Abstract
The development of an efficient and durable oxygen evolution reaction (OER) electrode is needed to solve the bottleneck in the application of an anion exchange membrane water electrolyzer (AEMWE). In this work, the self-supporting NiFe layered double hydroxides (NiFe LDHs) “nanoflower” cluster OER electrode directly grown on the surface of nickel fiber felt (Ni fiber) was synthesized by a one-step impregnation at ambient pressure and temperature. The self-supporting NiFe LDHs/Ni fiber electrode showed excellent activity and stability in a three-electrode system and as the anode of AEMWE. In a three-electrode system, the NiFe LDHs/Ni fiber electrode showed excellent OER performance with an overpotential of 208 mV at a current density of 10 mA cm−2 in 1 M KOH. The NiFe LDHs/Ni fiber electrode was used as the anode of the AEMWE, showing high cell performance with a current density of 0.5 A cm−2 at 1.68 V and a stability test for 200 h in 1 M KOH at 70 °C. The electrocatalytic performance of NiFe LDHs/Ni fiber electrode is due to the special morphological structure of “nanoflower” cluster petals stretching outward to produce the “tip effect,” which is beneficial for the exposure of active sites at the edge and mass transfer under high current density. The experimental results show that the NiFe LDHs/Ni fiber electrode synthesized by the one-step impregnation method has the advantages of good activity and low cost, and it is promising for industrial application.
1. Introduction
Water electrolysis is regarded as one of the most promising hydrogen production technologies because of its high catalytic efficiency, high purity, and environmental friendliness [1,2,3]. Alkaline anion exchange membrane water electrolyzer (AEMWE) has attracted widespread attention because it can use low-cost non-noble metal electrodes to replace the noble metal-based electrodes such as IrO2 and RuO2 [4,5], although its alkaline anion exchange membrane technology needs to be improved [5].
However, the large-scale application of water electrolysis is mainly hindered by the slow oxygen evolution reaction (OER) of the anode [4,6]. Therefore, a key goal is to design and explore OER electrodes with efficient catalytic activity and long stability [7,8]. NiFe-based layered double hydroxide materials have been widely studied because of their low cost, and show excellent performance comparable to noble metal-based OER electrodes in an alkaline medium [9,10].
Since the discovery that NiFe LDHs have high intrinsic OER performance [4,11,12], researchers have made many attempts to improve the catalytic performance of NiFe LDHs, such as optimizing the morphology structure and adjusting the electronic structure [3,4]. Although great progress has been made in the design of NiFe LDHs-based electrodes, there are still some shortcomings that hinder their large-scale commercial application. First, researchers try to design catalysts with low overpotential and pay less attention to stability. So far, it has been reported that NiFe-based nanomaterials are generally not particularly stable and can only run for about 10–12 h in a three-electrode system [2,4], which is far from the needs of industrial production. In addition, constructing self-supporting catalysts and more and more strategies needs to be explored to improve the stability of electrocatalysts at high current density in AEMWE.
In the process of application, precious noble metal-based powder catalysts need to be coated on the collector with the binder before the electrocatalytic process. However, the use of the binder will inevitably bury some active centers, increase the electrode resistance, and inhibit charge transfer [13]. In addition, the binder between the collector and the catalyst is weak, so the active centers are easy to peel off from the electrode, resulting in a decline in performance [13]. By comparison, the use of self-supporting electrodes can effectively avoid these problems.
At present, there are many methods for preparing self-supporting NiFe LDHs-based electrodes, such as constant pH coprecipitation, hydrothermal treatment, and electrodeposition [13,14]. However, the methods often use organic precipitant and high temperature and pressurized equipment, which increases the cost and limits the application [15].
Therefore, designing a simpler and energy-efficient method to prepare an efficient and durable OER self-supporting NiFe LDHs electrode can greatly reduce the cost of water electrolysis, and even meet the requirements of industrial hydrogen production [13]. Recently, Yan et al. [14] achieved the simple hydrolysis-promotion-deposition method for the preparation of NiFe LDHs nanosheets electrodes directly on nickel foam substrate at room temperature and ambient pressure. Li et al. [13] reported a simple and energy-efficient synthesis of NiFe LDHs directly growing on nickel foam substrates at room temperature and ambient pressure. These methods are simple and low-cost, and the synthesized NiFe LDHs electrode exhibits good performance in the OER reaction. However, these 2D nanosheets are easy to stack in synthesis, which greatly reduces the exposure of active sites and catalytic efficiency [15].
In this work, we selected nickel fiber felt (Ni fiber) with a relatively flat surface as the substrate, and obtained the self-supporting NiFe LDHs “nanoflower” cluster electrode directly and vertically grown on the Ni fiber substrate by a simple one-step impregnation method at room temperature and ambient pressure (in Figure 1). The results show that the self-supporting NiFe LDHs/Ni fiber electrode not only has remarkable catalytic activity and stability in a three-electrode system, but also serves assemble as the anode for the AEMWE single-cell.
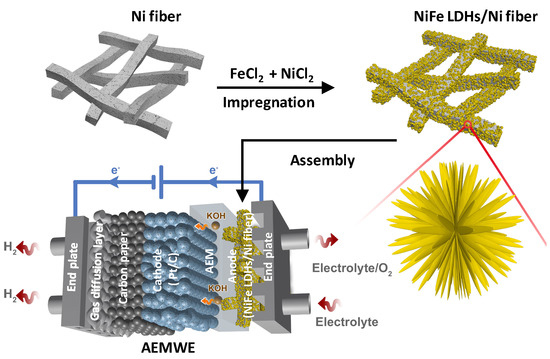
Figure 1.
Schematic preparation of NiFe LDHs/Ni fiber electrode and as the anode of the AEMWE.
2. Experimental
2.1. Chemical and Materials
The nickel fiber (Ni fiber felt, Ni ≥ 99.9%, thickness of 0.4 mm) was purchased from Aida, Co., Ltd. (Xinxiang, China). The nickel foam (Ni foam, thickness of 1 mm) was received from Lizhiyuan Co., Ltd. (Shanxi, China). Ferrous chloride (FeCl2∙4H2O, ≥98%), nickel chloride (NiCl2∙6H2O, ≥98%), cobalt chloride (CoCl2·6H2O, ≥99%), manganese chloride tetrahydrate (MnCl2·4H2O ≥ 99%), absolute ethanol (C2H5OH ≥ 99.7%), acetone (C3H6O ≥ 99%), and hydrochloric acid (HCl, 36–38%) were purchased from Bono Chemical Co., Ltd. (Dalian, China). Potassium hydroxide (KOH, ≥99.99%) was purchased from Aladdin Reagent Co., Ltd. (Shanghai, China). Pt/C (70 wt%) was purchased from Johnson Matthey Ltd. Iridium oxide (IrO2, 99.99%) was provided by Shanghai Chemical Reagent Co., Ltd. (Shanghai, China). All chemicals were used as received without any further purification. Millipore water was used in all experiments.
