Numerical Simulation of Gaseous Detonation Performance and Wall Reflection Effect of Acetylene-Rich Fuel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Numerical Modeling
2.1.1. Governing Equation
2.1.2. Reduced Chemical Kinetics Model
2.1.3. Numerical Methods
2.1.4. Initial and Boundary Condition
2.2. Numerical Method Verification
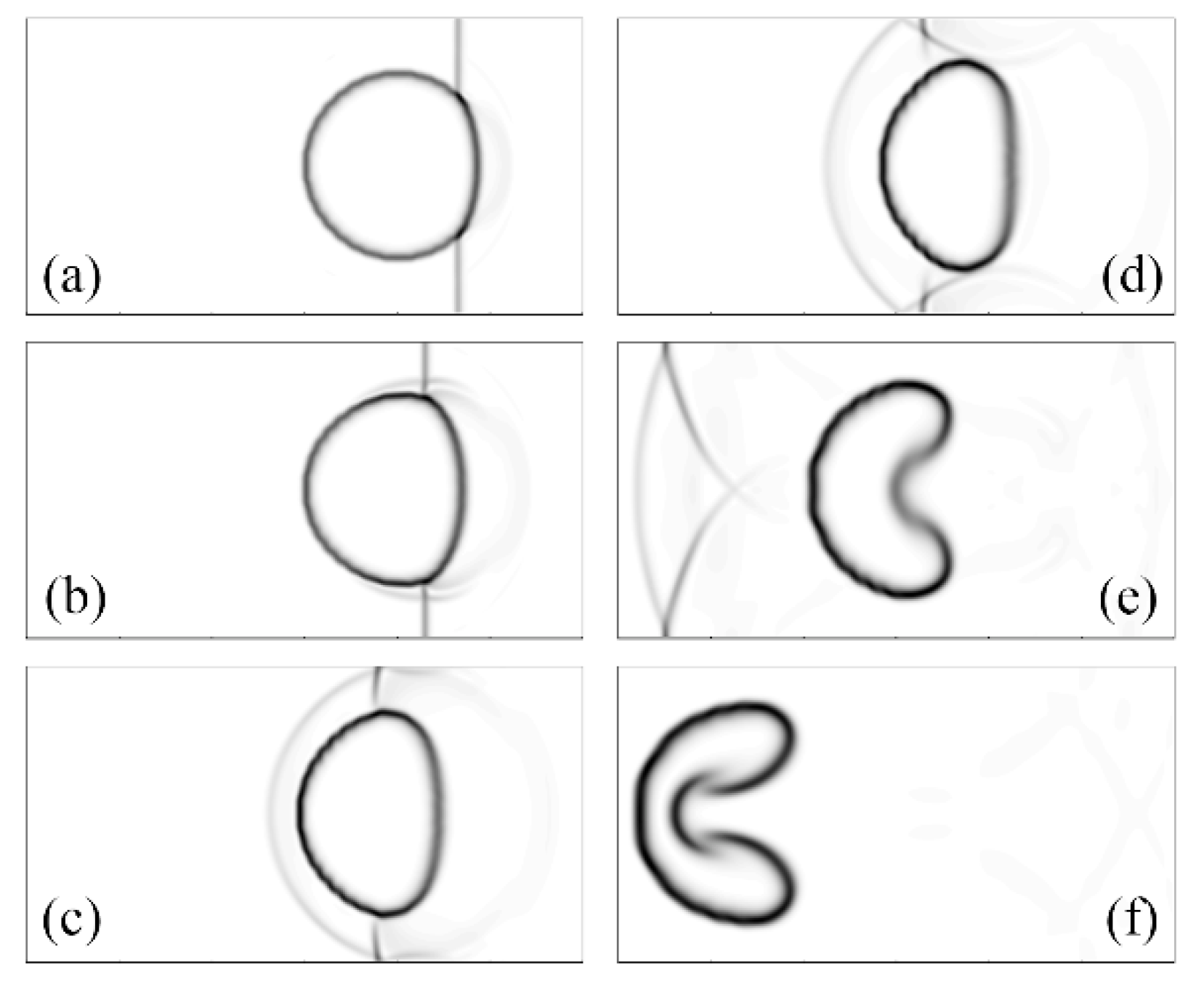
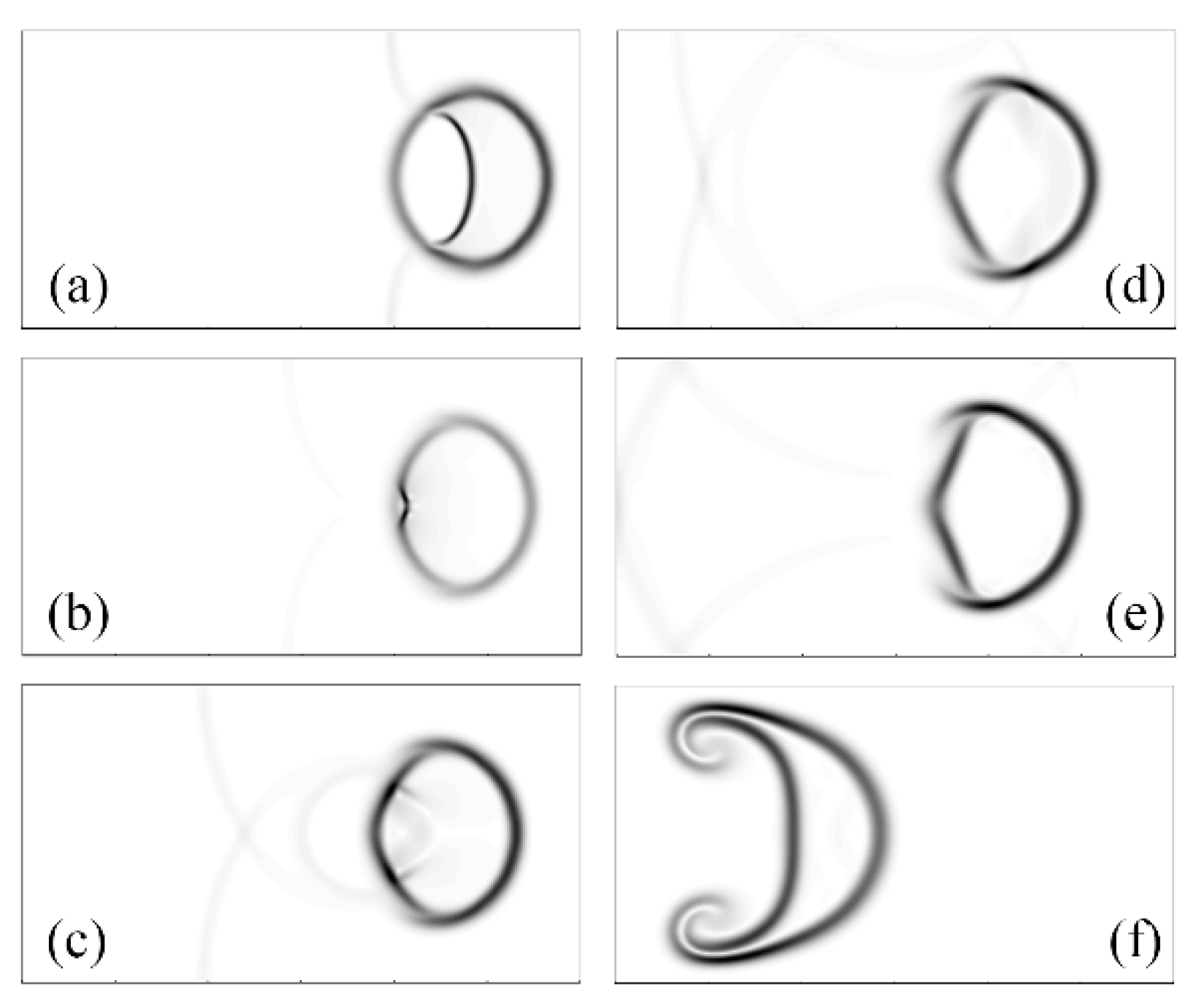
2.2.1. Interaction of Shock Waves and Bubbles
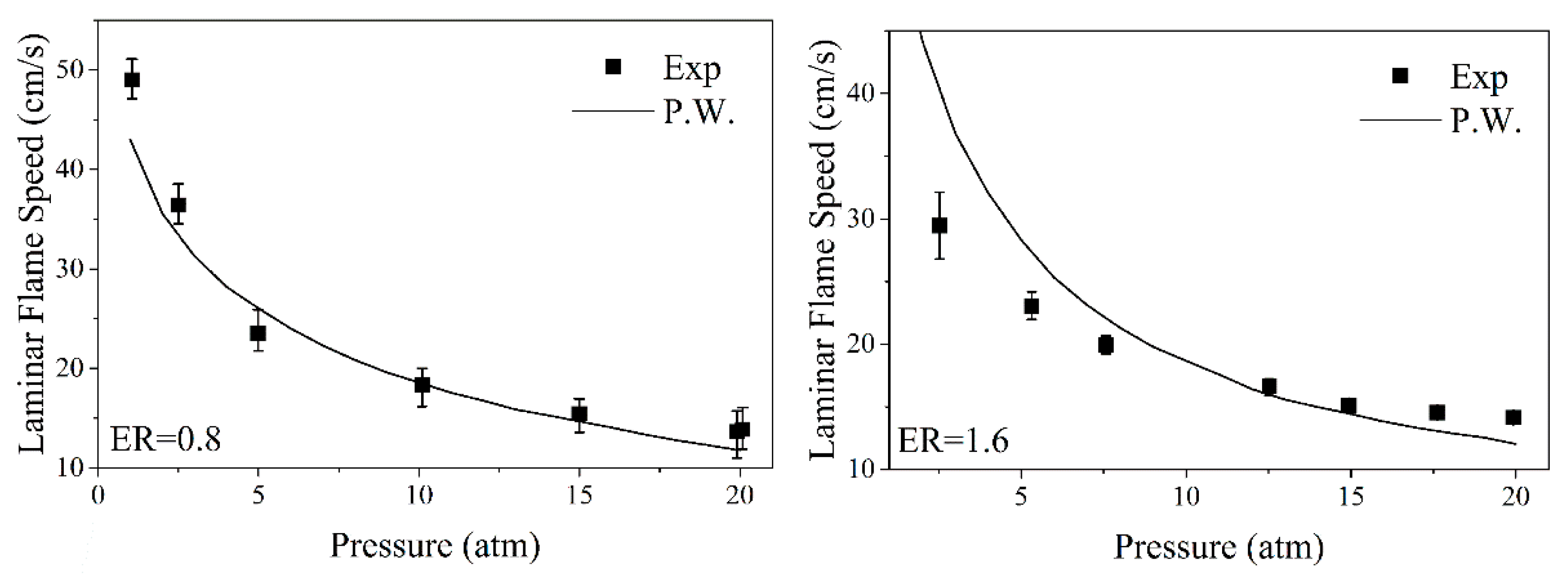
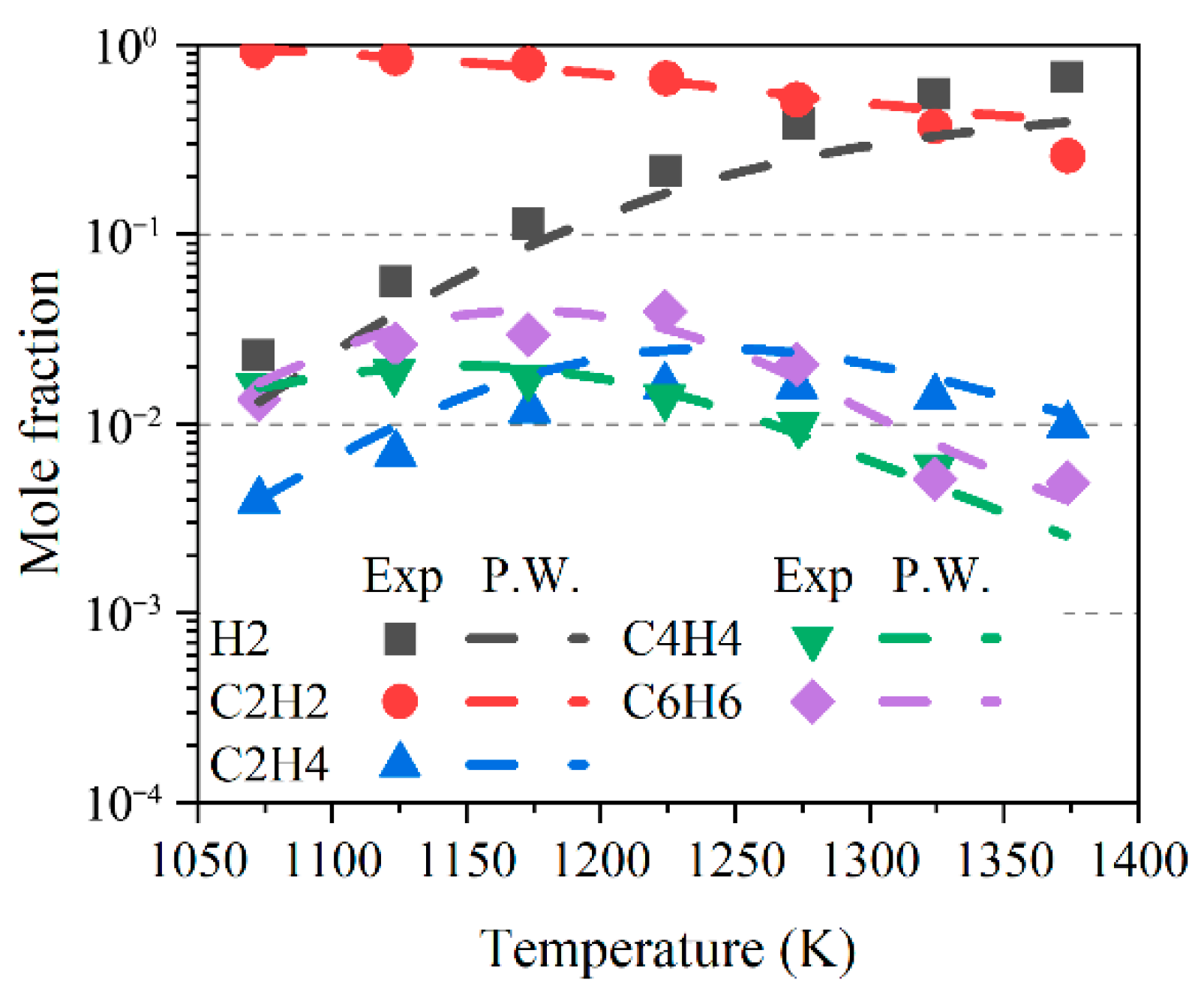
2.2.2. Acetylene Oxidation and Pyrolysis
2.2.3. Initial Detonation Cell Characteristic Parameters
3. Results and Discussion
3.1. Structure of Detonation
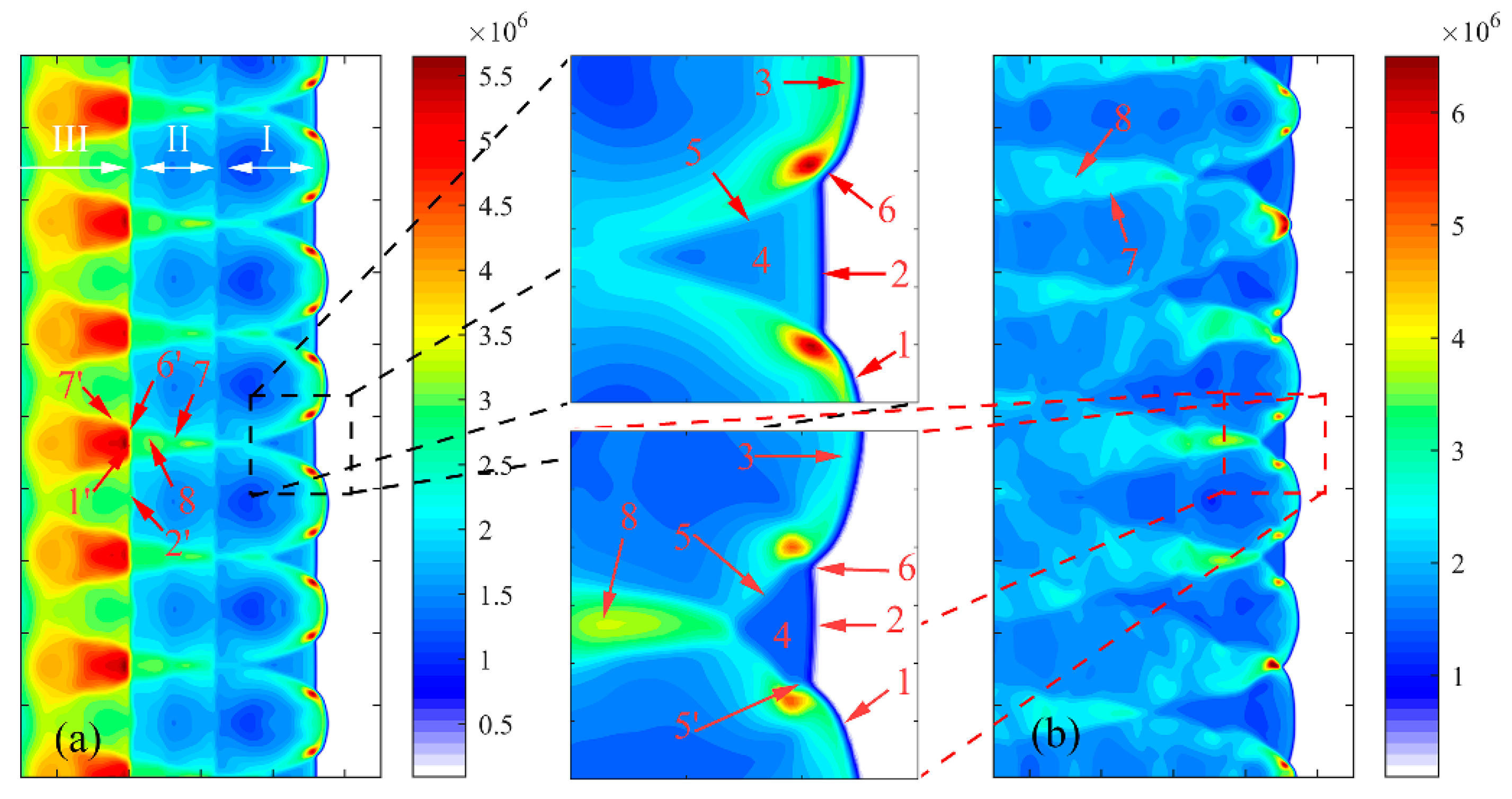
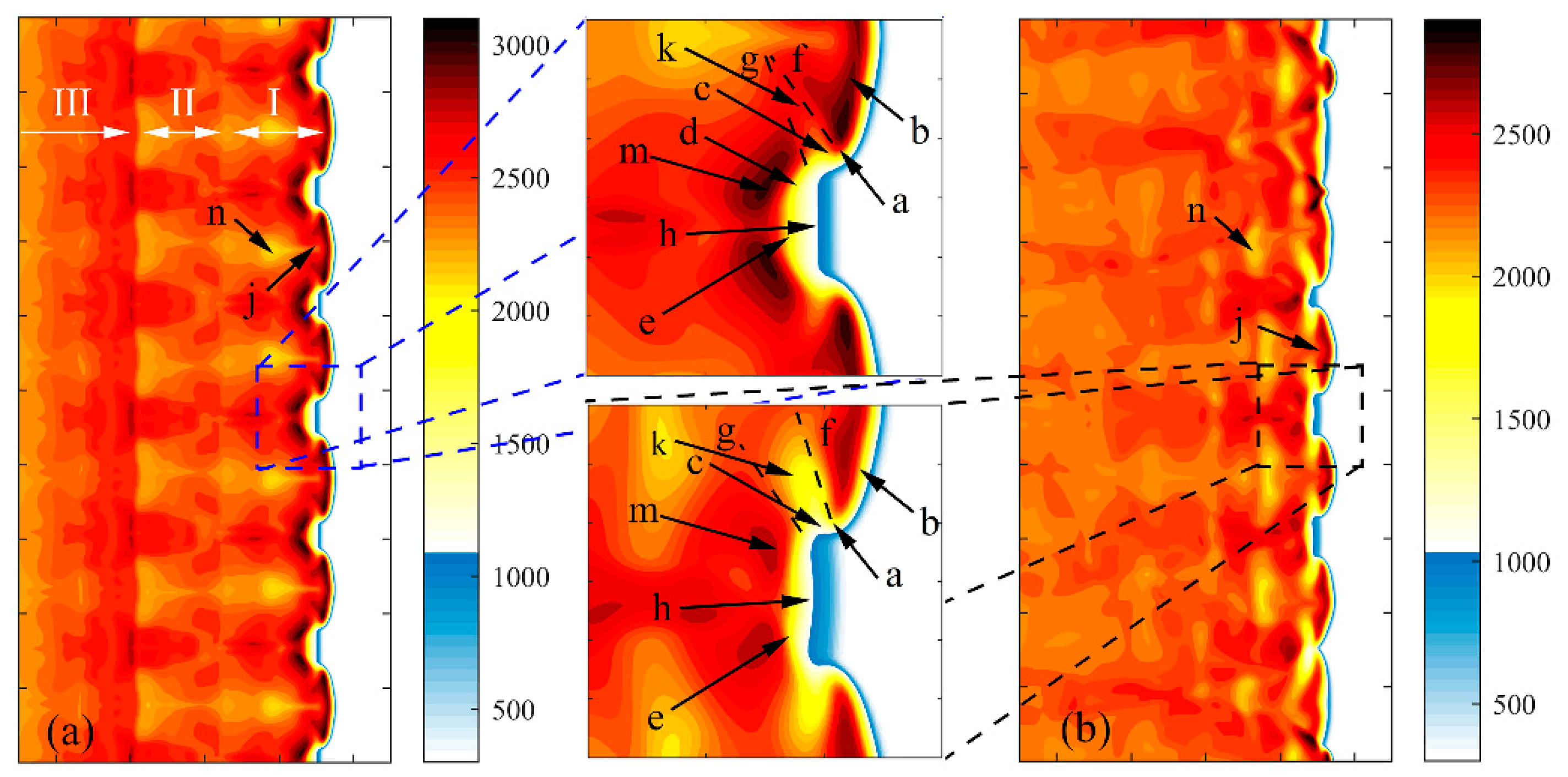
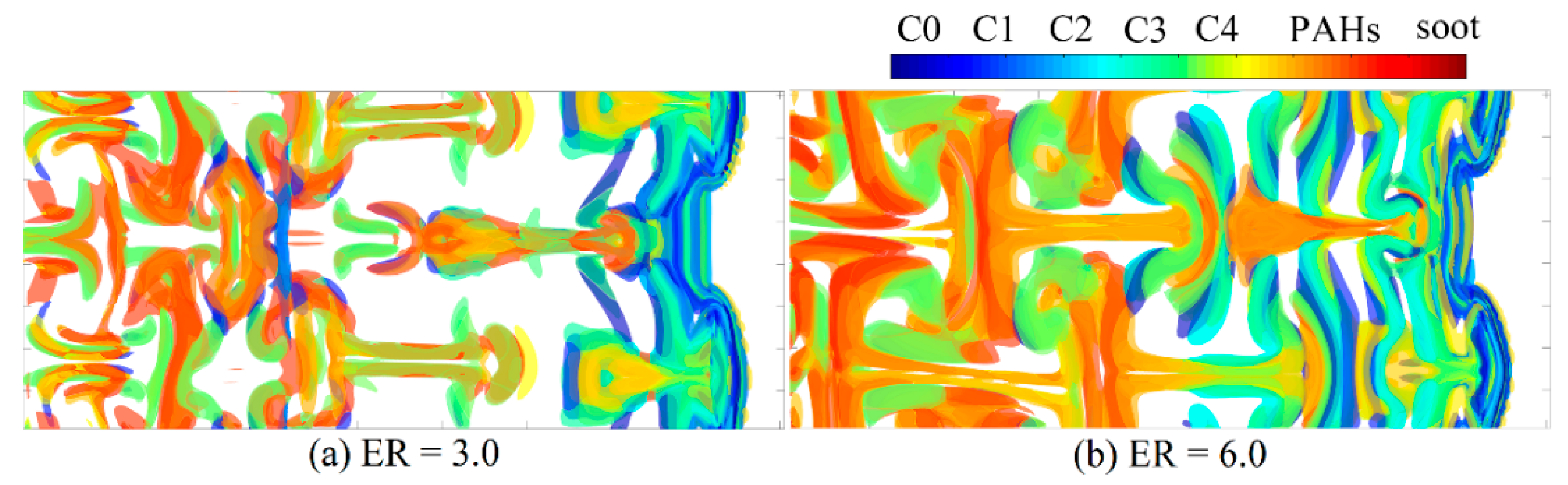
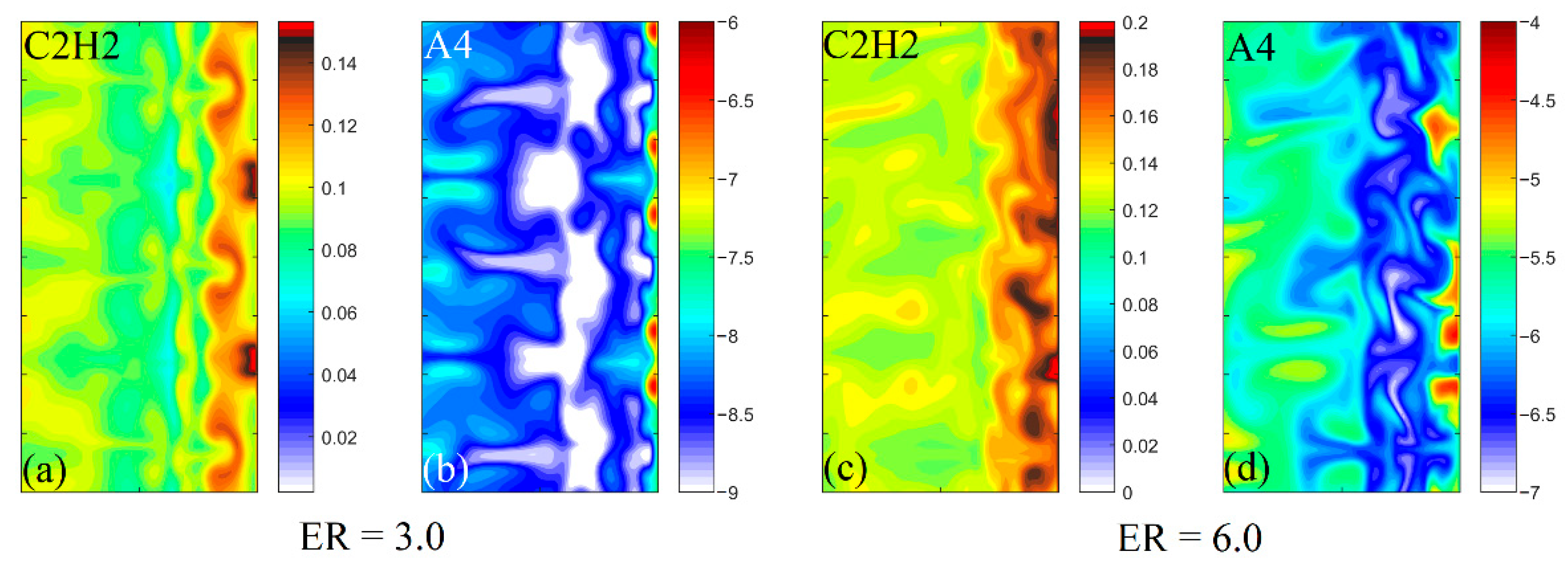
3.2. Characteristics of Reaction Dynamics
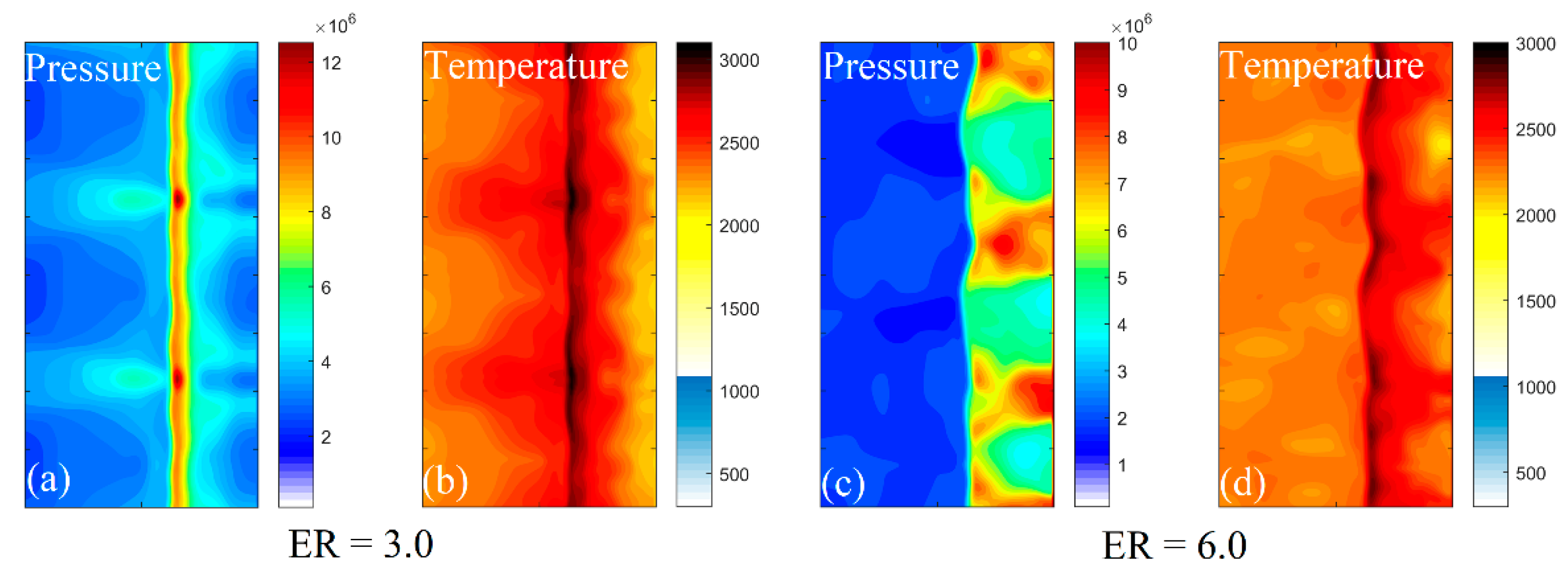
3.3. Wall Reflection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Emel’Yanov, A.V.; Eremin, A.V.; Fortov, V.E. Formation of a detonation wave in the process of chemical condensation of carbon nanoparticles. J. Eng. Phys. Rus 2010, 83, 1197–1209. [Google Scholar] [CrossRef]
