Kinetics Analysis of the NH3-SCR Denitration Reaction over Sintered Ore Catalysts
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
2.2. Kinetic Measurements
3. Results and Discussion
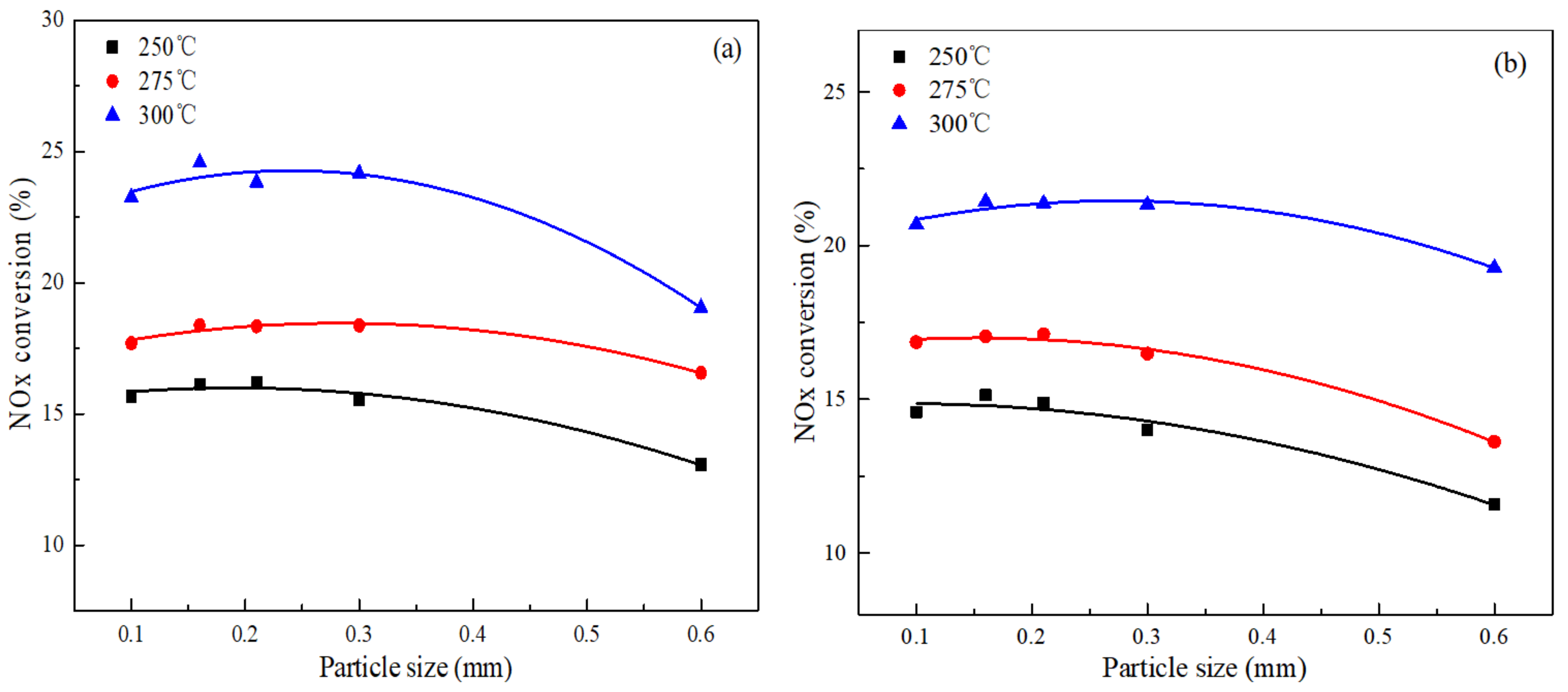
3.1. Elimination the External and Internal Diffusion Effect
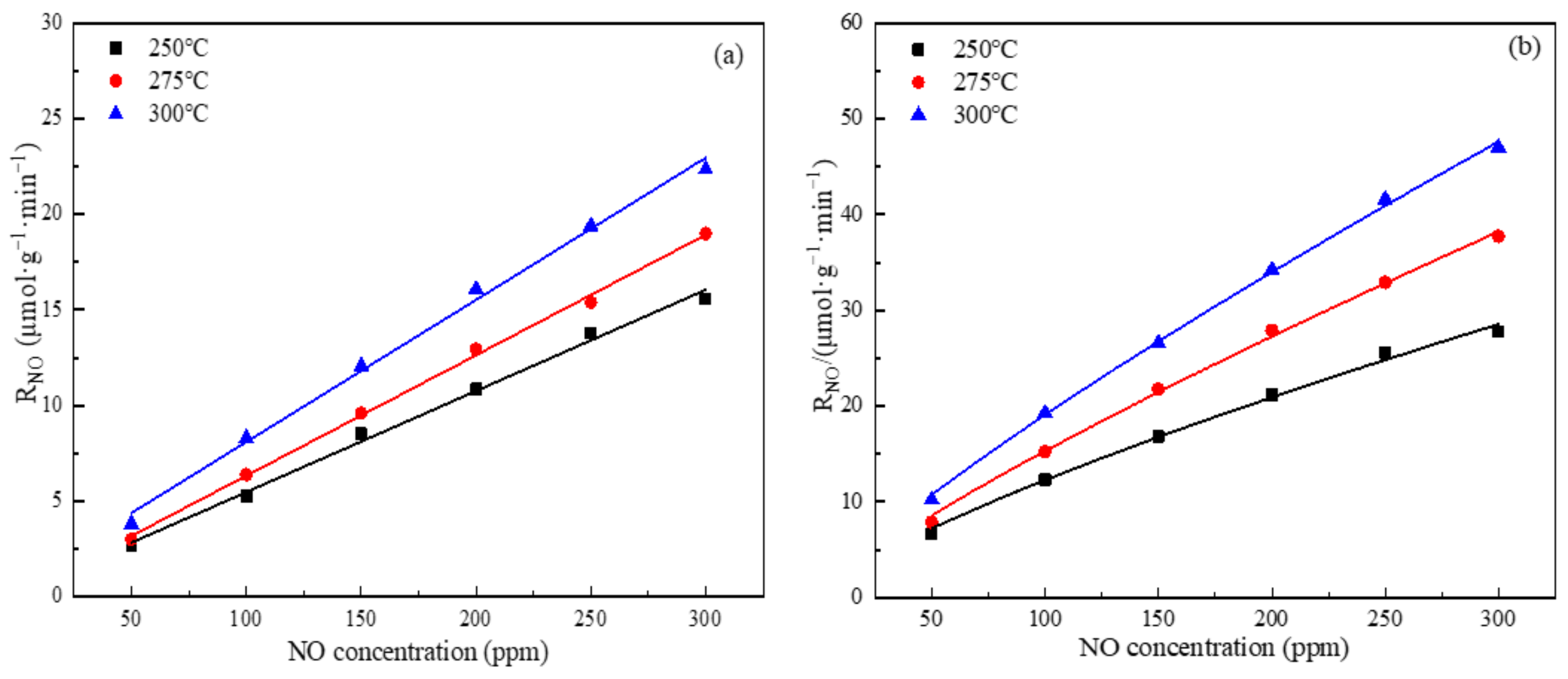
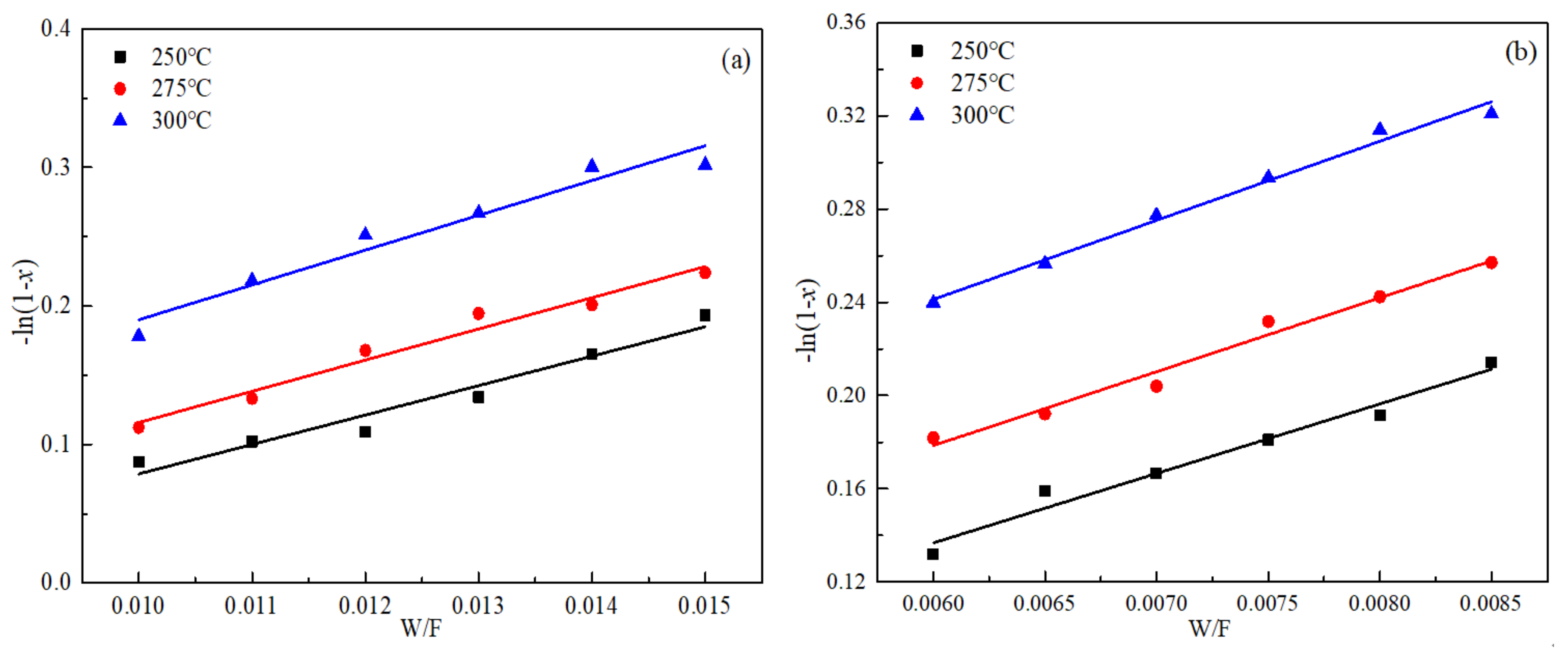
3.2. Effect of NO Concentration on the Rate of NO Conversion
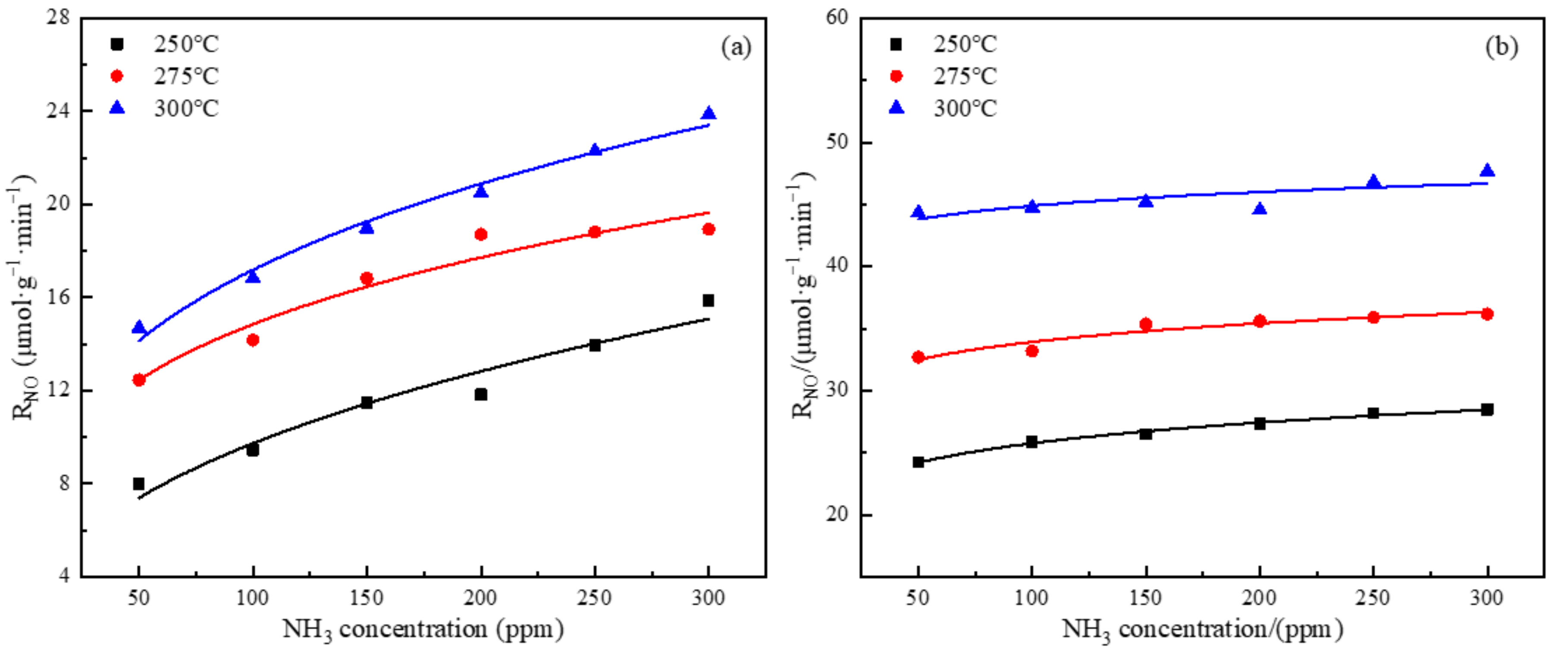
3.3. Effect of NH3 Concentration on the Rate of NO Conversion
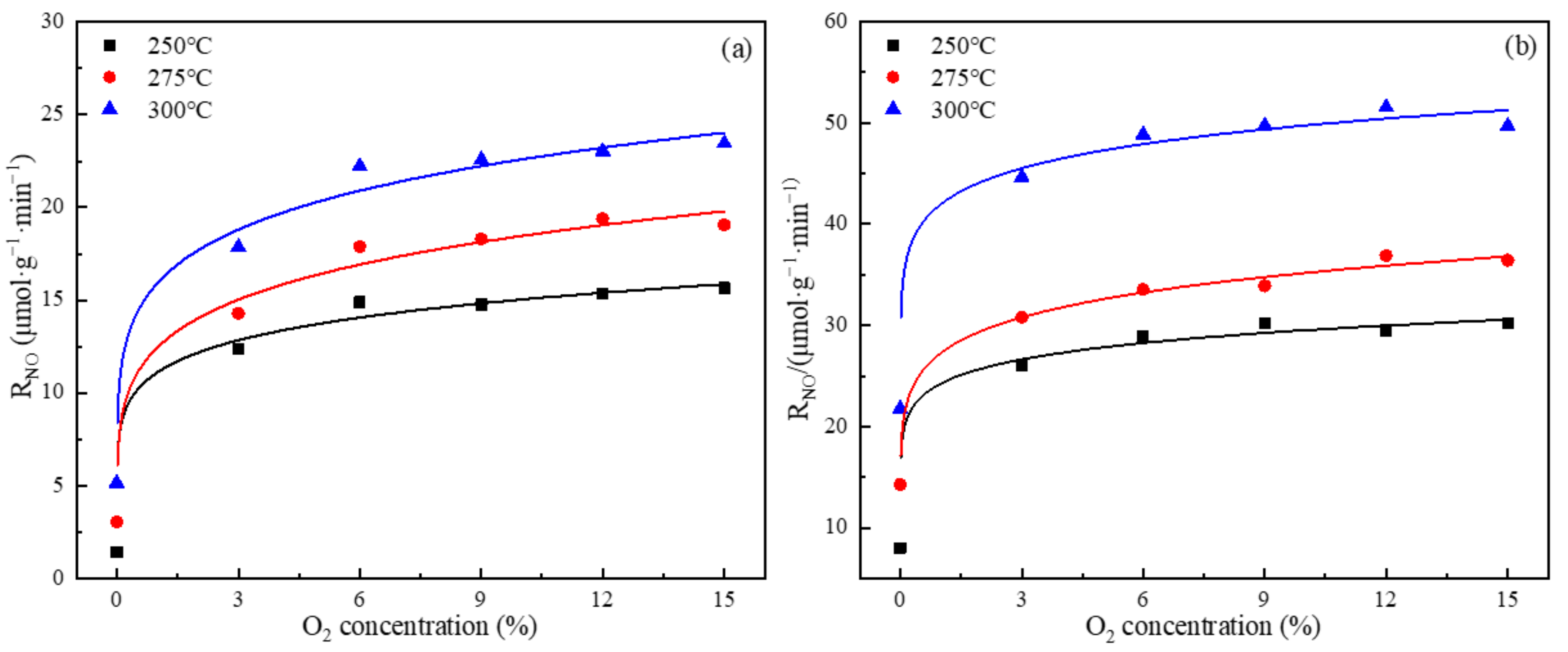
3.4. Effect of O2 Concentration on the Rate of NO Conversion
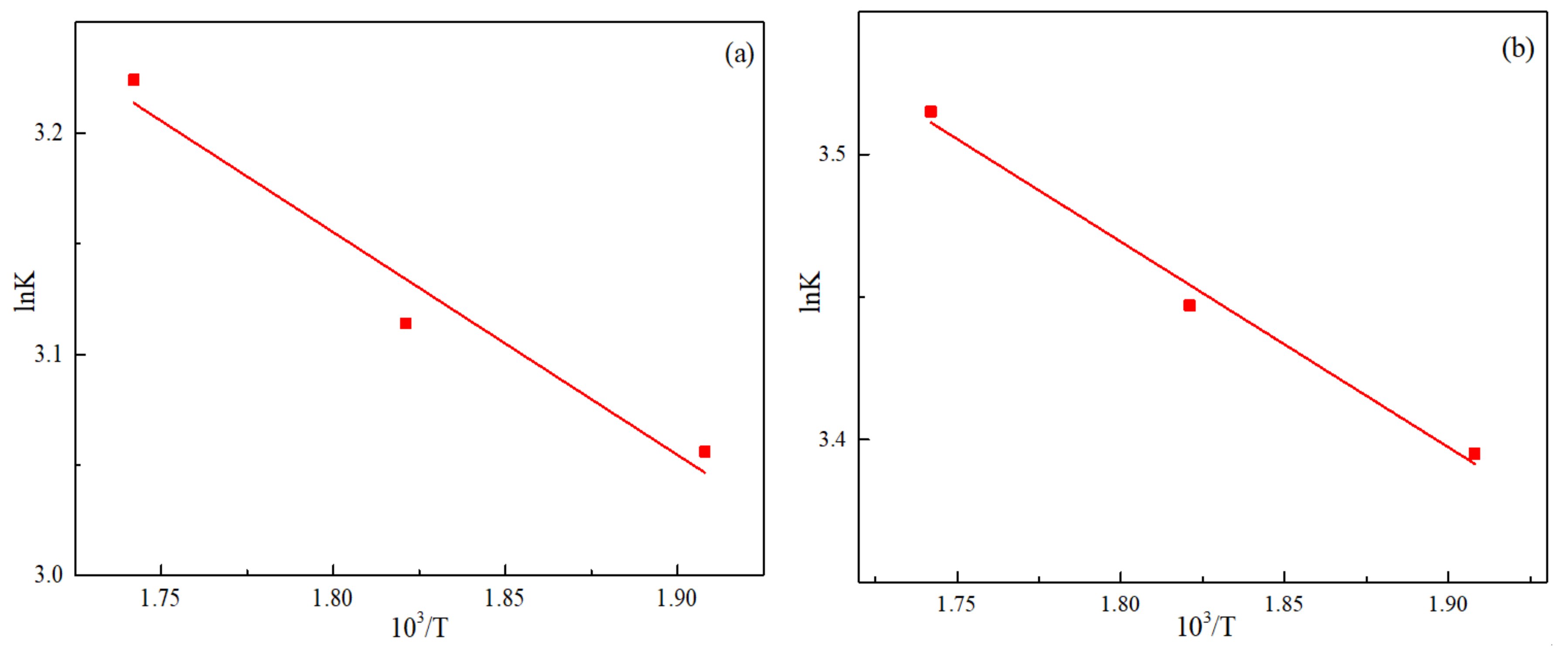
3.5. Reaction Rate Constant and Apparent Activation Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mousavi, S.M.; Panahi, P. Modeling and optimization of NH3-SCR performance of MnOx/γ-alumina nanocatalysts by response surface methodology. J. Taiwan Inst. Chem. Eng. 2016, 69, 68–77. [Google Scholar] [CrossRef]
