Development of Sustainable Biorefinery Processes Applying Deep Eutectic Solvents to Agrofood Wastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lignocellulosic Biomass
2.2. Chemicals
2.3. Synthesis of Deep Eutectic Solvent (DES)
2.4. DES Pretreatment
2.5. Lignocellulosic Analysis
2.6. Enzymatic Saccharification
2.7. Statistical Analysis
3. Results and Discussion
3.1. Compositional Analysis
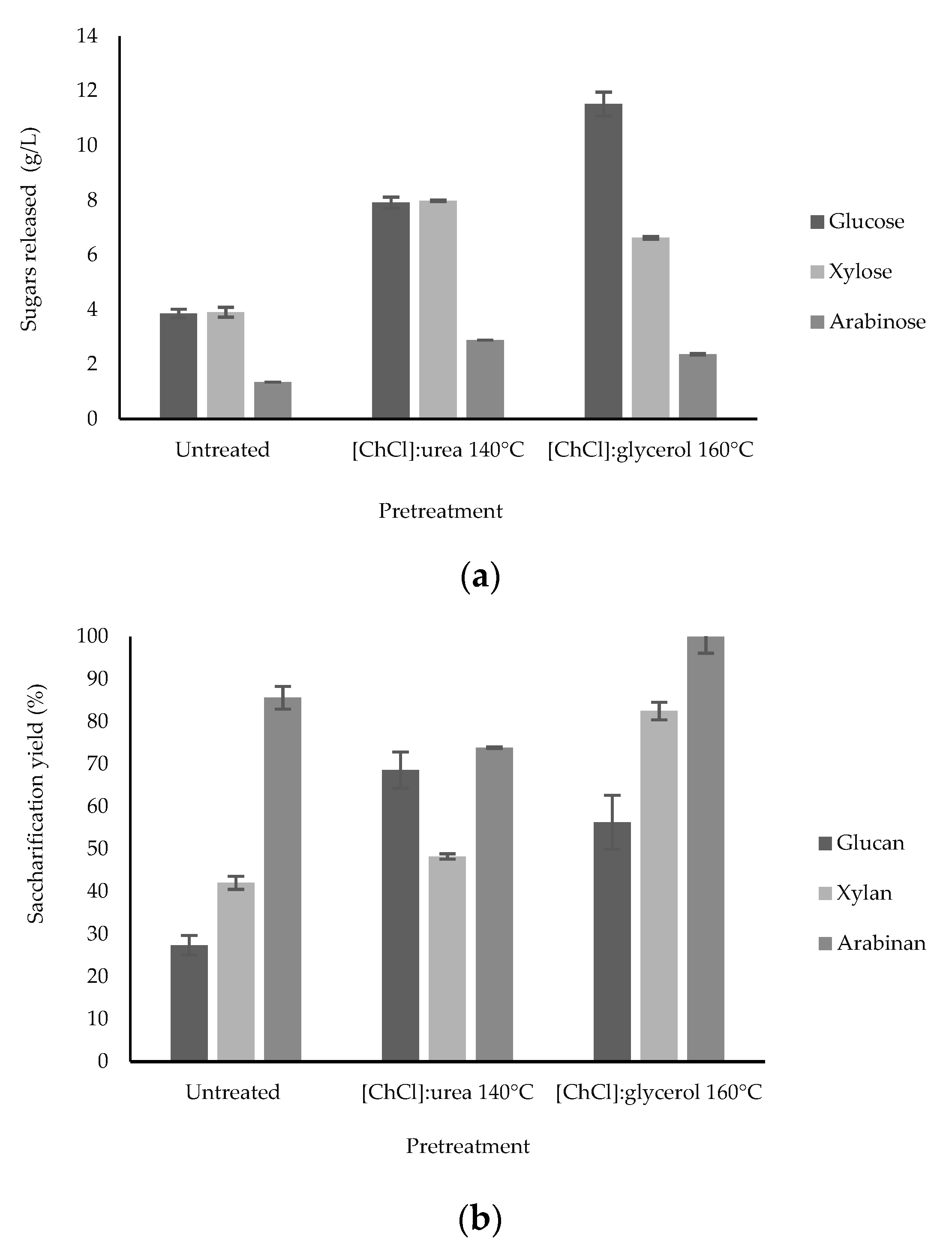
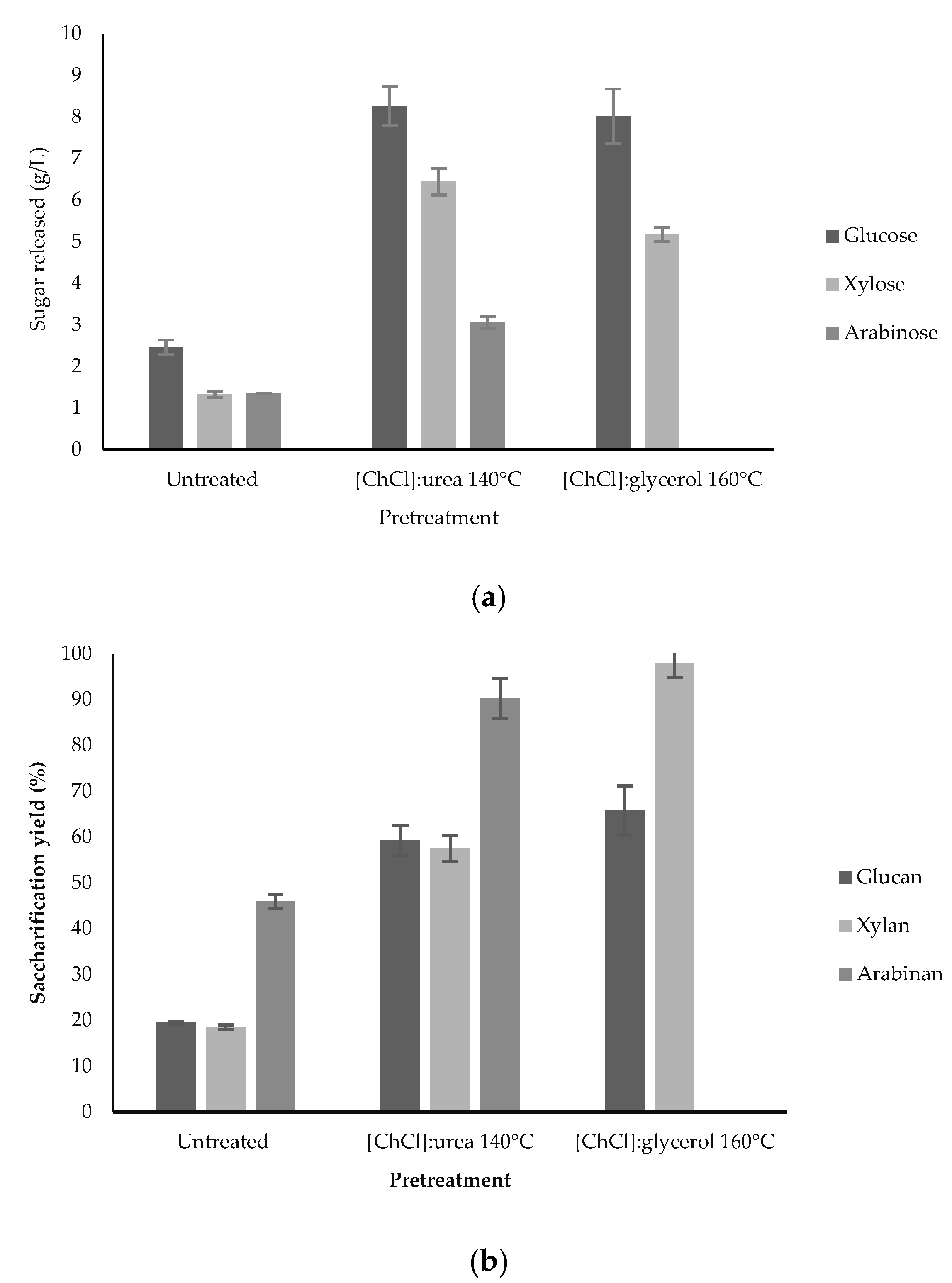
3.2. Effect of Enzymatic Digestibility in SCB and BSG after DES Pretreatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Xie, J.; Chen, J.; Cheng, Z.; Zhu, S.; Xu, J. Pretreatment of Pine Lignocelluloses by Recyclable Deep Eutectic Solvent for Elevated Enzymatic Saccharification and Lignin Nanoparticles Extraction. Carbohydr. Polym. 2021, 269, 118321. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.C.; Ding, J.C.; Han, R.Z.; Dong, J.J.; Ni, Y. Enhancing Cellulose Accessibility of Corn Stover by Deep Eutectic Solvent Pretreatment for Butanol Fermentation. Bioresour. Technol. 2016, 203, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.J. Lignocellulosic Biomass Pretreatment by Deep Eutectic Solvents on Lignin Extraction and Saccharification Enhancement: A Review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef] [PubMed]
- Haldar, D.; Purkait, M.K. A Review on the Environment-Friendly Emerging Techniques for Pretreatment of Lignocellulosic Biomass: Mechanistic Insight and Advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef]
- Aliyu, S.; Bala, M. Brewer’s Spent Grain: A Review of Its Potentials and Applications. Afr. J. Biotechnol. 2011, 10, 324–331. [Google Scholar]
- Mussatto, S.I. Brewer’s Spent Grain: A Valuable Feedstock for Industrial Applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [Green Version]
- Chandel, A.K.; da Silva, S.S.; Carvalho, W.; Singh, O.V. Sugarcane Bagasse and Leaves: Foreseeable Biomass of Biofuel and Bio-Products. J. Chem. Technol. Biotechnol. 2012, 87, 11–20. [Google Scholar] [CrossRef]
- Salmon, D.N.X.; Spier, M.R.; Soccol, C.R.; de Souza Vandenberghe, L.P.; Weingartner Montibeller, V.; Bier, M.C.J.; Faraco, V. Analysis of Inducers of Xylanase and Cellulase Activities Production by Ganoderma Applanatum LPB MR-56. Fungal Biol. 2014, 118, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Outeiriño, D.; Costa-Trigo, I.; Rodríguez, A.; Pérez Guerra, N.; Domínguez, J.M. Recovery and Reuse of Ionic Liquid Cholinium Glycinate in the Treatment of Brewery Spent Grain. Sep. Purif. Technol. 2021, 254, 117651. [Google Scholar] [CrossRef]
