Techno-Economic Assessment of the Integration of Direct Air Capture and the Production of Solar Fuels
Abstract
:1. Introduction
2. Materials and Methods
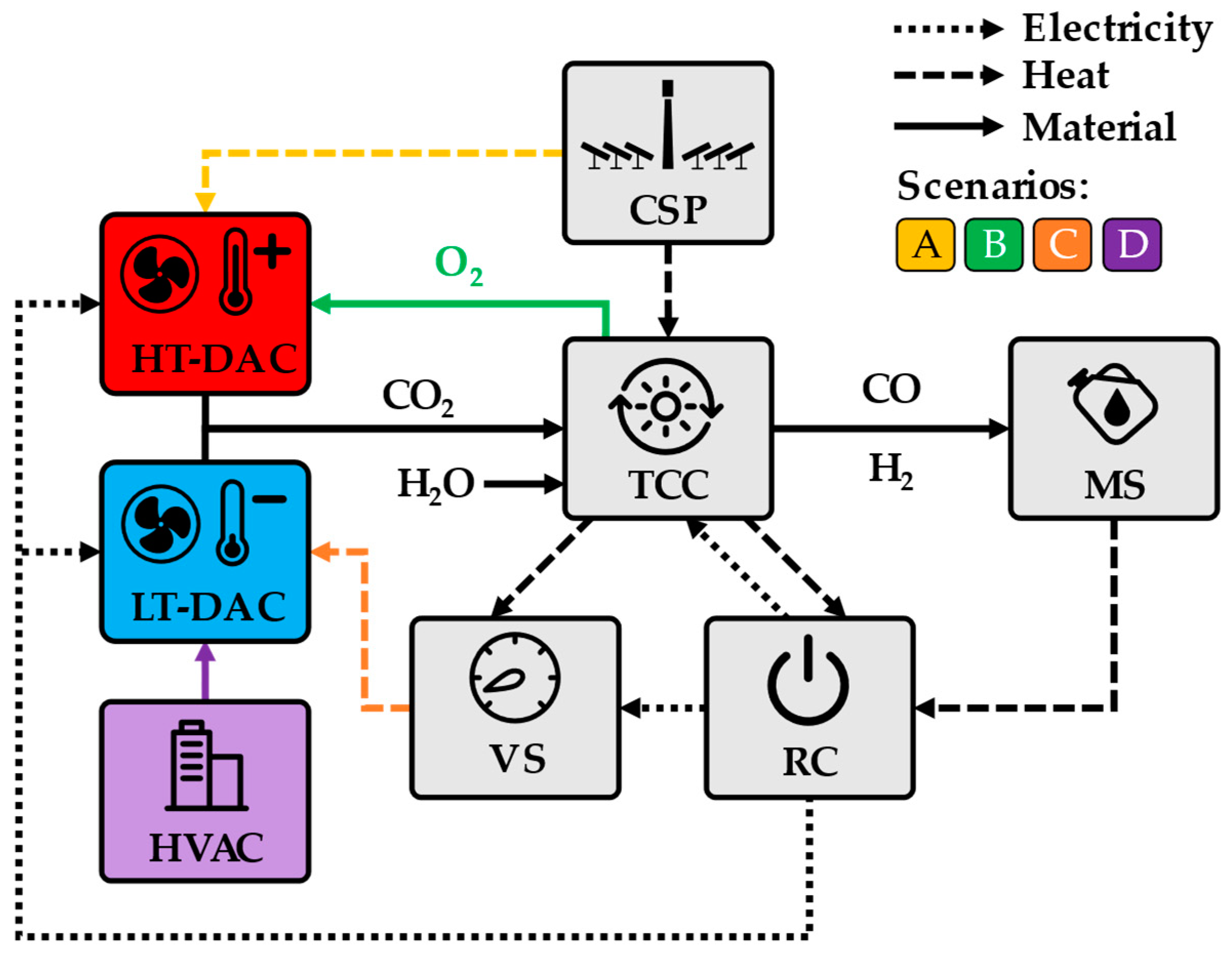
2.1. Model Modifications
- The reduction reactors are unified in a single reduction reactor;
- A closed circuit of supercritical CO2 is introduced for the high temperature heat transfer units;
- The pressure in the reduction reactor is increased from 0.5 to 1 mbar;
- The liquid ring pump in the vacuum system is replaced by two stages of steam jets;
- A heat pump is added to the vacuum system to allow steam condensation at temperatures below the ambient temperature;
- The CO separation technology is changed from absorption to VPSA (Vacuum Pressure Swing Adsorption).
2.2. Methods for Cost Estimation
2.3. Sizing Constraints
- The solar field is designed to produce 280 MWth at noon on solar equinox;
- Ten percent of the daily heat is lost to sensible heating of the plant;
- If operated in continuous, the annual CO2 outputs of the DAC unit are oversized by 10%;
- If operated intermittently, the nominal CO2 outputs of the DAC unit are oversized by 10%;
- The intermediate storage of synthesis gas is set to 48 h;
- When required, the intermediate storage of CO2 is set to 1 week;
- The methanol plant is operated continuously and at a maximum of 90% of its capacity.
