Experimental and Mechanistic Study of Synergistic Removal of Hg by Evaporation from Desulfurization Wastewater
Abstract
:1. Introduction
2. Experimental Equipment and Theoretical Methods
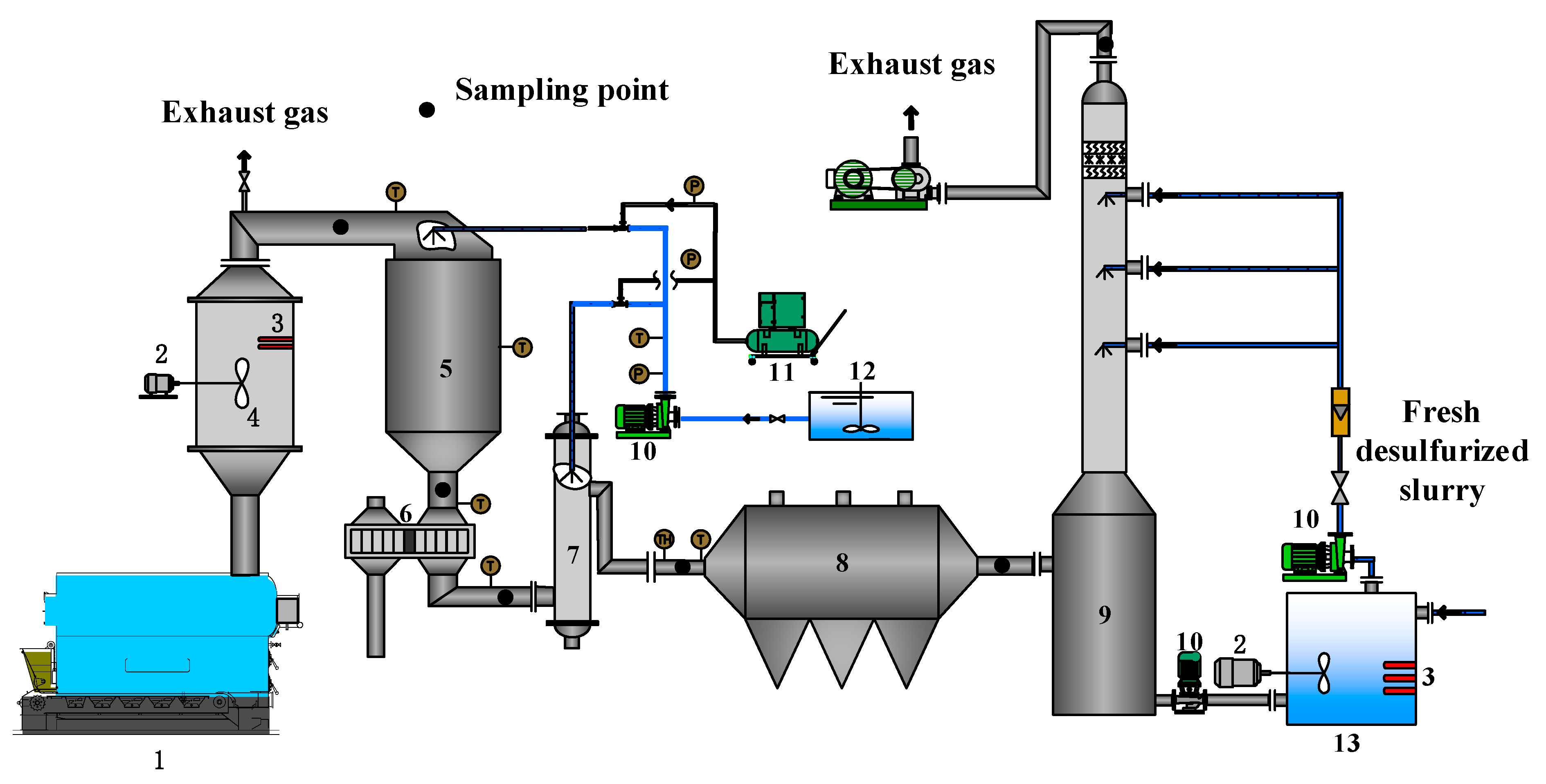
2.1. Experimental Device
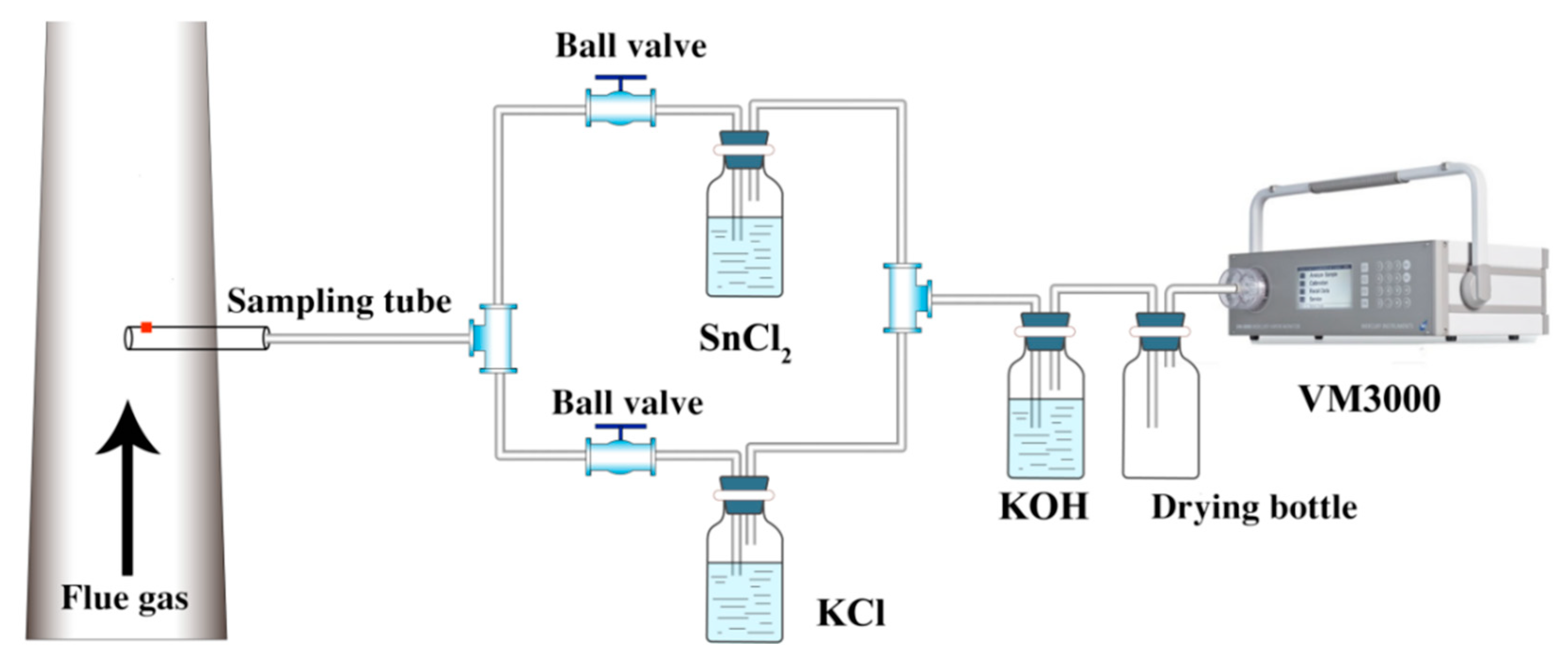
2.2. Test Methods
2.3. Experimental Materials
2.4. Calculation Methods and Calculation Models
3. Results and Discussion
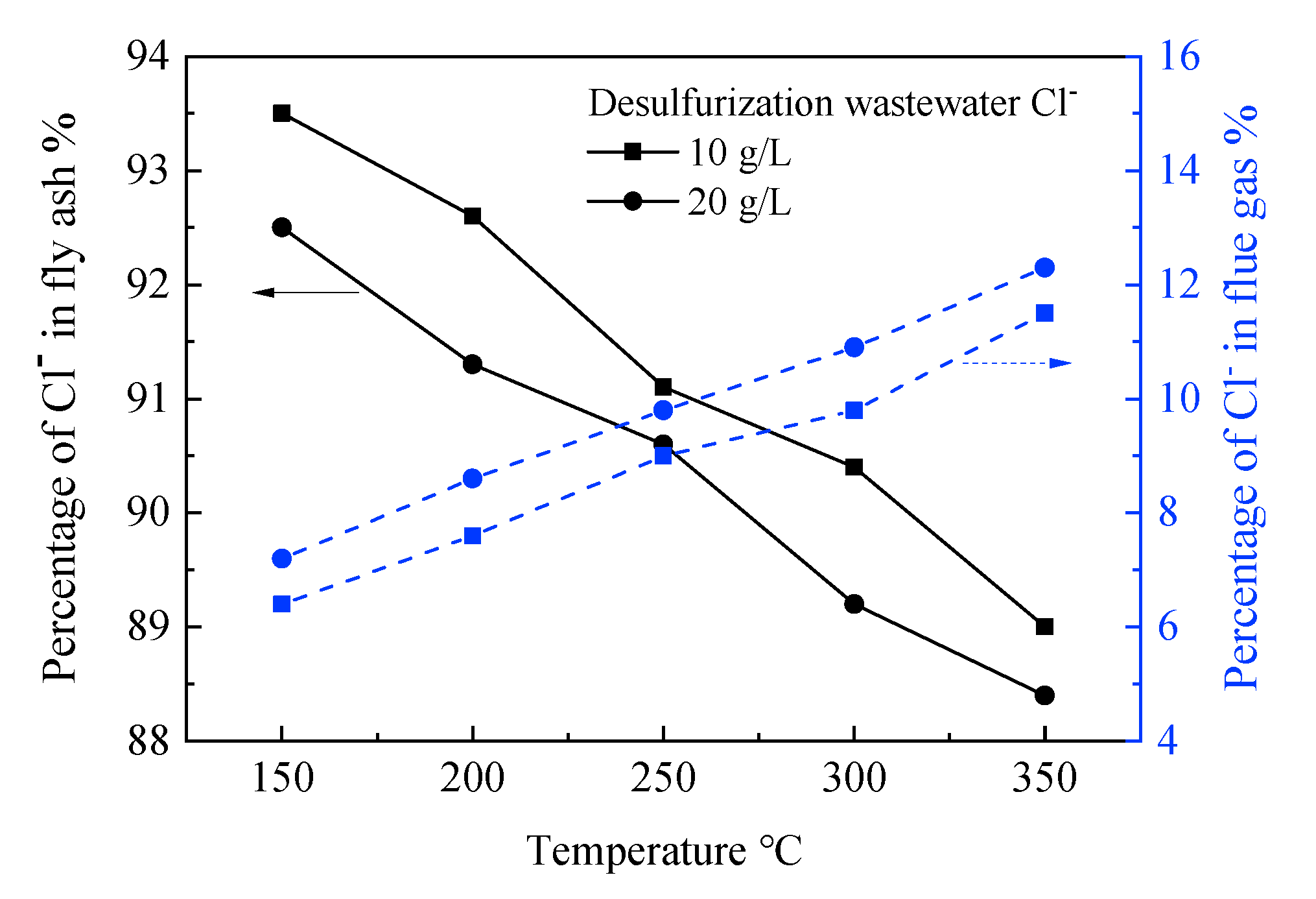
3.1. Characteristics of Chloride Ion Precipitation in Desulfurization Wastewater
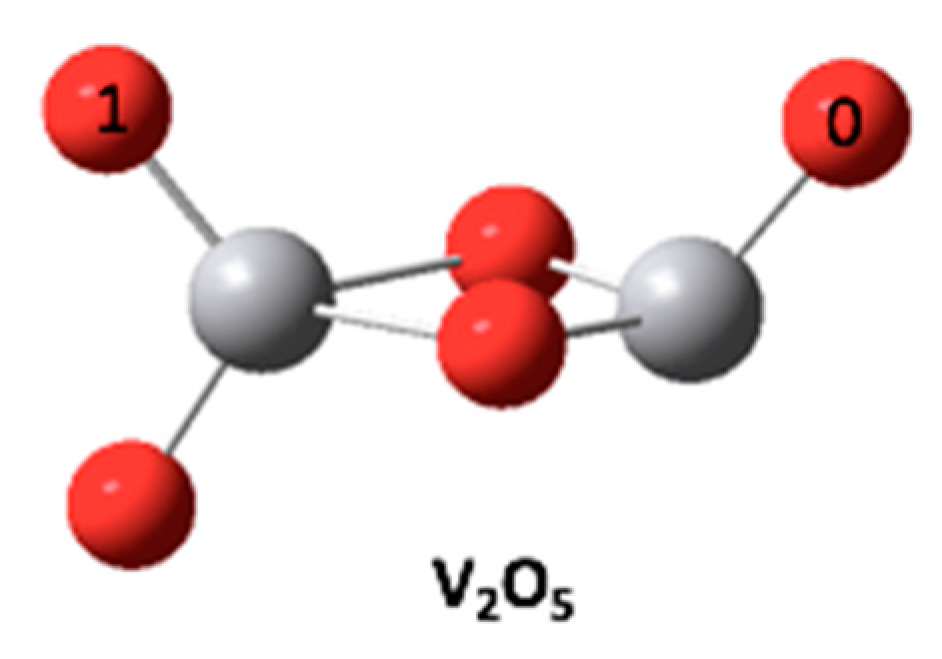
3.2. Mechanism of Enhanced SCR Oxidation of Hg0 by Evaporation of Desulfurization Wastewater