2.2. Catalyst Synthesis
2.2.1. Synthesis of NiFe LDHs/Ni Fiber
First, Ni fiber (2 × 5 cm2) was cleaned with C3H6O, 3 M HCl, C2H5OH, and deionized water in ultrasound for 5 minutes. In a typical procedure, NiCl2∙6H2O (1.19 g) and FeCl2∙4H2O (0.10 g) were dissolved in 50 mL of deionized water under continuous stirring for 1 h. The obtained solution was light green.
Afterward, the cleaned Ni fiber substrate was immersed in the light green solution for 7 days, and the obtained product was washed with deionized water several times. Finally, the product was continuously dried at 60 °C for 12 h by vacuum drying and was named NiFe LDHs/Ni fiber electrode.
2.2.2. Synthesis of NiCo LDHs/Ni Fiber
Similarly, Ni fiber (2 × 5 cm2) was cleaned with C3H6O, 3 M HCl, C2H5OH, and deionized water in ultrasound for 5 minutes. In a typical procedure, NiCl2∙6H2O (1.19 g) and CoCl2∙6H2O (0.12 g) were dissolved in 50 mL of deionized water under continuous stirring for 1 h. The obtained solution was red.
Afterward, the cleaned Ni fiber substrate was immersed in the red solution for 7 days, and the obtained product was washed with deionized water several times. Finally, the product was continuously dried at 60 °C for 12 h by vacuum drying and was named NiCo LDHs/Ni fiber electrode.
2.2.3. Synthesis of NiMn LDHs/Ni Fiber
Similarly, Ni fiber (2 × 5 cm2) was cleaned with C3H6O, 3 M HCl, C2H5OH, and deionized water in ultrasound for 5 minutes. In a typical procedure, NiCl2∙6H2O (1.19 g) and MnCl2∙4H2O (0.10 g) were dissolved in 50 mL of deionized water under continuous stirring for 1 h. The obtained solution was green.
Afterward, the cleaned Ni fiber substrate was immersed in the green solution for 7 days, and the obtained product was washed with deionized water several times. Finally, the product was continuously dried at 60 °C for 12 h by vacuum drying and was named NiMn LDHs/Ni fiber electrode.
2.2.4. Synthesis of NiFe LDHs/Ni Foam
For comparison, the Ni fiber was replaced with Ni foam (Figure S1). The Ni foam (2 × 5 cm2) was cleaned with C3H6O, 3 M HCl, C2H5OH, and deionized water in ultrasound for 5 minutes. In a typical procedure, NiCl2∙6H2O (1.19 g) and FeCl2∙4H2O (0.10 g) were dissolved in 50 mL of deionized water under continuous stirring for 1 h. The obtained solution was light green.
Afterward, the cleaned Ni foam substrate was immersed in the light green solution for 7 days, and the obtained product was washed with deionized water several times. Finally, the product was continuously dried at 60 °C for 12 h by vacuum drying and was named NiFe LDHs/Ni foam electrode.
2.2.5. Synthesis of IrO2/Ni Fiber
To prepare the IrO2/Ni fiber electrode, 5 mg of IrO2 was dispersed into 20 μL Nafion (5 wt%) and 980 μL ethanol and water (volume ratio 1:1), and obtained uniform ink through ultrasonic treatment [16]. Then IrO2 ink was dropped on the cleaned Ni fiber substrate and dried at 60 °C.
2.2.6. Preparation of the AEMWE Single-Cell
In a typical procedure, the AEMWE system consists of an anode (NiFe LDHs/Ni fiber, 2 × 2 cm2), a cathode (0.4 mg cm−2 of 70 wt.% Pt/C) coated on the anion exchange membrane (A201, Tokuyama, 2 × 2 cm2), and a gas diffusion layer (carbon paper, Toray) [17,18,19]. Then, the anode, the cathode, an alkaline anion exchange membrane, and carbon paper were hot-pressed at 60 °C and 0.1 MPa for 2 min to obtain membrane electrode assembly (NiFe LDHs/Ni fiber || Pt/C) [20,21]. The AEMWE single-cell was assembled as shown in Figure 1.
2.3. Material Characterizations
The morphology characterization was tested by a scanning electron microscope (SEM, JSM-7800F) instrument produced in Japan, and the microstructure was analyzed with a transmission electron microscope (TEM, JEM-2100F). A Miniflex600 (X-ray diffraction XRD) produced in Japan was used to characterize the object image structure of the material. The X-ray photoelectron spectroscopy (XPS) was measured on the ESCALAB 250Xi spectrometer (Thermo Fisher, Waltham, MA, USA).
2.4. Electrochemical Measurements
The electrochemical measurements were carried out on a Gamry Interface 1000 e electrochemical workstation in 1 M KOH (pH = ~14, O2 saturated) at room temperature. The graphite plate was used as the counter electrode, the standard Hg/HgO electrode was used as the reference electrode, and the prepared self-supported electrodes (1 × 1 cm2) were used as the working electrode. In a three-electrode system, the voltage used in the experiment has been converted to the reversible hydrogen electrode (RHE) based on the Formula (1) [22,23,24,25]:
The calculation of overpotential follows Equation (2) [25,26]:
The electrode was activated by cyclic voltammetry (CV). Linear sweep voltammetry (LSV) was used to evaluate the activity of the catalysts between 0–1 V (V vs. Hg/HgO) at a scan rate of 2 mV s−1. Electrochemical impedance spectroscopy (EIS) was tested at 0.6 V (V vs. Hg/HgO), with an amplitude of 5 mV in a frequency range from 0.1 Hz to 100 kHz. The chronopotentiometry curves were carried out at different current densities for 10, 50, 100, 200, 300, and 10 mA cm−2, respectively. The electrochemically surface area (ECSA) was measured by the CV method at different scan rates in the non-Faradaic region and calculated based on the following (3) [27,28]:
To further test the practical application of the NiFe LDHs/Ni fiber, it was assembled into an AEMWE (2 × 2 cm2). Current–voltage (I–V) curves were measured at 70 °C. 1 M KOH and the electrolyte were supplied into the anode at a flow rate of 5 mL min−1. The stability test was conducted at a current density of 0.5 A cm−2 for 200 h. The AEMWE charge transfer resistance and ohmic resistance were analyzed by measuring EIS at 1.7 V [19]. The amplitude was 10 mV and the frequency range was from 100 mHz to 10 kHz.