2.4. Dynamics
2.5. Economic Evaluation
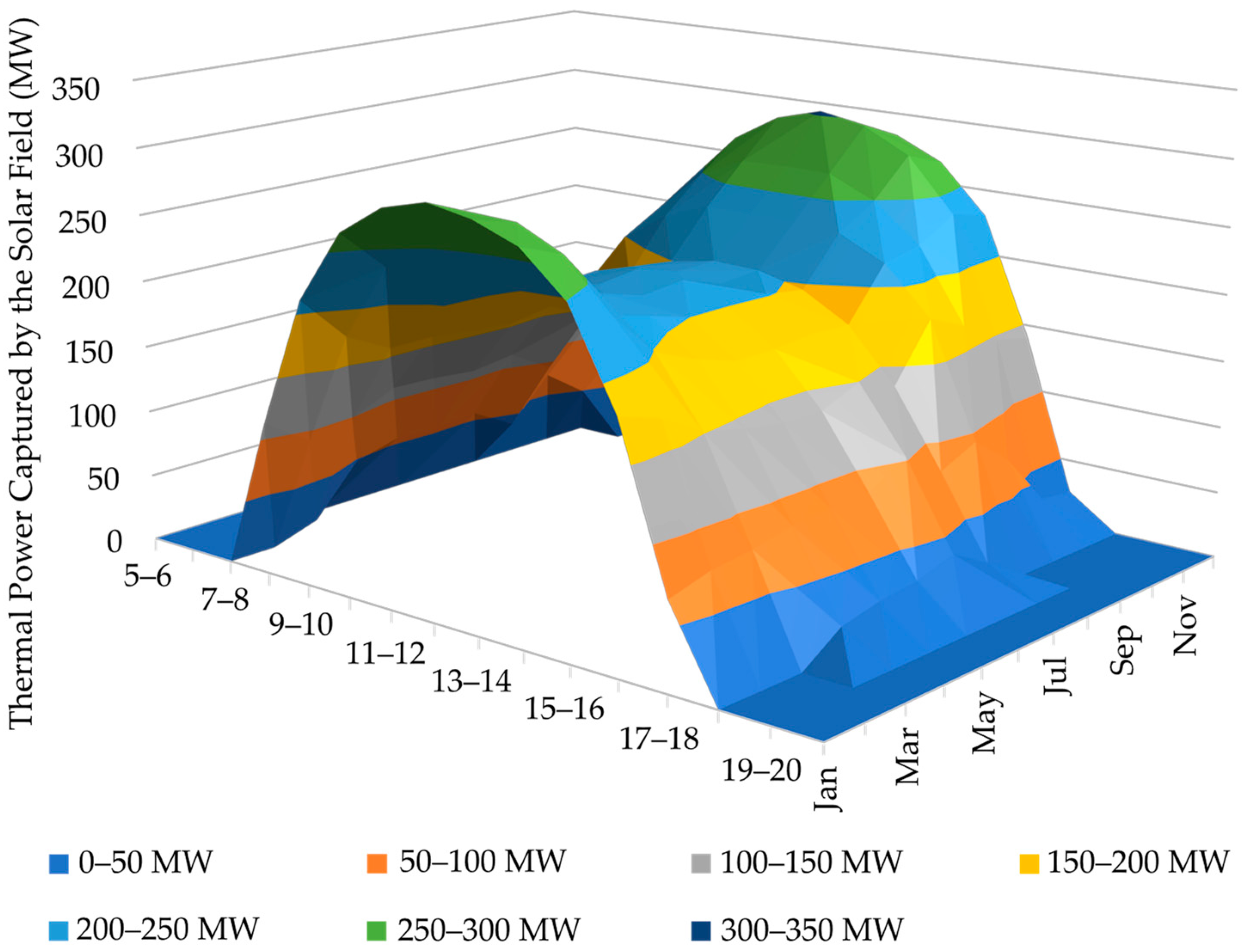
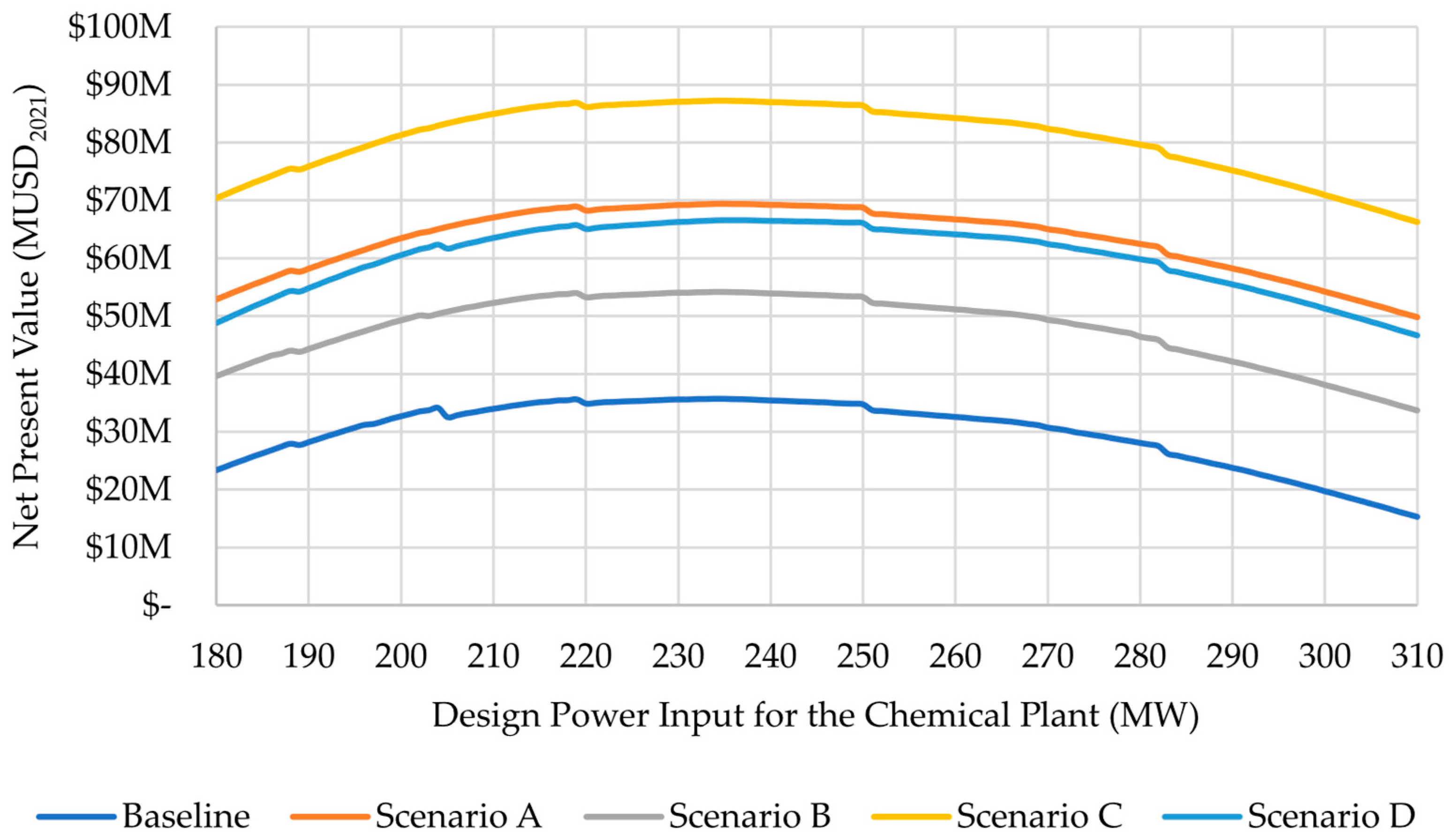
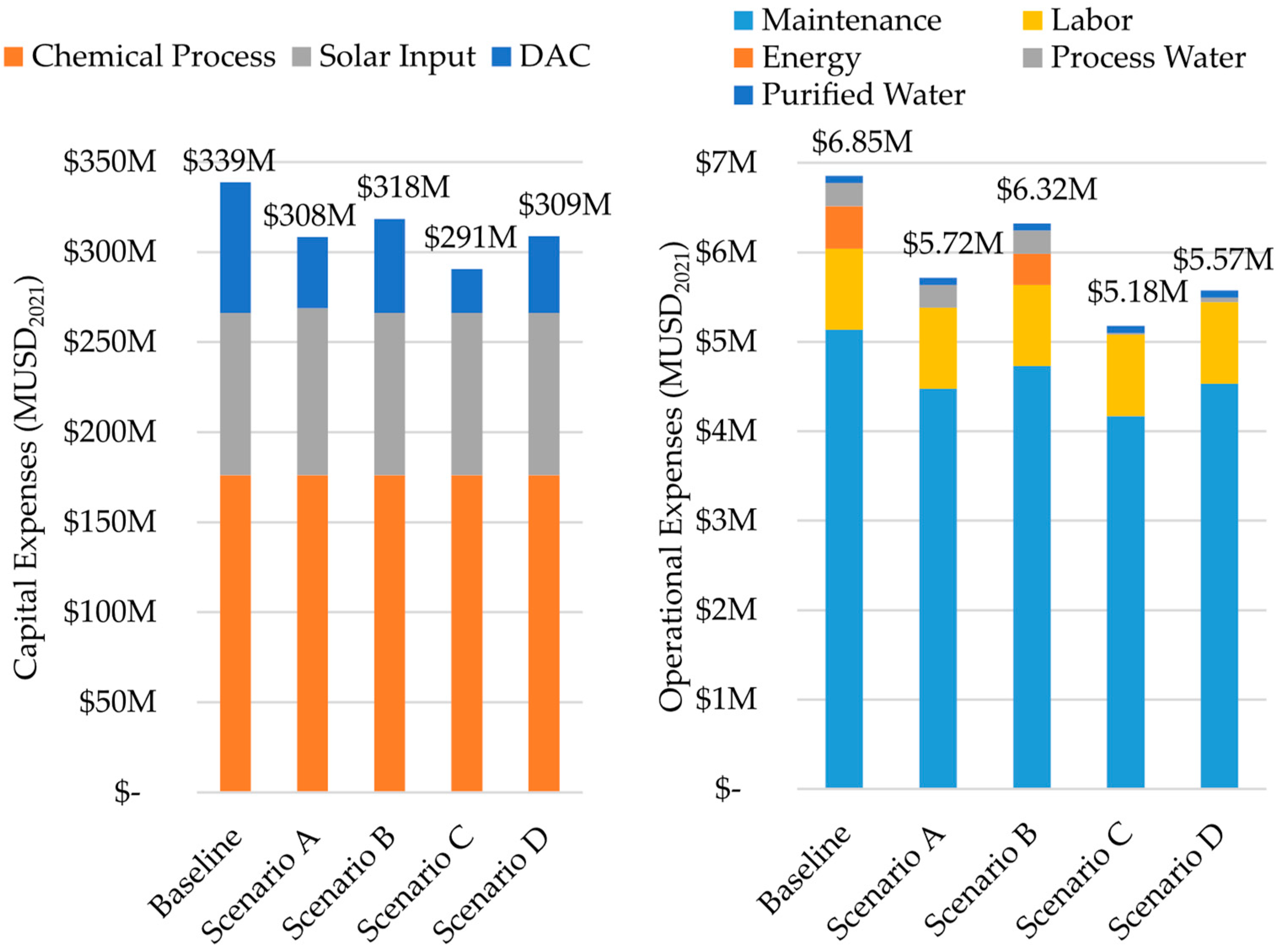
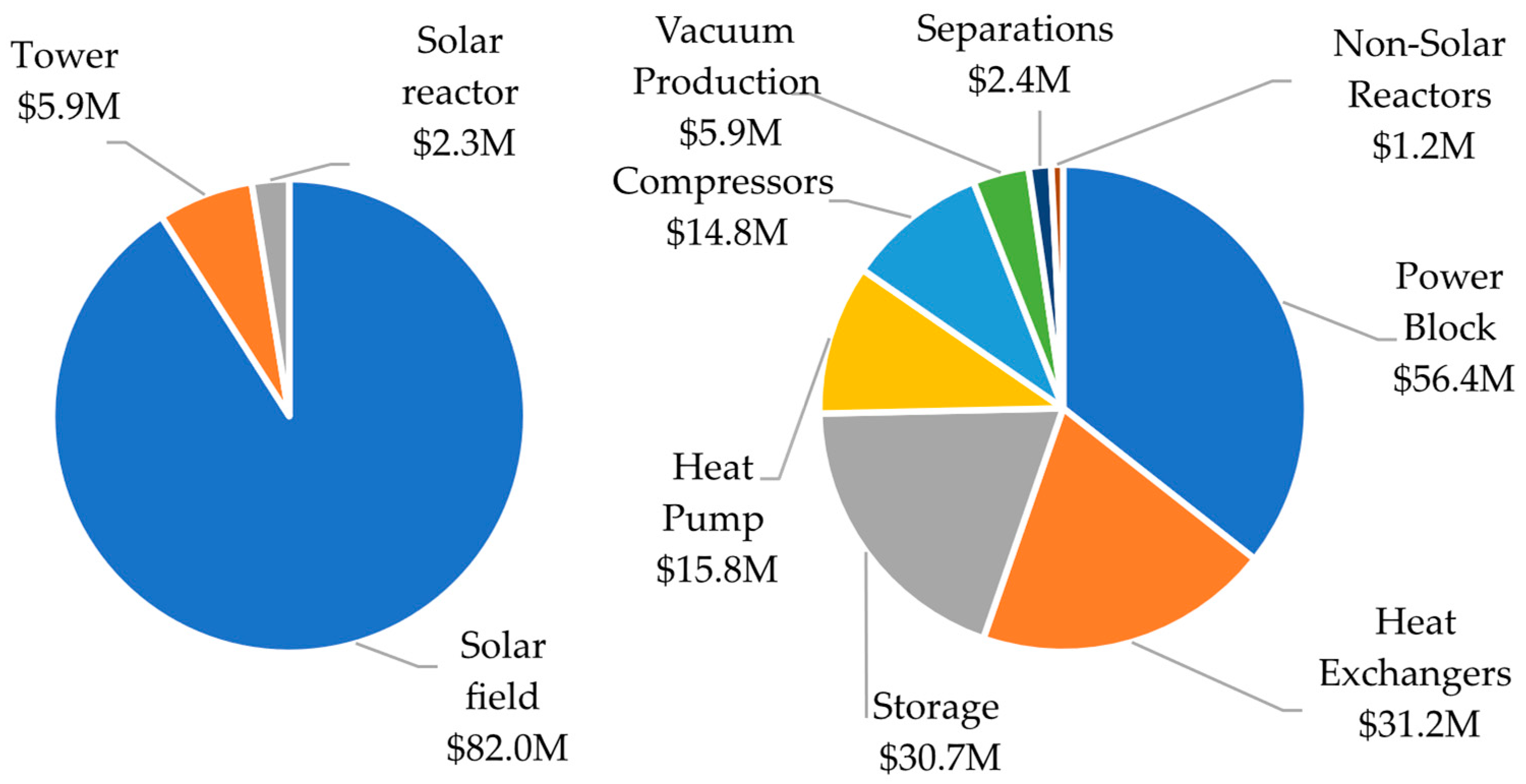
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
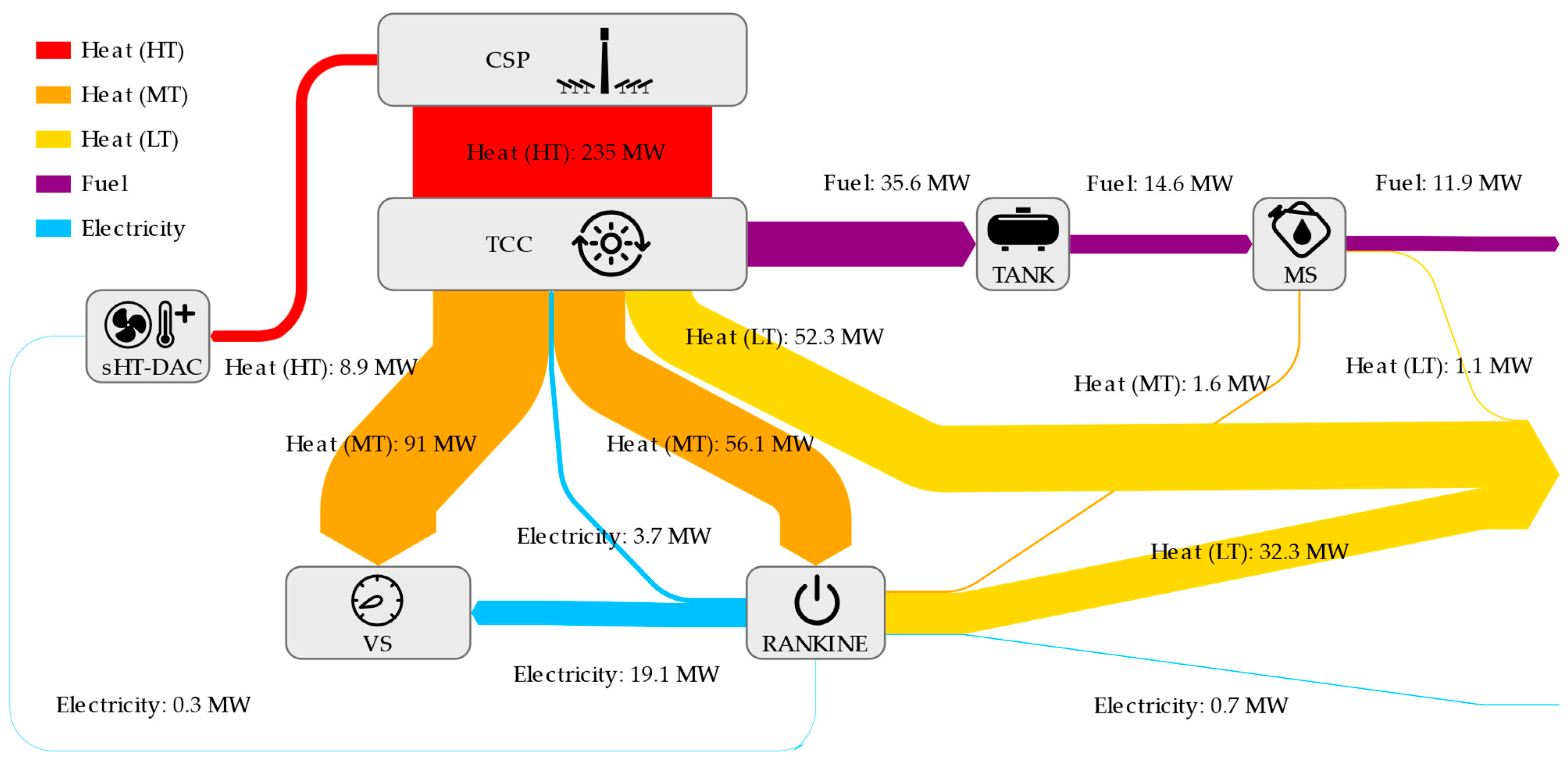
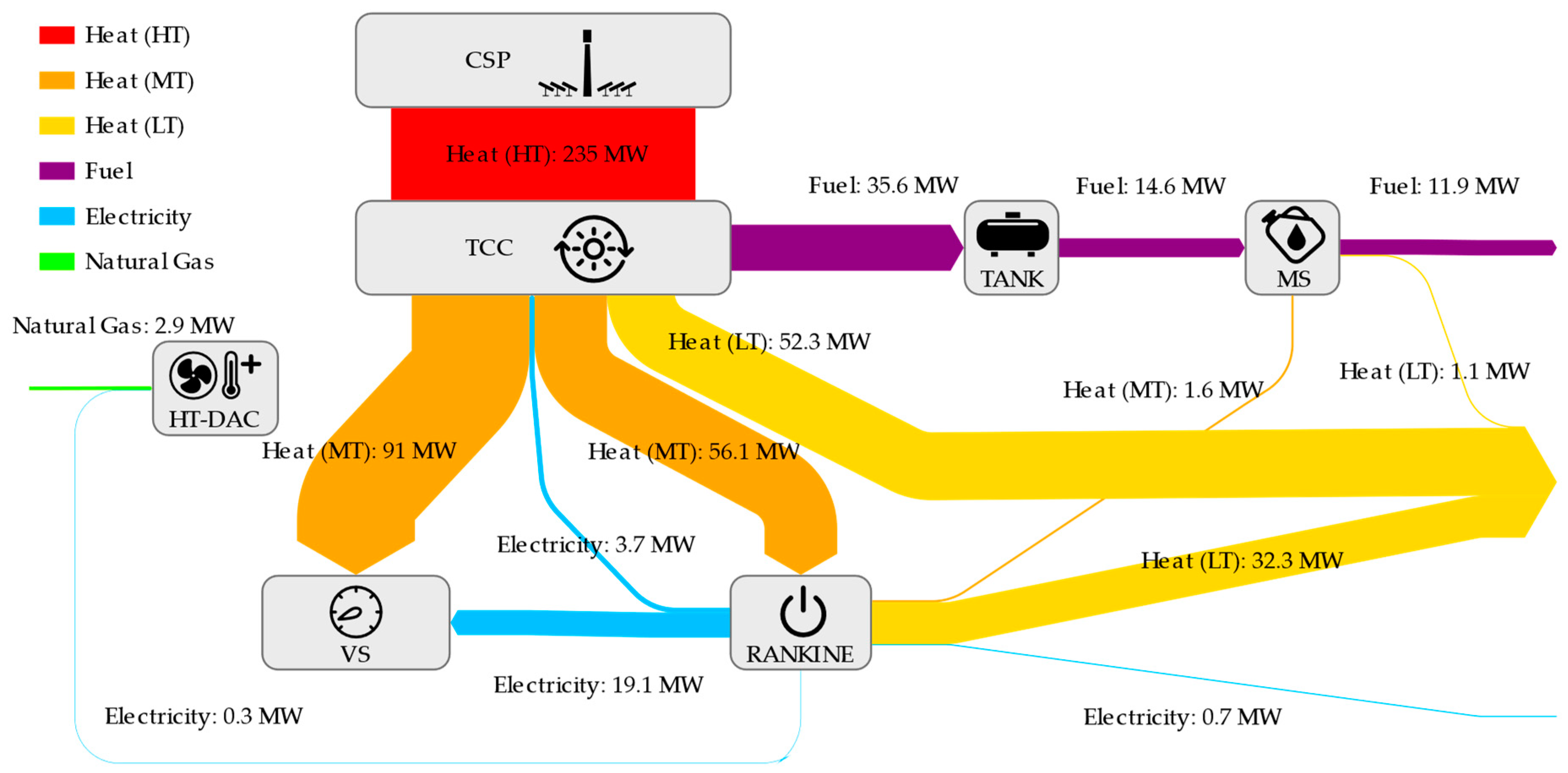
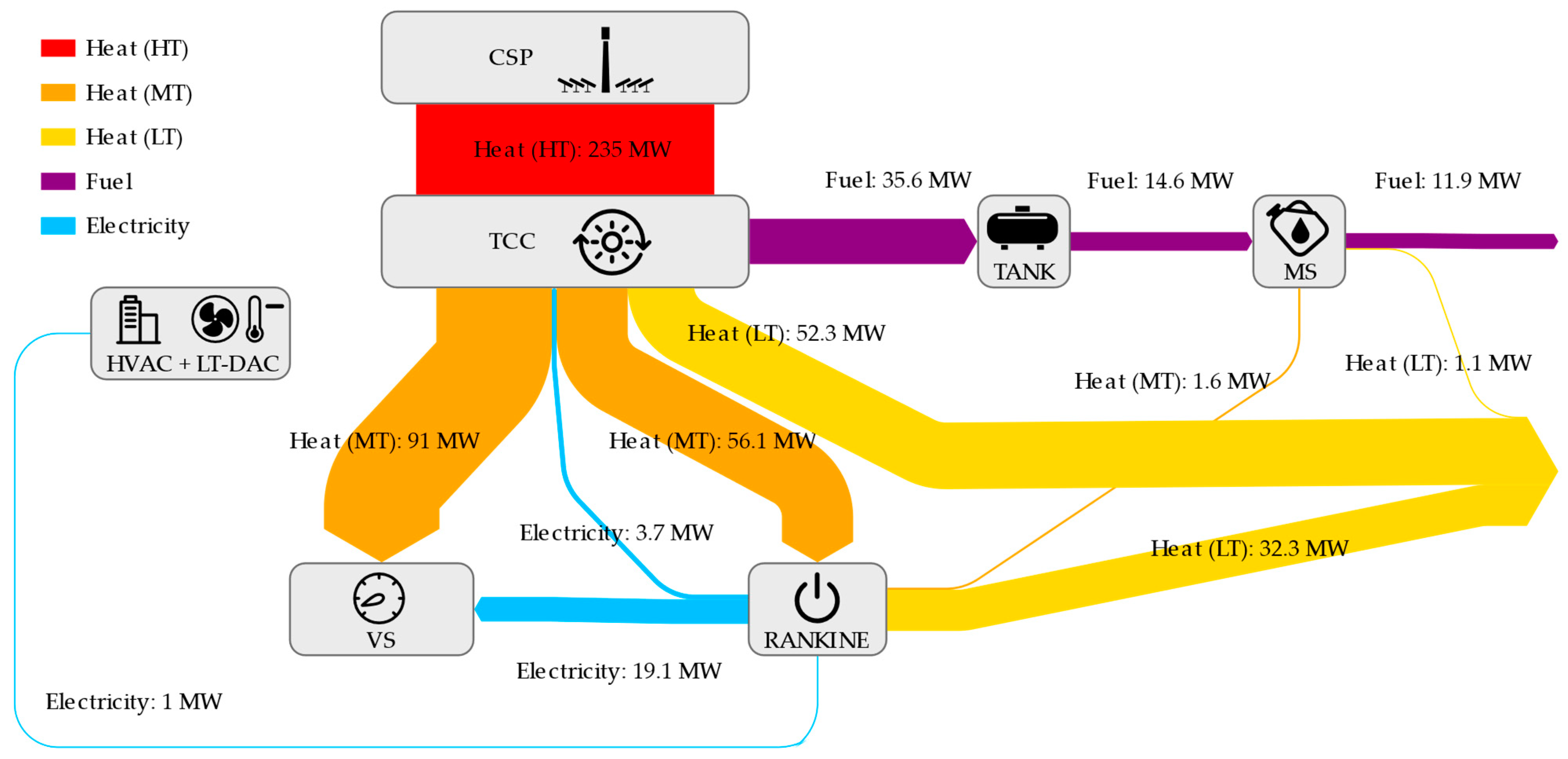
References
- The Emissions Gap Report 2020; United Nations Environment Programme: Nairobi, Kenya, 2020; ISBN 978-92-807-3812-4.
- United Nations Environment Programme. Emissions Gap Report 2021: The Heat Is On—A World of Climate Promises Not Yet Delivered; United Nations Environment Programme: Nairobi, Kenya, 2021. [Google Scholar]
- Blunden, J.; Arndt, D.S. State of the Climate in 2019. Bull. Am. Meteorol. Soc. 2020, 101, S1–S429. [Google Scholar] [CrossRef]
- Aresta, M. Carbon Dioxide as Chemical Feedstock; Wiley-VCH: Weinheim, Germany, 2010; ISBN 9783527324750. [Google Scholar]
- Lenton, T.M.; Held, H.; Kriegler, E.; Hall, J.W.; Lucht, W.; Rahmstorf, S.; Schellnhuber, H.J. Tipping elements in the Earth’s climate system. Proc. Natl. Acad. Sci. USA 2008, 105, 1786–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPCC. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2019. [Google Scholar]
- IPCC. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Cambridge University Press: Cambridge, UK, 2019; ISBN 9781009157964. [Google Scholar]
- SEI; IISD; ODI; E3G; UNEP. The Production Gap 2021 Report; Stockholm Environment Institute: Stockholm, Sweden, 2021. [Google Scholar]
- Bp. Full Report—Statistical Review of World Energy; BP: London, UK, 2021. [Google Scholar]
- U.S. Energy Information Administration. Proved Reserves of Crude Oil and Natural Gas in the United States, Year-End 2020; U.S. Department of Energy: Washington, DC, USA, 2022.
- IEA—International Energy Agency. Global Gas Security Review 2019; OECD: Paris, France, 2019; ISBN 9789264911765. [Google Scholar]