- Emelianov, A.; Eremin, A.; Jander, H.; Wagner, H. Carbon condensation wave in C3O2 and C2H2 initiated by a shock wave. Proc. Combust. Inst. 2011, 33, 525–532. [Google Scholar] [CrossRef]
- Knorre, V.G.; Kopylev, M.S.; Tesner, P.A. Role of soot in acetylene detonation. Combust. Explos. Shock. Waves 1977, 13, 732–736. [Google Scholar] [CrossRef]
- Golub, V.V.; Gurentsov, E.; Emel’Yanov, A.V.; Eremin, A.V.; Fortov, V.E. Energy gain of the detonation pyrolysis of acetylene. High Temp. 2015, 53, 363–369. [Google Scholar] [CrossRef]
- Frenklach, M.; Mebel, A.M. On the mechanism of soot nucleation. PCCP 2020, 22, 5314–5331. [Google Scholar] [CrossRef] [PubMed]
- Mikhalchenko, E.V.; Nikitin, V.F. Rotating detonation engine fed by acetylene-oxygen combustible mixture modeling. AIP Conf. Proc. 2020, 2304, 020022. [Google Scholar]
- Nabavi-Pelesaraei, A.; Bayat, R.; Hosseinzadeh-Bandbafha, H.; Afrasyabi, H.; Chau, K.-W. Modeling of energy consumption and environmental life cycle assessment for incineration and landfill systems of municipal solid waste management—A case study in Tehran Metropolis of Iran. J. Clean. Prod. 2017, 148, 427–440. [Google Scholar] [CrossRef]