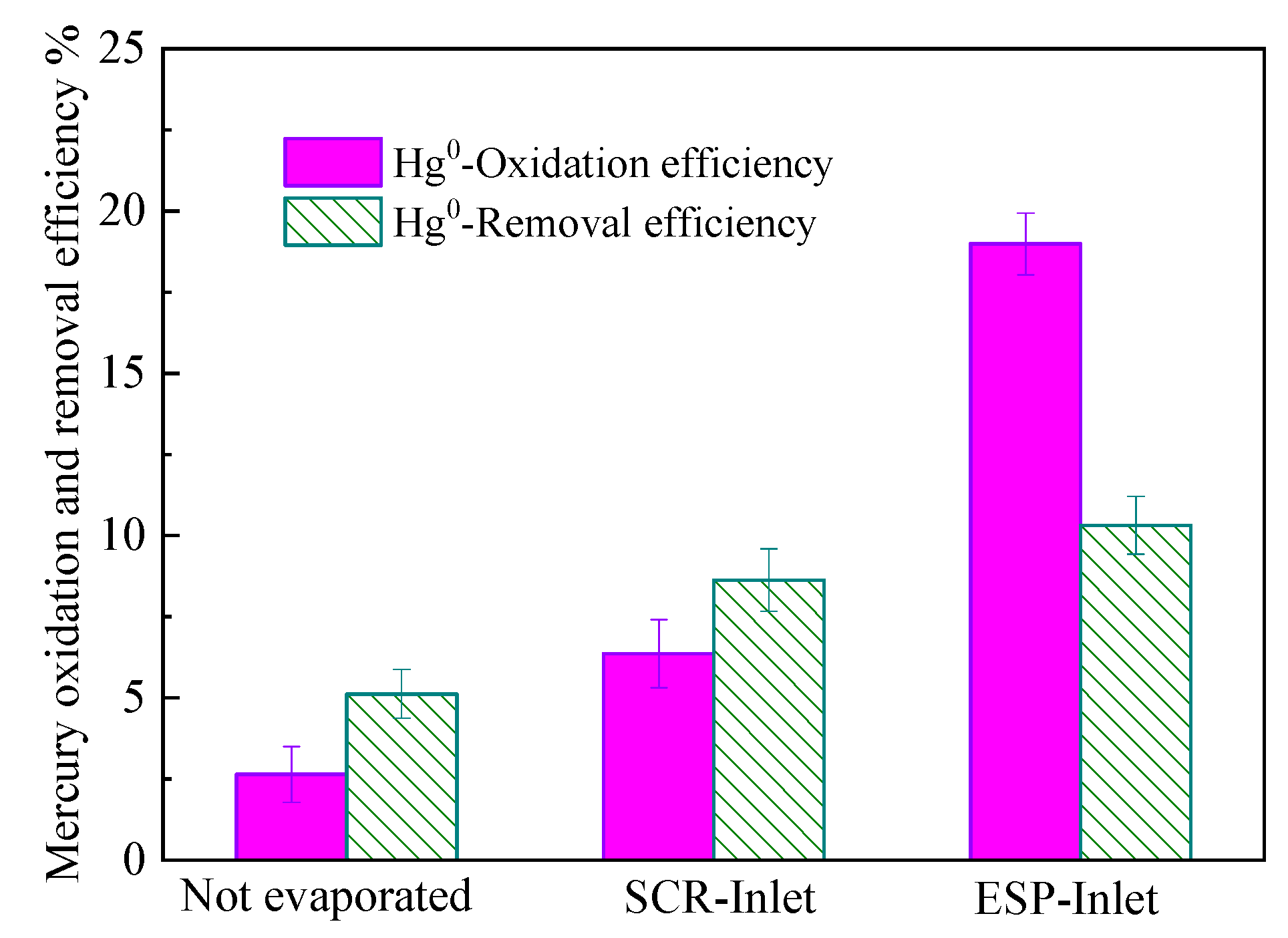
3.3. Desulfurization Wastewater Evaporation Enhanced Electrostatic Precipitation Removal of Hg
3.4. Evaporation of Desulfurization Wastewater Enhanced WFGD for Hg Removal
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, H.; Wan, D.; Che, Y.; Chang, J.; Zhao, J.; Hu, X.; Wang, L. Simultaneous magnesia regeneration and sulfur dioxide generation in magnesium-based flue gas desulfurization process. J. Clean. Prod. 2021, 284, 124720. [Google Scholar] [CrossRef]
- Yan, X.; Xu, Y. SO2 mitigation in China’s coal-fired power plants: A satellite-based assessment on compliance and enforcement. Atmos. Environ. 