- IEA—International Energy Agency. Security of Clean Energy Transitions; OECD: Paris, France, 2021; ISBN 9789264903395. [Google Scholar]
- Wan, C.; Zhou, L.; Xu, S.; Jin, B.; Ge, X.; Qian, X.; Xu, L.; Chen, F.; Zhan, X.; Yang, Y.; et al. Defect engineered mesoporous graphitic carbon nitride modified with AgPd nanoparticles for enhanced photocatalytic hydrogen evolution from formic acid. Chem. Eng. J. 2022, 429, 132388. [Google Scholar] [CrossRef]
- Mengis, N.; Kalhori, A.; Simon, S.; Harpprecht, C.; Baetcke, L.; Prats-Salvado, E.; Schmidt-Hattenberger, C.; Stevenson, A.; Dold, C.; Zohbi, J.; et al. Net-Zero CO2 Germany—A Retrospect from the Year 2050. Earth’s Future 2022, 10. [Google Scholar] [CrossRef]
- Liebich, A.; Fröhlich, T.; Münter, D.; Fehrenbach, H.; Simon, S.; Maier, S.; Albrecht, F.; Pregger, T.; Schillings, C.; Moser, M.; et al. System Comparison of Storable Energy Carriers from Renewable Energies; Umweltbundesamt: Dessau-Roßlau, Germany, 2021. [Google Scholar]
- Stechel, E.B.; Miller, J.E. Re-energizing CO2 to fuels with the sun: Issues of efficiency, scale, and economics. J. CO2 Util. 2013, 1, 28–36. [Google Scholar] [CrossRef]
- Fasihi, M.; Bogdanov, D.; Breyer, C. Long-Term Hydrocarbon Trade Options for the Maghreb Region and Europe—Renewable Energy Based Synthetic Fuels for a Net Zero Emissions World. Sustainability 2017, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Ram, M.; Galimova, T.; Bogdanov, D.; Fasihi, M.; Gulagi, A.; Breyer, C.; Micheli, M.; Crone, K. Powerfuels in a Renewable Energy World—Global Volumes, Costs, and Trading 2030 to 2050; Lappeenranta: Berlin, Germany, 2020. [Google Scholar]
- World Bank. Concentrating Solar Power: Clean Power on Demand 24/7; World Bank: Washington, DC, USA, 2020. [Google Scholar]
- IRENA. Renewable Power Generation Costs in 2020; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2021; ISBN 9789292603489. [Google Scholar]
- Agora Energiewende and AFRY Management Consulting. No-Regret Hydrogen: Charting Early Steps for H2 Infrastructure in Europe; Agora Energiewende: Berlin, Germany, 2021. [Google Scholar]