3. Results and Discussion
3.1. Structure and Characterization
The morphologies of the Ni fiber electrode and NiFe LDHs/Ni fiber electrode were observed with an SEM. Figure 2a is a bare Ni fiber substrate, which can be seen to be composed of many interlaced fibers. Figure 2b,c are SEM images of NiFe LDHs/Ni fiber electrode under different magnifications. It can be seen that a lot of “nanoflower” clusters grow uniformly on the Ni fiber substrate. The structure is similar to the microstructure of NiFe LDHs reported in the literature [29].
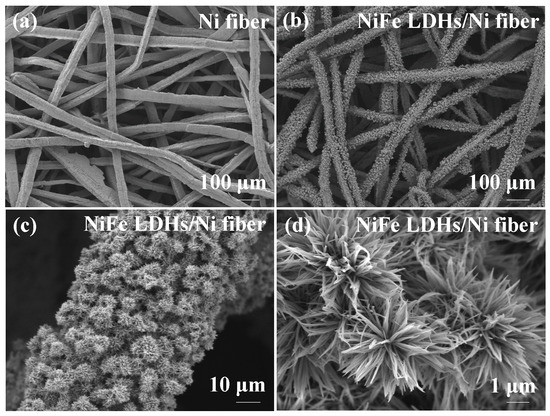
Figure 2.
SEM images of Ni fiber electrode, (a) and NiFe LDHs/Ni fiber electrode (b–d).
Further magnification can show that the nanoflower-like structure is composed of ordered nanowires and extends to the surrounding space as shown in Figure 2d. It can be seen from the further magnification in Figure 2d that the outward extension of each petal of the “nanoflower” cluster petals stretching outward produces the “tip effect”, which is more conducive to the exposure of active sites at the edge and mass transfer under high current density. The results are consistent with the research on the “cluster structure” in recently reported literature [5,30,31].
For comparison, the NiCo LDHs/Ni fiber electrode, the NiMn LDHs/Ni fiber electrode, and the NiFe LDHs/Ni foam electrode were also characterized with an SEM in Figure S2. It can be seen from Figure S2a,b that a dense layer of NiCo LDHs nanosheet is vertically grown on the Ni fiber substrate. Figure S2d–f shows that NiMn LDHs nanosheets are staggered and vertically grown on a Ni fiber substrate. Figure S2g–i shows that NiFe LDHs nanosheets are interlaced or part of the nanosheets form nanoflower balls, which are vertically arranged on the Ni foam substrate. The above results show that the special morphology and structure of NiFe LDHs/Ni fiber electrode are closely related to the substrate of Ni fiber.
To further investigate the microstructure of the NiFe LDHs/Ni fiber electrode, we used ultrasound to exfoliate NiFe LDHs from the Ni fiber framework and observed by the TEM. Figure 3a is the TEM diagram of NiFe LDHs “nanoflowers”. It can be seen that the “nanoflower” cluster petals stretching outward produce the “tip effect”. This result is consistent with the SEM image. The high-resolution TEM image of NiFe LDHs shows the clear lattice fringes are 0.26 nm, matching the (012) crystal planes in Figure 3b.
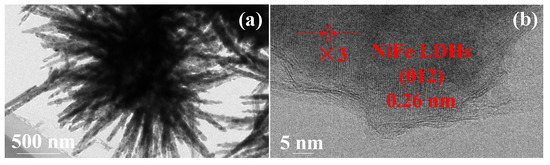
Figure 3.
TEM images of the NiFe LDHs (a,b).
The crystal phase characterization of the Ni fiber electrode and NiFe LDHs/Ni fiber electrode by XRD is shown in Figure 4a. The XRD pattern shows that the strong diffraction peaks at 44.3°, 51.7°, and 76.2° correspond to (111), (200), and (220) crystal planes of metal Ni (PDF#87-0712) fragments from Ni fiber [13,25]. The peaks at 11.4°, 23.1°, 34.3°, 38.8°, 46.3°, 59.8°, and 61.2° correspond to the (003), (006), (012), (015), (018), (110), and (113) crystal planes of NiFe LDHs (PDF#40-0215) [20,32,33]. These results prove the successful formation of the NiFe LDHs phase on Ni fiber substrate.
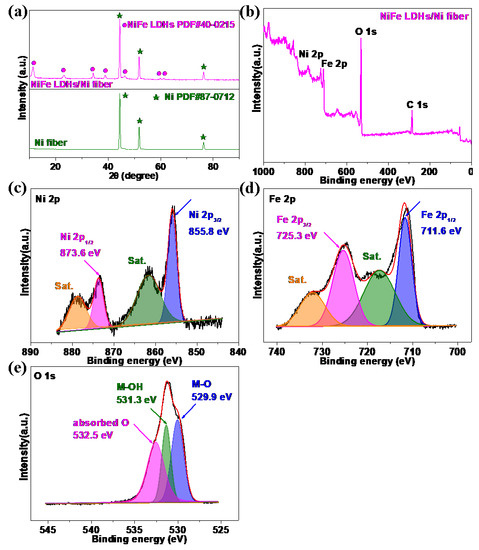
Figure 4.
(a) XRD patterns of NiFe LDHs/Ni fiber electrode; (b) the NiFe LDHs/Ni fiber electrode XPS spectra of survey; (c) the NiFe LDHs/Ni fiber electrode of Ni 2p XPS spectra; (d) the NiFe LDHs/Ni fiber electrode of Fe 2p XPS spectra; (e) the NiFe LDHs/Ni fiber electrode of O 1 s XPS spectra.