2021, 254, 118396. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.; Moon, I.; Lubomirsky, I.; Kaplan, V.; Kim, J.; Ahn, Y. Techno-economic assessment of carbonate melt flue gas desulfurization process. Comput. Chem. Eng. 2021, 146, 107227. [Google Scholar] [CrossRef]
- Li, R.; Zhao, C.; Yang, W.; Ma, W.; Jia, Z.; Wang, C.; Cui, X.; Jiao, H. Experimental Study of Flue Gas Desulfurization Wastewater Zero Discharge from Coal-fired Power Plant. AER-Adv. Eng. Res. 2016, 75, 1000–1005. [Google Scholar]
- Ma, S.; Chai, J.; Chen, G.; Wu, K.; Xiang, Y.; Wan, Z.; Zhang, J.; Zhu, H. Partitioning characteristic of chlorine ion in gas and solid phases in process of desulfurization wastewater evaporation: Model development and calculation. Environ. Sci. Pollut. Res. Int. 2019, 26, 8257–8265. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, H.; Zhao, N.; Feng, Y.; Liu, F.; Cai, C.; Che, G.; Yang, L. Experimental research and engineering application on the treatment of desulfurization wastewater from coal-fired power plants by spray evaporation. J. Water Process Eng. 2021, 40, 101960. [Google Scholar] [CrossRef]
- GB13223-2011; Emission Standard of Air Pollutants for Thermal Power Plants. Ministry of Environmental Protection of the People’s Republic of China, Standards Press of China: Beijing, China, 2015.