- Hinkley, J.T. A New Zealand Perspective on Hydrogen as an Export Commodity: Timing of Market Development and an Energy Assessment of Hydrogen Carriers. Energies 2021, 14, 4876. [Google Scholar] [CrossRef]
- Grube, T.; Reul, J.; Reuß, M.; Calnan, S.; Monnerie, N.; Schlatmann, R.; Sattler, C.; Robinius, M.; Stolten, D. A techno-economic perspective on solar-to-hydrogen concepts through 2025. Sustain. Energy Fuels 2020, 4, 5818–5834. [Google Scholar] [CrossRef]
- German Environment Agency. Power-to-Liquids: Potentials and Perspectives; Umweltbundesamt: Dessau-Roßlau, Germany, 2016. [Google Scholar]
- International Renewable Energy Agency (IRENA) and the Methanol Institute. Innovation Outlook: Renewable Methanol; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2021; ISBN 978-92-9260-320-5. [Google Scholar]
- Deutz, S.; Bardow, A. Life-cycle assessment of an industrial direct air capture process based on temperature–vacuum swing adsorption. Nat. Energy 2021, 6, 203–213. [Google Scholar] [CrossRef]
- Madhu, K.; Pauliuk, S.; Dhathri, S.; Creutzig, F. Understanding environmental trade-offs and resource demand of direct air capture technologies through comparative life-cycle assessment. Nat. Energy 2021, 6, 1035–1044. [Google Scholar] [CrossRef]
- Physics, A.P. Direct Air Capture of CO2 with Chemicals: A Technology Assessment for the APS Panel on Public Affairs; American Physical Society: College Park, MD, USA, 2011. [Google Scholar]
- Wilcox, J. Carbon Capture; Springer: New York, NY, USA, 2012; ISBN 978-1-4614-2214-3. [Google Scholar]
- Wilcox, J.; Psarras, P.C.; Liguori, S. Assessment of reasonable opportunities for direct air capture. Environ. Res. Lett. 2016, 12, 065001. [Google Scholar] [CrossRef] [Green Version]
- Blanco, M.; Ramirez Santigosa, L. Advances in Concentrating Solar Thermal Research and Technology; Woodhead Publishing: Oxford, UK, 2016; ISBN 9780081005163. [Google Scholar]
