Methane Adsorption Properties in Biomaterials: A Possible Route to Gas Storage and Transportation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gas Adsorption and Desorption Measurements
2.3. Pressure-Temperature and Raman Measurements
2.4. Microstructure Measurement
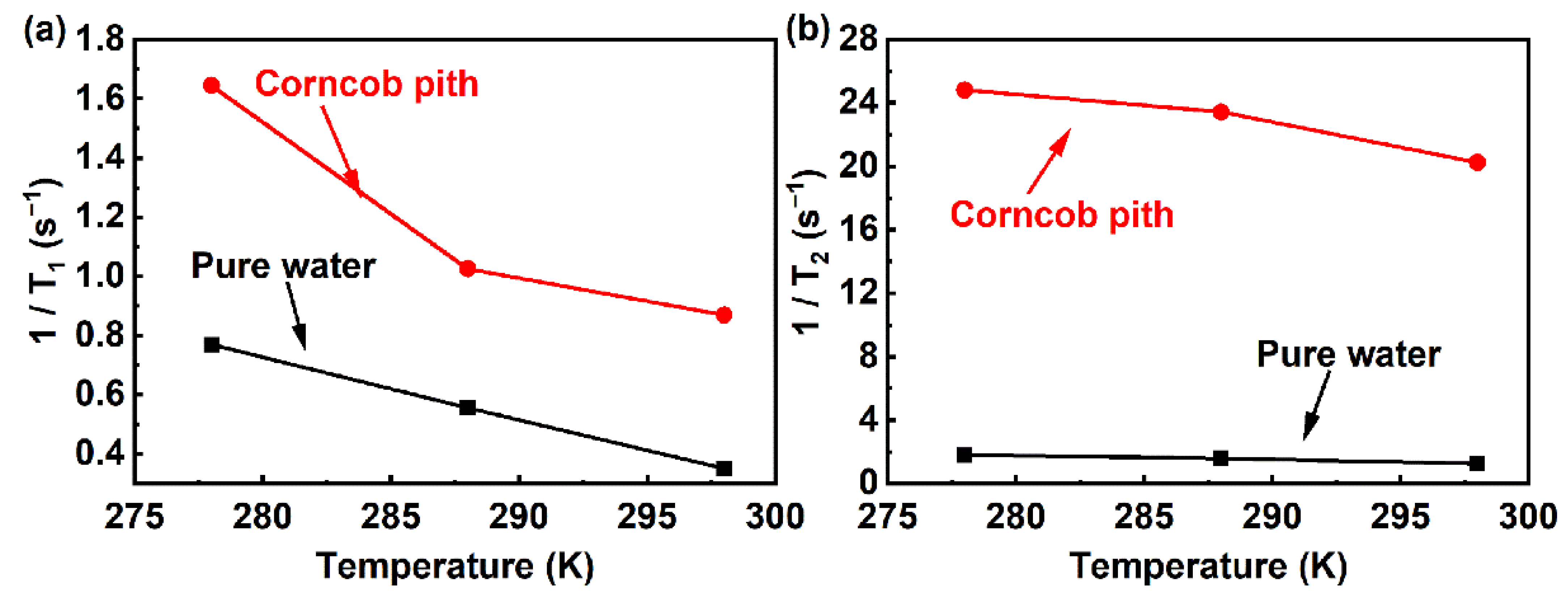
2.5. Relaxation Time Measurements
3. Results and Discussions
3.1. Structures of Corncob Pith and Hydrate within It
3.2. Gas Adsorption Characteristics