- Morán-Aguilar, M.G.; Santoyo, M.C.; Aguilar, J.M.D.M.G. Optimization of Cellulase and Xylanase Production by Aspergillus niger CECT 2700 Using Brewery Spent Grain Based on Taguchi Design. Biomass Convers. Biorefinery 2021, 1–9. [Google Scholar] [CrossRef]
- Morán-Aguilar, M.G.; Costa-Trigo, I.; Calderón-Santoyo, M.; Domínguez, J.M.; Aguilar-Uscanga, M.G. Production of Cellulases and Xylanases in Solid-State Fermentation by Different Strains of Aspergillus niger Using Sugarcane Bagasse and Brewery Spent Grain. Biochem. Eng. J. 2021, 172, 108060. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Duarah, P.; Haldar, D.; Purkait, M.K. Technological Advancement in the Synthesis and Applications of Lignin-Based Nanoparticles Derived from Agro-Industrial Waste Residues: A Review. Int. J. Biol. Macromol. 2020, 163, 1828–1843. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Zhang, J.; Zhou, J.; Shi, X.; Lv, C.; Geng, Z. A Subcritical Pretreatment Improved by Self-Produced Organic Acids to Increase Xylose Yield. Fuel Process. Technol. 2019, 195, 106148. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Monteagudo-Arrebola, M.J.; Dobado, J.A.; Isac-García, J. Green and Bio-Based Solvents. Top. Curr. Chem. 2018, 376, 1–40. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Spychaj, T. Deep Eutectic Solvents for Polysaccharides Processing. A Review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Xia, Q.; Guo, B.; Wang, Q.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Efficient Cleavage of Lignin–Carbohydrate Complexes and Ultrafast Extraction of Lignin Oligomers from Wood Biomass by Microwave-Assisted Treatment with Deep Eutectic Solvent. ChemSusChem 2017, 10, 1692–1700. [Google Scholar] [CrossRef] [Green Version]
- Lyu, G.; Li, T.; Ji, X.; Yang, G.; Liu, Y.; Lucia, L.A.; Chen, J. Characterization of Lignin Extracted from Willow by Deep Eutectic Solvent Treatments. Polymers 2018, 10, 869. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Kim, K.H.; Jeong, K.; Kim, N.; Yoo, C.G. Sustainable Biorefinery Processes Using Renewable Deep Eutectic Solvents. Green Sustain. Chem. 2021, 27, 100396. [Google Scholar] [CrossRef]
- Yiin, C.L.; Quitain, A.T.; Yusup, S.; Uemura, Y.; Sasaki, M.; Kida, T. Sustainable Green Pretreatment Approach to Biomass-to-Energy Conversion Using Natural Hydro-Low-Transition-Temperature Mixtures. Bioresour. Technol. 2018, 261, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Loow, Y.-L.; Wu, T.Y.; Yang, G.H.; Ang, L.Y.; New, E.K.; Siow, L.F.