Preparation of a Honeycomb-like FeNi(OH/P) Nanosheet Array as a High-Performance Cathode for Hybrid Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
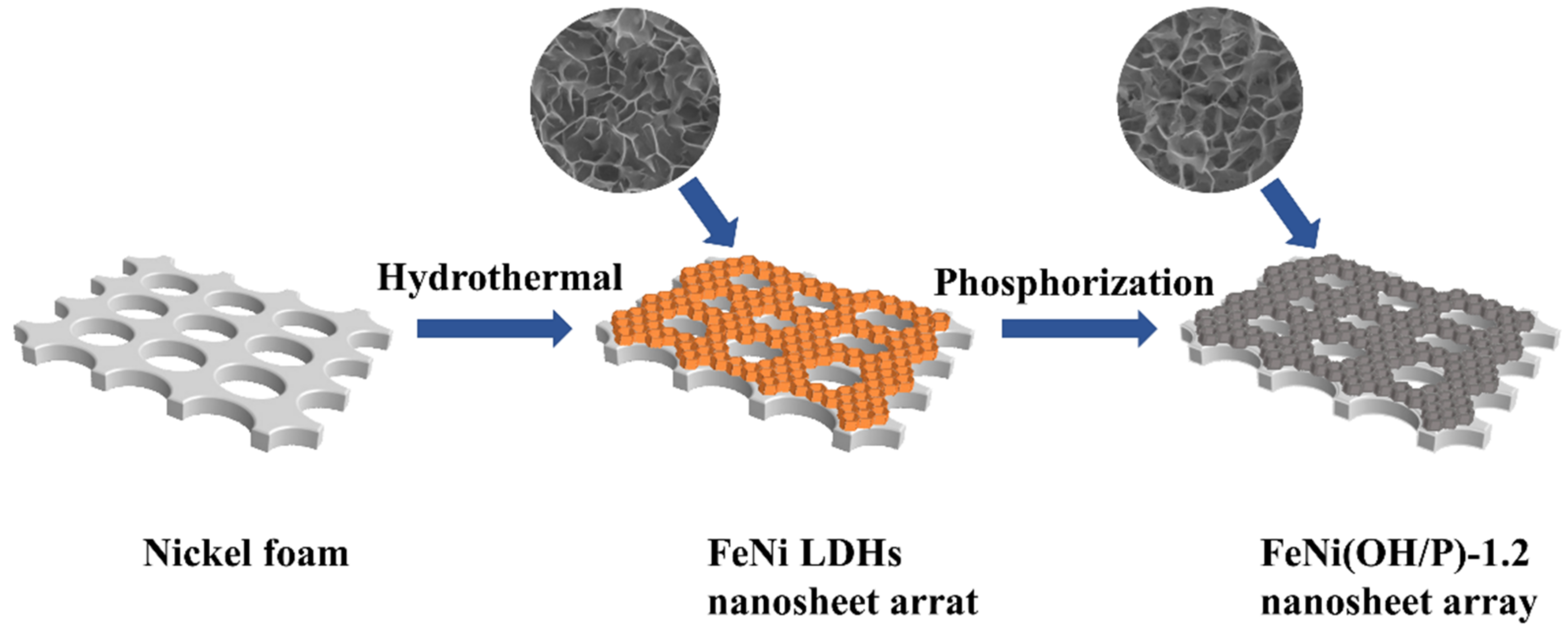
2.2. Synthesis of Materials
2.2.1. Synthesis of FeNi LDH’s Precursor
2.2.2. Synthesis of the FeNi(OH/P) Sheet Array Electrode
2.2.3. Assembly of Hybrid Supercapacitor Device
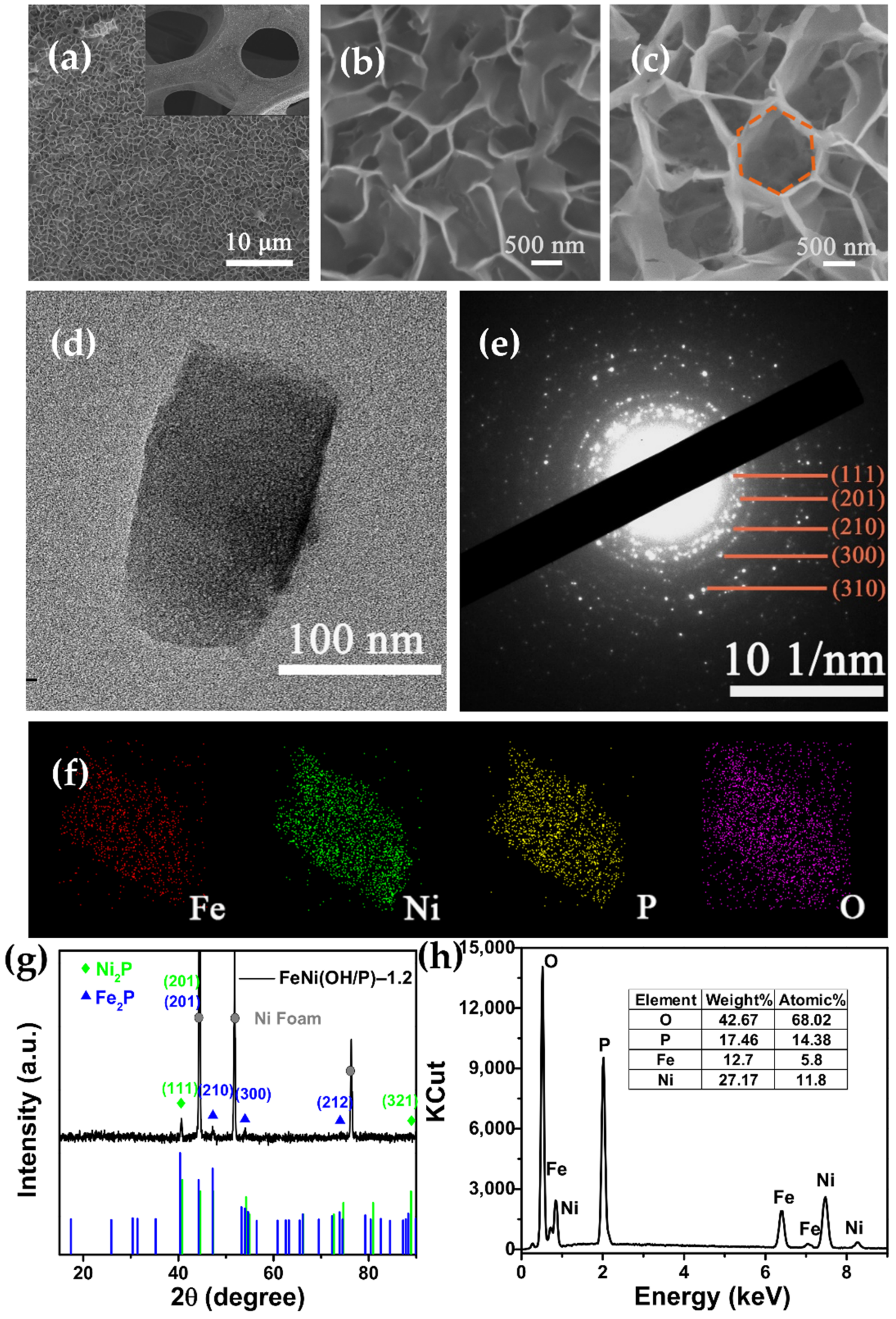
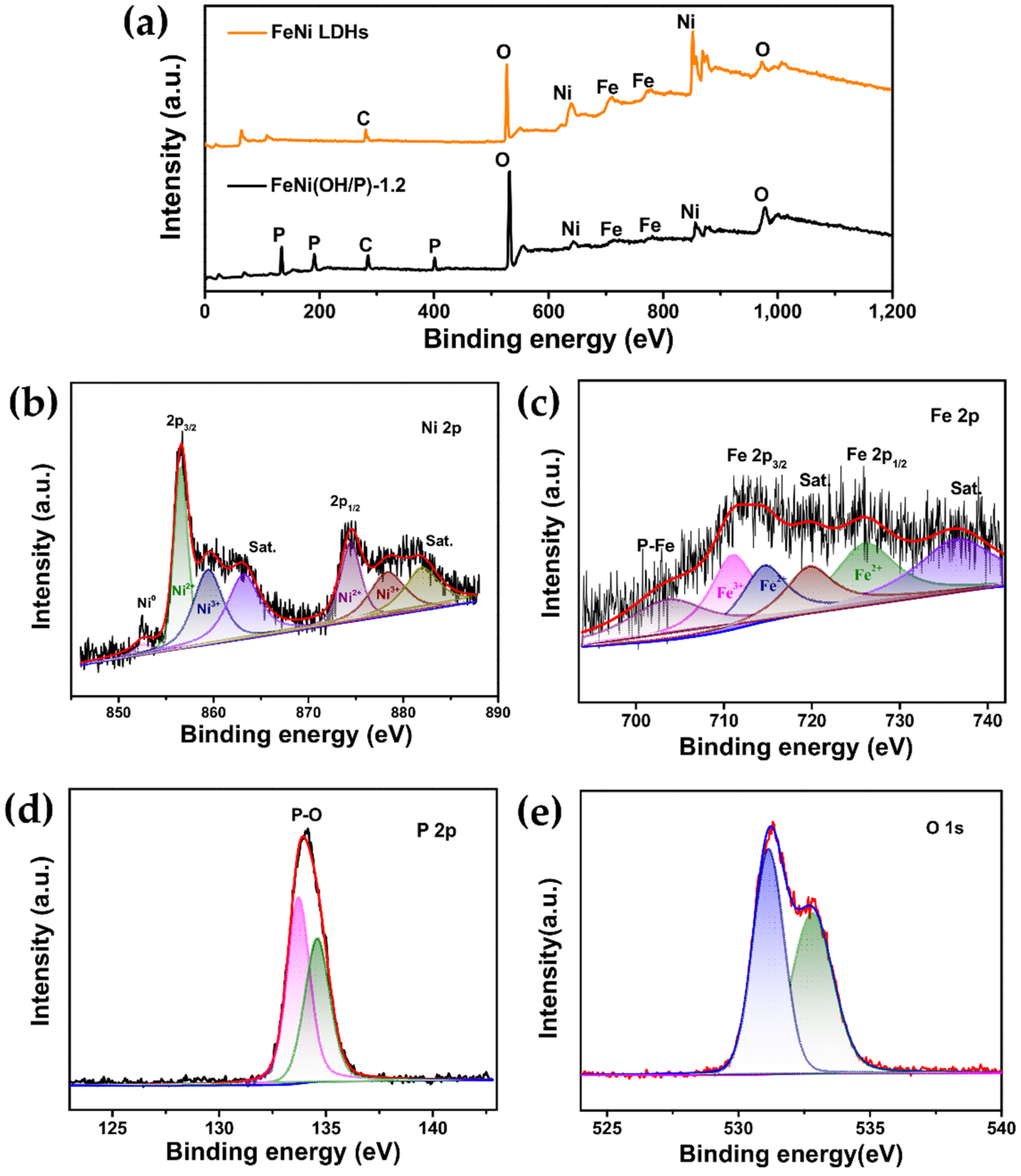
2.3. Morphology and Structure Characterization
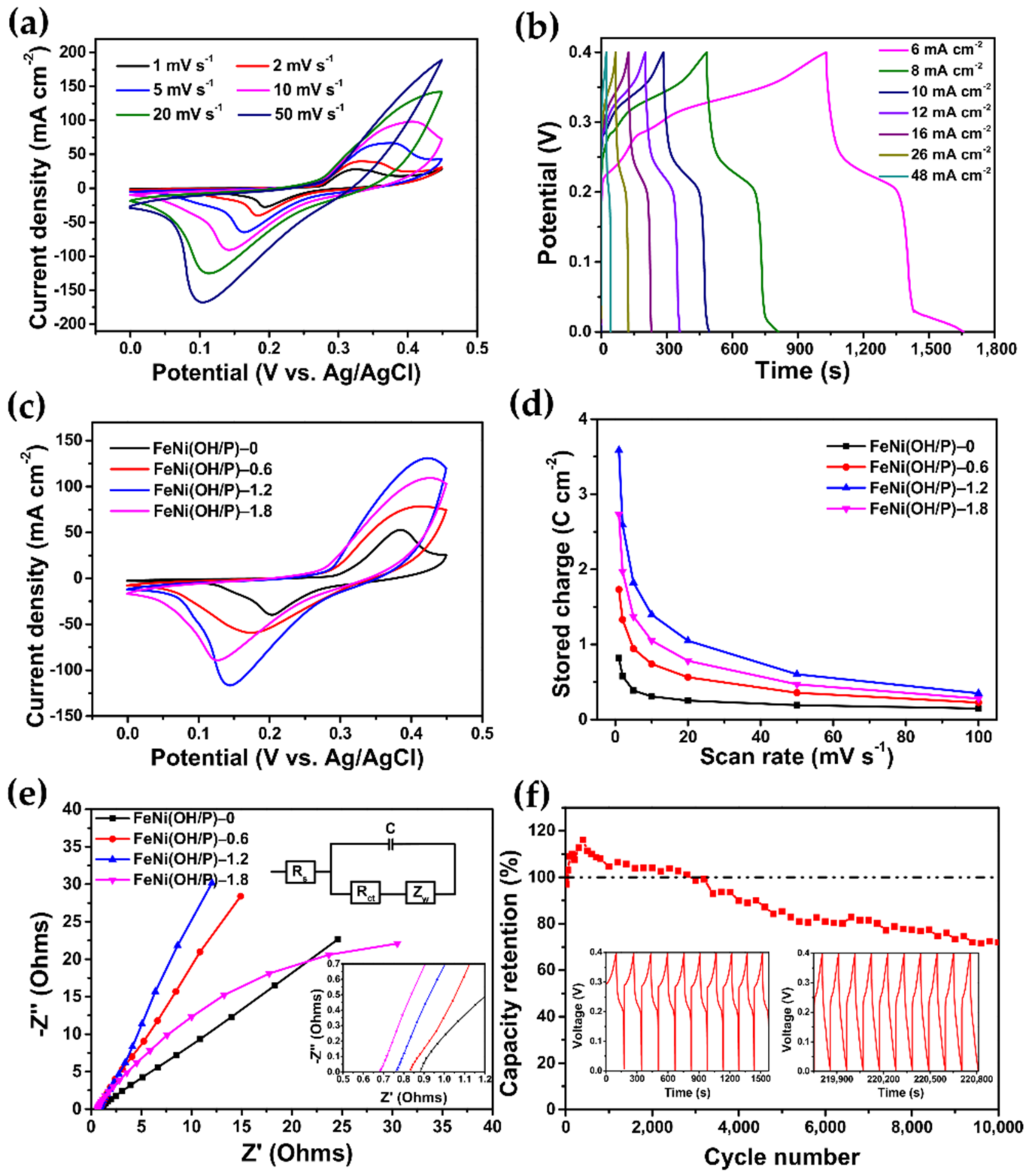
2.4. Electrochemical Measurements
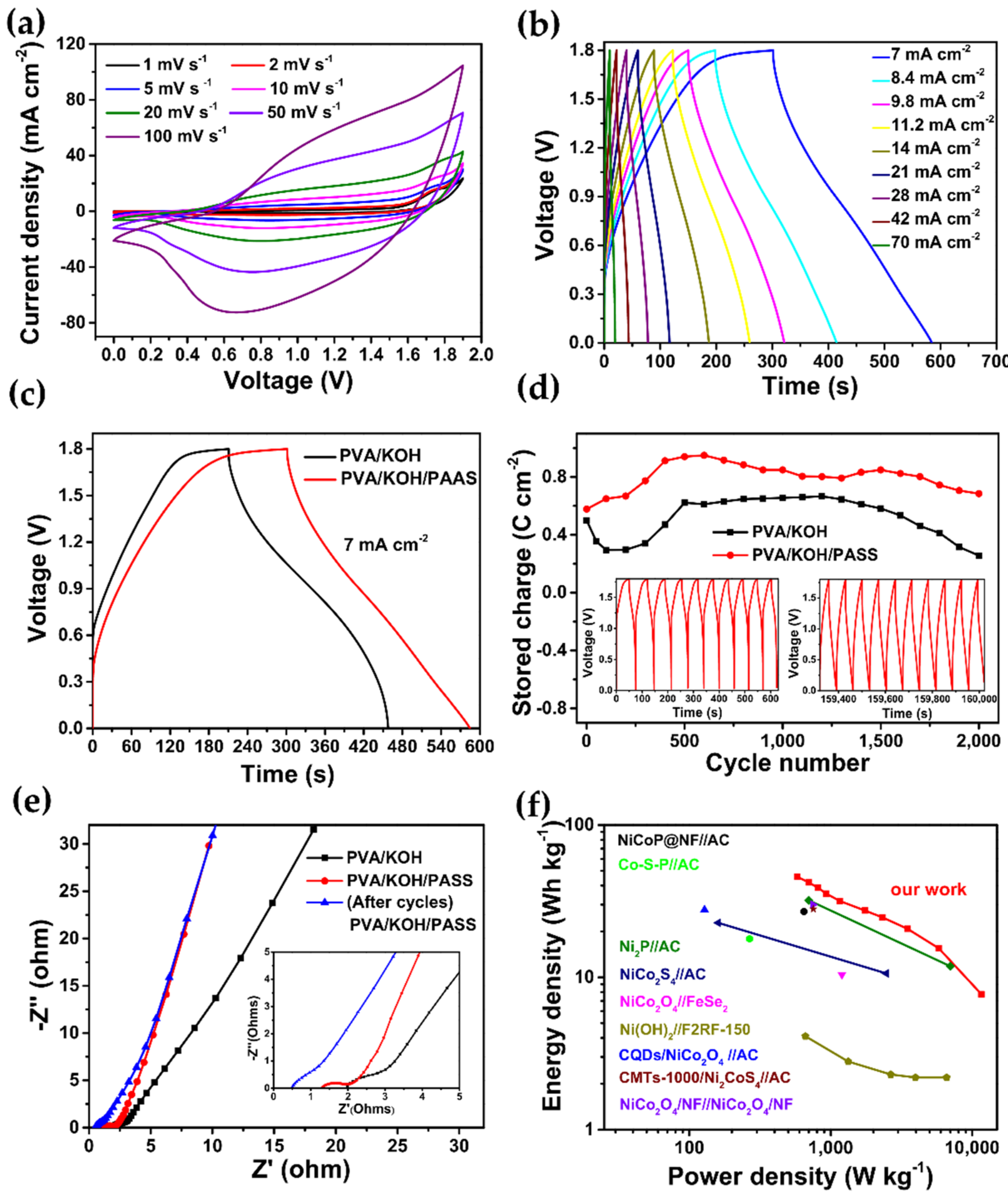
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gui, Q.; Ba, D.; Li, L.; Liu, W.; Li, Y.; Liu, J. Recent advances in materials and device technologies for aqueous hybrid supercapacitors. Sci. China Mater. 2022, 65, 10–31. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-Supercapacitor hybrid devices: Cecent progress and future prospects. Adv. Sci. 2017, 7, 1600539. [Google Scholar] [CrossRef] [PubMed]
- Theerthagiri, J.; Murthy, A.P.; Lee, S.J.; Karuppasamy, K.; Arumugam, S.R.; Yu, Y.; Hanafiah, M.M.; Kim, H.; Mittal, V.; Choi, M.Y. Recent progress on synthetic strategies and applications of transition metal phosphides in energy storage and conversion. Ceram. Int. 2020, 47, 4404–4425. [Google Scholar] [CrossRef]
- Zong, Q.; Liu, C.; Yang, H.; Zhang, Q.; Cao, G. Tailoring nanostructured transition metal phosphides for high-performance hybrid supercapacitors. Nano Today 2021, 38, 101201. [Google Scholar] [CrossRef]
- Oyama, S.T.; Clark, P.; Wang, X.; Shido, T.; Iwasawa, Y.; Hayashi, S.; Ramallo-López, J.M.; Requejo, F.G. Structural characterization of tungsten phosphide (WP) hydrotreating catalysts by X-ray absorption spectroscopy and nuclear magnetic resonance spectroscopy. J. Phys. Chem. B 2002, 106, 1913–1920. [Google Scholar] [CrossRef]
