Effects of Pore Structure and Pore Size of Expanded Graphite on the Properties of Paraffin Wax/Expanded Graphite Composite Phase Change Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of EG
2.3. Preparation of Paraffin/EG CPCMs
2.4. Characterization of CPCMs
3. Results
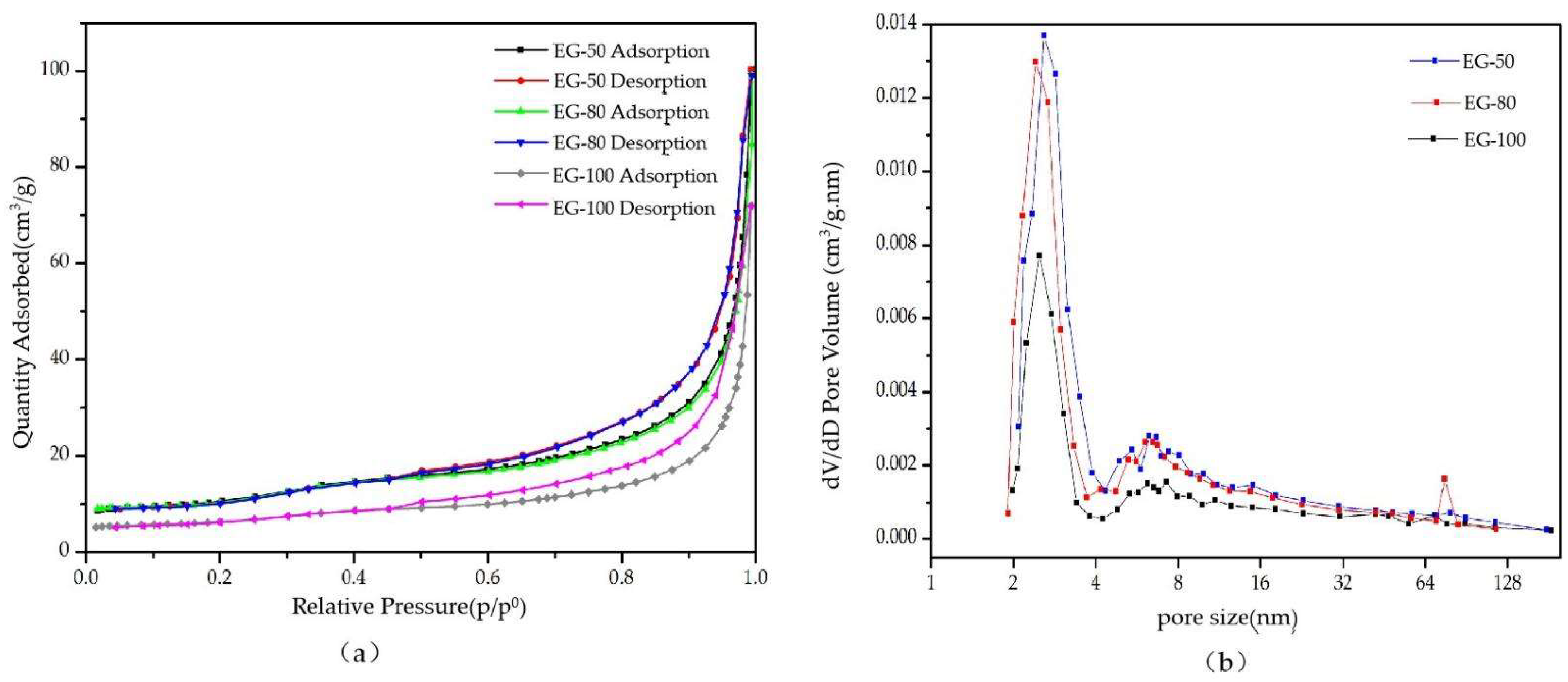
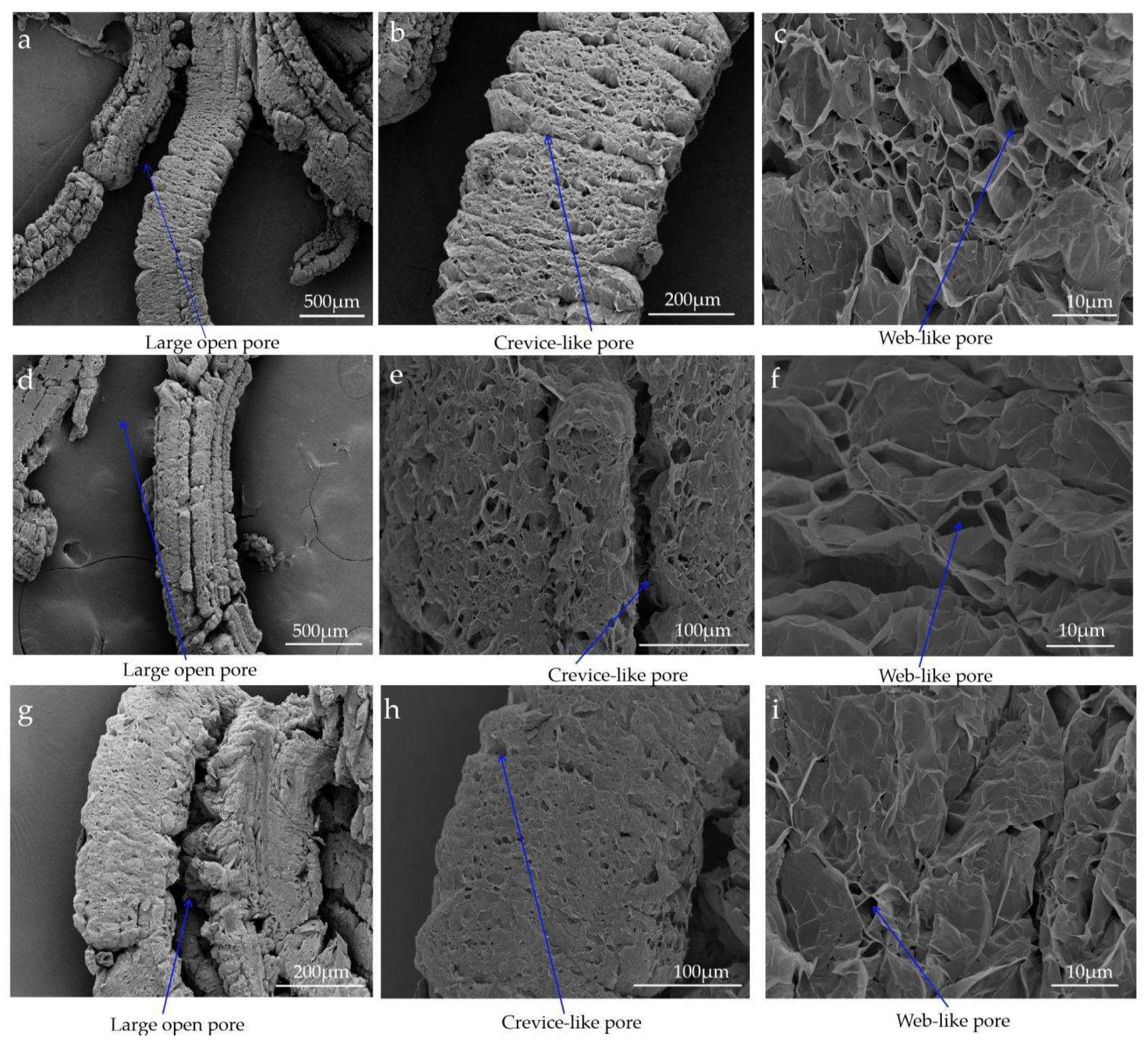
3.1. Pore Structure Analysis
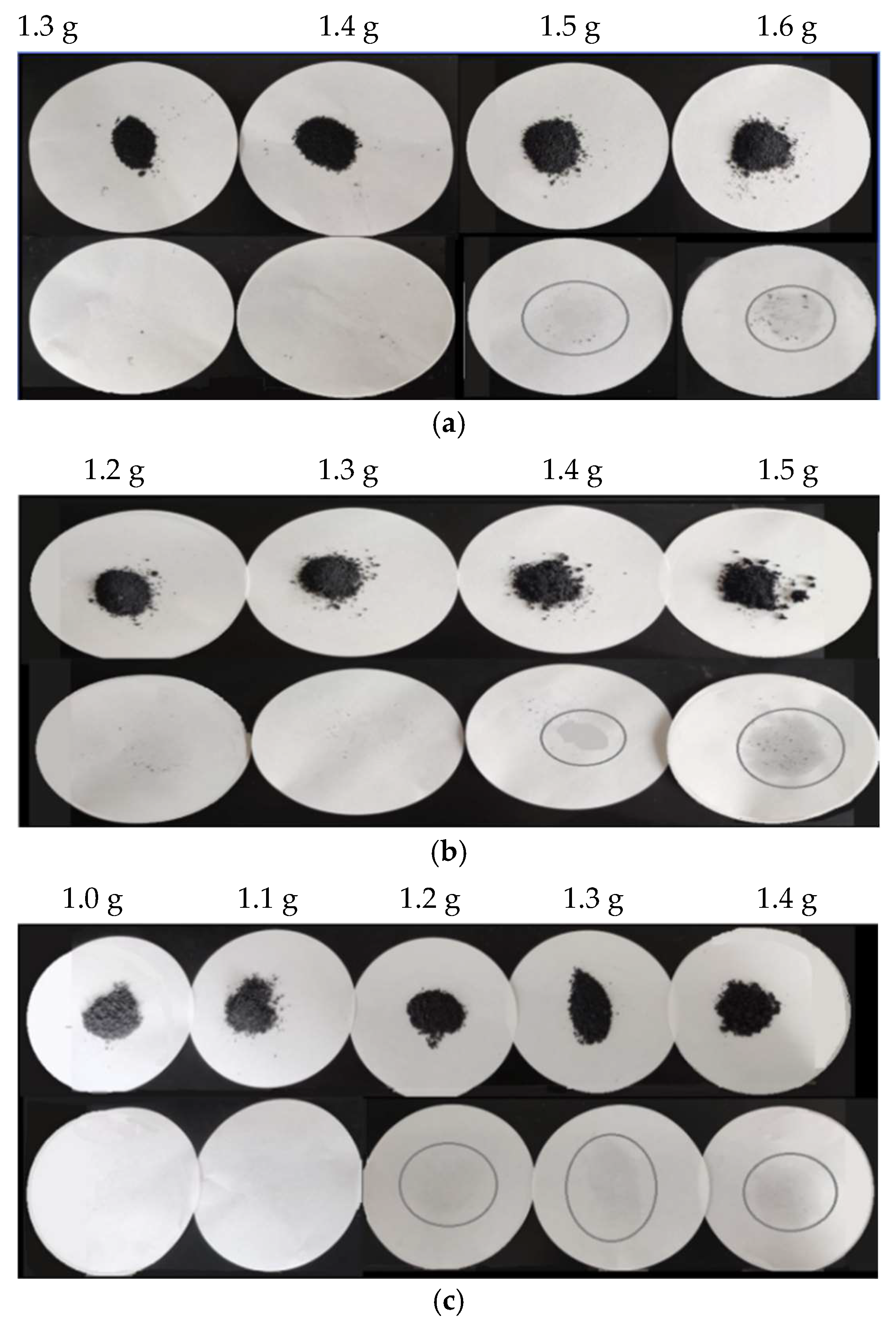
3.2. Leak Test Analysis