- Agrafiotis, C.; Roeb, M.; Sattler, C. 4.18 Solar Fuels. In Comprehensive Energy Systems; Dincer, I., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-814925-6. [Google Scholar]
- Bulfin, B.; Vieten, J.; Agrafiotis, C.; Roeb, M.; Sattler, C. Applications and Limitations of Two Step Metal Oxide Thermochemical Redox Cycles; A Review. J. Mater. Chem. 2017, 5, 18951–18966. [Google Scholar] [CrossRef]
- Lua, Y.; Zhua, L.; Agrafiotis, C.; Vieten, J.; Roeb, M.; Sattler, C. Solar fuels production: Two-step thermochemical cycles with cerium-based oxides. Progess Energy Combust. Sci. 2019, 75, 100785. [Google Scholar] [CrossRef]
- Marxer, D.; Furler, P.; Takacs, M.; Steinfeld, A. Solar thermochemical splitting of CO2 into separate streams of CO and O2 with high selectivity, stability, conversion, and efficiency. Energy Environ. Sci. 2017, 10, 1142–1149. [Google Scholar] [CrossRef] [Green Version]
- Moumin, G.; Neumann, N.C.; Roeb, M.; Rosenstiel, A.; Puga, E.V.; Dux, D.; Keuchel, B.; Walther, G.; Zeeb, P. CO2 Utilization in North Rhine-Westphalia: A Feasibility Study to Accelerate Implementation; German Aerospace Center (DLR): Cologne, Germany, 2021. [Google Scholar]
- Schäppi, R.; Rutz, D.; Dähler, F.; Muroyama, A.; Haueter, P.; Lilliestam, J.; Patt, A.; Furler, P.; Steinfeld, A. Drop-in fuels from sunlight and air. Nature 2022, 601, 63–68. [Google Scholar] [CrossRef]
- Committee on Developing a Research Agenda for Carbon Dioxide Removal and Reliable Sequestration; Board on Atmospheric Sciences and Climate; Board on Energy and Environmental Systems; Board on Agriculture and Natural Resources; Board on Earth Sciences and Resources; Board on Chemical Sciences and Technology; Ocean Studies Board; Division on Earth and Life Studies; National Academies of Sciences, Engineering, and Medicine. Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; The National Academis Press: Washington, DC, USA, 2018; ISBN 9780309484527. [Google Scholar]