- Nabavi-Pelesaraei, A.; Rafiee, S.; Mohammadkashi, N.; Chau, K.-W.; Mostashari-Rad, F. Principle of Life Cycle Assessment and Cumulative Exergy Demand for Biodiesel Production: Farm-To-Combustion Approach; Springer International Publishing: Cham, Switzerland, 2022; pp. 127–169. [Google Scholar]
- Martin, J.W.; Salamanca, M.; Kraft, M. Soot inception: Carbonaceous nanoparticle formation in flames. Prog. Energy Combust. 2022, 88, 100956. [Google Scholar] [CrossRef]
- Shtertser, A.; Rybin, D.; Ulianitsky, V.; Park, W.; Datekyu, M.; Wada, T.; Kato, H. Characterization of nanoscale detonation carbon produced in a pulse gas-detonation device. Diam. Relat. Mater. 2020, 101, 107553. [Google Scholar] [CrossRef]
- Premnath, N.; Mohanrasu, K.; Rao, R.G.R.; Dinesh, G.; Prakash, G.S.; Ananthi, V.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A crucial review on polycyclic aromatic Hydrocarbons - Environmental occurrence and strategies for microbial degradation. Chemosphere 2021, 280, 130608. [Google Scholar] [CrossRef]
- Poludnenko, A.Y.; Chambers, J.; Ahmed, K.; Gamezo, V.N.; Taylor, B.D. A unified mechanism for unconfined deflagration-to-detonation transition in terrestrial chemical systems and type Ia supernovae. Science 2019, 366, 7365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kistiakowsky, G.B.; Knight, H.T.; Malin, M.E. Gaseous Detonations. IV. The Acetylene-Oxygen Mixtures. J. Chem. Phys. 1952, 20, 884–887. [Google Scholar] [CrossRef]