- Agarwal, A.; Sankapal, B.R. Metal phosphides: Topical advances in the design of supercapacitors. J. Mater. Chem. A 2021, 9, 20241–20276. [Google Scholar] [CrossRef]
- Sun, M.; Liu, H.; Qu, J.; Li, J. Earth-Rich transition metal phosphide for energy conversion and storage. Adv. Energy Mater. 2016, 6, 1600087. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, B.; Fang, L.; Ling, F.; Wu, F.; Hu, B.; Meng, F.; Niu, K.; Lin, F.; Zheng, H. An investigation of ultrathin nickel-iron layered double hydroxide nanosheets grown on nickel foam for high-performance supercapacitor electrodes. J. Alloys Compd. 2017, 714, 63–70. [Google Scholar] [CrossRef]
- Li, M.; Jijie, R.; Barras, A.; Roussel, P.; Szunerits, S.; Boukherroub, R. NiFe layered double hydroxide electrodeposited on Ni foam coated with reduced graphene oxide for high-performance supercapacitors. Electrochim. Acta 2019, 302, 1–9. [Google Scholar] [CrossRef]
- Chen, C.; Dong, C.; Wang, B.; Huang, J.; Wang, Y. Synthesis and room temperature ferromagnetism in Fe-doped CuAlO2 semiconductor. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2013, 28, 500–503. [Google Scholar] [CrossRef]
- Varshney, D.; Kumar, A. Structural and optical properties of Ni substituted CaCu3Ti4-xNixO12. Opt. Int. J. Light Electron Opt. 2015, 126, 3437–3441. [Google Scholar] [CrossRef]
- Verma, S.; Arya, S.; Gupta, V.; Mahajan, S.; Furukawa, H.; Khosla, A. Performance analysis, challenges and future perspectives of nickel based nanostructured electrodes for electrochemical supercapacitors. J. Mater. Res. Technol. 2021, 11, 564–599. [Google Scholar] [CrossRef]