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Deep Eutectic Solvent and Inorganic Salt Pretreatment of Lignocellulosic Biomass for Improving Xylose Recovery. Bioresour. Technol. 2018, 249, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bai, X.; Lusi, A.; Jacoby, W.A.; Wan, C. One-Pot Selective Conversion of Lignocellulosic Biomass into Furfural and Co-Products Using Aqueous Choline Chloride/Methyl Isobutyl Ketone Biphasic Solvent System. Bioresour. Technol. 2019, 289, 121708. [Google Scholar] [CrossRef] [PubMed]
- Morais, A.R.C.; Pinto, J.V.; Nunes, D.; Roseiro, L.B.; Oliveira, M.C.; Fortunato, E.; Bogel-Łukasik, R. Imidazole: Prospect Solvent for Lignocellulosic Biomass Fractionation and Delignification. ACS Sustain. Chem. Eng. 2016, 4, 1643–1652. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Xiao, L.P.; Song, G. Unraveling the Structural Transformation of Wood Lignin During Deep Eutectic Solvent Treatment. Front. Energy Res. 2020, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Bubalo, M.C.; Curko, N.; Tomaševic, M.; Ganic, K.K.; Redovnikovic, I.R. Green Extraction of Grape Skin Phenolics by Using Deep Eutectic Solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- New, E.K.; Wu, T.Y.; Tien Loong Lee, C.B.; Poon, Z.Y.; Loow, Y.L.; Wei Foo, L.Y.; Procentese, A.; Siow, L.F.; Teoh, W.H.; Nik Daud, N.N.; et al. Potential Use of Pure and Diluted Choline Chloride-Based Deep Eutectic Solvent in Delignification of Oil Palm Fronds. Process Saf. Environ. Prot. 2019, 123, 190–198. [Google Scholar] [CrossRef]
- Loow, Y.; Wu, T.Y.; Tan, K.A.; Lim, Y.S.; Fong, L. Recent Advances in Application of Inorganic Salt Pretreatment for Transforming Lignocellulosic Biomass into Reducing Sugars. J. Agric. Food Chem. 2015, 63, 8349–8363. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H. Conversion of Xylan and Xylose into Furfural in Biorenewable Deep Eutectic Solvent with Trivalent Metal Chloride Added. BioResources 2013, 8, 6014–6025. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.; Verpoorte, R.; Choi, Y.H. Tailoring Properties of Natural Deep Eutectic Solvents with Water to Facilitate Their Applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Wang, Z.K.; Hong, S.; Wen, J.L.; Ma, C.Y.; Tang, L.; Jiang, H.; Chen, J.J.; Li, S.; Shen, X.J.; Yuan, T.Q. Lewis Acid-Facilitated Deep Eutectic Solvent (DES) Pretreatment for Producing High-Purity and Antioxidative Lignin. ACS Sustain. Chem. Eng. 2020, 8, 1050–1057. [Google Scholar] [CrossRef]