- Guilly, V.; Khasainov, B.; Presles, H.-N.; Desbordes, D. Simulation numérique des détonations à double structure cellulaire. Cr. Mec. 2006, 334, 679–685. [Google Scholar] [CrossRef]
- Smirnov, N.; Betelin, V.; Nikitin, V.; Phylippov, Y.; Koo, J. Detonation engine fed by acetylene–oxygen mixture. Acta Astronaut. 2014, 104, 134–146. [Google Scholar] [CrossRef]
- Batraev, I.S.; Vasil’Ev, A.A.; Ul’Yanitskii, V.Y.; Shtertser, A.A.; Rybin, D.K. Investigation of Gas Detonation in Over-Rich Mixtures of Hydrocarbons with Oxygen. Combust. Explos. Shock Waves 2018, 54, 207–215. [Google Scholar] [CrossRef]
- Mahmoudi, Y.; Mazaheri, K. High resolution numerical simulation of triple point collision and origin of unburned gas pockets in turbulent detonations. Acta Astronaut. 2015, 115, 40–51. [Google Scholar] [CrossRef]
- Yi, T.H.; Lu, F.K.; Wilson, D.R.; Emanuel, G. Numerical study of detonation wave propagation in a confined supersonic flow. Shock Waves 2017, 27, 395–408. [Google Scholar] [CrossRef]
- Ago, A.; Tsuboi, N.; Dzieminska, E.; Hayashi, A.K. Two-Dimensional Numerical Simulation of Detonation Transition with Multi-Step Reaction Model: Effects of Obstacle Height. Combust. Sci. Technol. 2019, 191, 659–675. [Google Scholar] [CrossRef]
