Challenges and Perspectives for Doping Strategy for Manganese-Based Zinc-ion Battery Cathode
Abstract
:1. Introduction
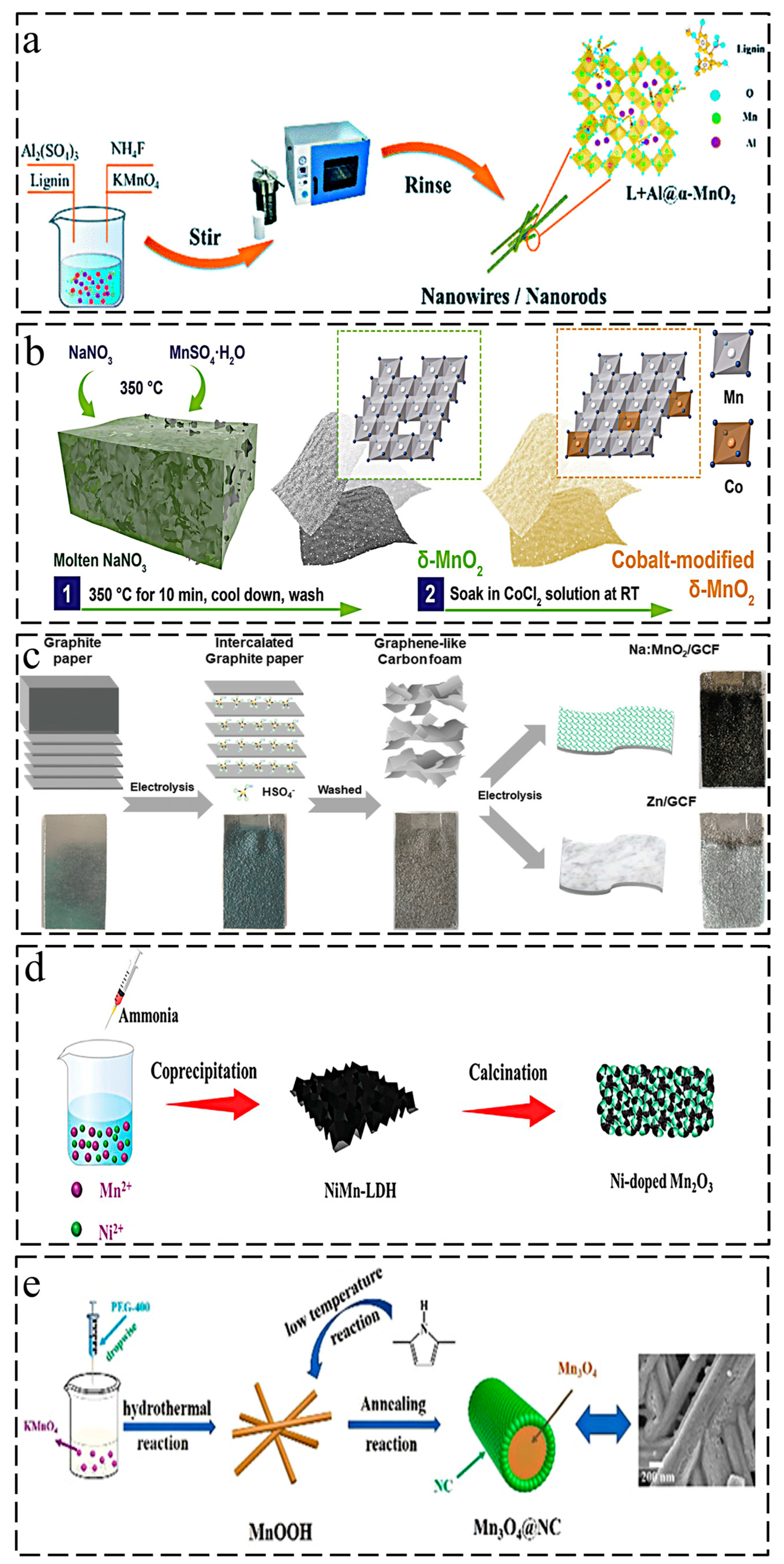
2. Synthesis Strategy for Ion-Doped Manganese-Based Oxides
2.1. Hydrothermal Method
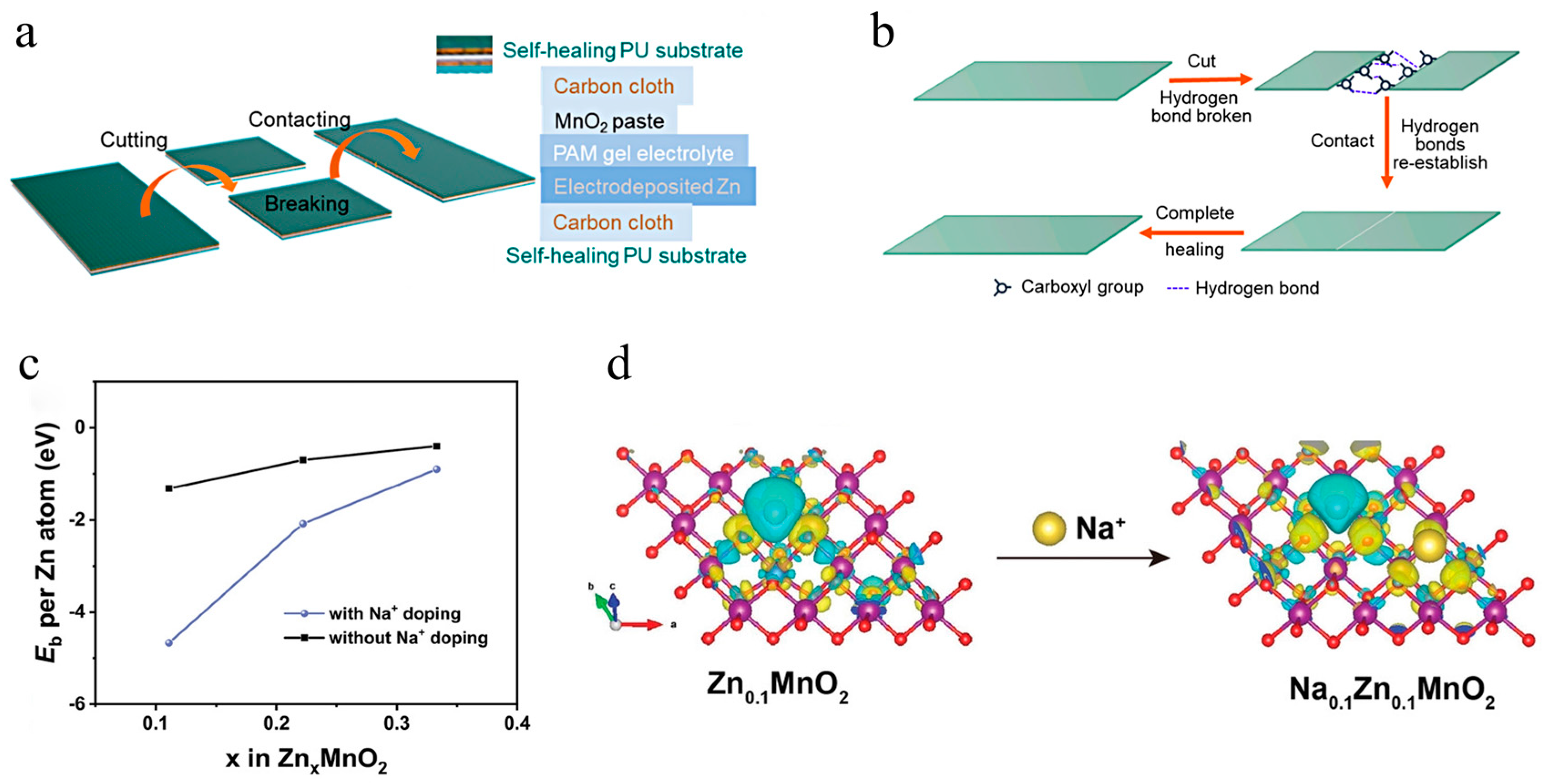
2.2. Ion Penetration/Exchange Method
2.3. Electrodeposition Method
2.4. Calcination Treatment
2.5. Other Methods
3. Valence-Dependent Dopant Ions in Manganese-Based Oxides
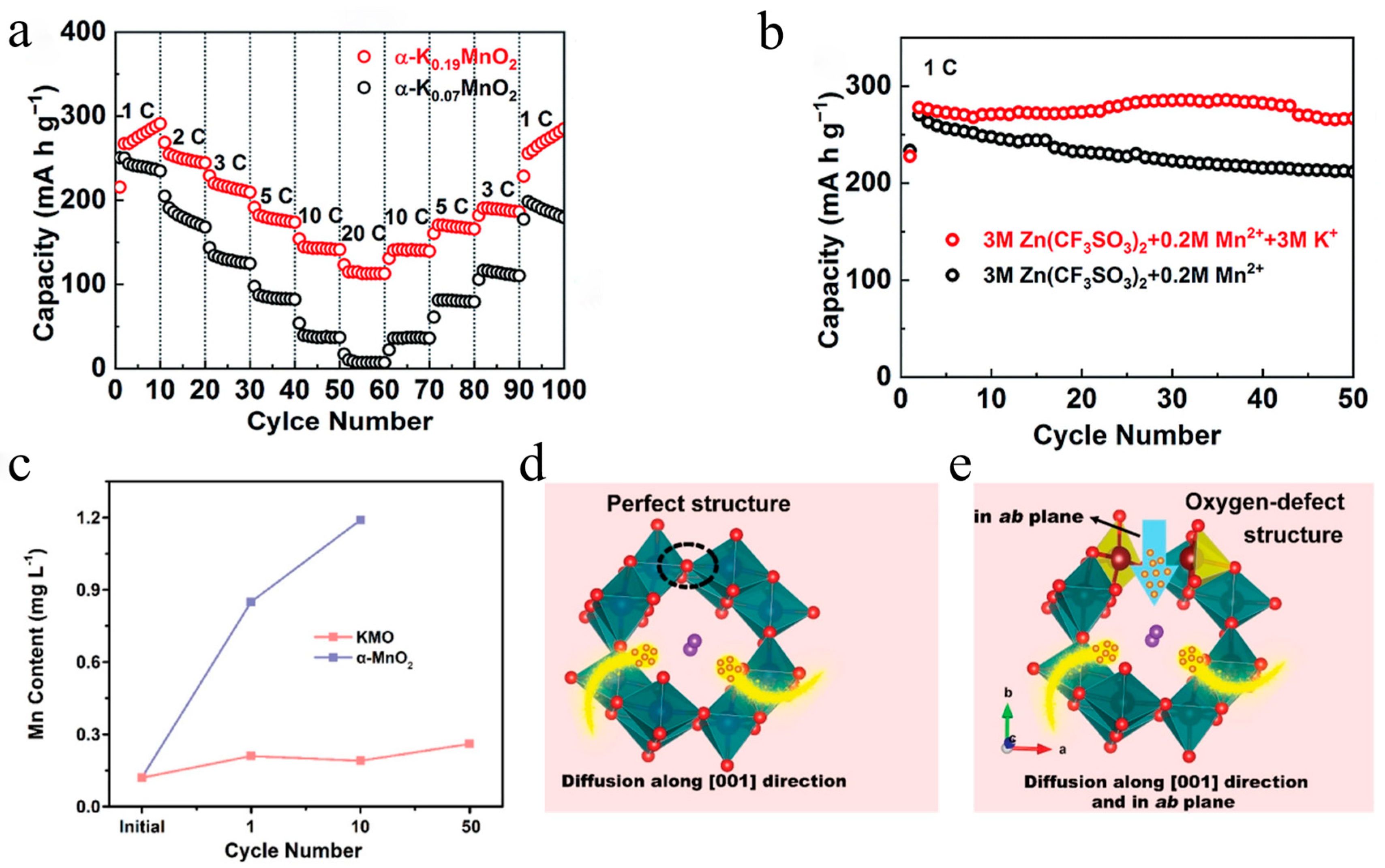
3.1. Monovalent Cation-Doped Manganese-Based Oxides
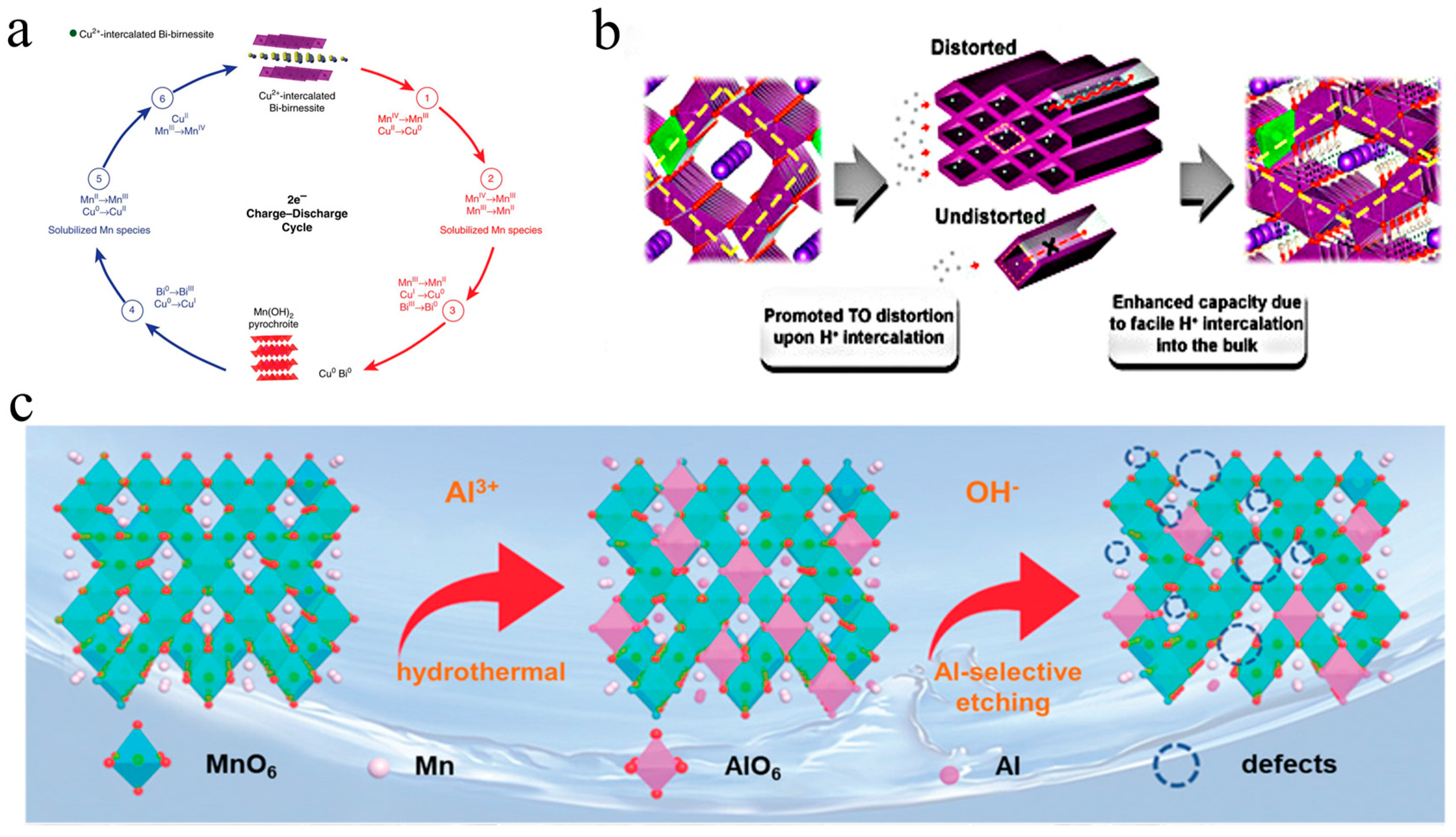
3.2. Multivalent Cation-Doped Manganese-Based Oxides
3.3. Anion-Doped Manganese-Based Oxides
4. Optimization Mechanism of Ion Doping in Zinc–Manganese Battery
4.1. Enlarged Interlayer Spacing for Improved Ion Diffusion Kinetics
4.2. Defect Engineering for Enhanced Electrical Conductivity
4.3. Pillar Effect to Stabilize the Host Structure
5. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Hosenuzzaman, M.; Rahim, N.A.; Selvaraj, J.; Hasanuzzaman, M.; Malek, A.B.M.A.; Nahar, A. Global prospects, progress, policies, and environmental impact of solar photovoltaic power generation. Renew. Sustain. Energy Rev. 2015, 41, 284–297. [Google Scholar] [CrossRef]
- Meng, J.; Guo, H.; Niu, C.; Zhao, Y.; Xu, L.; Li, Q.; Mai, L. Advances in structure and property optimizations of battery electrode materials. Joule 2017, 1, 522–547. [Google Scholar] [CrossRef] [Green Version]
- Sterl, S.; Vanderkelen, I.; Chawanda, C.J.; Russo, D.; Brecha, R.J.; van Griensven, A.; van Lipzig, N.P.M.; Thiery, W. Smart renewable electricity portfolios in West Africa. Nat. Sustain. 2020, 3, 710–719. [Google Scholar] [CrossRef]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Shan, L.; Liang, S.; Zhou, J. Issues and opportunities facing aqueous zinc-ion batteries. Energy Environ. Sci. 2019, 12, 3288–3304. [Google Scholar] [CrossRef]
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific challenges for the implementation of Zn-ion batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Cao, Y.; Li, M.; Lu, J.; Liu, J.; Amine, K. Bridging the academic and industrial metrics for next-generation practical batteries. Nat. Nanotechnol. 2019, 14, 200–207. [Google Scholar] [CrossRef]
- Liu, T.; Dai, A.; Lu, J.; Yuan, Y.; Xiao, Y.; Yu, L.; Li, M.; Gim, J.; Ma, L.; Liu, J.; et al. Correlation between manganese dissolution and dynamic phase stability in spinel-based lithium-ion battery. Nat. Commun. 2019, 10, 4721. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Sun, C.; Yu, Y.; Dong, Y.; Zhang, C.; Wang, C.; Voyles, P.M.; Mai, L.; Wang, X. Surface gradient Ti-doped MnO2 nanowires for high-rate and long-life lithium battery. ACS Appl. Mater. Interfaces 2018, 10, 44376–44384. [Google Scholar] [CrossRef] [PubMed]
- Evarts, E.C. Lithium batteries: To the limits of lithium. Nature 2015, 526, S93–S95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; An, Q.; Mai, L. Recent advances and prospects of cathode materials for rechargeable aqueous zinc-ion batteries. Adv. Mater. Interfaces 2019, 6, 1900387. [Google Scholar] [CrossRef]
- Huang, J.; Guo, Z.; Ma, Y.; Bin, D.; Wang, Y.; Xia, Y. Recent progress of rechargeable batteries using mild aqueous electrolytes. Small Methods 2019, 3, 1800272. [Google Scholar] [CrossRef] [Green Version]