- Ren, S.; Guo, F.; Yang, J.; Yao, L.; Zhao, Q.; Kong, M. Selection of carbon materials and modification methods in low-temperature sintering flue gas denitrification. Chem. Eng. Res. Des. 2017, 126, 278–285. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, J.; Zhou, M.; Cen, K. Experimental investigation on the mixing performance of heating gas into the low temperature sintering flue gas Selective Catalyst Reaction facilities. Appl. Therm. Eng. 2016, 115, 378–392. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, W. Simultaneous desulfurization and denitrification of sintering flue gas via composite absorbent. Chin. J. Chem. Eng. 2016, 24, 1104–1111. [Google Scholar] [CrossRef] [Green Version]
- Ogidiama, O.; Shamim, T. Performance Analysis of Industrial Selective Catalytic Reduction (SCR) Systems. Energy Procedia 2014, 61, 2154–2157. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Cao, Y.; Liu, S.; Chen, J.; Jia, W. Review on the latest developments in modified vanadium-titanium-based SCR catalysts. Chin. J. Catal. 2018, 39, 1347–1365. [Google Scholar] [CrossRef]
- Han, J.; Xiong, Z.; Zhao, B.; Liang, Y.; Wang, Y.; Qin, L. A prediction of arsenic and selenium emission during the process of bituminous and lignite coal co-combustion. Chem. Pap. 2020, 74, 2079–2089. [Google Scholar] [CrossRef]
- Kang, M.; Yeon, T.; Park, E.; Yie, J.; Kim, J.M. Novel MnOx Catalysts for NO Reduction at Low Temperature with Ammonia. Catal. Lett. 2006, 106, 77–80. [Google Scholar] [CrossRef]
- Qin, L.; Futang, X.; Zhao, B.; Chen, W.; Han, J. Reducing polycyclic aromatic hydrocarbon and its mechanism by porous alumina bed material during medical waste incineration. Chemosphere 2018, 212, 200–208. [Google Scholar] [CrossRef]