- Ma, L.; Sun, Y.; Guo, J. NiFeP nanocubes as advanced electrode material for hydrogen evolution and supercapacitor. Colloid Interface Sci. Commun. 2021, 45, 100520. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, B.; Li, D. Partial-sacrificial-template Synthesis of Fe/Ni Phosphides on Ni Foam: A Strongly Stabilized and Efficient Catalyst for Electrochemical Water Splitting. Electrochim. Acta 2017, 242, 260–267. [Google Scholar] [CrossRef]
- Liang, H.; Gandi, A.N.; Xia, C.; Hedhili, M.H.; Anjum, D.; Schwingenschlögl, U.N.; Alshareef, H. Amorphous NiFe-OH/NiFeP electrocatalyst fabricated at low temperature for water oxidation applications. ACS Energy Lett. 2017, 2, 1035–1042. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, K.; Si, Y.; Li, Z.; Jiang, L. High performance of multi-layered alternating Ni-Fe-P and Co-P films for hydrogen evolution. Green Energy Environ. 2020, 7, 75–85. [Google Scholar] [CrossRef]
- Baasanjav, E.; Bandyopadhyay, P.; Saeed, G.; Lim, S.; Jeong, S.M. Dual-ligand modulation approach for improving supercapacitive performance of hierarchical zinc–nickel–iron phosphide nanosheet-based electrode. J. Ind. Eng. Chem. 2021, 99, 299–308. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, Y.; Tang, Y.; Chi, L.; Xu, B. Synthesis and electrochemical properties of high-activity Fe2O3@Ni composite electrode. Inorg. Chem. Ind. 2019, 51, 24–27. [Google Scholar]
- Pan, C.; Liu, Z.; Huang, M. 2D iron-doped nickel MOF nanosheets grown on nickel foam for highly efficient oxygen evolution reaction. Appl. Surf. Sci. 2020, 529, 147201. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, B.; Zhou, Y. Three-dimensional polypyrrole derived N-doped carbon nanotube aerogel as a high performance metal free catalyst for oxygen reduction reaction. ChemCatChem 2019, 11, 5495–5504. [Google Scholar] [CrossRef]