- Chao, D.; Zhou, W.; Xie, F.; Ye, C.; Li, H.; Jaroniec, M.; Qiao, S.Z. Roadmap for advanced aqueous batteries: From design of materials to applications. Sci. Adv. 2020, 6, eaba4098. [Google Scholar] [CrossRef]
- Xiong, F.; Jiang, Y.; Cheng, L.; Yu, R.; Tan, S.; Tang, C.; Zuo, C.; An, Q.; Zhao, Y.; Gaumet, J.J. Low-strain TiP2O7 with three-dimensional ion channels as long-life and high-rate anode material for Mg-ion batteries. Interdiscip. Mater. 2022, 1, 140–147. [Google Scholar] [CrossRef]
- Li, C.; Xie, X.; Liang, S.; Zhou, J. Issues and future perspective on zinc metal anode for rechargeable aqueous zinc-ion batteries. Energy Environ. Mater. 2020, 3, 146–159. [Google Scholar] [CrossRef]
- Shin, J.; Lee, J.; Park, Y.; Choi, J.W. Aqueous zinc ion batteries: Focus on zinc metal anodes. Chem. Sci. 2020, 11, 2028–2044. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Zhu, J.; Tian, J.; Niu, Z. Recent progress in the electrolytes of aqueous zinc-ion batteries. Chem. Eur. J. 2019, 25, 14480–14494. [Google Scholar] [CrossRef]
- Shi, W.; Lee, W.S.V.; Xue, J. Recent development of Mn-based oxides as zinc-ion battery cathode. ChemSusChem 2021, 14, 1634–1658. [Google Scholar] [CrossRef]
- Zhao, Q.; Song, A.; Ding, S.; Qin, R.; Cui, Y.; Li, S.; Pan, F. Preintercalation strategy in manganese oxides for electrochemical energy storage: Review and prospects. Adv. Mater. 2020, 32, e2002450. [Google Scholar] [CrossRef] [PubMed]
- Ming, J.; Guo, J.; Xia, C.; Wang, W.; Alshareef, H.N. Zinc-ion batteries: Materials, mechanisms, and applications. Mater. Sci. Eng. R Rep. 2019, 135, 58–84. [Google Scholar] [CrossRef]
- Yu, P.; Zeng, Y.; Zhang, H.; Yu, M.; Tong, Y.; Lu, X. Flexible Zn-ion batteries: Recent progresses and challenges. Small 2019, 15, e1804760. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhuang, Z.; Zhao, H.; Lin, M.; Zhao, D.; Mai, L. Intricate Hollow Structures: Controlled Synthesis and Applications in Energy Storage and Conversion. Adv. Mater. 2017, 29, 1602914. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Liu, H.; Li, J.; Hou, Z.; Liang, J.; Zhou, J.; Zhu, Y.; Qian, Y. Ultrathin δ-MnO2 nanosheets as cathode for aqueous rechargeable zinc ion battery. Electrochim. Acta 2019, 304, 370–377. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.G.; Qin, X.; Wang, Y.; You, Z.; Liu, H.; Shao, M. Superfine MnO2 nanowires with rich defects toward boosted zinc ion storage performance. ACS Appl Mater. Interfaces 2020, 12, 34949–34958. [Google Scholar] [CrossRef]
- Baral, A.; Satish, L.; Zhang, G.; Ju, S.; Ghosh, M.K. A review of recent progress on nano MnO2: Synthesis, surface modification and applications. J. Inorg. Organomet. Polym Mater. 2020, 31, 899–922. [Google Scholar] [CrossRef]
- Xiong, T.; Zhang, Y.; Lee, W.S.V.; Xue, J. Defect engineering in manganese-based oxides for aqueous rechargeable zinc-ion batteries: A review. Adv. Energy Mater. 2020, 10, 2001769. [Google Scholar] [CrossRef]
- Xu, W.W.; Sun, C.L.; Wang, N.; Liao, X.B.; Zhao, K.N.; Yao, G.; Sun, Q.C.; Cheng, H.W.; Wang, Y.; Lu, X.G. Sn stabilized pyrovanadate structure rearrangement for zinc ion battery. Nano Energy 2021, 81, 105584. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, Y.; Castro, F.A.; Mai, L. Rational design of preintercalated electrodes for rechargeable batteries. ACS Energy Lett. 2019, 4, 771–778. [Google Scholar] [CrossRef]