- Chen, Z.; Reznicek, W.D.; Wan, C. Deep Eutectic Solvent Pretreatment Enabling Full Utilization of Switchgrass. Bioresour. Technol. 2018, 263, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, B.C.; Nam, K.; Choi, Y. Effect of Pretreatment Solutions and Conditions on Decomposition and Anaerobic Digestion of Lignocellulosic Biomass in Rice Straw. Biochem. Eng. J. 2018, 140, 108–114. [Google Scholar] [CrossRef]
- Shen, X.J.; Wen, J.L.; Mei, Q.Q.; Chen, X.; Sun, D.; Yuan, T.Q.; Sun, R.C. Facile Fractionation of Lignocelluloses by Biomass-Derived Deep Eutectic Solvent (DES) Pretreatment for Cellulose Enzymatic Hydrolysis and Lignin Valorization. Green Chem. 2019, 21, 275–283. [Google Scholar] [CrossRef]
- Procentese, A.; Johnson, E.; Orr, V.; Garruto Campanile, A.; Wood, J.A.; Marzocchella, A.; Rehmann, L. Deep Eutectic Solvent Pretreatment and Subsequent Saccharification of Corncob. Bioresour. Technol. 2015, 192, 31–36. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Biomass Anal. Technol. Team Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Chourasia, V.R.; Pandey, A.; Kishore, K.; Henry, R.J. Improving Enzymatic Digestibility of Sugarcane Bagasse from Different Varieties of Sugarcane Using Deep Eutectic Solvent Pretreatment. Bioresour. Technol. 2021, 337, 125480. [Google Scholar] [CrossRef]
- Hou, X.; Feng, G.; Ye, M.; Huang, C.; Zhang, Y. Significantly Enhanced Enzymatic Hydrolysis of Rice Straw via a High- Performance Two-Stage Deep Eutectic Solvents Synergistic Pretreatment. Bioresour. Technol. 2017, 238, 139–146. [Google Scholar] [CrossRef]
- Bustos, G.; Moldes, A.B.; Cruz, J.M. Revalorization of Hemicellulosic Trimming Vine Shoots Hydrolyzates Trough Continuous Production of Lactic Acid and Biosurfactants by L. pentosus. J. Food Eng. 2007, 78, 405–412. [Google Scholar] [CrossRef]
- Templeton, D.W.; Wolfrum, E.J.; Yen, J.H.; Sharpless, K.E.; Templeton, D.W. Compositional Analysis of Biomass Reference Materials: Results from an Interlaboratory Study. Bioenergy Res. 2016, 9, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yu, L.; Wu, Z. Delignification of Poplar Wood with Lactic-Based Deep Eutectic Solvents. Wood Res. 2019, 64, 499–514. [Google Scholar]
- Tian, D.; Guo, Y.; Hu, J.; Yang, G.; Zhang, J.; Luo, L.; Xiao, Y. Acidic Deep Eutectic Solvents Pretreatment for Selective Lignocellulosic Biomass Fractionation with Enhanced Cellulose Reactivity. Int. J. Biol. Macromol. 2020, 142, 288–297. [Google Scholar] [CrossRef]
- Shafie, M.H.; Yusof, R.; Gan, C. Synthesis of Citric Acid Monohydrate-Choline Chloride Based Deep Eutectic Solvents (DES) and Characterization of Their Physicochemical Properties. J. Mol. Liq. 2019, 5, 171667. [Google Scholar] [CrossRef]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2014, 10, 2416–2425. [Google Scholar] [CrossRef]