- Deng, X.; Xie, B.; Teng, H.; Xiao, F. High resolution multi-moment finite volume method for supersonic combustion on unstructured grids. Appl. Math. Model. 2019, 66, 404–423. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, Y.; Deng, X.; Teng, H. Structure of wedge-induced oblique detonation in acetylene-oxygen-argon mixtures. Phys. Fluids 2019, 31, 026108. [Google Scholar]
- Xiao, H.; Oran, E.S. Shock focusing and detonation initiation at a flame front. Combust. Flame 2019, 203, 397–406. [Google Scholar] [CrossRef]
- Lian, Y.; Xu, K. A Gas-Kinetic Scheme for Multimaterial Flows and Its Application in Chemical Reactions. J. Comput. Phys. 2000, 163, 349–375. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Cheng, J.; Wang, S.; Xu, K. A Two-Stage Fourth-Order Gas-Kinetic Scheme for Compressible Multicomponent Flows. Commun. Comput. Phys. 2017, 22, 1123–1149. [Google Scholar] [CrossRef]
- Yang, L.M.; Shu, C.; Chen, Z.; Liu, Y.Y.; Wu, J.; Shen, X. Gas kinetic flux solver based high-order finite-volume method for simulation of two-dimensional compressible flows. Phys. Rev. E 2021, 104, 015305. [Google Scholar] [CrossRef] [PubMed]