- Zheng, C.; Zheng, H.; Yang, Z.; Liu, S.; Li, X.; Zhang, Y.; Weng, W.; Gao, X. Experimental study on the evaporation and chlorine migration of desulfurization wastewater in flue gas. Environ. Sci. Pollut. Res. 2019, 26, 4791–4800. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, Z. Numerical study on evaporation characteristics of different substance droplet in low temperature flue gas. Proc. Csee 2010, 30, 62–68. [Google Scholar]
- Liang, Z.; Zhang, L.; Yang, Z.; Qiang, T.; Pu, G.; Ran, J. Evaporation and crystallization of a droplet of desulfurization wastewater from a coal-fired power plant. Appl. Therm. Eng. 2017, 119, 52–62. [Google Scholar] [CrossRef]
- Shuangchen, M.; Jin, C.; Gongda, C.; Weijing, Y.; Sijie, Z. Research on desulfurization wastewater evaporation: Present and future perspectives. Renew. Sustain. Energy Rev. 2016, 58, 1143–1151. [Google Scholar] [CrossRef]
- Chen, C.; Duan, Y.; Zhao, S.; Hu, B.; Li, N.; Yao, T.; Zhao, Y.; Wei, H.; Ren, S. Experimental study on mercury removal and regeneration of SO2 modified activated carbon. Ind. Eng. Chem. Res. 2019, 58, 13190–13197. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Ding, J.; Yu, Y.; Zhang, J. Mercury/oxygen reaction mechanism over CuFe2O4 catalyst. J. Hazard. Mater. 2021, 424, 127556. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Di, Y.; Tao, X.; Song, T.; Lu, P.; Xu, G.; Dong, L. Influence of Mo doping on mercury capture and SO2 tolerance of MoxFe6Mn1−xOy magnetic sorbent. Fuel 2022, 38, 121980. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, D.; Zhao, B.; Feng, L.; Ni, M.; Jin, J. Mercury removal based on adsorption and oxidation by fly ash: A review. Energy Fuels 2020, 34, 11840–11866. [Google Scholar] [CrossRef]
- Wang, H.; Duan, Y.; Ying, Z.; Xue, Y. Studies on mercury adsorption species and desorption activation energy on activated carbon under oxy combustion. Energy Fuels 2018, 32, 193–200. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Xue, Z.; Zhi, G.; Ma, J.; Liu, Y.; Gao, W. Research on synergistic mercury removal of coal-fired power plants. Chem. Ind. Eng. Prog. 2014, 33, 2187–2191. [Google Scholar]
- Yang, Y.; Liu, J.; Liu, F.; Wang, Z.; Ding, J. Comprehensive Hg/Br reaction chemistry over Fe2O3 surface during coal combustion. Combust. Flame 2018, 196, 210–222. [Google Scholar]
- Wu, S.; Ozaki, M.; Sasoka, E. Development of iron-based sorbents for Hg0 removal from coal derived fuel gas: Effect of hydrogen chloride. Fuel 2008, 87, 467–474. [Google Scholar] [CrossRef]
- She, M.; Duan, Y.; Zhu, C.; Jia, C.Q. Impact of nonoxidized sulfur species on elemental mercury removal by SO2 activated petroleum cokes. Energy Fuels 2020, 34, 14388–14399. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, B.; Zhou, Z.; Fang, W. Experimental and numerical investigations of desulfurization wastewater evaporation in a lab-scale flue gas duct: Evaporation and HCl release characteristics. Environ. Technol. 2021, 42, 1411–1427. [Google Scholar] [CrossRef]
- Bin, H.; Yang, Y.; Cai, L.; Yang, L.; Roszak, S. Enhancing mercury removal across air pollution control devices for coal-fired power plants by desulfurization wastewater evaporation. Environ. Technol. 2017, 40, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xu, H.; Mei, J.; Ma, Y.; Lou, T.; Qu, Z.; Yan, N. Ag-Mo modified SCR catalyst for a co-beneficial oxidation of elemental mercury at wide temperature range. Fuel 2017, 200, 236–243. [Google Scholar] [CrossRef]
- Chen, C.; Jia, W.; Liu, S.; Cao, Y.; Zhao, B.; Wang, J. Catalytic performance of CuCl2-modified V2O5-WO3/TiO2 catalyst for Hg0 oxidation in simulated flue gas. Korean J. Chem. Eng. 2018, 35, 637–644. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. Determination of Hydrogen Chloride in Ambient Air and Exhaust Gas-Ion Chromatography; Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2016.