- Kitchaev, D.A.; Dacek, S.T.; Sun, W.; Ceder, G. Thermodynamics of phase selection in MnO2 framework structures through alkali intercalation and hydration. J. Am. Chem. Soc. 2017, 139, 2672–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Kitchaev, D.A.; Wu, L.; Zhang, B.; Meng, Q.; Poyraz, A.S.; Marschilok, A.C.; Takeuchi, E.S.; Takeuchi, K.J.; Ceder, G. Revealing and rationalizing the rich polytypism of todorokite MnO2. J. Am. Chem. Soc. 2018, 140, 6961–6968. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Peng, H.; Zhao, K.; Zhang, Y.; Xia, F.; Lyu, J.; Van Tendeloo, G.; Sun, C.; Wu, J. Direct visualization of atomic-scale heterogeneous structure dynamics in MnO2 nanowires. ACS Appl. Mater. Interfaces 2021, 13, 33644–33651. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Huang, H.; Bi, R.; Xiao, X.; Ma, T.; Zhang, L. K+ pre-intercalated manganese dioxide with enhanced Zn2+ diffusion for high rate and durable aqueous zinc-ion batteries. J. Mater. Chem. A 2019, 7, 20806–20812. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Chen, X.; Yang, L.Y.; Chen, H.B.; Pan, F. Unravelling H+/Zn2+ synergistic intercalation in a novel phase of manganese oxide for high-performance aqueous rechargeable battery. Small 2019, 15, 1904545. [Google Scholar] [CrossRef]
- Ding, S.; Liu, L.; Qin, R.; Chen, X.; Song, A.; Li, J.; Li, S.; Zhao, Q.; Pan, F. Progressive “layer to hybrid spinel/layer” phase evolution with proton and Zn2+ co-intercalation to enable high performance of MnO2-based aqueous batteries. ACS Appl. Mater. Interfaces 2021, 13, 22466–22474. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, S.; Li, C.; Chen, X.; Wang, W.; Han, Y.; Lin, H.; Shi, Z.; Feng, S. Engineering the interplanar spacing of K-birnessite for ultra-long cycle Zn-ion battery through “hydrothermal potassium insertion” strategy. Chem. Eng. J. 2022, 435, 134754. [Google Scholar] [CrossRef]
- Peng, H.; Fan, H.; Yang, C.; Tian, Y.; Wang, C.; Sui, J. Ultrathin δ-MnO2 nanoflakes with Na+ intercalation as a high-capacity cathode for aqueous zinc-ion batteries. RSC Adv. 2020, 10, 17702–17712. [Google Scholar] [CrossRef]
- Sun, T.; Nian, Q.; Zheng, S.; Shi, J.; Tao, Z. Layered Ca0. 28MnO2·0.5H2O as a high performance cathode for aqueous zinc-Ion battery. Small 2020, 16, 2000597. [Google Scholar] [CrossRef]
- Zhao, Q.; Song, A.; Zhao, W.; Qin, R.; Ding, S.; Chen, X.; Song, Y.; Yang, L.; Lin, H.; Li, S.; et al. Boosting the energy density of aqueous batteries via facile grotthuss proton transport. Angew. Chem. Int. Ed. Engl. 2021, 60, 4169–4174. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Zhao, H.; Xu, L.; Xia, J.; Luo, M.; Yang, Y.; Du, Y. Superior-performance aqueous zinc ion battery based on structural transformation of MnO2 by rare earth doping. J. Mater. Chem. C 2019, 123, 22735–22741. [Google Scholar] [CrossRef]
- Ma, K.; Li, Q.; Hong, C.; Yang, G.; Wang, C. Bi doping-enhanced reversible-phase transition of alpha-MnO2 raising the cycle capability of aqueous Zn-Mn batteries. ACS Appl. Mater. Interfaces 2021, 13, 55208–55217. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Shi, M.; Zhao, Y.; Yang, C.; Zhao, L.; Yan, C. Al-intercalated MnO2 cathode with reversible phase transition for aqueous Zn-ion batteries. Chem. Eng. J. 2021, 422, 130375. [Google Scholar] [CrossRef]