- Van Leer, B. Towards the ultimate conservative difference scheme. V. A second-order sequel to Godunov’s method. J. Comput. Phys. 1979, 32, 101–136. [Google Scholar] [CrossRef]
- Van Albada, G.D.; van Leer, B.; Roberts, W.W. A Comparative Study of Computational Methods in Cosmic Gas Dynamics, in Upwind and High-Resolution Schemes; Springer: Berlin/Heidelberg, Germany, 1997; pp. 95–103. [Google Scholar]
- Yin, Z.; Linga, P. Methane hydrates: A future clean energy resource. Chin. J. Chem. Eng. 2019, 27, 2026–2036. [Google Scholar] [CrossRef]
- Reizer, E.; Viskolcz, B.; Fiser, B. Formation and growth mechanisms of polycyclic aromatic hydrocarbons: A mini-review. Chemosphere 2022, 291, 132793. [Google Scholar] [CrossRef]
- Tao, H.; Wang, H.-Y.; Ren, W.; Lin, K.C. Kinetic mechanism for modeling the temperature effect on PAH formation in pyrolysis of acetylene. Fuel 2019, 255, 115796. [Google Scholar] [CrossRef]
- Kathrotia, T.; Oßwald, P.; Naumann, C.; Richter, S. Combustion kinetics of alternative jet fuels, Part-II: Reaction model for fuel surrogate. Fuel 2021, 302, 120736. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. 2018, 67, 31–68. [Google Scholar] [CrossRef] [Green Version]