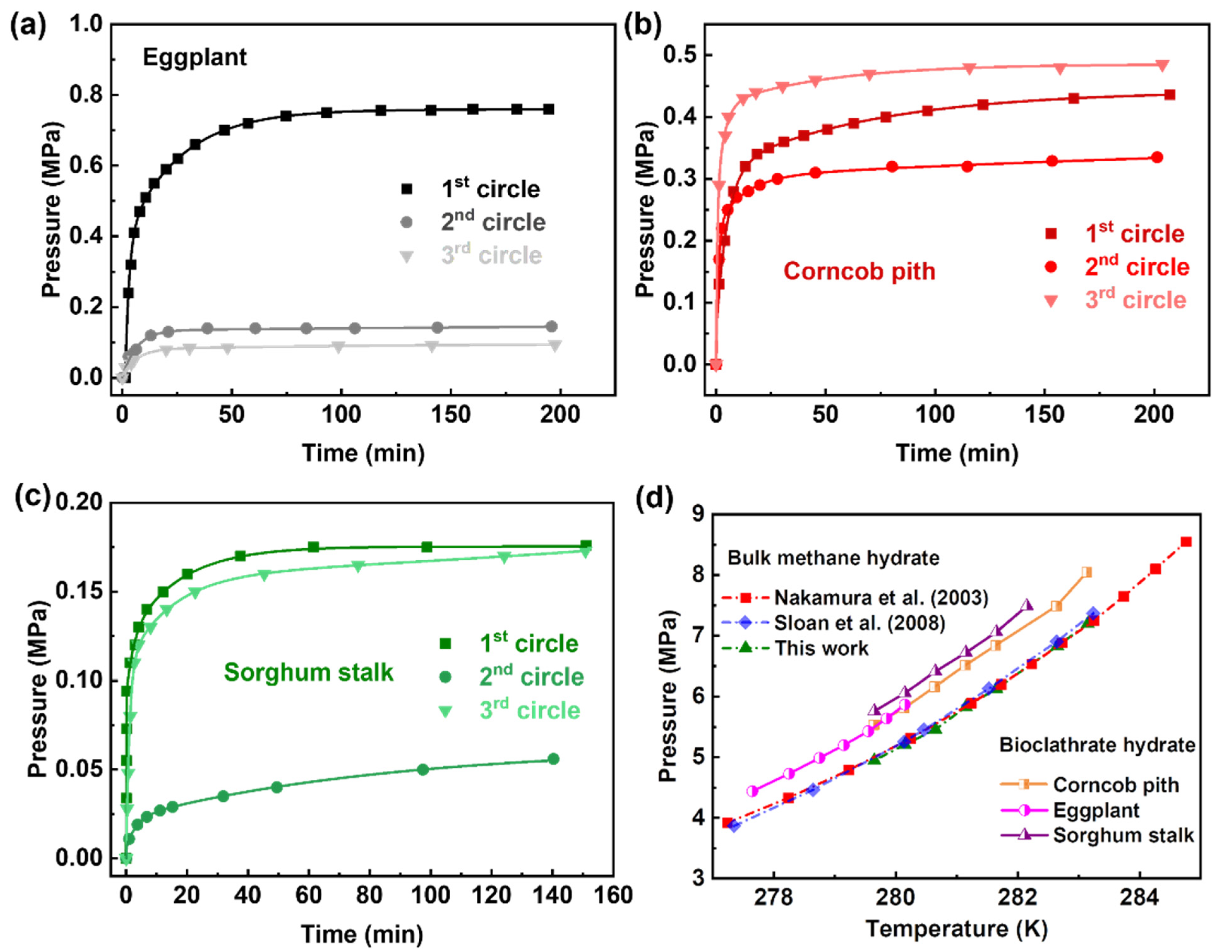
3.3. Gas Recovery and Thermodynamic Characteristics
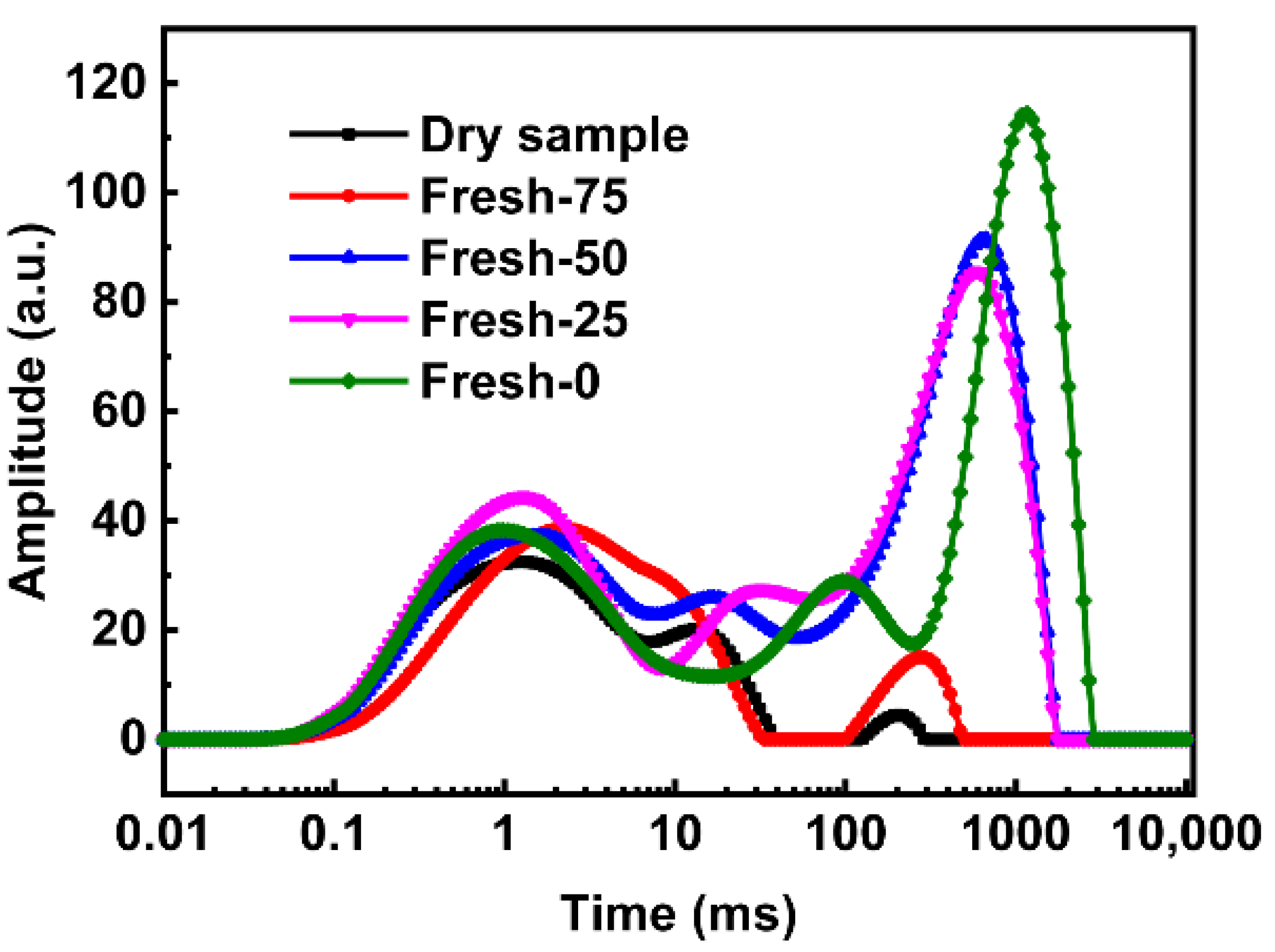
3.4. Hydrate Formation Kinetics Investigation
3.5. Thermodynamic Stability Investigation
4. Conclusions
- (1)
- Both two agricultural wastes (corncob pith and sorghum stalk) and eggplant exhibited much faster gas adsorption rates and higher adsorption capacities than a static water system. Regardless of high gas recovery, only corncob pith maintained high gas adsorption rate and adsorption capacity in multiple gas adsorption-desorption cycle measurements, showing excellent gas adsorption characteristics and high gas recovery.
- (2)
- The thermodynamic stability of methane hydrate in the three kinds of biomass (bioclathrate) did no change significantly compared with that of bulk hydrates. At 273.15 K, the equilibrium pressures of methane hydrate in biomass were only 0.6~0.8 MPa higher than that in bulk methane hydrate.
