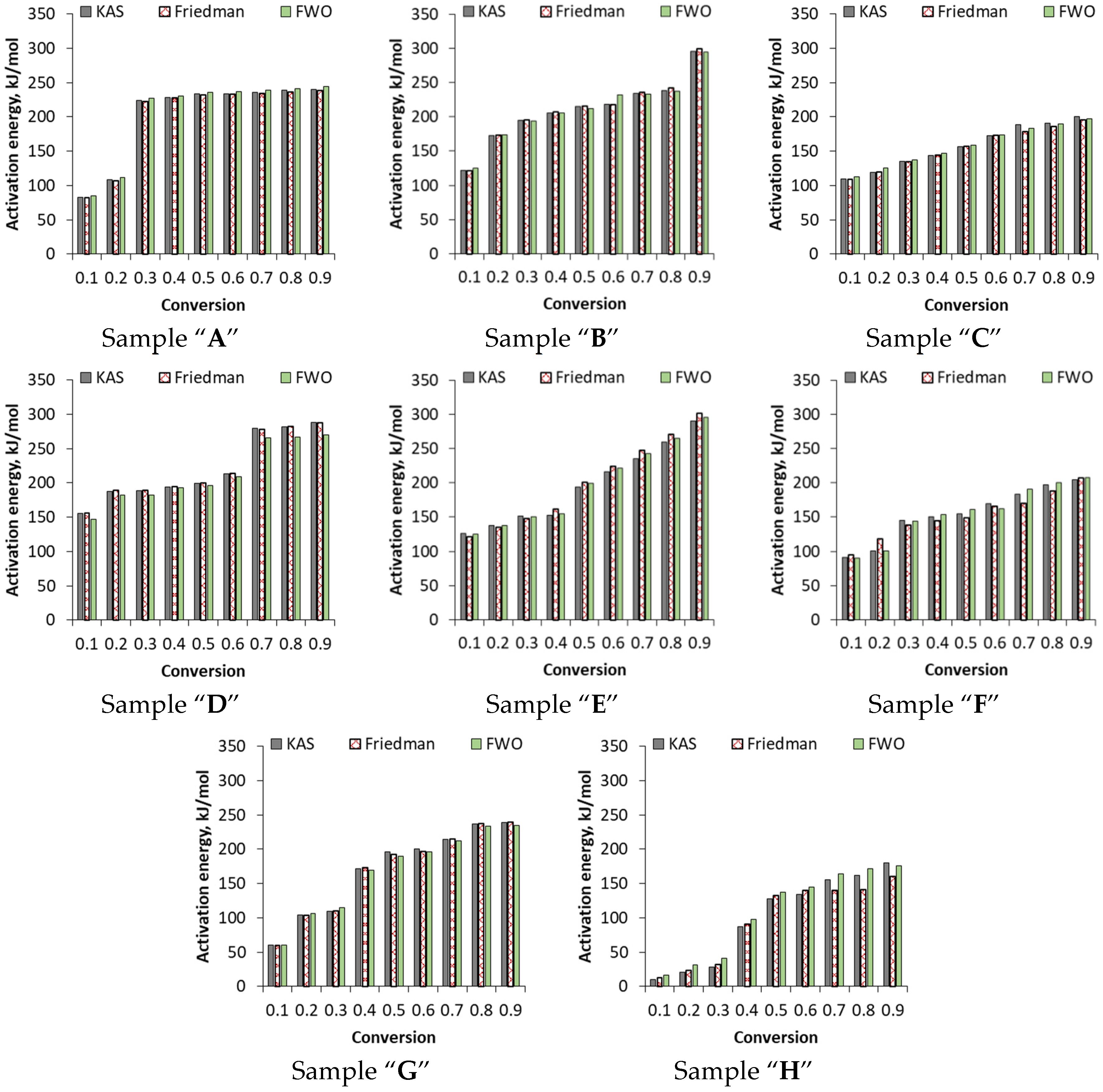
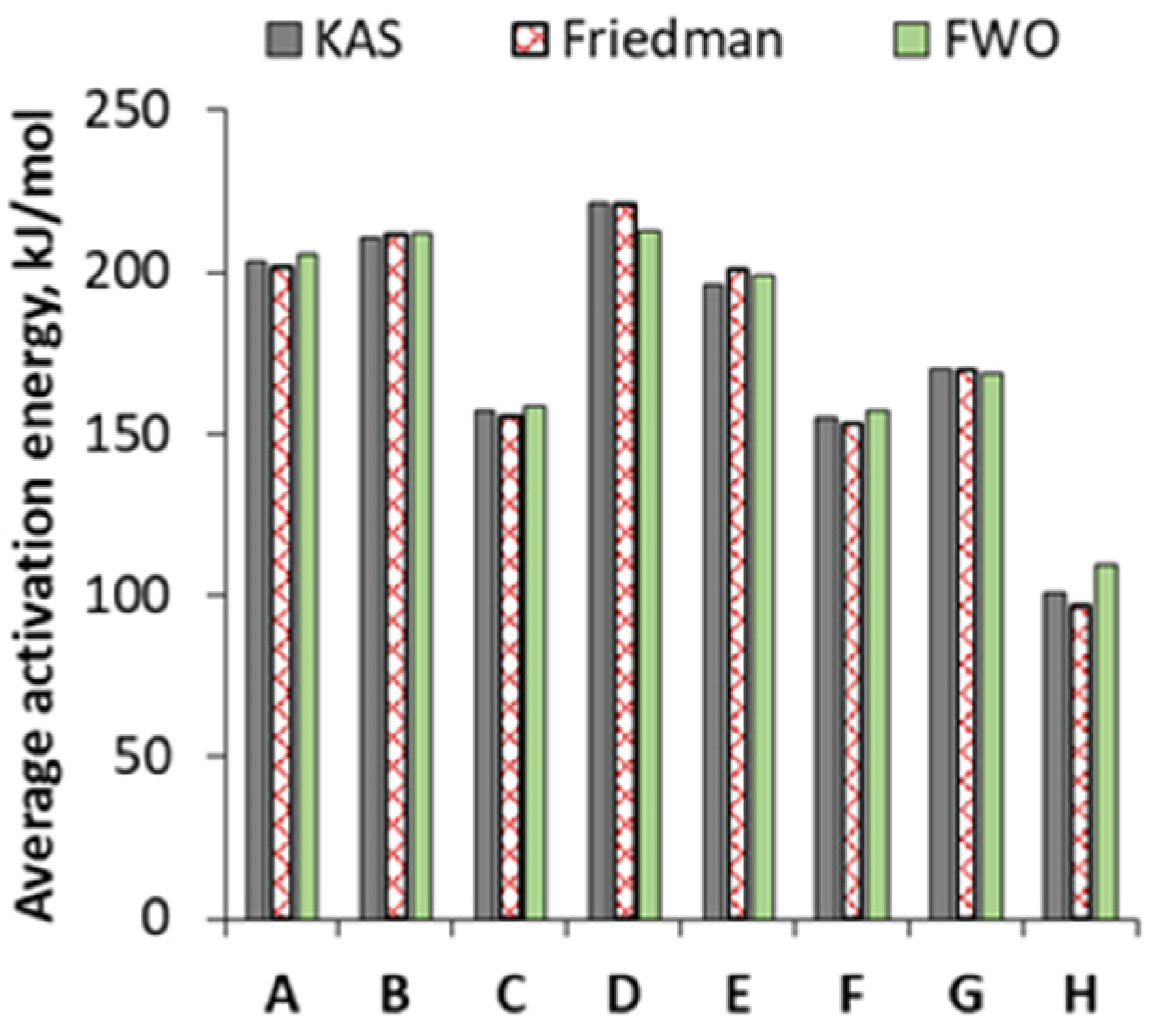
3.3.2. Kinetic Parameters on the Basis of FWO, Friedman and KAS Models
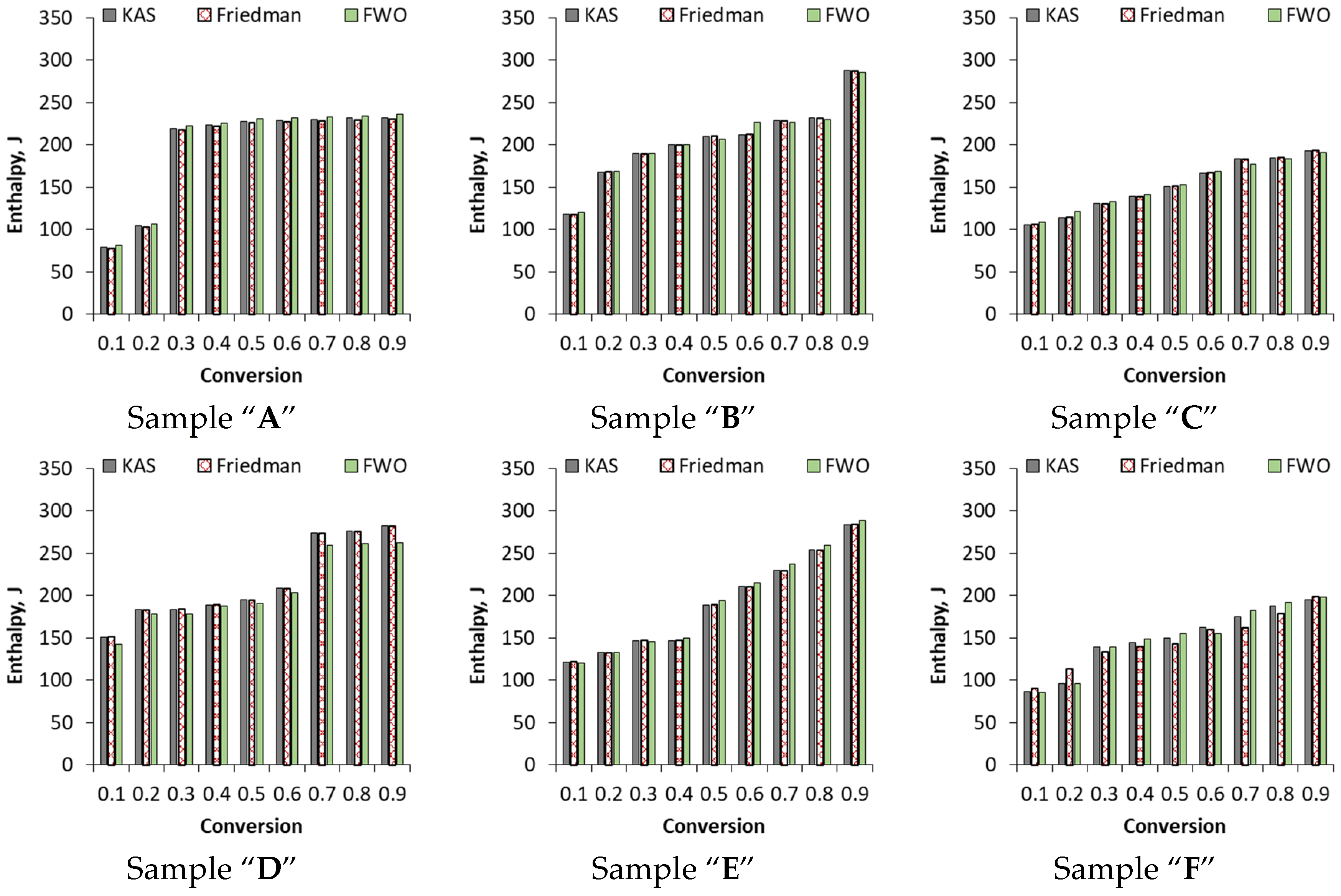
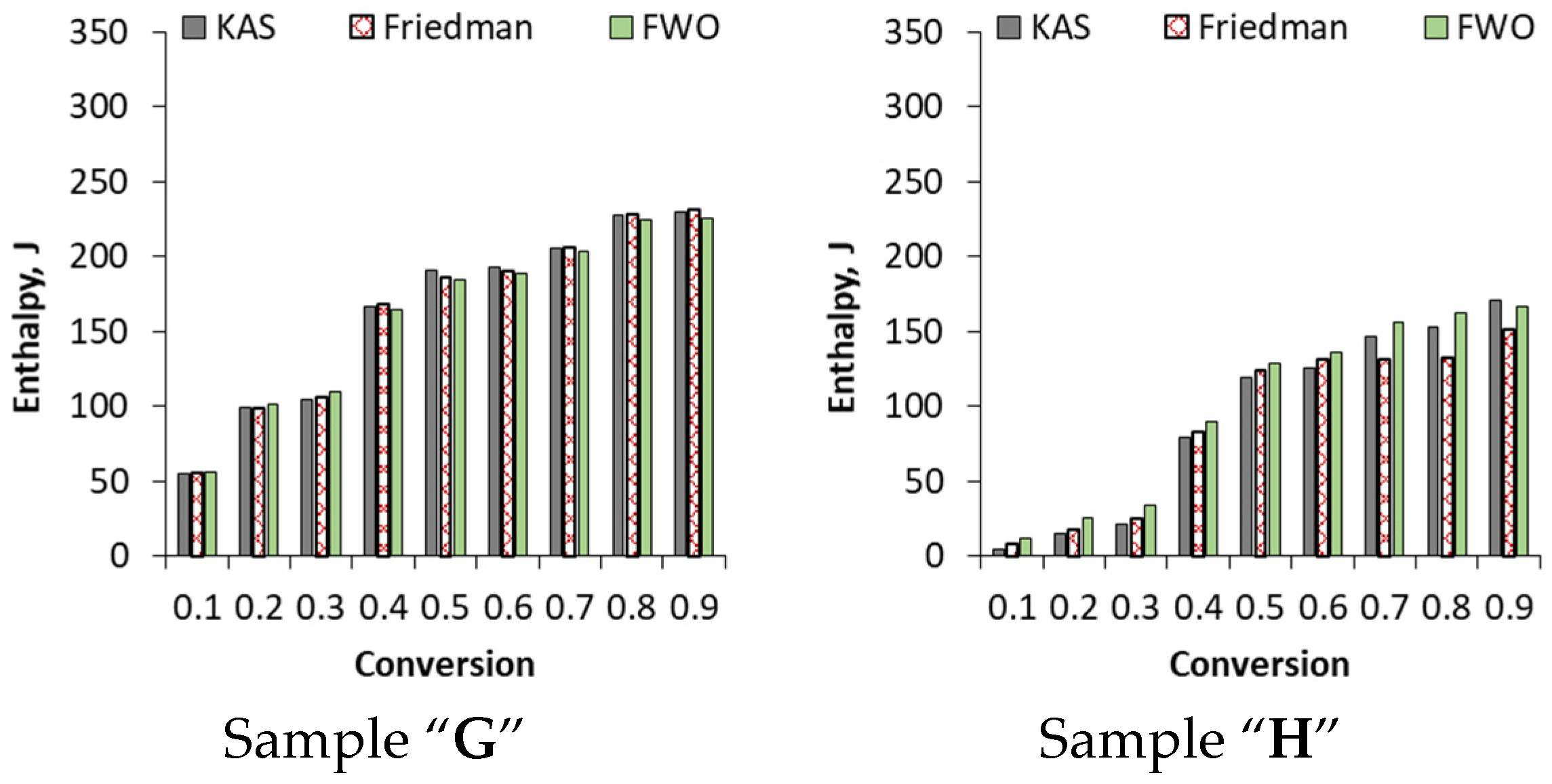
3.3.3. Enthalpy Calculated by KAS, Friedman and FWO Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.; Kim, Y.-M.; Siddiqui, M.Z.; Park, Y.-K. Different pyrolysis kinetics and product distribution of municipal and livestock manure sewage sludge. Environ. Pollut. 2021, 285, 117197. [Google Scholar] [CrossRef] [PubMed]
- Mateo-Sagasta, J.; Raschid-Sally, L.; Thebo, A. Global wastewater and sludge production, treatment and use. In Wastewater; Drechsel, P., Qadir, M., Wichelns, D., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 15–38. [Google Scholar] [CrossRef]
- Đurđević, D.; Blecich, P.; Jurić, Ž. Energy Recovery from Sewage Sludge: The Case Study of Croatia. Energies 2019, 12, 1927. [Google Scholar] [CrossRef] [Green Version]
- Dumontet, S.; Scopa, A.; Kerje, S.; Krovacek, K. The Importance of Pathogenic Organisms in Sewage and Sewage Sludge. J. Air Waste Manag. Assoc. 2011, 51, 848–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebreeyessus, G.D.; Jenicek, P. Thermophilic versus Mesophilic Anaerobic Digestion of Sewage Sludge: A Comparative Review. Bioengineering 2016, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Buta, M.; Hubeny, J.; Zieliński, W.; Harnisz, M.; Korzeniewska, E. Sewage sludge in agriculture—The effects of selected chemical pollutants and emerging genetic resistance determinants on the quality of soil and crops—A review. Ecotoxicol. Environ. Saf. 2021, 214, 112070. [Google Scholar] [CrossRef] [PubMed]
- Mininni, G.; Blanch, A.R.; Lucena, F.; Berselli, S. EU policy on sewage sludge utilization and perspectives on new approaches of sludge management. Environ. Sci. Pollut. Res. 2015, 22, 7361–7374. [Google Scholar] [CrossRef]