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef] [Green Version]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuss, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Davidson, N. Statistical Mechanics (McGraw Hill Series in Advanced Chemistry); Courier Corporation: New York, MA, USA, 1962. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Liu, J.; Qu, W.; Yuan, J.; Wang, S.; Qiu, J.; Zheng, C. Theoretical Studies of Properties and Reactions Involving Mercury Species Present in Combustion Flue Gases. Energy Fuels 2010, 24, 509–518. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.; Zhao, W.; Zhu, C.; Zhou, Q.; Ding, W. Study on Elemental Mercury Oxidation by Non-thermal Plasma with Calcium Chloride Enhancement. Plasma Chem. Plasma Processing 2018, 38, 573–586. [Google Scholar] [CrossRef]
- Liu, J.; He, M.; Zheng, C.; Chang, M. Density functional theory study of mercury adsorption on V2O5(001) surface with implications for oxidation. Proc. Combust. Inst. 2011, 33, 2771–2777. [Google Scholar] [CrossRef]
- Zhao, S.; Pudasainee, D.; Duan, Y.; Gupta, R.; Liu, M.; Lu, J. A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies. Prog. Energy Combust. Sci. 2019, 73, 26–64. [Google Scholar] [CrossRef]
- Zhu, C.; Duan, Y.; Wu, C.Y.; Zhou, Q.; She, M.; Yao, T.; Zhang, J. Mercury removal and synergistic capture of SO2/NO by ammonium halides modified rice husk char. Fuel 2016, 172, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.Z.; Chen, C.M.; Jiang, L.X.; Liu, S.T.; Wang, B. Study of mercury re-emission from simulated wet flue gas desulfurization liquors. Adv. Mater. Res. 2013, 610, 2033–2037. [Google Scholar]




| Analyte Name | Concentration mg·L−1 | Analyte Name | Concentration mg·L−1 |
|---|---|---|---|
| Ca2+ | 1204 | Pb | 0.52 |
| Mg2+ | 605 | Cd | 0.06 |
| Cl− | 5049 | Ni | 0.27 |
| F− | 12 | Hg | 0.12 |
| NH3/NH4 | 500 | Cu | 0.11 |
| Components | TiO2 | WO3 | SiO2 | SO3 | CaO | Al2O3 |
|---|---|---|---|---|---|---|
| Content% | 86.81 | 4.61 | 3.63 | 1.34 | 1.31 | 1.04 |
| Components | V2O5 | Fe2O3 | MgO | ZrO2 | Nb2O5 | |
| Content% | 0.89 | 0.08 | 0.06 | 0.05 | 0.04 |
| Basis Group | lanl2dz | lanl2mb | Experimental Value [32] |
|---|---|---|---|
| HgCl | 2.5744 | 2.6407 | 2.23 |
| HgCl2 | 2.4018 | 2.4176 | 1.84 |
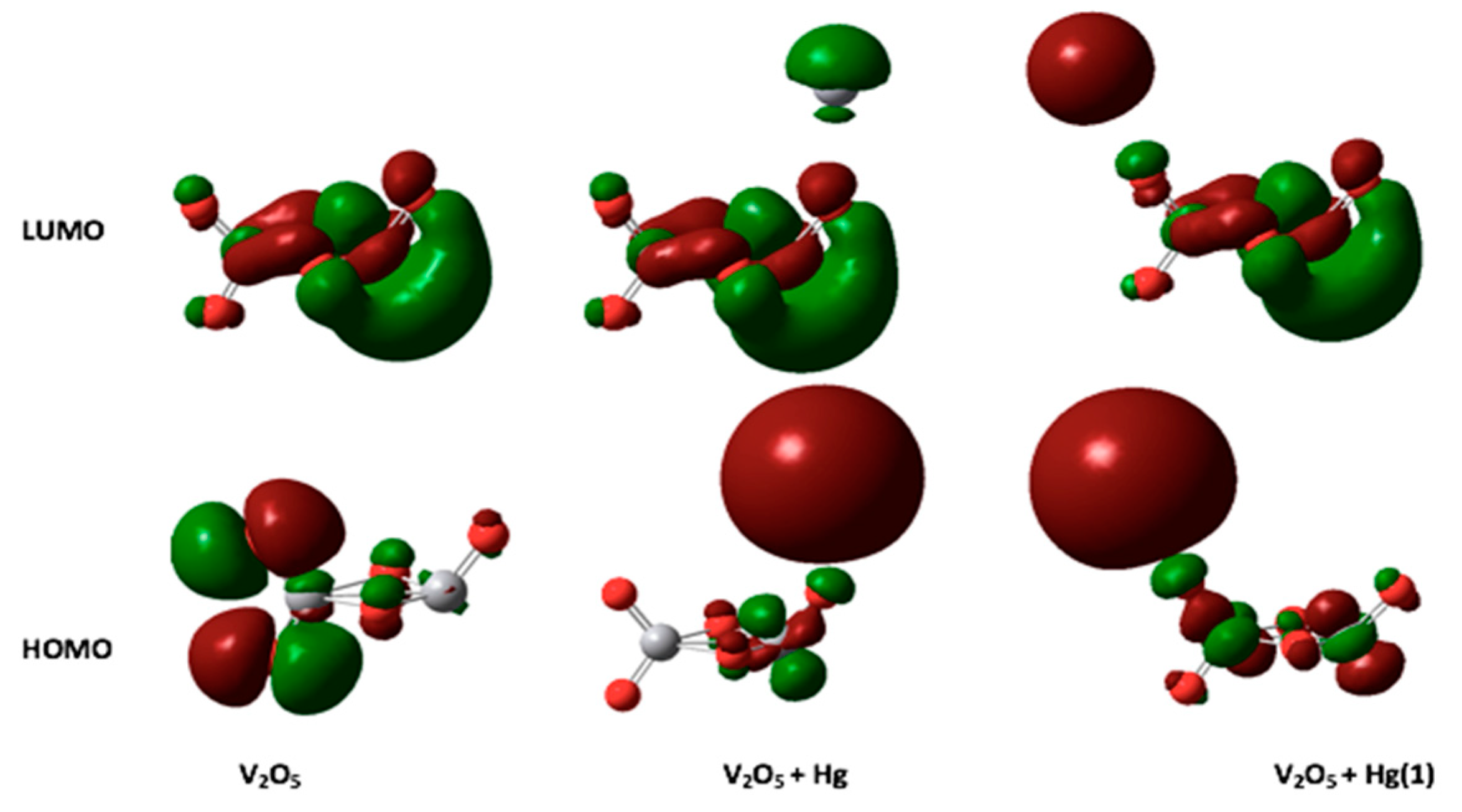
| QNBO (e) | EHOMO (eV) | ELUMO (eV) | Eg (eV) | |
|---|---|---|---|---|
| V2O5 | - | −8.657 | −5.715 | 2.942 |
| Hg | - | −6.483 | −0.421 | 6.062 |
| HgCl− | - | −14.275 | −11.714 | 2.562 |
| HgCl2 | - | −8.539 | −3.784 | 4.754 |
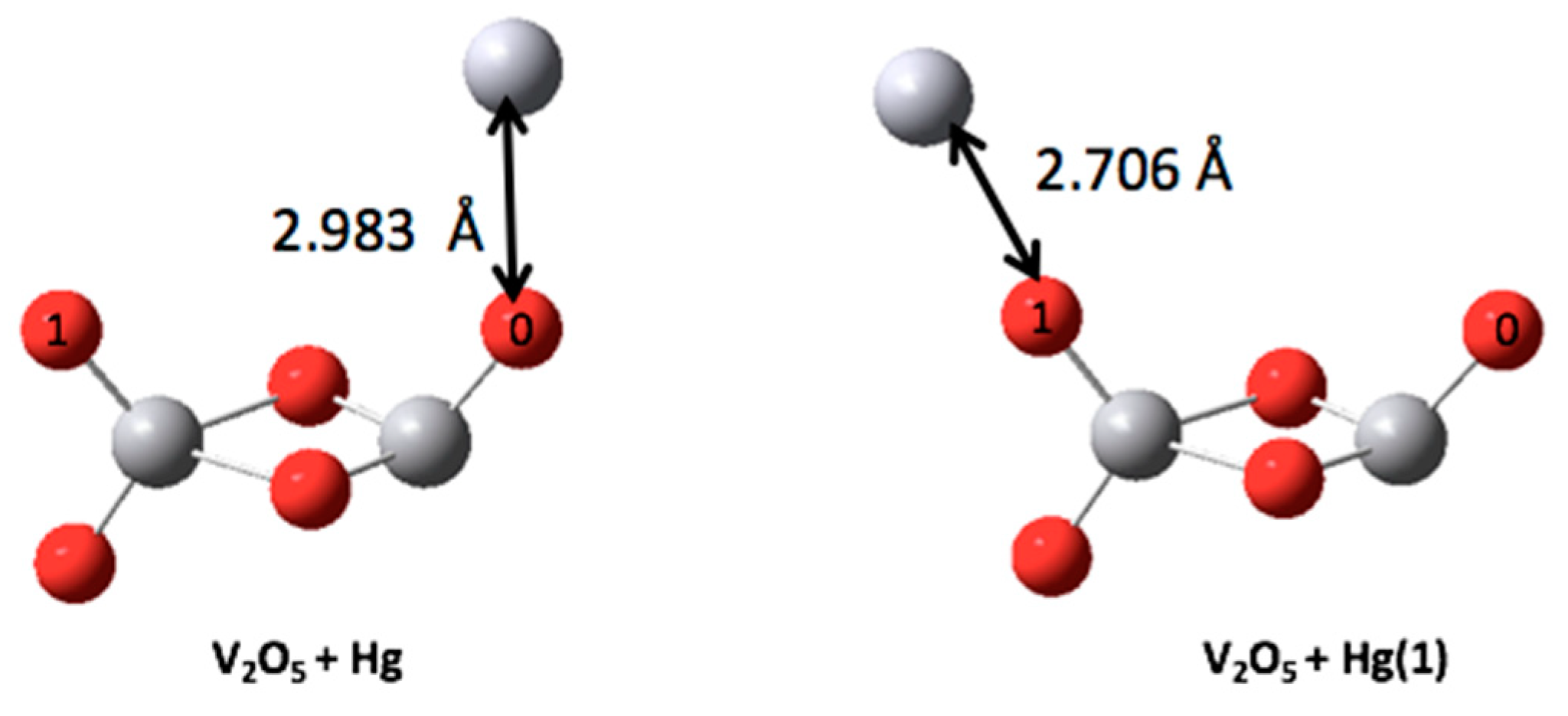
| V2O5-Hg | 0.142 | −6.550 | −5.500 | 1.050 |
| V2O5-Hg (1) | 0.210 | −6.225 | −5.466 | 0.760 |
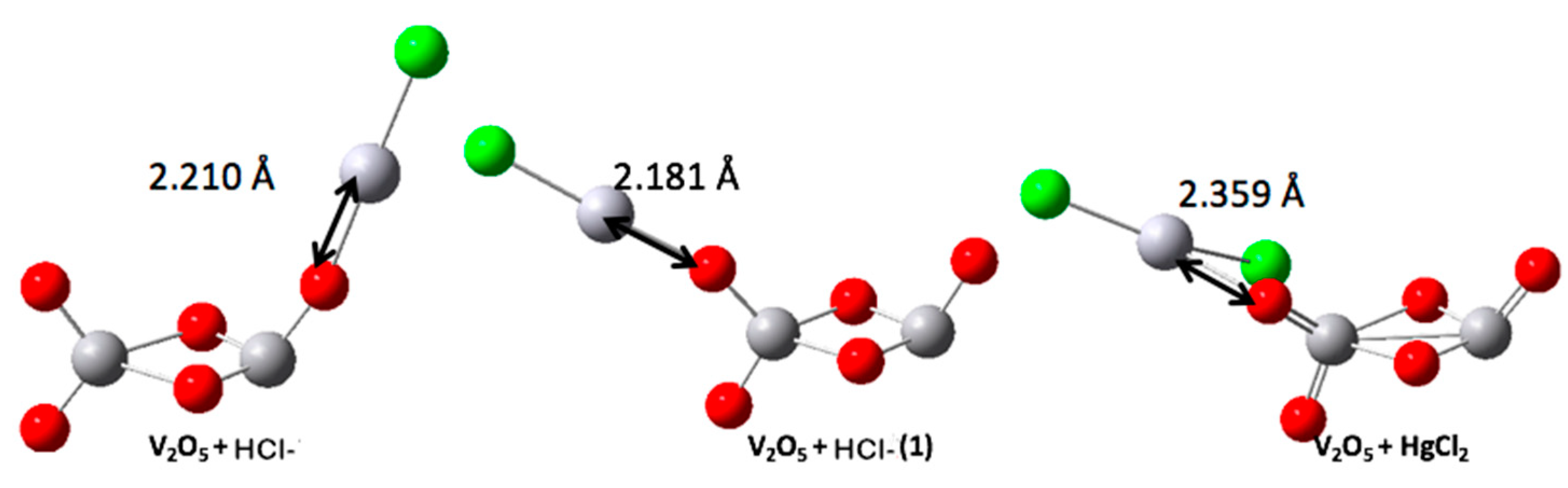
| V2O5-HgCl− | −0.401 | −12.548 | −10.142 | 2.406 |