- Zhou, K.; Zhou, W.; Yang, L.; Lu, J.; Cheng, S.; Mai, W.; Tang, Z.; Li, L.; Chen, S. Ultrahigh-performance pseudocapacitor electrodes based on transition metal phosphide nanosheets array via phosphorization: A general and effective approach. Adv. Funct. Mater. 2015, 25, 7530–7538. [Google Scholar] [CrossRef]
- Qu, M.; Jiang, Y.; Yang, M.; Liu, S.; Guo, Q.; Shen, W.; Li, M.; He, R. Regulating electron density of NiFe-P nanosheets electrocatalysts by a trifle of Ru for high-efficient overall water splitting. Appl. Catal. B Environ. 2020, 263, 118324. [Google Scholar] [CrossRef]
- Li, J.; Lian, R.; Wang, J.; He, S.; Rui, Z. Oxygen vacancy defects modulated electrocatalytic activity of iron-nickel layered double hydroxide on Ni foam as highly active electrodes for oxygen evolution reaction. Electrochim. Acta 2019, 331, 135395. [Google Scholar] [CrossRef]
- Luo, W.; Dahn, J.R. Preparation of Co1−zAlz(OH)2(NO3)z Layered Double Hydroxides and Li(Co1−zAlz)O2. Chem. Mater. 2009, 21, 56–62. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Penner, R.M. Energy Storage in Nanomaterials—Capacitive, Pseudocapacitive, or Battery-like? ACS Nano 2018, 12, 2081–2083. [Google Scholar] [CrossRef] [Green Version]
- Okhay, O.; Tkach, A. Graphene/Reduced Graphene Oxide-Carbon Nanotubes Composite Electrodes: From Capacitive to Battery-Type Behaviour. Nanomaterials 2021, 11, 1240. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, L.L.; Ji, H.; Li, Y.; Zhao, X.; Bai, X.; Fan, X.; Zhang, F.; Ruoff, R.S. Nanoporous Ni(OH)2 thin film on 3D ultrathin-graphite foam for asymmetric supercapacitor. Acs Nano 2013, 7, 6237–6243. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, M.; Ding, X.; Liu, Z.; Qiao, L. One-step colloid fabrication of nickel phosphides nanoplate/nickel foam hybrid electrode for high-performance asymmetric supercapacitors. Chem. Eng. J. 2019, 373, 1132–1143. [Google Scholar] [CrossRef]