3.3. XRD Patterns Analysis
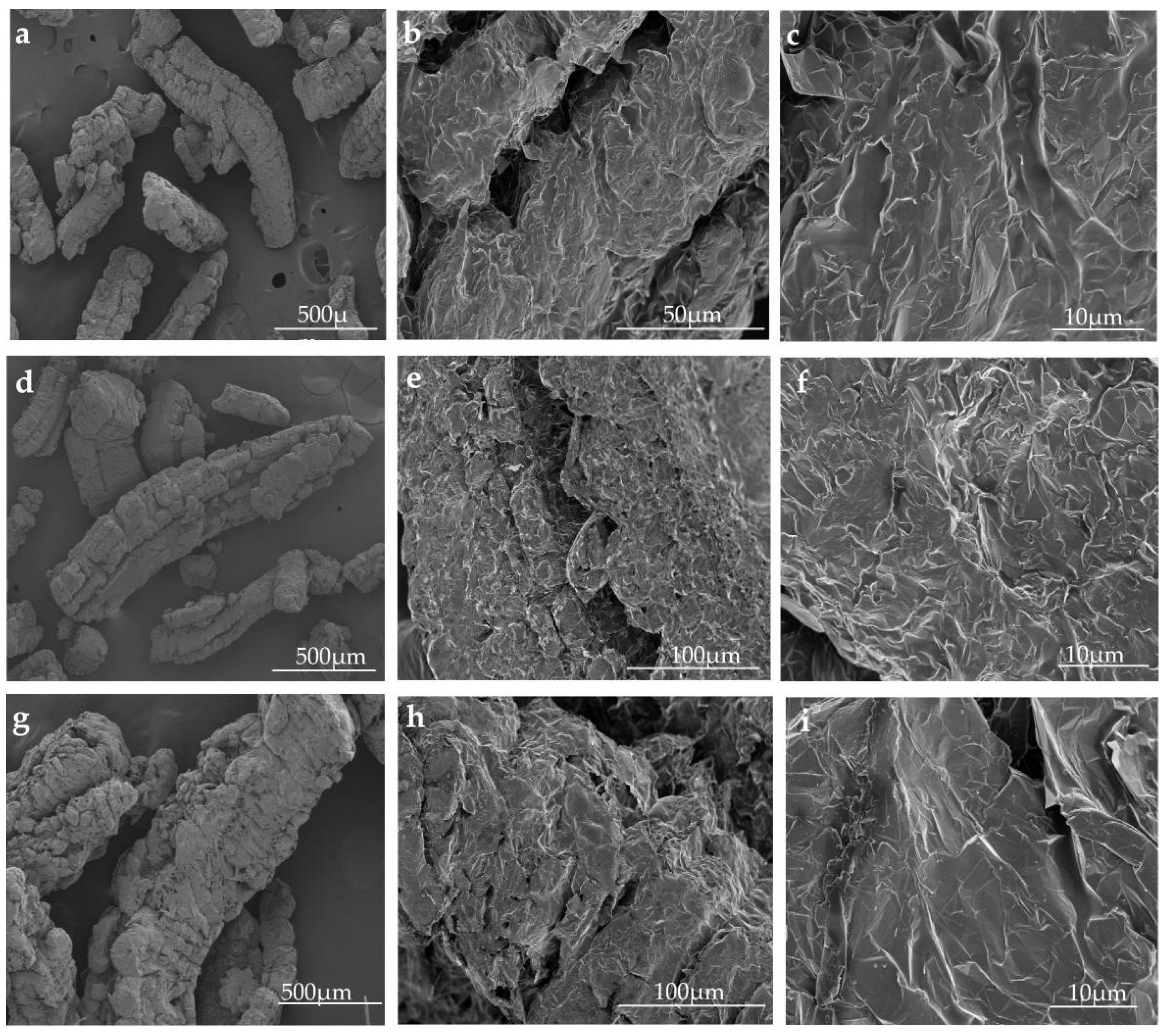
3.4. Morphological Analysis
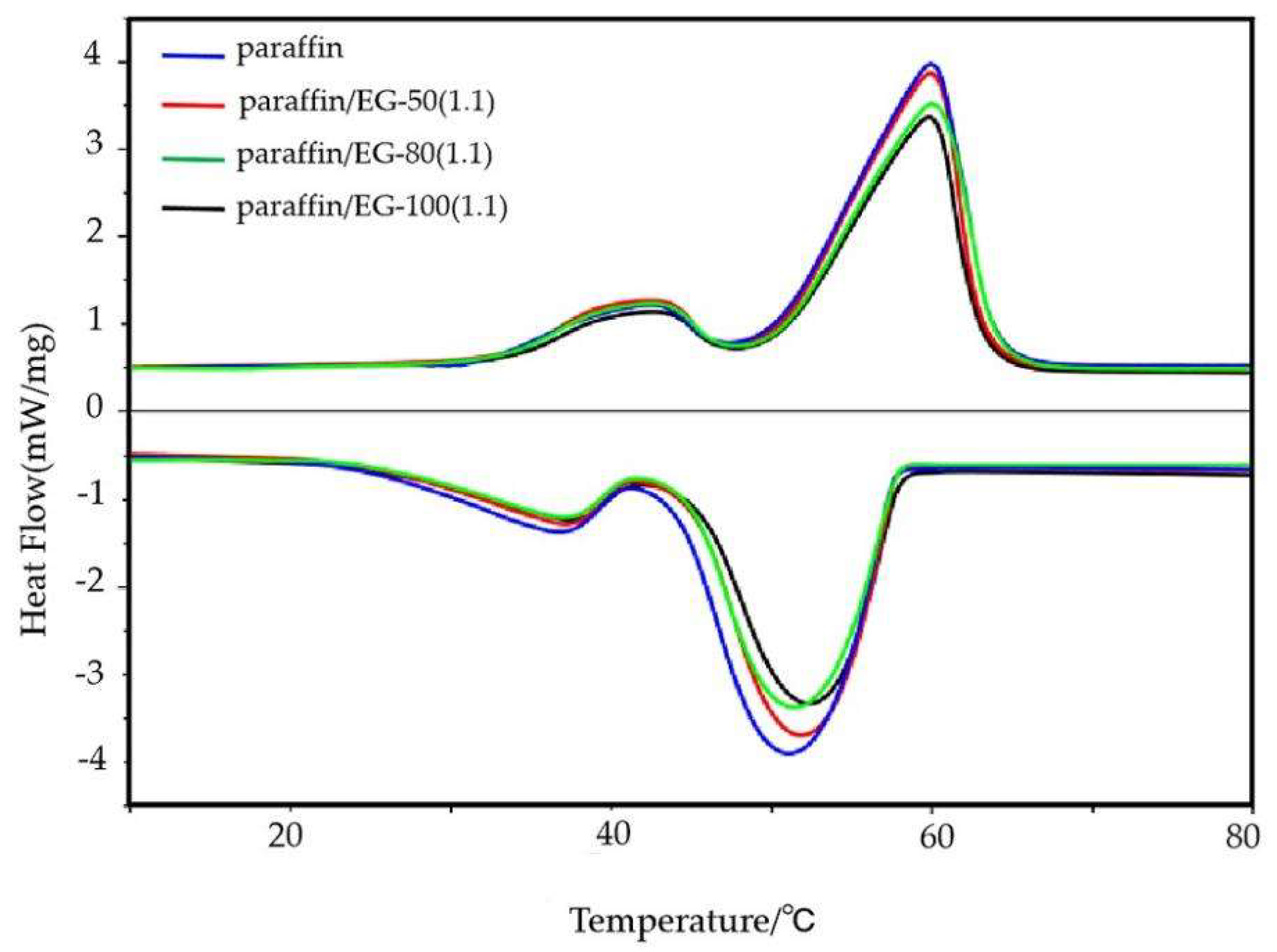
3.5. Thermal Characterization Analysis
3.6. Thermal Conductivity Analysis
4. Conclusions
- Under the same expansion conditions, the larger the particle size of graphite, the larger the expansion ratio, the larger the specific surface area and pore volume, and the smaller the pore diameter.
- After graphite with different particle sizes expanded, three pore structures were formed, namely, the large open pore of the graphite worm, the crevice-like pore of the graphite worm, and the web-like pore structure of the graphite worm. The pore size distribution of EG with three particle sizes was mainly in the range of 2~4 nm and 4~8 nm, which belonged to mesoporous, and there were some macropores with a particle size above 50 nm. EG-50 had a larger specific surface area, pore volume and web-like pore structure, and thus, higher adsorption capacity for paraffin wax. The different grades of pore played different roles in the adsorption of EG. Paraffin wax was only uniformly adsorbed in the web-like pore structure of EG through capillary force and surface tension, while the other two-pore structures only played the role of transporting adsorbate.
- Compared with paraffin wax, the addition of EG could reduce the supercooling degree of CPCMs and improve the thermal conductivity of CPCMs. the supercooling degree of EG-50 was lower than that of EG-80 and EG-100 due to its larger specific surface area, which could form more contact surfaces and angles to promote nucleation and reduce supercooling degree. In addition, CPCM with EG-50 had high thermal conductivity, because EG-50 had smaller interfacial resistance.