- Du, C.; Zhao, B.; Chen, X.; Birbilis, N.; Yang, H. Effect of Water Presence on Choline Chloride-2urea Ionic Liquid and Coating Platings from the Hydrated Ionic Liquid. Sci. Rep. 2016, 6, 29225. [Google Scholar] [CrossRef] [Green Version]
- Teles, A.R.R.; Capela, E.V.; Carmo, R.S.; Coutinho, J.A.P.; Silvestre, A.J.D.; Freire, M.G. Solvatochromic Parameters of Deep Eutectic Solvents Formed by Ammonium-Based Salts and Carboxylic Acids. Fluid Phase Equilib. 2017, 448, 15–21. [Google Scholar] [CrossRef]
- Soares, B.; Tavares, D.J.P.; Amaral, J.L.; Silvestre, A.J.D.; Freire, C.S.R.; Coutinho, J.A.P. Enhanced Solubility of Lignin Monomeric Model Compounds and Technical Lignins in Aqueous Solutions of Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 4056–4065. [Google Scholar] [CrossRef]
- Xu, H.; Peng, J.; Kong, Y.; Liu, Y.; Su, Z.; Li, B.; Song, X.; Liu, S.; Tian, W. Key Process Parameters for Deep Eutectic Solvents Pretreatment of Lignocellulosic Biomass Materials: A Review. Bioresour. Technol. 2020, 310, 123416. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Li, P.; Zhang, K.; Lian, H.; Liimatainen, H. Enhancement of the Nanofibrillation of Birch Cellulose Pretreated with Natural Deep Eutectic Solvent. Ind. Crops Prod. 2020, 154, 112677. [Google Scholar] [CrossRef]
- Kohli, K.; Katuwal, S.; Biswas, A.; Sharma, B.K. Effective Delignification of Lignocellulosic Biomass by Microwave Assisted Deep Eutectic Solvents. Bioresour. Technol. 2020, 303, 122897. [Google Scholar] [CrossRef]
- Guo, H.; Chang, Y.; Lee, D.J. Enzymatic Saccharification of Lignocellulosic Biorefinery: Research Focuses. Bioresour. Technol. 2018, 252, 198–215. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yu, J.; Lu, Y.; Jiang, B.; Fan, Y.; Wang, Z. High-Purity Lignin Isolated from Poplar Wood Meal through Dissolving Treatment with Deep Eutectic Solvents. R. Soc. Open Sci. 2019, 6, 181757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Huang, H.; Zhang, H.; Zhang, L.; Yan, L.; Chen, J. Ball Milling Pretreatment of Corn Stover for Enhancing the Efficiency of Enzymatic Hydrolysis. Appl. Biochem. Biotechnol. 2010, 162, 1872–1880. [Google Scholar] [CrossRef]
- Procentese, A.; Rehmann, L. Fermentable Sugar Production from a Coffee Processing By-Product after Deep Eutectic Solvent Pretreatment. Bioresour. Technol. Rep. 2018, 4, 174–180. [Google Scholar] [CrossRef]
- Thi, S.; Lee, K.M. Comparison of Deep Eutectic Solvents (DES) on Pretreatment of Oil Palm Empty Fruit Bunch (OPEFB): Cellulose Digestibility, Structural and Morphology Changes. Bioresour. Technol. 2019, 282, 525–529. [Google Scholar] [CrossRef]