- Xu, J.; Hu, X.; Alam, M.A.; Muhammad, G.; Lv, Y.; Wang, M.; Zhu, C.; Xiong, W. Al-doped α-MnO2 coated by lignin for high-performance rechargeable aqueous zinc-ion batteries. RSC Adv. 2021, 11, 35280–35286. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, K.; Chen, S.; Liu, Y.; Tao, Y.; Zhang, X.; Ding, Y.; Dai, S. Proton inserted manganese dioxides as a reversible cathode for aqueous Zn-ion batteries. ACS Appl. Energy Mater. 2019, 3, 319–327. [Google Scholar] [CrossRef]
- Yadav, G.G.; Gallaway, J.W.; Turney, D.E.; Nyce, M.; Huang, J.; Wei, X.; Banerjee, S. Regenerable Cu-intercalated MnO2 layered cathode for highly cyclable energy dense batteries. Nat. Commun. 2017, 8, 14424. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Xu, X.; Veder, J.P.; Shao, Z. Self-recovery chemistry and cobalt-catalyzed electrochemical deposition of cathode for boosting performance of aqueous zinc-Ion batteries. iScience 2020, 23, 100943. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, M.; Tao, Y.; Zhang, K.; Cai, M.; Ding, Y.; Liu, X.; Hayat, T.; Alsaedi, A.; Dai, S. Electrochemically derived graphene-like carbon film as a superb substrate for high-performance aqueous Zn-ion batteries. Adv. Funct. Mater. 2020, 30, 1907120. [Google Scholar] [CrossRef]
- Kataoka, F.; Ishida, T.; Nagita, K.; Kumbhar, V.; Yamabuki, K.; Nakayama, M. Cobalt-doped layered MnO2 thin film electrochemically grown on nitrogen-doped carbon cloth for aqueous zinc-ion batteries. ACS Appl. Energy Mater. 2020, 3, 4720–4726. [Google Scholar] [CrossRef]
- Ji, J.; Wan, H.; Zhang, B.; Wang, C.; Gan, Y.; Tan, Q.; Wang, N.; Yao, J.; Zheng, Z.; Liang, P.; et al. Co2+/3+/4+-regulated electron state of Mn-O for superb aqueous zinc-manganese oxide batteries. Adv. Energy Mater. 2020, 11, 2003203. [Google Scholar] [CrossRef]
- Long, J.; Gu, J.; Yang, Z.; Mao, J.; Hao, J.; Chen, Z.; Guo, Z. Highly porous, low band-gap NixMn3−xO4 (0.55 ≤ x ≤ 1.2) spinel nanoparticles with in situ coated carbon as advanced cathode materials for zinc-ion batteries. J. Mater. Chem. A 2019, 7, 17854–17866. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, J.; Zhang, X.; Insin, N.; Wang, S.; Han, J.; Zhao, Y.; Qin, J.; Huang, Y. Inhibition of manganese dissolution in Mn2O3 cathode with controllable Ni2+ incorporation for high-performance zinc ion battery. Adv. Funct. Mater. 2021, 31, 2009412. [Google Scholar] [CrossRef]
- Fu, Y.; Wei, Q.; Zhang, G.; Wang, X.; Zhang, J.; Hu, Y.; Wang, D.; Zuin, L.; Zhou, T.; Wu, Y. High-performance reversible aqueous Zn-ion battery based on porous MnOx nanorods coated by MOF-derived N-doped carbon. Adv. Energy Mater. 2018, 8, 1801445. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, S.; Luo, M.; Pan, G.; Zeng, Y.; Lu, X.; Ai, C.; Liu, Q.; Xiong, Q.; Wang, X.; et al. Defect promoted capacity and durability of N-MnO2-x branch arrays via low-temperature NH3 treatment for advanced aqueous zinc ion batteries. Small 2019, 15, e1905452. [Google Scholar] [CrossRef]