- Compared with paraffin wax, the ΔT of the CPCMs was slightly higher than that of paraffin wax. Because the paraffin wax and EG were combined through physical action, the physical action between them is very small, so the melting point of the CPCM showed a downward trend. On the contrary, the confinement effect of EG pores on the solid–liquid phase transition of paraffin wax made the phase transition temperature of the CPCMs increase. As a result of the comprehensive action, the phase transition temperature did not change much, and EG-50 had a small pore size, but the phase transition temperature of cpcm with EG-50 increased less, which was due to the complex composition of paraffin, which is inconsistent with the traditional change law. The change of latent heat of CPCMs was complex, which was mainly determined by the content of paraffin wax in CPCMs. At the same time, the pore diameter of EG and the complex composition of paraffin wax also play a regulatory role.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chriaa, I.; Karkri, M.; Trigui, A.; Jedidi, I.; Abdelmouleh, M.; Boudaya, C. The performances of expanded graphite on the phase change materials composites for thermal energy storage. Polymers 2021, 212, 123128. [Google Scholar] [CrossRef]
- Olabi, A.G.; Wilberforce, T.; Elsaid, K.; Sayed, E.T.; Ramadan, M.; Atiqure Rahman, S.M.; Abdelkareem, M.A. Recent progress on Carbon-based nanomaterial for phase change materials: Prospects and challenges. Therm. Sci. Eng. Prog. 2021, 23, 100920. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.M.; Sanjayan, J.; Petinakis, E.; Wilson, J. Development of thermal energy storage cementitious composites (TESC) containing a novel paraffin/hydrophobic expanded perlite composite phase change material. Sol. Energy. 2017, 158, 626–635. [Google Scholar] [CrossRef]
- Shi, J.b.; Li, M. Lightweight mortar with paraffin/expanded vermiculite-diatomite composite phase change materials: Development, characterization and year-round thermoregulation performance. Sol. Energy 2021, 220, 331–342. [Google Scholar] [CrossRef]
- Ran, X.F.; Wang, H.R.; Zhong, Y.J.; Zhang, F.; Lin, J.; Zou, H.; Dai, Z.M.; An, B.L. Thermal properties of eutectic salts/ceramics/expanded graphite composite phase change materials for high-temperature thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 225, 111047. [Google Scholar] [CrossRef]
- Ding, Z.; Yang, W.B.; He, F.F.; Jiang, Z.N.; He, R.; Fan, J.H.; Zhang, K. GO modified EPDM/paraffin shape-stabilized phase change materials with high elasticity and low leakage rate. Polymers 2020, 204, 122824. [Google Scholar] [CrossRef]
- Zhang, N.; Guo, H.J.; Xiong, L.; Zhang, H.R.; Chen, X.D. Preparation and characterization of paraffin/palygorskite shape-stable composite phase change materials for thermal energy storage. J. Energy Storage 2021, 34, 102189. [Google Scholar] [CrossRef]
- Li, X.F.; Li, H.; Kong, X.F.; Yang, H. Characterization and experimental investigation of composite phase change materials based on aluminum nitride/expanded graphite. J. Energy Storage 2021, 35, 102326. [Google Scholar] [CrossRef]
- Zeng, J.L.; Gan, J.; Zhu, F.R.; Yu, S.B.; Xiao, Z.L.; Yan, W.P.; Zhu, L.; Liu, Z.Q.; Sun, L.X.; Cao, Z. Tetradecanol/expanded graphite composite form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells. 2014, 127, 122–128. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.D.; Cao, J.Z. An energy-efficient composite by using expanded graphite stabilized paraffin as phase change material. Compos. Part A 2018, 107, 83–93. [Google Scholar] [CrossRef]