- (3)
- The rapid adsorption behavior in corncob pith can be attributed to its high-water content, porous structure, and chemical composition. The porous structure produces larger specific surface area, providing more nucleation sites for hydrate formation. The overall hydrophobic properties of corncob pith also contribute to hydrate formation. Moreover, the porous structure reduces the activity of water and, therefore, slightly decreases the thermodynamic stability of bioclathrate.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiri, R.N.E.; Gulbagca, F.; Aygun, A.; Cherif, A.; Sen, F. Biosynthesis of Ag–Pt bimetallic nanoparticles using propolis extract: Antibacterial effects and catalytic activity on NaBH4 hydrolysis. Environ. Res. 2022, 206, 112622. [Google Scholar] [CrossRef] [PubMed]
- Armaroli, N.; Balzani, V.V. The hydrogen issue. ChemSusChem 2011, 4, 21–36. [Google Scholar] [CrossRef]
- Crabtree, G.W.; Dresselhaus, M.S. The hydrogen fuel alternative. Mrs Bull. 2008, 33, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Demirbas, A. Methane gas hydrate: As a natural gas source. In Methane Gas Hydrate; Springer: London, UK, 2010; Volume 34, pp. 113–160. [Google Scholar] [CrossRef]
- Gouw, J.A.D.; Parrish, D.D.; Frost, G.J.; Trainer, M. Reduced emissions of CO2, NOx, and SO2 from U.S. power plants owing to switch from coal to natural gas with combined cycle technology. Earth’s Future 2014, 2, 75–82. [Google Scholar] [CrossRef]
- Kato, T.; Saeki, K.; Nishide, H.; Yamada, T. Development of CNG fueled engine with lean burn for small size commercial van. JSAE Rev. 2001, 22, 365–368. [Google Scholar] [CrossRef]
- Thomas, S.; Dawe, R.A. Review of ways to transport natural gas energy from countries which do not need the gas for domestic use. Energy 2003, 28, 1461–1477. [Google Scholar] [CrossRef]
- Veluswamy, H.P.; Kumar, A.; Seo, Y.; Lee, J.D.; Linga, P. A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates. Appl. Energ. 2018, 216, 262–285. [Google Scholar] [CrossRef]
- Casco, M.E.; Martinez-Escandell, M.; Gadea-Ramos, E.; Kaneko, K.; Silvestre-Albero, J.; Rodriguez-Reinoso, F. High-pressure methane storage in porous materials: Are carbon materials in the pole position? Chem. Mater. 2015, 27, 959–964. [Google Scholar] [CrossRef] [Green Version]
- Mason, J.A.; Veenstra, M.; Long, J.R. Evaluating metal-organic frameworks for natural gas storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef] [Green Version]
- Makogon, Y.F. Hydrates of Natural Gas; Penn Well Publishing: Tulsa, OK, USA, 1981; p. 237. [Google Scholar]
- Makogon, Y.F. Hydrates of Hydrocarbons; Penn Well Publishing Company: Tulsa, OK, USA, 1997; p. 504. [Google Scholar]
- Shui, B. Study and development of the applied storage technique for natural gas hydrate. Nat. Gas. Ind. 2000, 20, 93–97. [Google Scholar]