- Cheng, X.; Xiao, J.; Ye, M.; Zhang, Y.; Yang, Y.; Li, C.C. Achieving stable zinc-ion storage performance of manganese oxides by synergistic engineering of the interlayer structure and interface. ACS Appl. Mater. Interfaces 2022, 14, 10489–10497. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, P.; Liang, J.; Xia, X.; Ren, L.; Song, L.; Liu, W.; Sun, X. Uncovering sulfur doping effect in MnO2 nanosheets as an efficient cathode for aqueous zinc ion battery. Energy Storage Mater. 2022, 47, 424–433. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.-G.; Liu, H.; Wei, C.; Kang, F. Zinc ion stabilized MnO2 nanospheres for high capacity and long lifespan aqueous zinc-ion batteries. J. Mater. Chem. A 2019, 7, 13727–13735. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, W.; Luo, J.; Zhang, H.; Liu, J.; Liu, X.; Yang, Y.; Lu, X. A high-energy-density aqueous zinc–manganese battery with a La–Ca co-doped ε-MnO2 cathode. J. Mater. Chem. A 2020, 8, 11642–11648. [Google Scholar] [CrossRef]
- Sun, T.; Nian, Q.; Zheng, S.; Yuan, X.; Tao, Z. Water cointercalation for high-energy-density aqueous zinc-ion battery based potassium manganite cathode. J. Power Sources 2020, 478, 228758. [Google Scholar] [CrossRef]
- Lian, S.; Sun, C.; Xu, W.; Huo, W.; Luo, Y.; Zhao, K.; Yao, G.; Xu, W.; Zhang, Y.; Li, Z.; et al. Built-in oriented electric field facilitating durable Zn MnO2 battery. Nano Energy 2019, 62, 79–84. [Google Scholar] [CrossRef]
- Fenta, F.W.; Olbasa, B.W.; Tsai, M.-C.; Weret, M.A.; Zegeye, T.A.; Huang, C.-J.; Huang, W.-H.; Zeleke, T.S.; Sahalie, N.A.; Pao, C.-W. Electrochemical transformation reaction of Cu–MnO in aqueous rechargeable zinc-ion batteries for high performance and long cycle life. J. Mater. Chem. A 2020, 8, 17595–17607. [Google Scholar] [CrossRef]
- Sada, K.; Senthilkumar, B.; Barpanda, P. Cryptomelane K1.33Mn8O16 as a cathode for rechargeable aqueous zinc-ion batteries. J. Mater. Chem. A 2019, 7, 23981–23988. [Google Scholar] [CrossRef]
- Sun, M.; Li, D.S.; Wang, Y.F.; Liu, W.L.; Ren, M.M.; Kong, F.G.; Wang, S.J.; Guo, Y.Z.; Liu, Y.M. Mn3O4@NC composite nanorods as a cathode for rechargeable aqueous Zn-ion batteries. ChemElectroChem 2019, 6, 2510–2516. [Google Scholar] [CrossRef]
- Qiu, N.; Chen, H.; Yang, Z.; Sun, S.; Wang, Y. Low-cost birnessite as a promising cathode for high-performance aqueous rechargeable batteries. Electrochim. Acta 2018, 272, 154–160. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Liang, G.; Li, H.; Liu, Z.; Tang, Z.; Liang, J.; Zhi, C. A superior delta-MnO2 cathode and a self-healing Zn-delta-MnO2 battery. ACS Nano 2019, 13, 10643–10652. [Google Scholar] [CrossRef]
- Hou, Z.; Dong, M.; Xiong, Y.; Zhang, X.; Ao, H.; Liu, M.; Zhu, Y.; Qian, Y. A high-energy and long-life aqueous Zn/birnessite battery via reversible water and Zn2+ coinsertion. Small 2020, 16, e2001228. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Shi, H.; Zhong, Z.; Niu, X.; Zeng, P.; Long, Z.; Chen, X.; Peng, J.; Luo, Z. Electrochemical energy storage behavior of Na0. 44MnO2 in aqueous zinc-ion battery. ACS Sustain. Chem. Eng. 2020, 8, 10673–10681. [Google Scholar]