- Feng, L.J.; Wei, Y.H.; Yu, L.W.; Jie, X.Z.; Zheng, S.Z.; Jun, X.X.; Li, C.S. Numerical and experimental study on the heat transfer properties of the composite paraffin/expanded graphite phase change material. Int. J. Heat Mass Transfer. 2015, 84, 237–244. [Google Scholar]
- Liang, Y.J.; Tao, Z.C.; Guo, Q.G.; Liu, Z.J. Sponge gourd-bioinspired phase change material with high thermal conductivity and excellent shape-stability. J. Energy Storage 2021, 39, 102634. [Google Scholar] [CrossRef]
- Wu, Y.P.; Wang, T. Hydrated salts/expanded graphite composite with high thermal conductivity as a shape-stabilized phase change material for thermal energy storage. Energy Convers. Manag. 2015, 101, 164–171. [Google Scholar] [CrossRef]
- Cai, Z.D.; Liu, J.; Zhou, Y.X.; Dai, L.L.; Wang, H.X.; Liao, C.C.; Zou, X.L.; Chen, Y.F.; Xu, Y.J. Flexible phase change materials with enhanced tensile strength, thermal conductivity and photo-thermal performance. Sol. Energy Mater. Sol. Cells 2021, 219, 110728. [Google Scholar] [CrossRef]
- Avia, O.L.; Lavi, A.; Alatawna, A.; Ruse, E.; Ziskind, G.; Regev, O. Graphite-based shape-stabilized composites for phase change material applications. Renew. Energy 2021, 167, 580–590. [Google Scholar]
- Li, C.C.; Zhang, B.; Liu, Q.X. N-eicosane/expanded graphite as composite phase change materials for electro-driven thermal energy storage. J. Energy Storage 2020, 29, 101339. [Google Scholar] [CrossRef]
- Xua, T.; Chen, Q.l.; Huang, G.S.; Zhang, Z.G.; Gao, X.N.; Lu, S.S. Preparation and thermal energy storage properties of D-Mannitol/expanded graphite composite phase change material. Sol. Energy Mater. Sol. Cells 2016, 155, 141–146. [Google Scholar] [CrossRef]
- Pratap Singh, A.; Khanna, S.; Paneliya, S. Preparation and characterization of solid-state neopentyl glycol/expanded graphitemicro composite for thermal energy storage applications. Mater. Today Proc. 2020, 11, 403. [Google Scholar] [CrossRef]
- Yang, Y.N.; Pang, Y.; Liu, Y.; Guo, H.W. Preparation and thermal properties of polyethylene glycol/expanded graphite as novel form-stable phase change material for indoor energy saving. Mater. Lett. 2018, 216, 220–223. [Google Scholar] [CrossRef]
- Zhou, H.; Lv, L.Q.; Zhang, Y.Z.; Ji, M.T.; Cen, K.F. Preparation and characterization of a shape-stable xylitol/expanded graphite composite phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2021, 230, 111244. [Google Scholar] [CrossRef]
- Xie, N.; Li, Z.P.; Gao, X.N.; Fang, Y.T.; Zhang, Z.G. Preparation and performance of modified expanded graphite/eutecticsalt composite phase change cold storage material. Int. J. Refrig. 2020, 110, 178–186. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K.; Kahwash, F.; Makhkamova, I. Enhancing thermal conductivity of paraffin wax 53–57 °C using expanded graphite. Sol. Energy Mater. Sol. Cells 2019, 200, 110026. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Zhou, D.; Chen, Y.M.; Eames, P.; Wu, Z.G. Characterization and effects of thermal cycling on the properties of paraffin/expanded graphite composites. Renew. Energy 2020, 147, 1131–1138. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T. The dependence of phase change enthalpy on the pore structure and interfacial groups in hydrated salts/silica composites via sol-gel. J. Colloid Interface Sci. 2015, 448, 100–105. [Google Scholar] [CrossRef]
- Zou, T.; Liang, X.H.; Wang, S.F.; Gao, X.N.; Zhang, Z.G.; Fang, Y.T. Effect of expanded graphite size on performances of modified CaCl2·6H2Ophase change material for cold energy storage. Microporous Mesoporous Mater. 2020, 305, 110403. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Jin, L.; Zou, B.Y.; Qiao, G.; Zhang, T.T.; Cong, L.; Jiang, F.; Li, C.A.; Huang, Y.; Ding, Y.L. Expanded graphite-Paraffin composite phase change materials: Effect of particle size on the composite structure and properties. Appl. Therm. Eng. 2020, 171, 12. [Google Scholar] [CrossRef]