| V2O5-HgCl−(1) | −0.425 | −12.040 | −9.829 | 2.211 |
| Evaporation Location | Mercury Form | Before SCR μg∙m−3 | After SCR μg∙m−3 | After ESP μg∙m−3 | After WFGD μg∙m−3 |
|---|---|---|---|---|---|
| Evaporation before SCR | Hg0 | 40.12 | 15.96 | 12.42 | 14.23 |
| Hg2+ | 4.36 | 24.32 | 17.63 | 2.26 | |
| Evaporation before ESP | Hg0 | 40.12 | 27.63 | 20.98 | 24.63 |
| Hg2+ | 4.36 | 13.25 | 12.16 | 1.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, B.; Chen, C.; Yi, Y.; Jiang, S.; Liu, X. Experimental and Mechanistic Study of Synergistic Removal of Hg by Evaporation from Desulfurization Wastewater. Energies 2022, 15, 4541. https://doi.org/10.3390/en15134541
Hu B, Chen C, Yi Y, Jiang S, Liu X. Experimental and Mechanistic Study of Synergistic Removal of Hg by Evaporation from Desulfurization Wastewater. Energies. 2022; 15(13):4541. https://doi.org/10.3390/en15134541
Chicago/Turabian StyleHu, Bin, Cong Chen, Yang Yi, Shouxi Jiang, and Xiaosong Liu. 2022. "Experimental and Mechanistic Study of Synergistic Removal of Hg by Evaporation from Desulfurization Wastewater" Energies 15, no. 13: 4541. https://doi.org/10.3390/en15134541
APA StyleHu, B., Chen, C., Yi, Y., Jiang, S., & Liu, X. (2022). Experimental and Mechanistic Study of Synergistic Removal of Hg by Evaporation from Desulfurization Wastewater. Energies, 15(13), 4541. https://doi.org/10.3390/en15134541