The surface chemical composition and valence state of the NiFe LDHs/Ni fiber electrode were analyzed by XPS [13]. The survey spectrum further verified the existence of O, Fe, and Ni elements, respectively (in Figure 4b). The XPS spectrum of Ni 2p (in Figure 4c) shows that the peaks located at 855.8 eV and 873.6 eV belong to the Ni 2p3/2 and Ni 2p1/2, respectively, and the two surrounding satellites peaks (abbreviated as “sat.”), indicating that Ni2+ exists in NiFe LDHs/Ni fiber electrode [5,13,25]. As shown in Figure 4d, the Fe 2p XPS spectrum shows that the peaks at 711.6 and 725.3 eV belong to Fe 2p3/2 and Fe 2p1/2, respectively, and the two surrounding satellites (abbreviated as “sat.”), indicating that Fe3+ exists in NiFe LDHs/Ni fiber electrode [34,35,36]. O 1 s spectrum in Figure 4e, the peaks located at 529.2 eV, 530.8 eV, and 532.5 eV are attributed to the metal–oxygen bend (M–O), oxygen in the hydroxide group(M–OH), and chemisorbed water [34,37].
3.2. OER Performance in Alkaline Media
The OER electrocatalytic performance of the prepared self-supported NiFe LDHs/Ni fiber electrode was tested with a typical three-electrode in 1 M KOH solution at 25 °C (oxygen saturated). For comparison, the NiCo LDHs/Ni fiber electrode, NiMn LDHs/Ni fiber electrode and NiFe LDHs/Ni foam electrode, IrO2/Ni fiber electrode, and Ni fiber electrode were also investigated under the same conditions. Figure 5a shows the LSV curves (without iR compensation) of the Ni fiber electrode, NiCo LDHs/Ni fiber electrode, NiMn LDHs/Ni fiber electrode, NiFe LDHs/Ni foam electrode, and IrO2/Ni fiber electrode, respectively. It can be seen that the NiFe LDHs/Ni fiber electrode shows excellent OER catalytic activity in Figure 5a. The NiFe LDHs/Ni fiber at current densities of 10 and 50 mA cm−2 required overpotentials of 208 and 260 mV, respectively. This result is better than the NiCo LDHs/Ni fiber electrode (286 and 346 mV), NiMn LDHs/Ni fiber electrode (300 and 364 mV), NiFe LDHs/Ni foam electrode (246 and 300 mV), IrO2/Ni fiber electrode (300 and 356 mV), and Ni fiber electrode (340 and 416 mV), respectively (In Figure 5b). In Figure 5c, the NiFe LDHs/Ni fiber electrode shows a smaller Tafel slope of 50 mV dec−1, which is smaller than NiCo LDHs/Ni fiber electrode (69 mV dec−1), NiMn LDHs/Ni fiber electrode (78 mV dec−1), IrO2/Ni fiber electrode (75 mV dec−1), NiFe LDHs/Ni foam electrode (60 mV dec−1), and Ni fiber (89 mV dec−1). The kinetics of the electrodes are measured by EIS. As shown in Figure 5d, the NiFe LDHs/Ni fiber electrode has a smaller semicircle than the NiCo LDHs/Ni fiber electrode, NiMn LDHs/Ni fiber electrode, NiFe LDHs/Ni foam electrode, IrO2/Ni fiber electrode, and Ni fiber electrode, which indicates that it has the lowest charge transfer resistance (Rct) [25,38,39]. The results show that NiFe LDHs/Ni fiber electrode has fast charge transfer and ion diffusion. In addition, we evaluated the long-term stability of the NiFe LDHs/Ni fiber electrode through the chronopotentiometry test and studied different current densities (10, 50, 100, 200, 300, and 10 mA cm−2) for a total of 60 h (Figure 5e). Figure 5f summarizes the OER performance comparison between NiFe LDHs/Ni fiber electrode and the newly reported non-noble metal-based self-supporting electrodes (see Table 1) [5,6,40,41,42,43,44,45,46].
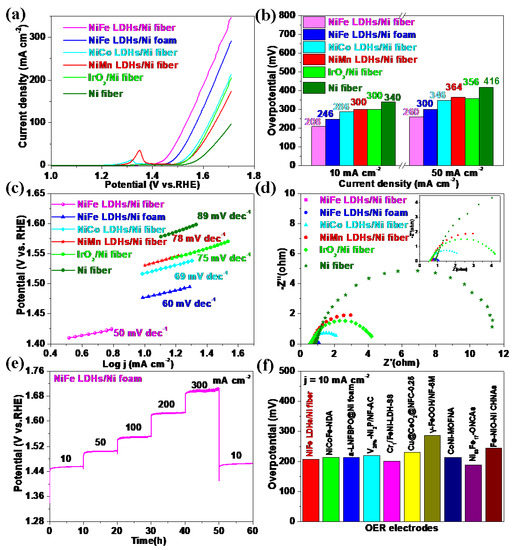
Figure 5.
The OER performances were tested in a three-electrode system with 1 M KOH. (a) LSV curves of Ni fiber electrode, NiCo LDHs/Ni fiber electrode, NiMn LDHs/Ni fiber electrode, IrO2/Ni fiber electrode, NiFe LDHs/Ni foam electrode, and NiFe LDHs/Ni fiber electrode with a scan rate of 2 mV s−1 at room temperature; (b) the comparison of overpotential at 10, 50, and 100 mA cm−2 for NiFe LDHs/Ni fiber electrode; (c) the corresponding Tafel plots; (d) Nuquist plots; (e) chronopotentiometry curves of NiFe LDHs/Ni fiber at various current densities; (f) comparison of the recently OER electrode and our work with the density of 10 mA cm−2.

Table 1.
Summary of various non-noble metal catalysts for OER in alkaline solution (25 °C).
To further study the catalytic performance of the electrodes, the electrochemical double-layer capacitance (Cdl) was calculated to evaluate the electrochemical active surface area (ECSA) in Figure 6. As shown in Figure 6f, the Cdl of NiFe LDHs/Ni fiber electrode is 5.57 mF cm−2, which is significantly higher than that NiFe LDHs/Ni foam electrode (5.02 mF cm−2), NiCo LDHs/Ni fiber electrode (4.61 mF cm−2), NiMn LDHs/Ni fiber electrode (4.49 mF cm−2), and Ni fiber electrode (3.51 mF cm−2). The NiFe LDHs/Ni fiber electrode has the largest Cdl, indicating that it has a large ECSA, which makes it easier to expose the catalytic active sites.