- Alekseev, V.A.; Bystrov, N.; Emelianov, A.; Eremin, A.; Yatsenko, P.; Konnov, A.A. High-temperature oxidation of acetylene by N2O at high Ar dilution conditions and in laminar premixed C2H2 + O2 + N2 flames. Combust. Flame 2022, 238, 111924. [Google Scholar] [CrossRef]
- Burke, M.P.; Chaos, M.; Ju, Y.; Dryer, F.L.; Klippenstein, S.J. Comprehensive H2/O2 kinetic model for high-pressure combustion. Int. J. Chem. Kinet. 2012, 44, 444–474. [Google Scholar] [CrossRef]
- Lopez, J.G.; Rasmussen, C.L.; Alzueta, M.U.; Gao, Y.; Marshall, P.; Glarborg, P. Experimental and Kinetic Modeling Study of C2H2 Oxidation at High Pressure. Int. J. Chem. Kinet. 2016, 48, 724–738. [Google Scholar] [CrossRef]
- Shen, X.; Yang, X.; Santner, J.; Sun, J.; Ju, Y. Experimental and kinetic studies of acetylene flames at elevated pressures. Combust. Inst. 2015, 35, 721–728. [Google Scholar] [CrossRef]
- Norinaga, K.; Janardhanan, V.M.; Deutschmann, O. Detailed chemical kinetic modeling of pyrolysis of ethylene, acetylene, and propylene at 1073–1373 K with a plug-flow reactor model. Int. J. Chem. Kinet. 2008, 40, 199–208. [Google Scholar] [CrossRef]
- Liu, M.; Chu, T.-C.; Jocher, A.; Smith, M.C.; Lengyel, I.; Green, W.H. Predicting polycyclic aromatic hydrocarbon formation with an automatically generated mechanism for acetylene pyrolysis. Int. J. Chem. Kinet. 2021, 53, 27–42. [Google Scholar] [CrossRef]