- Djandja, O.S.; Wang, Z.-C.; Wang, F.; Xu, Y.-P.; Duan, P.-G. Pyrolysis of Municipal Sewage Sludge for Biofuel Production: A Review. Ind. Eng. Chem. Res. 2020, 59, 16939–16956. [Google Scholar] [CrossRef]
- Moško, J.; Pohořelý, M.; Skoblia, S.; Beňo, Z.; Jeremiáš, M. Detailed Analysis of Sewage Sludge Pyrolysis Gas: Effect of Pyrolysis Temperature. Energies 2020, 13, 4087. [Google Scholar] [CrossRef]
- Agar, D.A.; Kwapinska, M.; Leahy, J.J. Pyrolysis of wastewater sludge and composted organic fines from municipal solid waste: Laboratory reactor characterisation and product distribution. Environ. Sci. Pollut. Res. 2018, 25, 35874–35882. [Google Scholar] [CrossRef]
- Hanif, M.U.; Zwawi, M.; Capareda, S.C.; Iqbal, H.; Algarni, M.; Felemban, B.F.; Bahadar, A.; Waqas, A. Influence of Pyrolysis Temperature on Product Distribution and Characteristics of Anaerobic Sludge. Energies 2020, 13, 79. [Google Scholar] [CrossRef] [Green Version]
- Gbouri, I.; Yu, F.; Wang, X.; Wang, J.; Cui, X.; Hu, Y.; Yan, B.; Chen, G. Co-Pyrolysis of Sewage Sludge and Wetland Biomass Waste for Biochar Production: Behaviors of Phosphorus and Heavy Metals. Int. J. Environ. Res. Public Health 2022, 19, 2818. [Google Scholar] [CrossRef]
- Tang, S.; Tian, S.; Zheng, C.; Zhang, Z. Effect of Calcium Hydroxide on the Pyrolysis Behavior of Sewage Sludge: Reaction Characteristics and Kinetics. Energy Fuels 2017, 31, 5079–5087. [Google Scholar] [CrossRef]
- Chan, W.P.; Wang, J.-Y. Formation of synthetic sludge as a representative tool for thermochemical conversion modelling and performance analysis of sewage sludge–Based on a TG-FTIR study. J. Anal. Appl. Pyrolysis 2018, 133, 97–106. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Soria-Verdugo, A. Pyrolysis of sludge and biomass residues. In Wastewater Treatment Residues as Resources for Biorefinery Products and Biofuels; Elsevier: Amsterdam, The Netherlands, 2020; Volume 8, pp. 155–181. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Tariq, R.; Hameed, Z.; Ali, I.; Naqvi, M.; Chen, W.-H.; Ceylan, S.; Rashid, H.; Ahmad, J.; Taqvi, S.A.; et al. Pyrolysis of high ash sewage sludge: Kinetics and thermodynamic analysis using Coats-Redfern method. Renew. Energy 2019, 131, 854–860. [Google Scholar] [CrossRef]
- Sobek, S.; Werle, S. Isoconversional determination of the apparent reaction models governing pyrolysis of wood, straw and sewage sludge, with an approach to rate modelling. Renew. Energy 2020, 161, 972–987. [Google Scholar] [CrossRef]
- González-Arias, J.; Gil, M.V.; Fernández, R.; Martínez, E.J.; Fernández, C.; Papaharalabos, G.; Gómez, X. Integrating anaerobic digestion and pyrolysis for treating digestates derived from sewage sludge and fat wastes. Environ. Sci. Pollut. Res. 2020, 27, 32603–32614. [Google Scholar] [CrossRef]
- Wen, S.; Yan, Y.; Liu, J.; Buyukada, M.; Evrendilek, F. Pyrolysis performance, kinetic, thermodynamic, product and joint optimization analyses of incense sticks in N2 and CO2 atmospheres. Renew. Energy 2019, 141, 814–827. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ma, J.; Luo, H.; Li, Y.; Liu, Z.; Li, D.; Gai, C.; Jiao, W. Pyrolysis kinetics and thermodynamic parameters of the hydrochars derived from co-hydrothermal carbonization of sawdust and sewage sludge using thermogravimetric analysis. Bioresour. Technol. 2019, 282, 133–141. [Google Scholar] [CrossRef]
- Xu, Q.; Tang, S.; Wang, J.; Ko, J.H. Pyrolysis kinetics of sewage sludge and its biochar characteristics. Process Saf. Environ. Prot. 2018, 115, 49–56. [Google Scholar] [CrossRef]
- Hayhurst, A.N. The kinetics of the pyrolysis or devolatilisation of sewage sludge and other solid fuels. Combust. Flame 2013, 160, 138–144. [Google Scholar] [CrossRef]