- Su, Y.; Huang, C.; Lai, C.; Yong, Q. Green Solvent Pretreatment for Enhanced Production of Sugars and Antioxidative Lignin from Poplar. Bioresour. Technol. 2021, 321, 124471. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiong, L.; Chen, X.; Li, H.; Qi, G.; Huang, C.; Luo, M.; Chen, X. Enhanced Enzymatic Hydrolysis and Acetone-Butanol-Ethanol Fermentation of Sugarcane Bagasse by Combined Diluted Acid with Oxidate Ammonolysis Pretreatment. Bioresour. Technol. 2017, 228, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Park, S.; Jung, D.; Oh, K.K.; Lee, S.H. Effect of Hydrogen Bond Donor on the Choline Chloride-Based Deep Eutectic Solvent-Mediated Extraction of Lignin from Pine Wood. Int. J. Biol. Macromol. 2020, 165, 187–197. [Google Scholar] [CrossRef]
- Zhao, J.; Meng, Z.; Zhao, Z.; Zhao, L. Ultrasound-Assisted Deep Eutectic Solvent as Green and Efficient Media Combined with Functionalized Magnetic Multi-Walled Carbon Nanotubes as Solid-Phase Extraction to Determine Pesticide Residues in Food Products. Food Chem. 2020, 310, 125863. [Google Scholar] [CrossRef]
- Xu, H.; Kong, Y.; Peng, J.; Song, X.; Liu, Y.; Su, Z.; Li, B. Comprehensive Analysis of Important Parameters of Choline Chloride-Based Deep Eutectic Solvent Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2021, 319, 124209. [Google Scholar] [CrossRef]
- Sai, Y.W.; Lee, K.M. Enhanced Cellulase Accessibility Using Acid-Based Deep Eutectic Solvent in Pretreatment of Empty Fruit Bunches. Cellulose 2019, 26, 9517–9528. [Google Scholar] [CrossRef]
| Conventional Type | Pretreatment Methods | Advantages | Disadvantages |
|---|---|---|---|
| Biological |
|
|
|
| Physical |
|
|
|
| Chemical |
|
|
|
| Physicochemical |
|
|
| Composition (%) | Lignin (%) | Lignin Remotion (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Biomass | DES | Temperature | Glucan | Xylan | Arabinan | ASL | KL | ASL * | KL * |
| SCB | Untreated | - | 41.00 ± 1.53 | 27.80 ± 0.89 | 2.49 ± 1.78 | 3.07 ± 0.05 | 19.14 ± 1.23 | - | - |
| [ChCl]:U | 140 °C | 35.52 ± 2.58 | 31.99 ± 1.05 | 10.32 ± 1.69 | 2.80 ± 0.35 | 17.69 ± 1.25 | 2.80 ± 0.08 | 7.57 ± 2.21 | |
| [ChCl]:G | 160 °C | 53.58 ± 5.78 | 19.97 ± 1.52 | 5.56 ± 0.78 | 0.75 ± 0.02 | 16.29 ± 2.45 | 75.57 ± 1.89 | 14.89 ± 3.65 | |
| BSG | Untreated | - | 35.73 ± 0.25 | 17.06 ± 1.54 | 7.84 ± 2.54 | 6.37 ± 0.45 | 18.75 ± 1.23 | - | - |
| [ChCl]:U | 140 °C | 37.67 ± 1.47 | 29.53 ± 2.54 | 8.95 ± 0.69 | 2.89 ± 0.04 | 15.60 ± 3.21 | 54.63 ± 1.12 | 16.80 ± 3.25 | |