- Wan, L.; Wang, Y.; Zhang, Y.; Du, C.; Xie, M. FeCoP nanosheets@Ni-Co carbonate hydroxide nanoneedles as free-standing electrode material for hybrid supercapacitors. Chem. Eng. J. 2021, 415, 128995. [Google Scholar] [CrossRef]
- Wan, L.; Chen, D.; Liu, J.; Zhang, Y.; Du, C. Construction of FeNiP@CoNi-layered double hydroxide hybrid nanosheets on carbon cloth for high energy asymmetric supercapacitors. J. Power Sources 2020, 465, 228293. [Google Scholar] [CrossRef]
- Wan, L.; He, C.; Chen, D.; Liu, J.; Chen, J. In situ grown NiFeP@NiCo2S4 nanosheet arrays on carbon cloth for asymmetric supercapacitors. Chem. Eng. J. 2020, 399, 125778. [Google Scholar] [CrossRef]
- Shuai, M.; Lin, J.; Wu, W.; Kuang, H.; Zhang, W.; Ling, Q.; Chen, H.; Komarneni, S. Metallic nickel–cobalt phosphide/multilayer graphene composite for high-performance supercapacitors. New J. Chem. 2020, 44, 8796–8804. [Google Scholar] [CrossRef]
- He, S.; Li, Z.; Mi, H.; Ji, C.; Qiu, J. 3D nickel-cobalt phosphide heterostructure for high-performance solid-state hybrid supercapacitors. J. Power Sources 2020, 467, 228324. [Google Scholar] [CrossRef]
- Parveen, N.; Hilal, M.; Han, J.I. Newly design porous/sponge red phosphorus@graphene and highly conductive Ni2P electrode for asymmetric solid state supercapacitive device with excellent performance. Nano Micro Lett. 2020, 12, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Chen, F.; Kannan, P.; Ji, S.; Wang, H. Nickel phosphate nanowires directly grown on Ni foam as binder-free electrode for pseudocapacitors. Mater. Lett. 2019, 257, 126742. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, T.; Wang, Y.; Liu, Q.; Xiao, D. Through a hydrothermal phosphatization method synthesized NiCo and Fe-based electrodes for high-performance battery-supercapacitor hybrid device. Appl. Surf. Sci. 2019, 475, 729–739. [Google Scholar] [CrossRef]