- Chen, X.; Jeyaseelan, S. Study of Sewage Sludge Pyrolysis Mechanism and Mathematical Modeling. J. Environ. Eng. 2001, 127, 585–593. [Google Scholar] [CrossRef]
- Lin, Y.; Tian, Y.; Xia, Y.; Fang, S.; Liao, Y.; Yu, Z.; Ma, X. General distributed activation energy model (G-DAEM) on co-pyrolysis kinetics of bagasse and sewage sludge. Bioresour. Technol. 2019, 273, 545–555. [Google Scholar] [CrossRef]
- Lai, Z.; Ma, X.; Tang, Y.; Lin, H. A study on municipal solid waste (MSW) combustion in N2/O2 and CO2/O2 atmosphere from the perspective of TGA. Energy 2011, 36, 819–824. [Google Scholar] [CrossRef]
- Naqvi, S.R.; Tariq, R.; Shahbaz, M.; Naqvi, M.; Aslam, M.; Khan, Z.; Mackey, H.; Mckay, G.; Al-Ansari, T. Recent developments on sewage sludge pyrolysis and its kinetics: Resources recovery, thermogravimetric platforms, and innovative prospects. Comput. Chem. Eng. 2021, 150, 107325. [Google Scholar] [CrossRef]
- Petrovič, A.; Vohl, S.; Predikaka, T.C.; Bedoić, R.; Simonič, M.; Ban, I.; Čuček, L. Pyrolysis of Solid Digestate from Sewage Sludge and Lignocellulosic Biomass: Kinetic and Thermodynamic Analysis, Characterization of Biochar. Sustainability 2021, 13, 9642. [Google Scholar] [CrossRef]
- Gou, X.; Zhao, X.; Singh, S.; Qiao, D. Tri-pyrolysis: A thermo-kinetic characterisation of polyethylene, cornstalk, and anthracite coal using TGA-FTIR analysis. Fuel 2019, 252, 393–402. [Google Scholar] [CrossRef]
- Giwa, A.S.; Xu, H.; Wu, J.; Li, Y.; Chang, F.; Zhang, X.; Jin, Z.; Huang, B.; Wang, K. Sustainable recycling of residues from the food waste (FW) composting plant via pyrolysis: Thermal characterization and kinetic studies. J. Clean. Prod. 2018, 180, 43–49. [Google Scholar] [CrossRef]