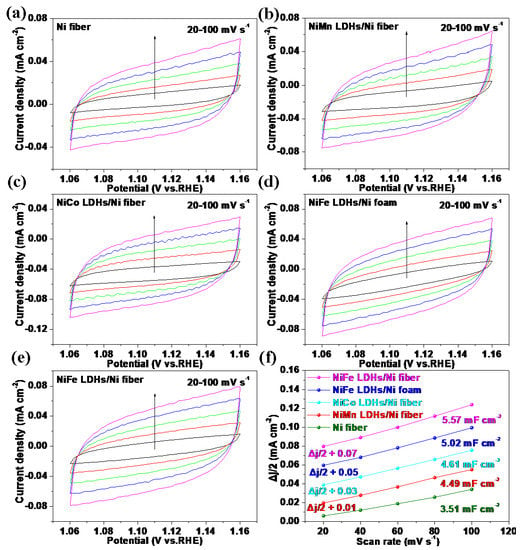
Figure 6.
CV curves of Ni fiber (a), NiMn LDHs/Ni fiber (b), NiCo LDHs/Ni fiber (c), NiFe LDHs/Ni foam (d), and NiFe LDHs/Ni fiber (e) in the double-layer region at scan rates of 20, 40, 60, 80, and 100 mV s−1 in 1 M KOH; the Cdl of (f) determined by the plots of Δj/2 = (ja − jc)/2 as a function of the scan rate for with different shells. ja represents the anode current density, jc represents the cathode current density.
To investigate the changes of NiFe LDHs/Ni fiber electrode after the long-term stability OER test, the morphology structure and element analysis were measured by SEM (Figure S3), and XPS (Figure S4) shows that the morphology of the NiFe LDHs/Ni fiber electrode continues to remain original after the long-term stability test. In addition, the XPS spectra before and after the stability experiment were compared. It can be seen that the chemical components of Ni, Fe, and O still exist. This result proves that the NiFe LDHs/Ni fiber electrode has excellent stability.
The results further prove that the NiFe LDHs/Ni fiber electrode has excellent OER electrocatalytic performance, which is due to the special morphological structure of the “nanoflowers” cluster petal stretching outward to produce the “tip effect”, which is more conducive to the exposure of active sites at the edge and mass transfer under high current density [5,30,31].
3.3. Performance of NiFe LDHs/Ni Fiber Electrode as the Anode of the AEMWE
The application of the NiFe LDHs/Ni fiber electrode as an AEMWE anode under industrial conditions for large currents (>200 mA cm−2) at alkaline water electrolysis [25,47] was further investigated. The assembly of AEMWE (NiFe LDHs/Ni fiber || Pt/C) (in Figure S6) was tested at 70 °C in 1 M KOH with used a NiFe LDHs/Ni fiber electrode as an anode and commercial Pt/C as a cathode, respectively. As shown in Figure 7a, the AEMWE (NiFe LDHs/Ni fiber || Pt/C) cell current densities were 0.5 and 1 A cm−2, and the cell voltages only 1.68 and 1.87 V (without iR correction), corresponding to the energy efficiency of 88.1 and 79.1%, respectively. This proves that the self-supporting structure of the NiFe LDHs/Ni fiber electrode is beneficial to the gas diffusion of bubbles and the transportation of the electrolytes, resulting in excellent water electrolysis performance under high current density [25]. Figure 7b shows that the long-term stability of the AEMWE was measured at 0.5 A cm−2 for 200 h. In addition, the decrease in voltage is due to the following reasons: (1) after a long-term stability test, some NiFe LDHs catalysts fell off from the Ni fiber substrate; (2) to imitate the start-up and shutdown in the actual application process, the start-up and shutdown are conducted every 12 h, and the newly prepared KOH electrolyte is replaced at the same time; and (3) the in-depth research of Liu et al. on the OER loss mechanism of the layered NiFe LDHs catalyst revealed that the layered structure is not conducive to long-term stability [48]. Ohmic resistance and charge transfer resistance were evaluated by measuring EIS at 1.7 V (about 0.5 A cm−2) [4]. As shown in Figure 7d, it can be seen that the Rct of the AEMWE is small, which means that the NiFe LDHs/Ni fiber electrode has an efficient charge transfer capability in AEMWE [20]. Figure 7d summarizes the performance comparison between the NiFe LDHs/Ni fiber electrode and the newly reported non-noble metal-based self-supporting electrode as the AEMWEs electrode (see Table 2) [4,17,18,20,21,32,49,50]. The above results reveal that the NiFe LDHs/Ni fiber electrode has excellent catalytic activity and electrochemical stability in AEMWE. In addition, we also enlarged the NiFe LDHs/Ni fiber electrode (20 × 20 cm) as shown in Figure S5. This further proves that the NiFe LDHs/Ni fiber electrode has industrialized application prospects.
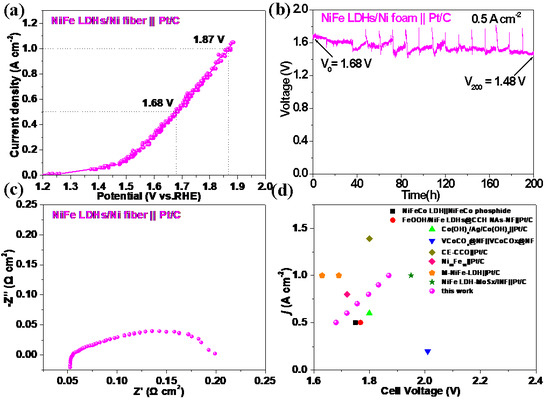
Figure 7.
(a) Current-voltage curves of the NiFe LDHs/Ni fiber electrode as the anode for AEMWE at 70 °C, (b) stability test at 0.5 A cm−2, (c) Nyquist plots were evaluated at 1.7 V, (d) comparison of the NiFe LDHs/Ni fiber || Pt/C AEMWE with that recently reported.

Table 2.
Comparison of the AEMWE electrode performance 1 M KOH.