- Quan, Z.; Hui, Y.; Wang, Q.; Zhang, Q.; Shen, Q. NiCo2O4/NiCoP nanoflake-nanowire arrays: A homogeneous hetero-structure for high performance asymmetric hybrid supercapacitors. Dalton Trans. 2018, 47, 16320–16328. [Google Scholar]
- Yong, Y.; Zhao, H.; Zong, Y.; Li, X.; Sun, Y.; Feng, J.; Wang, Y.; Zheng, X.; Du, Y. Phosphorization boosts the capacitance of mixed metal nanosheet arrays for high performance supercapacitor electrodes. Nanoscale 2018, 10, 11775–11781. [Google Scholar]
- Shi, F.; Xie, D.; Zhong, Y.; Wang, D.H.; Xia, X.H.; Gu, C.D.; Wang, X.L.; Tu, J.P. Facile synthesis of self-supported Ni2P nanosheet@Ni sponge composite for high-rate battery. J. Power Sources 2016, 328, 405–412. [Google Scholar] [CrossRef]
- Bandgar, S.B.; Vadiyar, M.M.; Jambhale, C.L.; Ye, Z.; Kolekar, S.S. Construction of dual metal ferrite-based core-shell nanostructures as low-cost multimetal electrode for boosting energy density of flexible asymmetric supercapattery. J. Energy Storage 2021, 36, 102379. [Google Scholar] [CrossRef]
- Liu, M.; Guo, T. Preparation and swelling properties of crosslinked sodium polyacrylate. J. Appl. Polym. Sci. 2010, 82, 1515–1520. [Google Scholar] [CrossRef]
- Ji, C.; Liu, F.; Xu, L.; Yang, S. Urchin-like NiCO2O4 hollow microspheres and FeSe2 micro-snowflakes for flexible solid-state asymmetric supercapacitors. J. Mater. Chem. A Mater. Energy Sustain. 2017, 5, 5568–5576. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, Z.; Jing, M.; Hou, H.; Ji, X. Porous NiCo2O4 spheres tuned through carbon quantum dots utilised as advanced materials for an asymmetric supercapacitor. J. Mater. Chem. A 2014, 3, 866–877. [Google Scholar] [CrossRef]