- Gudmundsson, J.S.; Mork, M.; Graff, O.F. Hydrate non-pipeline technology. In Proceedings of the 4th International Conference on Gas Hydrates, Yokohama, Japan, 19–23 May 2002. [Google Scholar]
- Kanda, H. Economic study on natural gas transportation with natural gas hydrate (NGH) pellets. In Proceedings of the 23rd World Gas Conference, Amsterdam, The Netherlands, 5–9 June 2006. [Google Scholar]
- Linga, P.; Haligva, C.; Nam, S.C.; Ripmeester, J.A.; Englezos, P. Gas hydrate formation in a variable volume bed of silica sand particles. Energ. Fuel. 2009, 23, 5496–5507. [Google Scholar] [CrossRef]
- Carter, B.O.; Wang, W.; Adams, D.J.; Cooper, A.I. Gas storage in “dry water” and “dry gel” clathrates. Langmuir 2010, 26, 3186–3193. [Google Scholar] [CrossRef] [PubMed]
- Siangsai, A.; Rangsunvigit, P.; Kitiyanan, B.; Kulprathipanja, S.; Linga, P. Investigation on the roles of activated carbon particle sizes on methane hydrate formation and dissociation. Chem. Eng. Sci. 2015, 126, 383–389. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, Y.; Zhou, Y. Enhancement of the methane storage on activated carbon by preadsorbed water. AIChE J. 2002, 48, 2412–2416. [Google Scholar] [CrossRef]
- Wang, W.; Bray, C.L.; Adams, D.J.; Cooper, A.I. Methane storage in dry water gas hydrates. J. Am. Chem. Soc. 2008, 130, 11608–11609. [Google Scholar] [CrossRef]
- Pasieka, J.; Coulombe, S.; Servio, P. Investigating the effects of hydrophobic and hydrophilic multi-wall carbon nanotubes on methane hydrate growth kinetics. Chem. Eng. Sci. 2013, 104, 998–1002. [Google Scholar] [CrossRef]
- Wang, W.; Ma, C.; Lin, P.; Sun, L.; Cooper, A.I. Gas storage in renewable bioclathrates. Energ. Environ. Sci. 2013, 6, 105–107. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, P.; Long, X.; Huang, J.; Liu, Y.; Tan, B.; Sun, L. Methane storage in tea clathrates. Chem. Commun. 2014, 50, 1244–1246. [Google Scholar] [CrossRef]
- Takada, M.; Niu, R.; Minami, E.; Saka, S. Characterization of three tissue fractions in corn (Zea mays) cob. Biomass Bioenerg. 2018, 115, 130–135. [Google Scholar] [CrossRef]
- Tulk, C.A.; Ripmeester, J.A.; Klug, D.D. The application of Raman spectroscopy to the study of gas hydrates. Ann. N. Y. Acad. Sci. 2000, 912, 859–872. [Google Scholar] [CrossRef]
- Babaee, S.; Hashemi, H.; Mohammadi, A.H.; Naidoo, P.; Ramjugernath, D. Kinetic study of hydrate formation for argon plus TBAB plus SDS aqueous solution system. J. Chem. Thermodyn. 2018, 116, 121–129. [Google Scholar] [CrossRef]
- Babaee, S.; Hashemi, H.; Mohammadi, A.H.; Naidoo, P.; Ramjugernath, D. Kinetic and thermodynamic behaviour of CF4 clathrate hydrates. J. Chem. Thermodyn. 2015, 81, 52–59. [Google Scholar] [CrossRef]