- Hou, B.; Sun, H.J.; Peng, T.J.; Zhang, X.Y.; Ren, Y.Z. Rapid preparation of expanded graphite at low temperature. New Carbon Mater. 2020, 35, 262–268. [Google Scholar] [CrossRef]
- Kholmanov, I.; Kim, J.; Ou, E. Continuous carbon nanotube-ultrathin graphite hybrid foams for increased thermal conductivity and suppressed subcooling in composite phase change materials. Am. Chem. Soc. Nano. 2015, 9, 11699–11707. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Gubbinns, K.E. Free energy studies of freezing in slit pores: An order-parameter approach using Monte Carlo simulation. Mol. Phys. 1999, 96, 1249. [Google Scholar] [CrossRef]
- El Id, M.M.; Karkri, M.; Kraiem, M. Preparation and effective thermal conductivity of a Paraffin/Metal Foam composite. J. Energy Storage 2021, 33, 102077. [Google Scholar] [CrossRef]
- Zuo, X.C.; Zhao, X.G.; Li, J.W.; Hu, Y.Q.; Yang, H.M.; Chen, D.L. Enhanced thermal conductivity of form-stable composite phase-change materials with graphite hybridizing expanded perlite/paraffin. Solar Energy 2020, 209, 85–95. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, B.; Qu, Z.G.; Zhang, J.F.; Jin, Z.G. Effects of graphite microstructure evolution on the anisotropic thermal conductivity of expanded graphite/paraffin phase change materials and their thermal energy storage performance. Int. J. Heat Mass Transfer. 2020, 155, 119853. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, P.; Wang, R.Z. Preparation and thermal characterization of expanded graphite/paraffin composite phase change material. Carbon 2010, 48, 2538–2548. [Google Scholar] [CrossRef]


| Sample | SBET (m2/g) | Vp (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| EG-50 | 36 | 0.1551 | 15.9800 |
| EG-80 | 35 | 0.1531 | 16.2377 |
| EG-100 | 21 | 0.1112 | 19.8323 |
| Sample | Melt | Solidification | ||||||
|---|---|---|---|---|---|---|---|---|
| Tm−1 | Tm−2 | ΔHc | ΔHt | Ts−1 | Ts−2 | ΔHs | Tm−2–Ts−2 | |
| (°C) | (°C) | (J/g) | (J/g) | (°C) | (°C) | (J/g) | (°C) | |
| Paraffin/EG-50 (1.1) | 34 | 50.3 | 170.66 | 172.42 | 28.9 | 45.4 | 171.11 | 5 |
| Paraffin/EG-80 (1.1) | 34.2 | 50.5 | 169.09 | 172.42 | 28.7 | 45 | 170.39 | 5.3 |
| Paraffin/EG-100 (1.1) | 34.3 | 50.7 | 143.63 | 172.42 | 28.1 | 45.1 | 145.4 | 5.5 |
| paraffin | 33.8 | 50 | 188.1 | - | 28 | 44.1 | 183.71 | 6.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, L.; Wu, J.; Sun, W.; Cai, W. Effects of Pore Structure and Pore Size of Expanded Graphite on the Properties of Paraffin Wax/Expanded Graphite Composite Phase Change Materials. Energies 2022, 15, 4201. https://doi.org/10.3390/en15124201
Feng L, Wu J, Sun W, Cai W. Effects of Pore Structure and Pore Size of Expanded Graphite on the Properties of Paraffin Wax/Expanded Graphite Composite Phase Change Materials. Energies. 2022; 15(12):4201. https://doi.org/10.3390/en15124201
Chicago/Turabian StyleFeng, Lipin, Jian Wu, Wenjuan Sun, and Weimeng Cai. 2022. "Effects of Pore Structure and Pore Size of Expanded Graphite on the Properties of Paraffin Wax/Expanded Graphite Composite Phase Change Materials" Energies 15, no. 12: 4201. https://doi.org/10.3390/en15124201
APA StyleFeng, L., Wu, J., Sun, W., & Cai, W. (2022). Effects of Pore Structure and Pore Size of Expanded Graphite on the Properties of Paraffin Wax/Expanded Graphite Composite Phase Change Materials. Energies, 15(12), 4201. https://doi.org/10.3390/en15124201