4. Conclusions
In conclusion, the highly efficient and stable self-supporting NiFe LDHs/Ni fiber “nanoflower” cluster electrode was successfully prepared by a facile one-step impregnation method at room temperature and ambient pressure. Benefiting from the synergistic effect between NiFe LDH “nanoflower” cluster petals stretching outward to produce the “tip effect” and the Ni fiber substrate, the NiFe LDHs/Ni fiber electrode has significant inherent activity and structure. The NiFe LDHs/Ni fiber electrode has excellent OER performance with a low overpotential of 208 mV at the current density of 10 mA cm−2 in 1 M KOH. When the NiFe LDHs/Ni fiber electrode is used as the anode of AEMWE (NiFe LDHs/Ni fiber || Pt/C), it shows high energy conversion efficiency of 88.1% with the cell voltage of 1.68 V at the current density of 0.5 A cm−2 under industrial conditions of alkaline water electrolysis. In addition, the NiFe LDHs/Ni fiber electrode was tested by stability at 0.5 A cm−2 for 200 h, indicating that it has good stability. The self-supporting NiFe LDHs/Ni fiber electrode has the advantage of simple, low-cost synthesis method that yields high-efficient catalytic activity and durability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en15134645/s1, Figure S1: The SEM images of Ni foam. Figure S2: The SEM images of NiCo LDHs/Ni fiber (a–c), NiMn LDHs/Ni fiber (d–f), NiFe LDHs/Ni foam (g–i). Figure S3: The SEM images of NiFe LDHs/Ni fiber after the durability test. Figure S4: The images of NiFe LDHs/Ni fiber after the durability test. XPS spectra of survey (a); Ni 2p XPS spectra (b); Fe 2p XPS spectra (c); O 1 s XPS spectra (d). Figure S5: The optical image of NiFe LDHs grown on large-scale Ni fiber. Figure S6. The digital photo of the AEMWE cell.
Author Contributions
Conceptualization, D.G.; methodology, D.G. and G.J.; software, D.G. and G.J.; validation, D.G.; formal analysis, D.G.; investigation, D.G.; resources, D.G., H.Y., J.C. and Z.S.; data curation, D.G.; writing—original draft preparation, D.G.; writing—review and editing, D.G., H.Y. and J.C.; visualization, H.Y.; supervision, H.Y.; project administration, H.Y. and Z.S.; funding acquisition, H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Key Program of the National Natural Science Foundation of China (No. 22090032, 22090030), the Joint Fund of the Yulin University and the Dalian National Laboratory for Clean Energy (Grant. YLU-DNL Fund 2021001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Key Program of the National Natural Science Foundation of China (No. 22090032, 22090030), the Joint Fund of the Yulin University and the Dalian National Laboratory for Clean Energy (Grant. YLU-DNL Fund 2021001). In addition, we sincerely appreciate Dalian Key Laboratory of Electrolysis for Hydrogen Production’s support of our work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, W.; Gao, Y.; Chen, Z.; Zhao, Y.; Wu, Z.; Wang, L. Strategies on improving the electrocatalytic hydrogen evolution performances of metal phosphides. Chin. J. Catal. 2021, 42, 1876–1902. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Li, Z.; Bu, X. Recent progress on NiFe-based electrocatalysts for the oxygen evolution reaction. Small 2020, 16, 2003916–2003938. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, W.; Shao, W.; Bai, M.; Zhou, M.; Li, S.; Ma, T.; Ma, L.; Cheng, C.; Liu, X. Synthesis and electronic modulation of nanostructured layered double hydroxides for efficient electrochemical oxygen evolution. ChemSusChem 2021, 14, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.S.; Lim, J.; Kang, P.W.; Lee, J.W.; Kang, G.; Lee, H. Design principles of NiFe-layered double hydroxide anode catalysts for anion exchange membrane water electrolyzers. ACS Appl. Mater. Interfaces 2021, 13, 37179–37186. [Google Scholar] [CrossRef]
- Liu, P.; Chen, B.; Liang, C.; Yao, W.; Cui, Y.; Hu, S.; Zou, P.; Zhang, H.; Fan, H.; Yang, C. Tip-enhanced electric field: A new mechanism promoting mass transfer in oxygen evolution reactions. Adv. Mater. 2021, 33, 2007377–2007385. [Google Scholar] [CrossRef]
- Yue, K.; Liu, J.; Zhu, Y.; Xia, C.; Wang, P.; Zhang, J.; Kong, Y.; Wang, X.; Yan, Y.; Xia, B.Y. In situ ion-exchange preparation and topological transformation of trimetal-organic frameworks for efficient electrocatalytic water oxidation. Energy Environ. Sci. 2021, 14, 6546–6553. [Google Scholar] [CrossRef]
- Gong, L.; Koh, J.; Yeo, B.S. Mechanistic study of the synergy between iron and transition metals for the catalysis of the oxygen evolution reaction. ChemSusChem 2018, 11, 3790–3795. [Google Scholar] [CrossRef]
- Chen, Y.; Rui, K.; Zhu, J.; Dou, S.; Sun, W. Recent progress on nickel-based oxide/(oxy)hydroxide electrocatalysts for the oxygen evolution reaction. Chem. Eur. J. 2019, 25, 703–713. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, X.; Wan, H.; Wang, S.; Zhao, Y.; Zhang, J.; Zhou, D.; Gao, W.; Ma, R.; Sasaki, T.; et al. Interface modulation of two-dimensional superlattices for efficient overall water splitting. Nano Lett. 2019, 19, 4518–4526. [Google Scholar] [CrossRef]
- Mohammed-Ibrahim, J. A review on NiFe-based electrocatalysts for efficient alkaline oxygen evolution reaction. J. Power Sources 2020, 448, 227375–227424. [Google Scholar] [CrossRef]