- Huang, X.; Shen, Y. Calculation of natural gas compression factor based on Redlieh-Kwong contrast state equation. Control Instrum. Chem. Ind. 2019, 46, 107–110. [Google Scholar]
- Zou, Y.; Fu, J.; Chen, Z.; Ren, L. Field decomposition of corn cob in seasonally frozen soil and its intrinsic influencing factors: The case of northeast China. Agriculture 2021, 11, 556. [Google Scholar] [CrossRef]
- Zou, Y.; Fu, J.; Chen, Z.; Ren, L. The effect of microstructure on mechanical properties of corn cob. Micron 2021, 146, 103070. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Qian, H.; Lu, Z.; Cui, S. Revealing the hydrophobicity of natural cellulose by single-molecule experiments. Macromolecules 2015, 48, 3685–3690. [Google Scholar] [CrossRef]
- Heiner, A.P.; Kuutti, L.; Teleman, O. Comparison of the interface between water and four surfaces of native crystalline cellulose by molecular dynamics simulations. Carbohyd. Res. 1998, 306, 205–220. [Google Scholar] [CrossRef]
- Yeap, R.Y. The Potential of Lignin to Increase the Hydrophobicity of Micro/Nanofibrillated Cellulose (MNFC). Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2020. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, A.V.; Steel, K.M.; Dang, L.X.; Galib, M. Interfacial gas enrichment at hydrophobic surfaces and the origin of promotion of gas hydrate formation by hydrophobic solid particles. J. Phys. Chem. C 2017, 121, 3830–3840. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, A.V. Hydrophobic effect on gas hydrate formation in the presence of additives. Energy Fuel. 2017, 31, 10311–10323. [Google Scholar] [CrossRef]
- Kang, D.; Yun, T.S.; Kim, K.Y.; Jang, J. Effect of hydrate nucleation mechanisms and capillarity on permeability reduction in granular media. Geophys. Res. Lett. 2016, 43, 9018–9025. [Google Scholar] [CrossRef]
- Azimi, A.; Javanmardi, J.; Mohammadi, A.H. Development of thermodynamic frameworks for modeling of clathrate hydrates stability conditions in porous media. J. Mol. Liq. 2021, 329, 115463. [Google Scholar] [CrossRef]
- Hills, B.P.; Manning, C.E.; Ridge, Y.; Brocklehurst, T. NMR water relaxation, water activity and bacterial survival in porous media. J. Sci. Food Agric. 1996, 71, 185–194. [Google Scholar] [CrossRef]
- Fukatsu, Y.; Morikawa, K.; Ikeda, Y.; Tsukahara, T. Temperature and size effects on structural and dynamical properties of water confined in 1–10 nm-scale pores using proton NMR spectroscopy. Anal. Sci. 2017, 33, 903–909. [Google Scholar] [CrossRef] [Green Version]
- Tsukahara, T.; Hibara, A.; Ikeda, Y.; Kitamori, T. NMR study of water molecules confined in extended nanospaces. Angew. Chem. 2007, 119, 1199–1202. [Google Scholar] [CrossRef]