- Fang, G.; Zhu, C.; Chen, M.; Zhou, J.; Tang, B.; Cao, X.; Zheng, X.; Pan, A.; Liang, S. Suppressing manganese dissolution in potassium manganate with rich oxygen defects engaged high-energy-density and durable aqueous zinc-ion battery. Adv. Funct. Mater. 2019, 29, 1808375. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Q.; Abraham, A.; West, P.J.; Housel, L.M.; Singh, G.; Sadique, N.; Quilty, C.D.; Wu, D.; Takeuchi, E.S. Silver-containing α-MnO2 nanorods: Electrochemistry in rechargeable aqueous Zn-MnO2 batteries. J. Electrochem. Soc. 2019, 166, A3575. [Google Scholar] [CrossRef]
- Liu, N.; Wu, X.; Yin, Y.; Chen, A.; Zhao, C.; Guo, Z.; Fan, L.; Zhang, N. Constructing the efficient ion diffusion pathway by introducing oxygen defects in Mn2O3 for high-performance aqueous zinc-ion batteries. ACS Appl Mater. Interfaces 2020, 12, 28199–28205. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q.; Wang, J.; Chen, K.; Xue, D.; Liu, J.; Lu, X. Boosting the Zn-ion storage capability of birnessite manganese oxide nanoflorets by La3+ intercalation. J. Mater. Chem. A 2019, 7, 22079–22083. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, S.; Li, C.; Chen, X.; Zhang, W.; Ge, X.; Lin, H.; Shi, Z.; Feng, S. High-performance aqueous zinc-ion battery based on an Al0.35Mn2.52O4 cathode: A design Strategy from defect engineering and atomic composition tuning. Small 2022, 18, 2105970. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Gao, Q.L.; Xia, Y.M.; Lin, X.S.; Liu, W.L.; Ren, M.M.; Kong, F.G.; Wang, S.J.; Lin, C. High-performance reversible aqueous zinc-ion battery based on iron-doped alpha-manganese dioxide coated by polypyrrole. J. Colloid Interface Sci. 2021, 598, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Alfaruqi, M.H.; Islam, S.; Mathew, V.; Song, J.; Kim, S.; Tung, D.P.; Jo, J.; Kim, S.; Baboo, J.P.; Xiu, Z.; et al. Ambient redox synthesis of vanadium-doped manganese dioxide nanoparticles and their enhanced zinc storage properties. Appl. Surf. Sci. 2017, 404, 435–442. [Google Scholar] [CrossRef]
- Tao, Y.; Li, Z.; Tang, L.; Pu, X.; Cao, T.; Cheng, D.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Nickel and cobalt co-substituted spinel ZnMn2O4@N-rGO for increased capacity and stability as a cathode material for rechargeable aqueous zinc-ion battery. Electrochim. Acta 2020, 331, 135296. [Google Scholar] [CrossRef]
- Qiu, W.; Li, Y.; You, A.; Zhang, Z.; Li, G.; Lu, X.; Tong, Y. High-performance flexible quasi-solid-state Zn–MnO2 battery based on MnO2 nanorod arrays coated 3D porous nitrogen-doped carbon cloth. J. Mater. Chem. A 2017, 5, 14838–14846. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; Qin, H.; Zeng, X.; Meng, J. Advanced electrochemical performance of ZnMn2O4/N-doped graphene hybrid as cathode material for zinc ion battery. J. Power Sources 2019, 425, 162–169. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.; Cui, F.; Meng, J.; Jiang, Y.; Long, J.; Zeng, X. Ultrathin MnO2 nanoflakes grown on N-doped hollow carbon spheres for high-performance aqueous zinc ion batteries. Mater. Chem. Front. 2020, 4, 213–221. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, S.; Pan, G.; Zhang, H.; Liu, B.; Wang, X.L.; Zheng, X.; Liu, Q.; Wang, X.; Xia, X.; et al. Introducing oxygen defects into phosphate ions intercalated manganese dioxide/vertical multilayer graphene arrays to boost flexible zinc ion storage. Small Methods 2020, 4, 1900828. [Google Scholar] [CrossRef]