- Menares, T.; Herrera, J.; Romero, R.; Osorio, P.; Arteaga-Pérez, L.E. Waste tires pyrolysis kinetics and reaction mechanisms explained by TGA and Py-GC/MS under kinetically-controlled regime. Waste Manag. 2020, 102, 21–29. [Google Scholar] [CrossRef]
- Yan, J.; Yang, Q.; Zhang, L.; Lei, Z.; Li, Z.; Wang, Z.; Ren, S.; Kang, S.; Shui, H. Investigation of kinetic and thermodynamic parameters of coal pyrolysis with model-free fitting methods. Carbon Resour. Convers. 2020, 3, 173–181. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Zhang, X.; Che, D. A Study on Coal Properties and Combustion Characteristics of Blended Coals in Northwestern China. Energy Fuels 2011, 25, 3634–3645. [Google Scholar] [CrossRef]
- Leroy, V.; Cancellieri, D.; Leoni, E.; Rossi, J.-L. Kinetic study of forest fuels by TGA: Model-free kinetic approach for the prediction of phenomena. Thermochim. Acta 2010, 497, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Xu, G.; Li, G. Pyrolysis characteristic and kinetic analysis of sewage sludge using model-free and master plots methods. Process Saf. Environ. Prot. 2021, 149, 48–55. [Google Scholar] [CrossRef]

| Model | Description | Reference |
|---|---|---|
| Coats and Redfern model | Integrated kinetic model using simplified Arrhenius equation. | [17] |
| Friedman model | Determination of activation energies for different conversions. | [18] |
| Flynn–Wall–Ozawa model | Determination of activation energies for different conversions. Typically used for solid samples. | [19] |
| Horowitz–Metzger method | Suitable for different types of waste (biomass, plastic, sewage sludge). | [16,20] |
| Kissinger method | Used for reaction kinetic characterization of mixtures. | [21] |
| Kissinger–Akira–Sunose model | Uses the appropriate temperature for each conversion, instead of the peak maximum. | [22] |
| Single reaction model | Thermal decomposition processes take place through the same thermal reactivity. The reliability of the final result is limited and largely depends on the description of the gross process. | [23] |