- Kong, W.; Lu, C.; Zhang, W.; Pu, J.; Wang, Z. Homogeneous core-shell NiCo2S4 nanostructures supported on nickel foam for supercapacitors. J. Mater. Chem. A 2015, 3, 12452–12460. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, C.; Huang, Y.; Gao, Y.; Wang, Y.; Li, Z.; Yan, Y.; Zhang, M. Oxygen-vacancy-rich NiCo2O4 nanoneedles electrode with poor crystallinity for high energy density all-solid-state symmetric supercapacitors. J. Power Sources 2020, 449, 227571. [Google Scholar] [CrossRef]
- Zhao, X.; Zhu, J.; Lu, Y.; Zhang, Z.; Hong, Y.; Wang, W.; Karimov, K.; Murtaza, I.; Wang, Q.; Dong, X. Three-dimensional Co–S–P nanoflowers as highly stable electrode materials for asymmetric supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 11448–11454. [Google Scholar] [CrossRef]
- Wang, K.; Yan, R.; Tian, X.; Wang, Y.; Lei, S.; Li, X.; Yang, T.; Wang, X.; Song, Y.; Liu, Y.; et al. Multi-scale biomass-based carbon microtubes decorated with Ni-Co sulphides nanoparticles for supercapacitors with high rate performance. Electrochim. Acta 2019, 302, 78–91. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, X.; Zhang, Y.; Qian, X. Fe2O3/RGO/Fe3O4 composite in-situ grown on Fe foil for high performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 30133–30142. [Google Scholar] [CrossRef]
| Electrode Materials | Current Collector | Electrolyte | Specific Capacitance | Capacitance Retention | Year Ref. |
|---|---|---|---|---|---|
| ZnNiFe(OH/P) nanosheet | Nickel foam | 3 M KOH | 1708 F g−1/5.64 F cm−2 (1 A g−1) | 83% (4000) | 2021 [17] |
| FeCoP@NiCo nanosheet | Carbon foam | 2 M KOH | 795.5 C g−1 (1 A g−1) | 89.7% (5000) | 2021 [30] |
| FeNiP@CoNi LDHs nanosheet | Nickel foam | 3 M KOH | 2280 F g−1 (1 A g−1) | 70% (5000) | 2020 [31] |