- Li, J.; McColl, K.; Lu, X.; Sathasivam, S.; Dong, H.; Kang, L.; Li, Z.; Zhao, S.; Kafizas, A.G.; Wang, R.; et al. Multi-scale investigations of δ-Ni0.25V2O5·nH2O cathode materials in aqueous zinc-ion batteries. Adv. Energy Mater. 2020, 10, 2000058. [Google Scholar] [CrossRef]
- Kundu, D.; Hosseini Vajargah, S.; Wan, L.; Adams, B.; Prendergast, D.; Nazar, L.F. Aqueous vs. nonaqueous Zn-ion batteries: Consequences of the desolvation penalty at the interface. Energy Environ. Sci. 2018, 11, 881–892. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; Jiang, H.; Parkin, I.P.; Shearing, P.R.; Brett, D.J.L. Cathode design for aqueous rechargeable multivalent ion batteries: Challenges and opportunities. Adv. Funct. Mater. 2021, 31, 2010445. [Google Scholar] [CrossRef]
- Yan, M.; He, P.; Chen, Y.; Wang, S.; Wei, Q.; Zhao, K.; Xu, X.; An, Q.; Shuang, Y.; Shao, Y. Water-lubricated intercalation in V2O5·nH2O for high-capacity and high-rate aqueous rechargeable zinc batteries. Adv. Mater. 2018, 30, 1703725. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Ye, Y.; Pan, F. ‘Structure units’ as material genes in cathode materials for lithium-ion batteries. Natl. Sci. Rev. 2020, 7, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Young, M.J.; Holder, A.M.; George, S.M.; Musgrave, C.B. Charge storage in cation incorporated α-MnO2. Chem. Mater. 2015, 27, 1172–1180. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Liu, C.; Neale, Z.G.; Yang, J.; Cao, G. Active materials for aqueous zinc ion batteries: Synthesis, crystal structure, morphology, and electrochemistry. Chem. Rev. 2020, 120, 7795–7866. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, J.; Wang, L.; Long, X.; Liu, X.; Li, F.; Chen, J. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Alfaruqi, M.H.; Mathew, V.; Gim, J.; Kim, S.; Song, J.; Baboo, J.P.; Choi, S.H.; Kim, J. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system. Chem. Mater. 2015, 27, 3609–3620. [Google Scholar] [CrossRef]
- Kobayashi, S.; Kottegoda, I.R.; Uchimoto, Y.; Wakihara, M. XANES and EXAFS analysis of nano-size manganese dioxide as a cathode material for lithium-ion batteries. J. Mater. Chem. 2004, 14, 1843–1848. [Google Scholar] [CrossRef]
- Berg, H.; Göransson, K.; Noläng, B.; Thomas, J.O. Electronic structure and stability of the LixMn2O4 (0 < x < 2) system. J. Mater. Chem. 1999, 9, 2813–2820. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. Sect. A. 1976, 32, 751–767. [Google Scholar] [CrossRef]

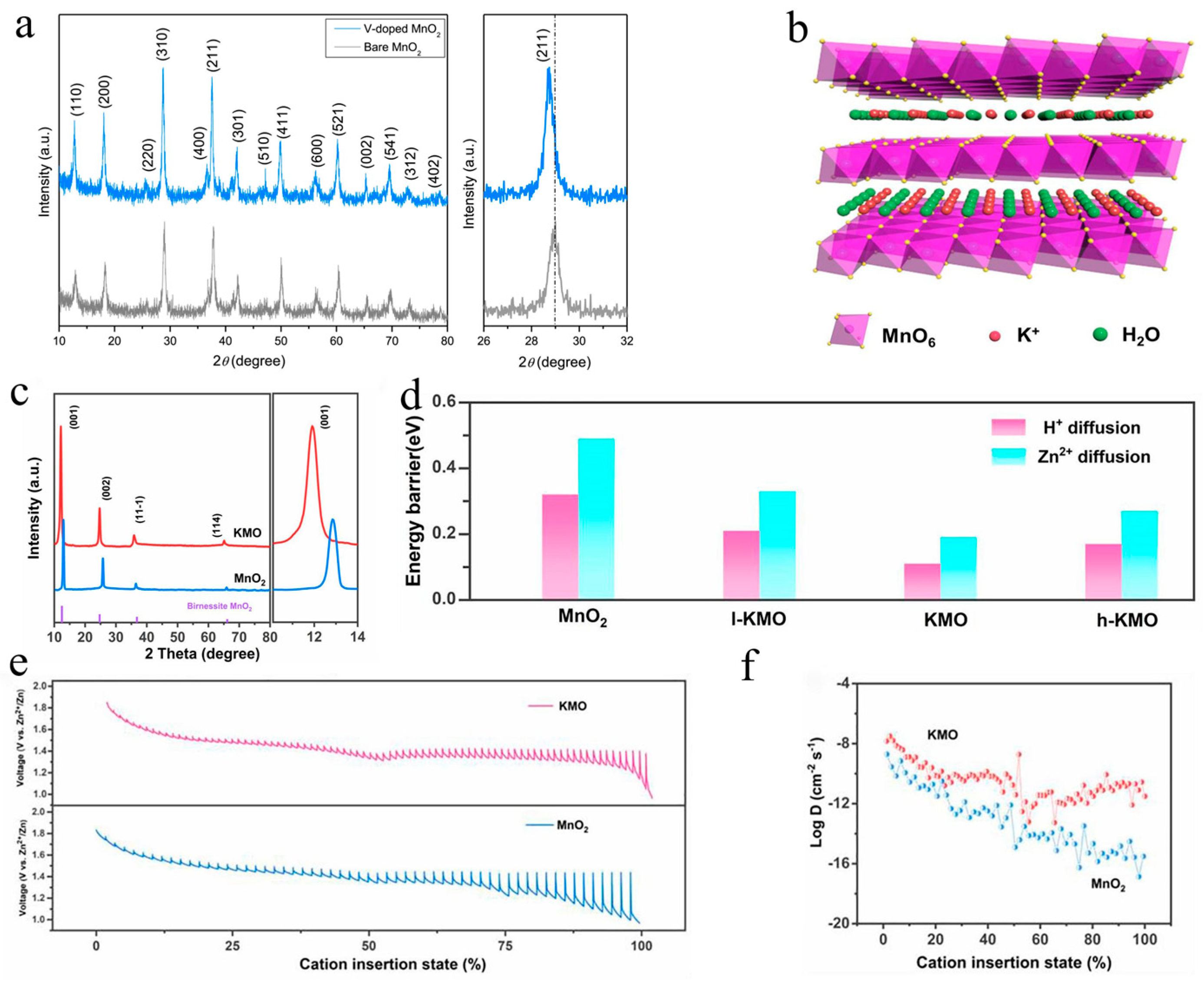
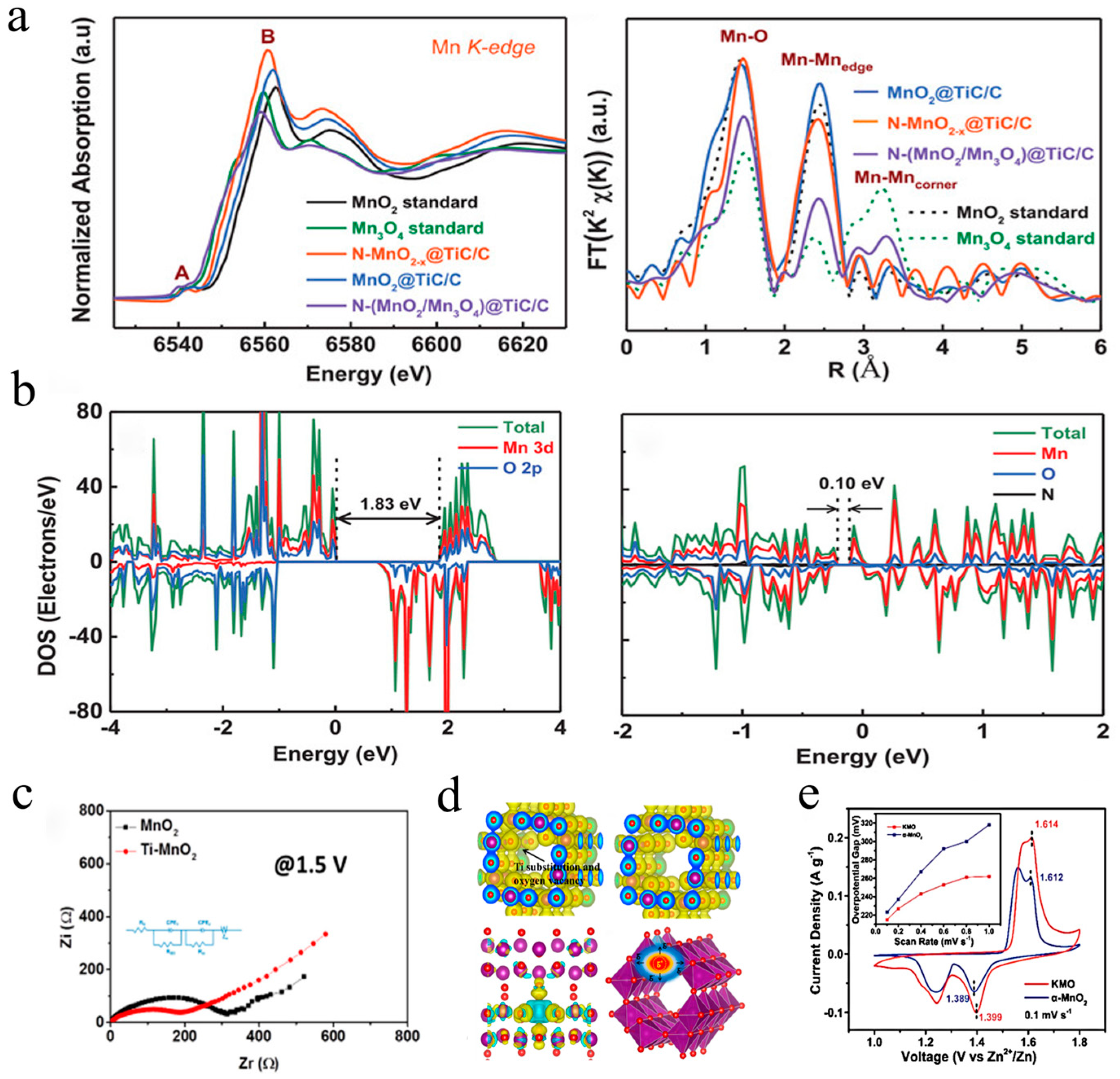
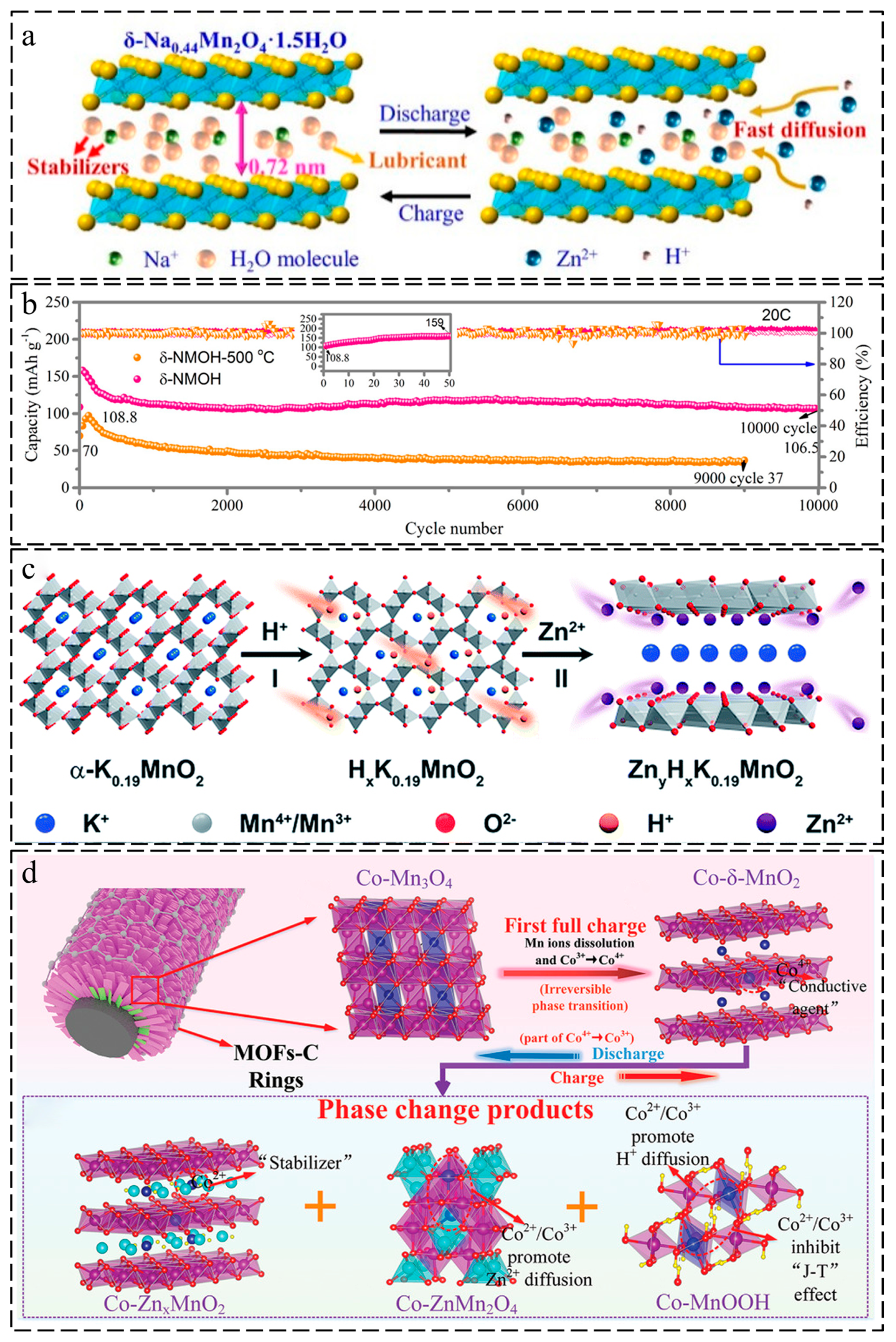
| Cathode Material | Synthesis Method | Capacity (mAh·g−1/mA·g−1) | Rate Performance (mAh·g−1/mA·g−1) | Cycle Performance (X %/cycles) | Ref. |
|---|---|---|---|---|---|
| MnO2–birnessite | Other | 266/100 | 134/2000 | 83.7%/2000 | [64] |
| δ-Na0.44Mn2O4·1.5H2O | Other | 278/300 | 106/6000 | 98%/10,000 | [65] |
| Na:MnO2/GCF | Electrodeposition | 381.8/100 | 175/2000 | 75.2%/1000 | [48] |
| Na+–δ-MnO2 | Hydrothermal | 335/500 | 194/4000 | 93%/1000 | [38] |
| Na0.1MnO2·0.5H2O | Other | 270/30 | 100/3000 | 98%/5000 | [66] |
| Na0.44MnO2 | Other | 301.3/100 | 80/1000 | 69.3%/800 | [67] |
| N–Na2Mn3O7 | Calcination treatment | 300/200 | 100/10,000 | 78.9%/550 | [55] |
| α-K0.19MnO2 | Hydrothermal | 266/300 | 113/6000 | 90%/400 | [34] |