- Fasihi, M.; Efimova, O.; Breyer, C. Techno-economic assessment of CO2 direct air capture plants. J. Clean. Prod. 2019, 224, 957–980. [Google Scholar] [CrossRef]
- McQueen, N.; Gomes, K.V.; McCormick, C.; Blumanthal, K.; Pisciotta, M.; Wilcox, J. A review of direct air capture (DAC): Scaling up commercial technologies and innovating for the future. Prog. Energy 2021, 3, 32001. [Google Scholar] [CrossRef]
- Kaya, A.; Tok, M.; Koc, M. A Levelized Cost Analysis for Solar-Energy-Powered Sea Water Desalination in The Emirate of Abu Dhabi. Sustainability 2019, 11, 1691. [Google Scholar] [CrossRef] [Green Version]
- Prats-Salvado, E.; Monnerie, N.; Sattler, C. Synergies between Direct Air Capture Technologies and Solar Thermochemical Cycles in the Production of Methanol. Energies 2021, 14, 4818. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Klumpp, M.; Kant, P.; Ozin, G. Crowd oil not crude oil. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Remer, D.S.; Chai, L.H. Process Equipment, Cost Scale-Up; Marcel Dekker, Inc.: New York, NY, USA, 1993; ISBN 9780824724511. [Google Scholar]
- Peters, M.S.; Timmerhaus, K.D.; West, R.E. Plant Design and Economics for Chemical Engineers, 5th ed.; Peters, M.S., Timmerhaus, K.D., West, R.E., Eds.; McGraw-Hill: New York, NY, USA, 2003; ISBN 9780072392661. [Google Scholar]
- Lim, Y.-I.; Choi, J.; Moon, H.-M.; Kim, G.-H. Techno-economic Comparison of Absorption and Adsorption Processes for Carbon Monoxide (CO) Separation from Linze-Donawitz Gas (LDG). Korean Chem. Eng. Res. 2015, 54, 320–331. [Google Scholar] [CrossRef] [Green Version]
- Cheang, V.T.; Hedderwick, R.A.; McGregor, C. Benchmarking supercritical carbon dioxide cycles against steam Rankine cycles for Concentrated Solar Power. Solar Energy 2015, 113, 199–211. [Google Scholar] [CrossRef]
- Marina, A.; Spoelstra, S.; Zondag, H.A.; Wemmers, A.K. An estimation of the European industrial heat pump market potential. Renew. Sustain. Energy Rev. 2021, 139, 110545. [Google Scholar] [CrossRef]