- Putluru, S.; Schill, L.; Godiksen, A.; Poreddy, R.; Mossin, S.; Jensen, A.; Fehrmann, R. Promoted V2O5/TiO2 catalysts for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2016, 85, 48–51. [Google Scholar]
- Yao, X.; Kong, T.; Yu, S.; Li, L.; Yang, F.; Dong, L. Influence of different supports on the physicochemical properties and denitration performance of the supported Mn-based catalysts for NH3-SCR at low temperature. Appl. Surf. Sci. 2017, 402, 208–217. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Haller, G.; Li, Y. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B-Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Chen, L.; Xiaoyu, N.; Li, Z.; Dong, Y.; Zhang, Z.; Yuan, F.; Zhu, Y. Promoting catalytic performances of Ni-Mn spinel for NH3-SCR by treatment with SO2 and H2O. Catal. Commun. 2016, 85, 48–51. [Google Scholar] [CrossRef]
- Pasel, J.; Käßner, P.; Montanari, B.; Gazzano, M.; Vaccari, A.; Makowski, W.; Łojewski, T.; Dziembaj, R.; Papp, H. Transition metal oxides supported on active carbons as low temperature catalysts for the selective catalytic reduction (SCR) of NO with NH3. Appl. Catal. B Environ. 1998, 18, 199–213. [Google Scholar] [CrossRef]
- Han, J.; He, X.; Qin, L.; Chen, W.; Yu, F. NOx removal coupled with energy recovery in sintering plant. Ironmak. Steelmak. 2014, 41, 350–354. [Google Scholar] [CrossRef]
- Chen, W.; Luo, J.; Qin, L.; Han, J. Selective autocatalytic reduction of NO from sintering flue gas by the hot sintered ore in the presence of NH3. J. Environ. Manag. 2015, 164, 146–150. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Hu, F.; Qin, L.; Han, J.; Wu, G. In-situ DRIFTS investigation on the selective catalytic reduction of NO with NH3 over the sintered ore catalyst. Appl. Surf. Sci. 2018, 439, 75–81. [Google Scholar] [CrossRef]