- Xu, D.; Stevens, M.B.; Cosby, M.R.; Oener, S.Z.; Smith, A.M.; Enman, L.J.; Ayers, K.E.; Capuano, C.B.; Renner, J.N.; Danilovic, N.; et al. Earth-abundant oxygen electrocatalysts for alkaline anion exchange-membrane water electrolysis: Effects of catalyst conductivity and comparison with performance in three-electrode cells. ACS Catal. 2019, 9, 7–15. [Google Scholar] [CrossRef]
- Zignani, S.C.; Faro, M.L.; Trocino, S.; Aricò, A.S. Investigation of NiFe-Based catalysts for oxygen evolution in anion-exchange membrane electrolysis. Energies 2020, 13, 1720. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, C.; Fang, Z.; Xu, L.; Lu, C.; Hou, W. Ultrafast room-temperature synthesis of self-supported NiFe-layered double hydroxide as large-current-density oxygen evolution electrocatalyst. Small 2021, 18, 2104354–2104363. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, E.; Gao, J.; Yang, J.; Wu, C.; Jiang, L.; Zhu, M.; Sun, G. An exceptionally facile synthesis of high efficient oxygen evolution electrodes for zinc-oxygen batteries. ChemElectroChem 2017, 4, 2190–2195. [Google Scholar] [CrossRef]
- Cao, D.; Xu, H.; Cheng, D. Branch-leaf-shaped CuNi@NiFeCu nanodendrites as highly efficient electrocatalysts for overall water splitting. Appl. Catal. B 2021, 398, 120600–120609. [Google Scholar] [CrossRef]
- Zhang, R.; Duan, J.; Feng, J.; Mei, L.; Zhang, Q.; Wang, A. Walnut kernel-like iron-cobalt-nickel sulfide nanosheets directly grown on nickel foam: A binder-free electrocatalyst for high-efficiency oxygen evolution reaction. J. Colloid Interface Sci. 2021, 587, 141–149. [Google Scholar] [CrossRef]
- Guo, W.; Kim, J.; Kim, H.; Han, G.H.; Jang, H.W.; Kim, S.Y.; Ahn, S.H. Sandwich-like Co(OH)x/Ag/Co(OH)2 nanosheet composites for oxygen evolution reaction in anion exchange membrane water electrolyzer. J. Alloys Compd. 2021, 889, 161674–161681. [Google Scholar] [CrossRef]
- Lee, J.; Jung, H.; Park, Y.S.; Kwon, N.; Woo, S.; Selvam, N.C.S.; Han, G.S.; Jung, H.S.; Yoo, P.J.; Choi, S.M.; et al. Chemical transformation approach for high-performance ternary NiFeCo metal compound-based water splitting electrodes. Appl. Catal. B 2021, 294, 120246–120254. [Google Scholar] [CrossRef]
- Kim, J.-C.; Kim, J.; Park, J.C.; Ahn, S.H.; Kim, D.-W. Ru2P nanofibers for high-performance anion exchange membrane water electrolyzer. Chem. Eng. J. 2021, 420, 130491–130498. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, G.; Liu, X.; Ning, B.; Shi, C.; Pan, L.; Zhang, X.; Huang, Z.-F.; Zou, J.-J. Self-supporting NiFe LDH-MoSx integrated electrode for highly efficient water splitting at the industrial electrolysis conditions. Chin. J. Catal. 2021, 42, 1732–1741. [Google Scholar] [CrossRef]
- Meena, A.; Thangavel, P.; Nissimagoudar, A.S.; Singh, A.N.; Jana, A.; Jeong, D.S.; Im, H.; Kim, K.S. Bifunctional oxovanadate doped cobalt carbonate for high-efficient overall water splitting in alkaline-anion-exchange-membrane water-electrolyzer. Chem. Eng. J. 2022, 430, 132623–132630. [Google Scholar] [CrossRef]
- Zhai, P.; Xia, M.; Wu, Y.; Zhang, G.; Gao, J.; Zhang, B.; Cao, S.; Zhang, Y.; Li, Z.; Fan, Z.; et al. Engineering single-atomic ruthenium catalytic sites on defective nickel-iron layered double hydroxide for overall water splitting. Nat. Commun. 2021, 12, 4587–4597. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhang, X.; Gao, H.; Chen, S.; Cheng, P.; Wang, P.; Zhao, Z.; Dang, R.; Wang, G. In situ semi-sacrificial template-assisted growth of ultrathin metal-organic framework nanosheets for electrocatalytic oxygen evolution. Chem. Eng. J. 2021, 426, 131348–131358. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Ma, X.; Liu, F.; Xiao, H.; Zhang, J.; Lin, Z.; Hao, Z. Engineering ultrafine NiFe-LDH into self-supporting nanosheets: Separation-and-reunion strategy to expose additional edge sites for oxygen evolution. Small 2021, 17, 2103785–2103792. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Kuang, P.; Wang, L.; Yu, J. Hierarchical porous nickel supported NiFeOxHy nanosheets for efficient and robust oxygen evolution electrocatalyst under industrial condition. Appl. Catal. B 2021, 299, 120668–120675. [Google Scholar] [CrossRef]
- Dou, Y.; He, C.; Zhang, L.; Yin, H.; Al-Mamun, M.; Ma, J.; Zhao, H. Approaching the activity limit of CoSe2 for oxygen evolution via Fe doping and Co vacancy. Nat. Commun. 2020, 11, 1664–1672. [Google Scholar] [CrossRef] [Green Version]
- Lv, J.; Wang, L.; Li, R.; Zhang, K.; Zhao, D.; Li, Y.; Li, X.; Huang, X.; Wang, G. Constructing a hetero-interface composed of oxygen vacancy enriched Co3O4 and crystalline-amorphous NiFe-LDH for oxygen evolution reaction. ACS Catal. 2021, 11, 14338–14351. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, H.; Hu, W.; Yin, H.; Cao, G.; Wen, H.; Wang, J.; Wang, P. Highly dispersed Ni2−xMoxP nanoparticles on oxygen-defect-rich NiMoO4−y nanosheets as an active electrocatalyst for alkaline hydrogen evolution reaction. J. Power Sources 2019, 444, 227311–227317. [Google Scholar] [CrossRef]
- Luo, Q.; Peng, M.; Sun, X.; Luo, Y.; Asiri, A.M. Efficient electrochemical water splitting catalyzed by electrodeposited NiFe nanosheets film. Int. J. Hydrogen Energy 2016, 41, 8785–8792. [Google Scholar] [CrossRef]
- Yang, J.; Li, W.; Xu, K.; Tan, S.; Wang, D.; Li, Y. Regulating the tip effect on single-atom and cluster catalysts: Forming reversible oxygen species with high efficiency in chlorine evolution reaction. Angew. Chem. Int. Ed. 2022, 61, 202200366–202200373. [Google Scholar] [CrossRef]