- Peletiri, S.P.; Rahmanian, N.; Mujtaba, I.M. CO2 Pipeline Design: A Review. Energies 2018, 11, 2184. [Google Scholar] [CrossRef] [Green Version]
- Keith, D.; Holmes, G.; St. Angelo, D.; Heidel, K. A Process for Capturing CO2 from the Atmosphere. Joule 2018, 2, 006. [Google Scholar] [CrossRef] [Green Version]
- National Academies of Sciences, Engineering, and Medicine. Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Falter, C.; Valente, A.; Habersetzer, A.; Iribarren, D.; Dufour, J. An integrated techno-economic, environmental and social assessment of the solar thermochemical fuel pathway. Sustain. Energy Fuels 2020, 4, 3992–4002. [Google Scholar] [CrossRef]
- SolarPACES. Atacama I/Cerro Dominador 110 MW CSP + 100 MW PV CSP Project. Available online: https://solarpaces.nrel.gov/project/atacama-i-cerro-dominador-110mw-csp-100-mw-pv (accessed on 11 May 2021).
- Schwarzbözl, P.; Pitz-Paal, R.; Schmitz, M. Visual HFLCAL—A Software Tool for Layout and Optimisation of Heliostat Fields. In Proceedings of the 15th International SolarPACES Symposium, Berlin, Germany, 15–18 September 2009. [Google Scholar]
- Brealey, R.A.; Myers, S.C.; Allen, F. Principles of Corporate Finance, 10th ed.; McGraw-Hill/Irwin: New York, NY, USA, 2011. [Google Scholar]
- Monnerie, N.; Gan, P.G.; Roeb, M.; Sattler, C. Methanol production using hydrogen from concentrated solar energy. Hydrog. Energy Publ. 2020, 45, 26117–26125. [Google Scholar] [CrossRef]
- Kim, J.; Henao, C.A.; Johnson, T.A.; Dedrick, D.E.; Miller, J.E.; Stechel, E.B.; Maravelias, C.T. Methanol production from CO2 using solar-thermal energy: Process development and techno-economic analysis. Energy Environ. Sci. 2011, 4, 3122. [Google Scholar] [CrossRef]
- Kim, J.; Johnson, T.A.; Miller, J.E.; Stechel, E.B.; Maravelias, C.T. Fuel production from CO2 using solar-thermal energy: System level analysis. Energy Environ. Sci. 2012, 5, 8417–8429. [Google Scholar] [CrossRef]
- Falter, C.; Pitz-Paal, R. Water Footprint and Land Requirement of Solar Thermochemical Jet-Fuel Production. Environ. Sci. Technol. 2017, 51, 12938–12947. [Google Scholar] [CrossRef]