- Haas, J.-F.; Sturtevant, B. Interaction of weak shock waves with cylindrical and spherical gas inhomogeneities. J. Fluid Mech. 1987, 181, 41–76. [Google Scholar] [CrossRef] [Green Version]

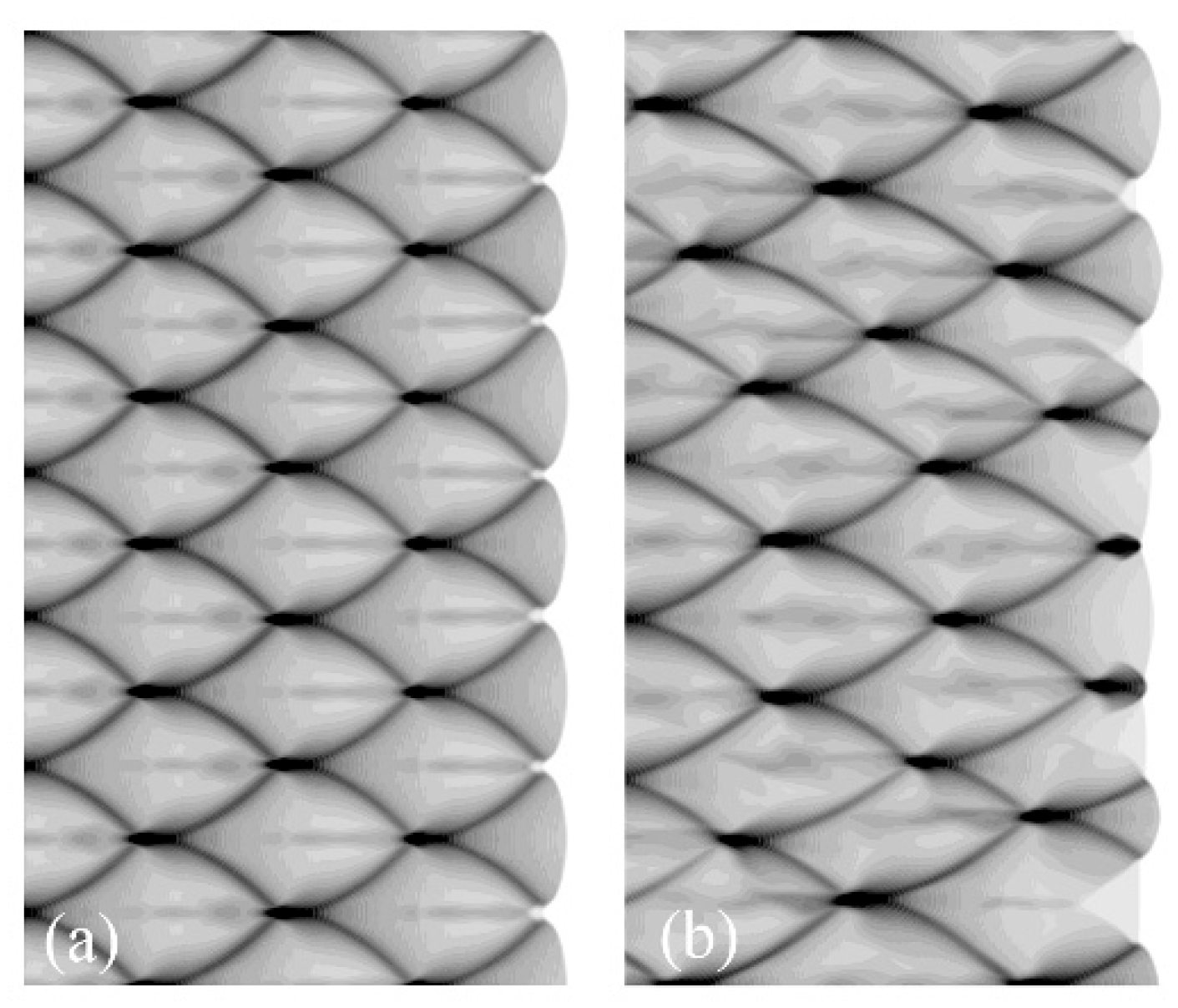
| Characteristic Parameters | Computation Results | |
|---|---|---|
| ER = 3.0 | ER = 6.0 | |
| λ/l | 0.53 | 0.44~0.5 |
| α | 11.50 | 10.68~15.35 |
| β | 37.94 | 28.78~36.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Tang, H. Numerical Simulation of Gaseous Detonation Performance and Wall Reflection Effect of Acetylene-Rich Fuel. Energies 2022, 15, 4985. https://doi.org/10.3390/en15144985
Gao H, Tang H. Numerical Simulation of Gaseous Detonation Performance and Wall Reflection Effect of Acetylene-Rich Fuel. Energies. 2022; 15(14):4985. https://doi.org/10.3390/en15144985
Chicago/Turabian StyleGao, He, and Hao Tang. 2022. "Numerical Simulation of Gaseous Detonation Performance and Wall Reflection Effect of Acetylene-Rich Fuel" Energies 15, no. 14: 4985. https://doi.org/10.3390/en15144985
APA StyleGao, H., & Tang, H. (2022). Numerical Simulation of Gaseous Detonation Performance and Wall Reflection Effect of Acetylene-Rich Fuel. Energies, 15(14), 4985. https://doi.org/10.3390/en15144985