- Chen, W.; Hu, F.; Qin, L.; Han, J.; Zhao, B.; Tu, Y.; Yu, F. Mechanism and Performance of the SCR of NO with NH3 over Sulfated Sintered Ore Catalyst. Catalysts 2019, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Beeckman, J.; Hegedus, L. Design of monolith catalysts for power plant NOx emission control. Ind. Eng. Chem. Res. 1991, 30, 969–978. [Google Scholar] [CrossRef]
- Huang, H.; Long, R.; Yang, R.-T. Kinetics of selective catalytic reduction of NO with NH3 on Fe-ZSM-5 catalyst. Appl. Catal. A-Gen. 2002, 235, 241–251. [Google Scholar] [CrossRef]
- Peng, J.; Wang, D.; Zhang, X.; Lu, C.; Niu, S.; Li, J.; Xu, L. Kinetic study of selective catalytic reduction of NOx by NH3 on magnetic γ-Fe2O3 catalyst. Proc. CSEE 2015, 35, 4690–4696. [Google Scholar]
- Li, Z.; Shen, L.; Huang, W.; Xie, K. Kinetics of selective catalytic reduction of NO by NH3 on Fe-Mo/ZSM-5 catalyst. J. Environ. Sci. 2007, 19, 1516–1519. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Huang, D.; Zhao, B.; Hu, F.; Qin, L.; Wang, S. Kinetics Analysis of the NH3-SCR Denitration Reaction over Sintered Ore Catalysts. Energies 2022, 15, 4522. https://doi.org/10.3390/en15134522
Chen W, Huang D, Zhao B, Hu F, Qin L, Wang S. Kinetics Analysis of the NH3-SCR Denitration Reaction over Sintered Ore Catalysts. Energies. 2022; 15(13):4522. https://doi.org/10.3390/en15134522
Chicago/Turabian StyleChen, Wangsheng, Dongping Huang, Bo Zhao, Fali Hu, Linbo Qin, and Shijie Wang. 2022. "Kinetics Analysis of the NH3-SCR Denitration Reaction over Sintered Ore Catalysts" Energies 15, no. 13: 4522. https://doi.org/10.3390/en15134522
APA StyleChen, W., Huang, D., Zhao, B., Hu, F., Qin, L., & Wang, S. (2022). Kinetics Analysis of the NH3-SCR Denitration Reaction over Sintered Ore Catalysts. Energies, 15(13), 4522. https://doi.org/10.3390/en15134522