- Cao, Y.; Su, Y.; Xu, L.; Yang, X.; Han, Z.; Cao, R.; Li, G. Oxygen vacancy-rich amorphous FeNi hydroxide nanoclusters as an efficient electrocatalyst for water oxidation. J. Energy Chem. 2022, 71, 167–173. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H.; Jiang, G.; Jia, J.; Qin, B.; Yi, B.; Shao, Z. Construction of orderly hierarchical FeOOH/NiFe layered double hydroxides supported on cobaltous carbonate hydroxide nanowire arrays for a highly efficient oxygen evolution reaction. J. Mater. Chem. A 2018, 6, 3397–3401. [Google Scholar] [CrossRef]
- Minakshi, M. Lithium intercalation into amorphous FePO4 cathode in aqueous solutions. Electrochim. Acta 2010, 55, 9174–9178. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Zou, P.; Nairan, A.; Zhang, Y.; Liu, J.; Liu, K.; Hu, S.; Kang, F.; Fan, H.; Yang, C. Exceptional performance of hierarchical Ni-Fe oxyhydroxide@NiFe alloy nanowire array electrocatalysts for large current density water splitting. Energy Environ. Sci. 2020, 13, 86–95. [Google Scholar] [CrossRef]
- Shi, G.; Yu, C.; Fan, Z.; Li, J.; Yuan, M. Graphdiyne-supported NiFe layered double hydroxide nanosheets as functional electrocatalysts for oxygen evolution. ACS Appl. Mater. Interfaces 2019, 11, 2662–2669. [Google Scholar] [CrossRef]
- Yang, Y.; Dang, L.; Shearer, M.J.; Sheng, H.; Li, W.; Chen, J.; Xiao, P.; Zhang, Y.; Hamers, R.J.; Jin, S. Highly active trimetallic NiFeCr layered double hydroxide electrocatalysts for oxygen evolution reaction. Adv. Energy Mater. 2018, 8, 1703189–1703197. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, F.; Ma, X.; Zhu, C.; Wang, Y.; Xie, Y.; Chou, S.; Huang, Y.; Chen, Y. Regulation of morphology and electronic structure of FeCoNi layered double hydroxides for highly active and stable water oxidization catalysts. Adv. Energy Mater. 2021, 11, 2102141–2102151. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, S.; Jia, Y.; Xiong, X.; Yang, H.; Liu, S.; Tang, J.; Zhang, J.; Liu, D.; Zheng, L.; et al. NiFe hydroxide lattice tensile strain: Enhancement of adsorption of oxygenated intermediates for efficient water oxidation catalysis. Angew. Chem. Int. Edit. 2019, 58, 736–740. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Zhao, X.; Wang, Y.; Li, Q.; Wang, Q.; Tang, Y.; Lei, Y. Trimetallic oxyhydroxides as active sites for large-current-density alkaline oxygen evolution and overall water splitting. J. Mater. Sci. Technol. 2022, 110, 128–135. [Google Scholar] [CrossRef]
- Kwon, J.; Han, H.; Jo, S.; Choi, S.; Chung, K.Y.; Ali, G.; Park, K.; Paik, U.; Song, T. Amorphous nickel-iron borophosphate for a robust and efficient oxygen evolution reaction. Adv. Energy Mater. 2021, 11, 2100624–2100633. [Google Scholar] [CrossRef]
- Zhao, T.; Shen, X.; Wang, Y.; Hocking, R.K.; Li, Y.; Rong, C.; Dastafkan, K.; Su, Z.; Zhao, C. In situ reconstruction of V-doped Ni2P pre-catalysts with tunable electronic structures for water oxidation. Adv. Funct. Mater. 2021, 31, 2100614–2100623. [Google Scholar] [CrossRef]
- Xie, X.; Cao, C.; Wei, W.; Zhou, S.; Wu, X.; Zhu, Q. Ligand-assisted capping growth of self-supporting ultrathin FeNi-LDH nanosheet arrays with atomically dispersed chromium atoms for efficient electrocatalytic water oxidation. Nanoscale 2020, 12, 5817–5823. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhao, H.; Huang, B.; Xu, L.; Luo, M.; Wang, J.; Luo, F.; Du, Y.; Yan, C. Efficient optimization of electron/oxygen pathway by constructing ceria/hydroxide interface for highly active oxygen evolution reaction. Adv. Funct. Mater. 2020, 30, 1908367–1908375. [Google Scholar] [CrossRef]
- Wang, K.; Du, H.; He, S.; Liu, L.; Yang, K.; Sun, J.; Liu, Y.; Du, Z.; Xie, L.; Ai, W.; et al. Kinetically Controlled, Scalable synthesis of γ-FeOOH nanosheet arrays on nickel foam toward efficient oxygen evolution: The key role of in-situ-generated γ-NiOOH. Adv. Mater. 2021, 33, 2005587–2005596. [Google Scholar] [CrossRef]
- Huang, L.; Gao, G.; Zhang, H.; Chen, J.; Fang, Y.; Dong, S. Self-dissociation-assembly of ultrathin metal-organic framework nanosheet arrays for efficient oxygen evolution. Nano Energy 2020, 68, 104296–104304. [Google Scholar] [CrossRef]
- Lei, Y.; Xu, T.; Ye, S.; Zheng, L.; Liao, P.; Xiong, W.; Hu, J.; Wang, Y.; Wang, J.; Ren, X.; et al. Engineering defect-rich Fe-doped NiO coupled Ni cluster nanotube arrays with excellent oxygen evolution activity. Appl. Catal. B 2021, 285, 119809–119818. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, L.; Khan, U.; Yu, Q.; Cheng, H.; Zou, X.; Liu, B. Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density. Nat. Commun. 2019, 10, 269–277. [Google Scholar] [CrossRef]
- Chen, R.; Hung, S.; Zhou, D.; Gao, J.; Yang, C.; Tao, H.; Yang, H.; Zhang, L.; Zhang, L.; Xiong, Q.; et al. Layered structure causes bulk NiFe layered double hydroxide unstable in alkaline oxygen evolution reaction. Adv. Mater. 2019, 31, 1903909–1903915. [Google Scholar] [CrossRef]
- Park, Y.S.; Yang, J.; Lee, J.; Jang, M.J.; Jeong, J.; Choia, W.-S.; Kim, Y.; Yin, Y.; Seo, M.H.; Chen, Z.; et al. Superior performance of anion exchange membrane water electrolyzer: Ensemble of producing oxygen vacancies and controlling mass transfer resistance. Appl. Catal. B 2020, 278, 119276–119287. [Google Scholar] [CrossRef]
- Cossar, E.; Barnett, A.O.; Seland, F.; Safari, R.; Botton, G.A.; Baranova, E.A. Ionomer content optimization in nickel-iron-based anodes with and without ceria for anion exchange membrane water electrolysis. J. Power Sources 2021, 514, 230563–230574. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).