| [ChCl]:G | 160 °C | 32.91 ± 2.85 | 13.94 ± 3.54 | 0.00 ± 0.00 | 1.32 ± 0.07 | 46.44 ± 5.35 | 79.27 ± 4.35 | - | |
| DES | Biomass | Operating Conditions MR (mol/mol)—T (°C)—SLR (w/w)—t (h) | Saccharification Yield (%) | References |
|---|---|---|---|---|
| [ChCl]:glycerol | Corncob | 1:2—150—1:16—15 | 91.5 glucan 95.5 xylan | [35] |
| [ChCl]:urea | 58.6 glucan 31.2 xylan | |||
| [ChCl]:glycerol | Switchgrass | 1:2—120—1:10—1 | 79.9 glucan | [32] |
| [ChCl]:glycerol | Brewery spent grain | 1:2—150—1:32—3 | 94 glucan | [54] |
| [ChCl]:lactic acid | Oil palm empty fruit bunch | 1:2—120—1:10—3 | 20.7 glucan and xylan mainly | [55] |
| [ChCl]:urea | 20 glucan and xylan mainly | |||
| [ChCl]:glycerol | 16.9 glucan and xylan mainly | |||
| [ChCl]:glycerol | Sugarcane bagasse | 1:2—80—1:20—12 | 95.84 glucan | [37] |
| [ChCl]:lactic acid | Poplar sawdust | 1:2—130—NR—1.5 | 75.8 glucan | [56] |
| [ChCl]:glycerol | Sugarcane bagasse | 1:2—160—1:16—15 | 56.30 glucan 82.47 xylan 100 arabinan | Present study |
| [ChCl]:urea | 1:2—140—1:16—15 | 56.67 glucan 65.37 xylan 73.87 arabinan | ||
| [ChCl]:glycerol | Brewery spent grain | 1:2—160—1:16—15 | 65.77 glucan 97.96 xylan | |
| [ChCl]:urea | 1:2—140—1:16—15 | 59.22 glucan 57.60 xylan 90.21 arabinan |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morán-Aguilar, M.G.; Costa-Trigo, I.; Ramírez-Pérez, A.M.; de Blas, E.; Calderón-Santoyo, M.; Aguilar-Uscanga, M.G.; Domínguez, J.M. Development of Sustainable Biorefinery Processes Applying Deep Eutectic Solvents to Agrofood Wastes. Energies 2022, 15, 4101. https://doi.org/10.3390/en15114101
Morán-Aguilar MG, Costa-Trigo I, Ramírez-Pérez AM, de Blas E, Calderón-Santoyo M, Aguilar-Uscanga MG, Domínguez JM. Development of Sustainable Biorefinery Processes Applying Deep Eutectic Solvents to Agrofood Wastes. Energies. 2022; 15(11):4101. https://doi.org/10.3390/en15114101
Chicago/Turabian StyleMorán-Aguilar, María Guadalupe, Iván Costa-Trigo, Alexandra María Ramírez-Pérez, Esther de Blas, Montserrat Calderón-Santoyo, María Guadalupe Aguilar-Uscanga, and José Manuel Domínguez. 2022. "Development of Sustainable Biorefinery Processes Applying Deep Eutectic Solvents to Agrofood Wastes" Energies 15, no. 11: 4101. https://doi.org/10.3390/en15114101
APA StyleMorán-Aguilar, M. G., Costa-Trigo, I., Ramírez-Pérez, A. M., de Blas, E., Calderón-Santoyo, M., Aguilar-Uscanga, M. G., & Domínguez, J. M. (2022). Development of Sustainable Biorefinery Processes Applying Deep Eutectic Solvents to Agrofood Wastes. Energies, 15(11), 4101. https://doi.org/10.3390/en15114101