| Parallel reactions model | Multiple parallel reaction models. Usually three-step. Used for biomass. In the case of sewage sludge, modification is needed. | [24] |
| Consecutive reactions model | Sequential reactions. Generally an isothermal kinetic approach. Used for biomass, plastic waste and sewage sludge. | [25] |
| Distributed activation energy model | It monitors the ongoing processes as a complex system of reactions with constantly changing activation energies, but with a constant pre-exponential factor. Used for biomass, plastic waste and sewage sludge. | [26] |
| Vyazovkin method | Non-isothermal method independent from heating rate. | [19,27] |
| Starink’s model | Non-isothermal method independent from heating rate. | [28] |
| A | B | C | D | E | F | G | H | |
|---|---|---|---|---|---|---|---|---|
| Source | Municipal sewage sludge | Municipal sewage sludge | Municipal sewage sludge | Municipal sewage sludge | Distillery sludge | Cattle manure | Chicken manure | Brook mud |
| Water content, % (1) | 55.36 | 51.86 | 37.88 | 22.83 | 47.42 | 46.57 | 35.28 | 86.71 |
| Fixed carbon, % | 7.75 | 12.44 | 12.83 | 4.47 | 23.23 | 5.89 | 15.07 | 12.67 |
| Ash content, % | 47.61 | 39.42 | 25.05 | 18.36 | 24.19 | 40.68 | 20.21 | 74.04 |
| Volatiles, % | 44.64 | 48.14 | 62.12 | 78.66 | 52.28 | 53.43 | 64.72 | 13.29 |
| C | 23.4 | 31.2 | 35.4 | 50.5 | 42.3 | 24.8 | 34.7 | 9.6 |
| H | 3.4 | 4.0 | 5.5 | 7.8 | 5.2 | 3.0 | 4.5 | 1.0 |
| N | 3.8 | 4.4 | 5.5 | 4.5 | 0.8 | 2.9 | 4.6 | 0.7 |
| S | 2.0 | 1.4 | 2.6 | 0.0 | 0.0 | 1.3 | 1.6 | 0.0 |
| O | 52.9 | 44.7 | 41.6 | 28.6 | 31.7 | 54.5 | 40.1 | 66.6 |
| Na | 0.7 | 0.3 | 0.6 | 0.1 | 1.5 | 0.4 | 0.8 | 0.5 |
| Mg | 1.0 | 0.5 | 0.5 | 0.4 | 1.4 | 0.4 | 0.5 | 1.1 |
| Al | 1.1 | 2.2 | 1.6 | 0.2 | 1.9 | 0.3 | 0.2 | 2.2 |