- Kornyshev, A.A.; Kuznetsov, A.M.; Spohr, E.; Ulstrup, J. Kinetics of proton transport in water. J. Phys. Chem. B 2003, 107, 3351–3366. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Qiu, Z.; Zhang, Z.; Zhang, Y. Relationship between the gas hydrate suppression temperature and water activity in the presence of thermodynamic hydrate inhibitor. Fuel 2020, 264, 116776. [Google Scholar] [CrossRef]
- Nihous, G.C.; Kinoshita, C.K.; Masutani, S.M. A determination of the activity of water in water-alcohol mixtures using mobile order thermodynamics. Chem. Eng. Sci. 2009, 64, 2767–2771. [Google Scholar] [CrossRef]
- Fantazzini, P.; Bortolotti, V.; Brown, R.J.S.; Camaiti, M.; Garavaglia, C.; Viola, R.; Giavaresi, G. Two 1H-nuclear magnetic resonance methods to measure internal porosity of bone trabeculae: By solid–liquid signal separation and by longitudinal relaxation. J. Appl. Phys. 2004, 95, 339–343. [Google Scholar] [CrossRef]
- Borgia, G.C.; Brown, R.J.S.; Fantazzini, P. Nuclear magnetic resonance relaxivity and surface-to-volume ratio in porous media with a wide distribution of pore sizes. J. Appl. Phys. 1996, 79, 3656–3664. [Google Scholar] [CrossRef]
- Maccotta, A.; Fantazzini, P.; Garavaglia, C.; Donato, I.D.; Perzia, P.; Brai, M.; Morreale, F. Preliminary 1H NMR study on archaeological waterlogged wood. Ann. Di Chim. 2005, 95, 117–124. [Google Scholar] [CrossRef]

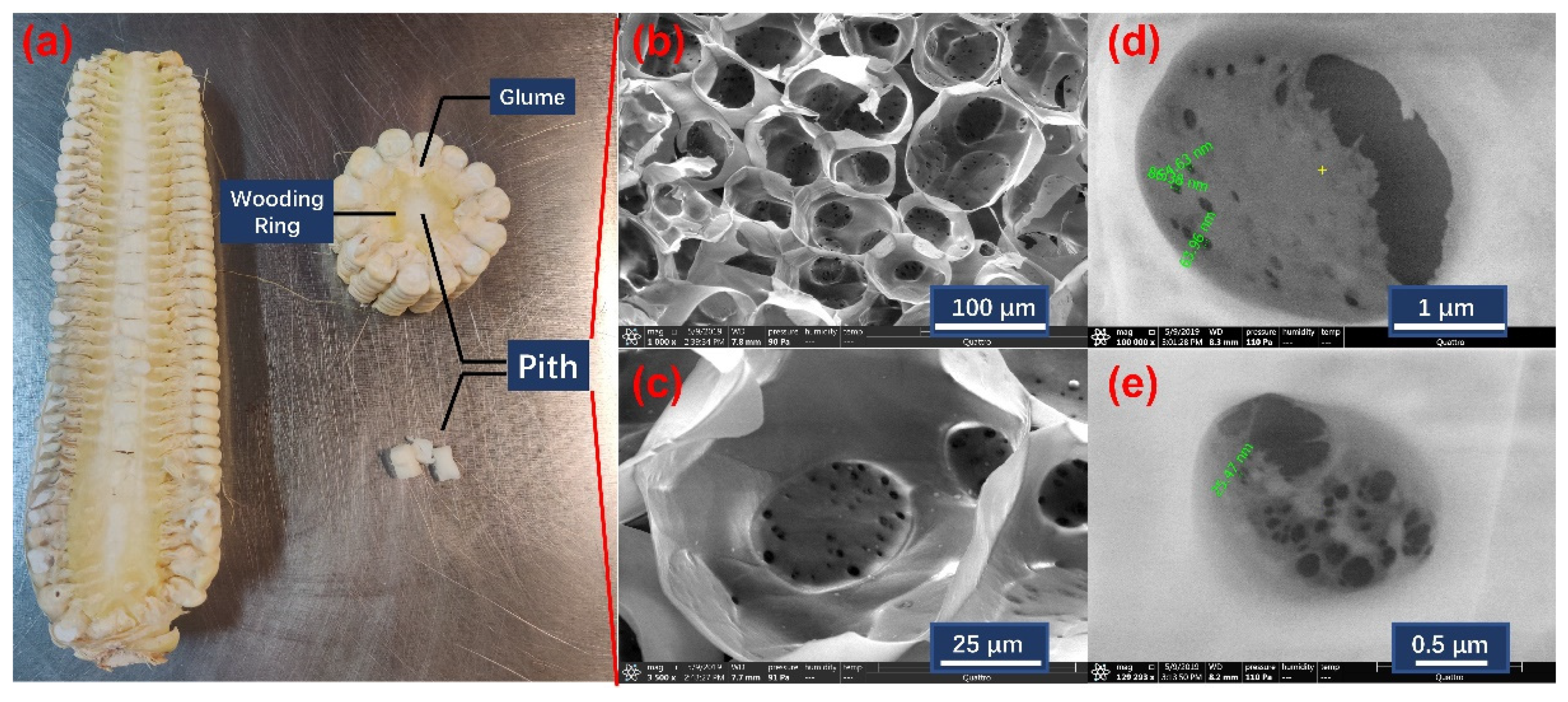



| Pore Width (nm) | Cumulative Volume (cm3/g) | Incremental Volume (cm3/g) | Cumulative Area (m2/g) | Incremental Area (m2/g) |
|---|---|---|---|---|
| 0~2 | 0 | 0 | 0 | 0 |
| 2~50 | 0.02196 | 0.02196 | 7.272 | 7.272 |
| 50~220 | 0.02690 | 0.00494 | 7.347 | 0.075 |
| Eggplant | Corncob Pith | Sorghum Stalk | ||||
|---|---|---|---|---|---|---|
| 1st cycle | 152.25 | 100% | 89.78 | 100% | 54.28 | 100% |
| 2nd cycle | 72.32 | 47.50% | 60.66 | 67.57% | 36.19 | 66.67% |
| 3rd cycle | 30.13 | 19.19% | 65.73 | 73.22% | 36.19 | 66.67% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Qu, Y.; Li, H.; Yu, X. Methane Adsorption Properties in Biomaterials: A Possible Route to Gas Storage and Transportation. Energies 2022, 15, 4261. https://doi.org/10.3390/en15124261
Du S, Qu Y, Li H, Yu X. Methane Adsorption Properties in Biomaterials: A Possible Route to Gas Storage and Transportation. Energies. 2022; 15(12):4261. https://doi.org/10.3390/en15124261
Chicago/Turabian StyleDu, Sanya, Yixin Qu, Hui Li, and Xiaohui Yu. 2022. "Methane Adsorption Properties in Biomaterials: A Possible Route to Gas Storage and Transportation" Energies 15, no. 12: 4261. https://doi.org/10.3390/en15124261
APA StyleDu, S., Qu, Y., Li, H., & Yu, X. (2022). Methane Adsorption Properties in Biomaterials: A Possible Route to Gas Storage and Transportation. Energies, 15(12), 4261. https://doi.org/10.3390/en15124261