| Equipment Class | Design Parameter | Estimation Method | Source |
|---|---|---|---|
| Compressors | Power | Correlation | [45] |
| HX: Shell & Tube | Area | Correlation | [45] |
| HX: Air-cooled | Area | Correlation | [45] |
| HX: Furnace | Power | Correlation | [45] |
| Reactors (non-solar) | Area (heat exchange) | Correlation | [45] |
| Flash separators | Volume | Correlation | [45] |
| VPSA | Volume | Correlation | [45,46] |
| Storage tanks | Metal mass | Correlation | [45] |
| Steam jets | Gas flowrate | Correlation | [45] |
| Power blocks | Energy input | Seven-tenths rule | [47] |
| Heat pump | Energy output | Linear | [48] |
| CO2 Pipeline | Length & Diameter | Linear | [49] |
| HT-DAC 1 | Annual CO2 output | Seven-tenths rule | [50] |
| LT-DAC 1 | Annual CO2 output | Seven-tenths rule | [51] |
| Solar reactors | Thermal power input | Power Law | [52] |
| Heliostat field | Heliostat area | Linear | [52] |
| Solar tower | Thermal power input | Linear | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prats-Salvado, E.; Monnerie, N.; Sattler, C. Techno-Economic Assessment of the Integration of Direct Air Capture and the Production of Solar Fuels. Energies 2022, 15, 5017. https://doi.org/10.3390/en15145017
Prats-Salvado E, Monnerie N, Sattler C. Techno-Economic Assessment of the Integration of Direct Air Capture and the Production of Solar Fuels. Energies. 2022; 15(14):5017. https://doi.org/10.3390/en15145017
Chicago/Turabian StylePrats-Salvado, Enric, Nathalie Monnerie, and Christian Sattler. 2022. "Techno-Economic Assessment of the Integration of Direct Air Capture and the Production of Solar Fuels" Energies 15, no. 14: 5017. https://doi.org/10.3390/en15145017
APA StylePrats-Salvado, E., Monnerie, N., & Sattler, C. (2022). Techno-Economic Assessment of the Integration of Direct Air Capture and the Production of Solar Fuels. Energies, 15(14), 5017. https://doi.org/10.3390/en15145017