| Si | 2.6 | 3.1 | 0.4 | 0.6 | 7.2 | 0.7 | 0.3 | 11.6 |
| P | 3.4 | 2.6 | 2.1 | 1.3 | 1.6 | 1.2 | 2.1 | 0.1 |
| K | 0.4 | 1.6 | 0.3 | 0.3 | 0.5 | 1.8 | 4.8 | 0.6 |
| Ca | 1.9 | 1.9 | 2.2 | 2.3 | 3.8 | 7.9 | 5.1 | 4.6 |
| Fe | 3.4 | 1.7 | 1.3 | 2.5 | 1.8 | 0.4 | 0.0 | 1.3 |
| Other | 0.1 | 0.2 | 0.3 | 0.7 | 0.3 | 0.4 | 0.6 | 0.2 |
| Sample | Heating Rate, °C/min | 1st Step | Residue 1st Step, % | 2nd Step | Residue (2nd Step), % | 3rd Step | Residue (3rd Step), % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | Tm | Tf | Ti | Tm | Tf | Ti | Tm | Tf | |||||
| A | 25 | 185 | 298 | 398 | 73.54 | 410 | - | 541 | 64.64 | 661 | 753 | 791 | 55.36 |
| 50 | 191 | 302 | 404 | 73.21 | 419 | - | 554 | 63.51 | 668 | 757 | 794 | 54.71 | |
| 100 | 198 | 315 | 411 | 71.38 | 427 | - | 562 | 61.65 | 681 | 762 | 799 | 51.86 | |
| B | 25 | 197 | 292 | 403 | 72.06 | 411 | - | 554 | 61.81 | 654 | 728 | 791 | 51.83 |
| 50 | 205 | 298 | 410 | 70.37 | 417 | - | 559 | 60.25 | 662 | 735 | 794 | 49.55 | |
| 100 | 217 | 306 | 415 | 70.15 | 419 | - | 567 | 60.48 | 679 | 746 | 800 | 50.60 | |
| C | 25 | 205 | 292 | 400 | 57.16 | 400 | - | 556 | 44.57 | - | - | - | 37.88 |
| 50 | 229 | 303 | 405 | 57.56 | 470 | - | 562 | 45.73 | - | - | - | 38.91 | |
| 100 | 217 | 320 | 410 | 56.53 | 410 | - | 574 | 43.99 | - | - | - | 38.89 | |
| D | 25 | 211 | 316 | 405 | 45.01 | 405 | 456 | 518 | 29.28 | - | - | - | 21.34 |
| 50 | 220 | 336 | 416 | 48.16 | 416 | 476 | 536 | 28.33 | - | - | - | 22.83 | |
| 100 | 222 | 338 | 426 | 44.91 | 426 | 465 | 539 | 30.89 | - | - | - | 25.44 | |
| E | 25 | 217 | 354 | 429 | 58.54 | 442 | 435 | 571 | 51.06 | - | - | - | 47.72 |
| 50 | 229 | 362 | 435 | 45.42 | 451 | 453 | 586 | 33.38 | - | - | - | 28.05 | |
| 100 | 233 | 267 | 441 | 49.19 | 458 | 459 | 602 | 38.72 | - | - | - | 35.45 | |