| K1.33Mn8O16 | Other | 312/30 | 80/1500 | 80%/650 | [62] |
| K0.8Mn8O16 | Hydrothermal | 300/100 | 154/1000 | 100%/1000 | [68] |
| K0.36H0.26MnO2·0.28H2O | Hydrothermal | 329.8/30 | 100.1/3000 | 93.4%/3000 | [36] |
| K0.29MnO2·0.67H2O | Hydrothermal | 300/200 | 158/2000 | 98%/12,000 | [37] |
| Ag1.5Mn8O16 | Other | 240/50 | - | - | [69] |
| MnO2H0.16(H2O)0.27 | Hydrothermal | 275.6/30 | 115.1/3000 | 79%/2000 | [35] |
| H1.57Mn2O4 | Ion exchange | 281/100 | 133.4/1000 | 93.5%/1000 | [45] |
| Cu–MnO | Other | 320/150 | 156/900 | 70%/1000 | [61] |
| Ocu–Mn2O3 | Other | 246/50 | 112/1000 | 88%/600 | [70] |
| NixMn3−xO4 | Calcination treatment | 139.7/50 | 98.5/1200 | 93.5%/800 | [51] |
| Ni-doped Mn2O3 | Calcination treatment | 252/100 | 132/1000 | 85.6%/2500 | [52] |
| Ni0.052K0.119Mn0.948O2·0.208H2O | Hydrothermal | 303/30 | 175/1200 | 71.4%/2000 | [40] |
| Zn2+-δ-MnO2 | Other | 358/300 | 124/3000 | 80%/2000 | [57] |
| Co-Mn3O4 | Electrodeposition | 362/100 | 90/4000 | 80%/1100 | [50] |
| Ca0.28MnO2·0.5H2O | Hydrothermal | 298/175 | 124.5/3500 | 100%/5000 | [39] |
| Ce-doped MnO2 | Hydrothermal | 270/150 | 134/1500 | 70%/100 | [41] |
| La3+-δ-MnO2 | Other | 278.5/100 | 121.8/1600 | 71%/200 | [71] |
| Bi-α-MnO2 | Hydrothermal | 325/300 | 150/5000 | 90.9%/2000 | [42] |
| Al0.35Mn2.52O4 | Hydrothermal | 302/100 | 147/1500 | 95%/1000 | [72] |
| Al-intercalated MnO2 | Hydrothermal | 401/100 | 229/4000 | 94.5%/2000 | [43] |
| Fe/α-MnO2@PPy | Other | 270/100 | 96/800 | 75%/1000 | [73] |
| Ti–MnO2 | Other | 259/100 | 179/1000 | 80%/4000 | [60] |
| V-doped MnO2 | Other | 266/66 | 67/1064 | - | [74] |
| La–Ca co-doped ε-MnO2 | Other | 297.3/200 | 161/1600 | 76.8%/200 | [58] |
| ZnNixCoyMn2−x−yO4@N-rGO | Other | 200.5/10 | 93.5/1500 | 79%/900 | [75] |
| N–CC@MnO2 | Other | 353/500 | 201/6000 | 93.6%/1000 | [76] |
| N–C@MnOx | Calcination treatment | 305/500 | 100/2000 | 100%/1600 | [53] |
| Mn3O4@NC | Other | 280/100 | 97/1000 | 100%/700 | [63] |
| ZnMn2O4/N | Other | 221/100 | 110/1000 | 97.4%/2500 | [77] |
| MnO2–NHCS | Other | 349/100 | 100/2000 | 78.7%/2000 | [78] |
| N–MnO2–x | Calcination treatment | 285/200 | 155.2/2000 | 85.7%/1000 | [54] |
| P–MnO2−x@VMG | Other | 302.8/500 | 150.1/10,000 | Above 90%/1000 | [79] |
| S–MnO2 | Calcination treatment | 324/200 | 205/2000 | 95%/1000 | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Chen, J.; Sun, W.; Shao, Y.; Zhang, L.; Zhao, K. Challenges and Perspectives for Doping Strategy for Manganese-Based Zinc-ion Battery Cathode. Energies 2022, 15, 4698. https://doi.org/10.3390/en15134698
Zhang B, Chen J, Sun W, Shao Y, Zhang L, Zhao K. Challenges and Perspectives for Doping Strategy for Manganese-Based Zinc-ion Battery Cathode. Energies. 2022; 15(13):4698. https://doi.org/10.3390/en15134698
Chicago/Turabian StyleZhang, Bomian, Jinghui Chen, Weiyi Sun, Yubo Shao, Lei Zhang, and Kangning Zhao. 2022. "Challenges and Perspectives for Doping Strategy for Manganese-Based Zinc-ion Battery Cathode" Energies 15, no. 13: 4698. https://doi.org/10.3390/en15134698
APA StyleZhang, B., Chen, J., Sun, W., Shao, Y., Zhang, L., & Zhao, K. (2022). Challenges and Perspectives for Doping Strategy for Manganese-Based Zinc-ion Battery Cathode. Energies, 15(13), 4698. https://doi.org/10.3390/en15134698