| F | 25 | 154 | 333 | 378 | 76.10 | 390 | - | 547 | 69.01 | 665 | 804 | 866 | 46.57 |
| 50 | 180 | 342 | 379 | 74.89 | 390 | - | 560 | 66.42 | 678 | 810 | 880 | 45.01 | |
| 100 | 181 | 355 | 391 | 75.18 | 395 | - | 570 | 67.47 | 710 | 824 | 894 | 40.98 | |
| G | 25 | 208 | 282 | 367 | 60.34 | 390 | 443 | 540 | 45.17 | 679 | 744 | 827 | 35.28 |
| 50 | 218 | 296 | 379 | 60.39 | 399 | 447 | 545 | 46.62 | 683 | 770 | 889 | 35.40 | |
| 100 | 234 | 316 | 400 | 59.69 | 415 | 457 | 565 | 45.14 | 690 | 792 | 891 | 34.91 | |
| H | 25 | 220 | 318 | 530 | 95.88 | - | - | - | - | 650 | 772 | 810 | 86.71 |
| 50 | 228 | 337 | 542 | 95.21 | - | - | - | - | 662 | 780 | 820 | 86.24 | |
| 100 | 250 | 351 | 558 | 96.80 | - | - | - | - | 680 | - | - | 81.34 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miskolczi, N.; Tomasek, S. Investigation of Pyrolysis Behavior of Sewage Sludge by Thermogravimetric Analysis Coupled with Fourier Transform Infrared Spectrometry Using Different Heating Rates. Energies 2022, 15, 5116. https://doi.org/10.3390/en15145116
Miskolczi N, Tomasek S. Investigation of Pyrolysis Behavior of Sewage Sludge by Thermogravimetric Analysis Coupled with Fourier Transform Infrared Spectrometry Using Different Heating Rates. Energies. 2022; 15(14):5116. https://doi.org/10.3390/en15145116
Chicago/Turabian StyleMiskolczi, Norbert, and Szabina Tomasek. 2022. "Investigation of Pyrolysis Behavior of Sewage Sludge by Thermogravimetric Analysis Coupled with Fourier Transform Infrared Spectrometry Using Different Heating Rates" Energies 15, no. 14: 5116. https://doi.org/10.3390/en15145116
APA StyleMiskolczi, N., & Tomasek, S. (2022). Investigation of Pyrolysis Behavior of Sewage Sludge by Thermogravimetric Analysis Coupled with Fourier Transform Infrared Spectrometry Using Different Heating Rates. Energies, 15(14), 5116. https://doi.org/10.3390/en15145116