| NiFeP@NiCo2S4 nanosheet | Carbon cloth | 2 M KOH | 874.4 C g−1 (1 A g−1) | 85.6% (5000) | 2020 [32] |
| NiCoP-MLG nanosheet (powder) | - | 3 M KOH | 1419.6 F g−1 (1 A g−1) | 91% (3000) | 2020 [33] |
| NiCoP nanoplate (powder) | - | 2 M KOH | 1306 F g−1 (1 A g−1) | - | 2020 [34] |
| Ni2P@GO (powder) | - | 2 M KOH | 1526.66 F g−1 (1 A g−1) | 86.3% (2500) | 2020 [35] |
| NiHPO4 nanowire | Nickel foam | 1 M KOH | 1472 F g−1 (1 A g−1) | 90.3% (6000) | 2019 [36] |
| NiCoP nanowire | Nickel foam | 6 M KOH | 19.9 F cm−2 (50 mA cm−2) | 92% (2000) | 2019 [37] |
| NixP nanosphere | Nickel foam | 6 M KOH | 382.7 F g−1 (0.45 A g−1) | 110.9% (4000) | 2018 [11] |
| NiCo2O4-NiCoP nanosheet/nanowire | Nickel foam | 3 M KOH | 2288.8 F g−1 (1 A g−1) | 71% (2000) | 2018 [38] |
| NiCoP nanosheet | Nickel foam | 6 M KOH | 2143 F g−1 (1 A g−1) | 73% (2000) | 2018 [39] |
| NiP nanosheet | Nickel Sponge | 6 M KOH | 430.3 mAh g−1 (1 A g−1) | - | 2016 [40] |
| Ni2P nanosheet | Nickel foam | 6 M KOH | 2141 F g−1 (50 mV s−1) | - | 2015 [21] |
| CuFe2O4 NR@NiFe2O4-NS nanosheet/nanowire | Stainless-steel mesh | 6 M KOH | 1366 C g−1 (1 A g−1) | 94% (10,000) | 2021 [41] |
| NiFe-LDH nanosheet | Nickel foam | 1 M KOH | 2708 F g−1 (5 A g−1) | 42.6% (500) | 2017 [9] |
| FeNi(OH/P)-1.2 nanosheet | Nickel foam | 3 M KOH | 3.6 C cm−2/1469.9 C g−1/408.3 mAh g−1 (1 mV s−1) | 72.6% (10,000) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Li, R.; Zhou, Y. Preparation of a Honeycomb-like FeNi(OH/P) Nanosheet Array as a High-Performance Cathode for Hybrid Supercapacitors. Energies 2022, 15, 3877. https://doi.org/10.3390/en15113877
Li C, Li R, Zhou Y. Preparation of a Honeycomb-like FeNi(OH/P) Nanosheet Array as a High-Performance Cathode for Hybrid Supercapacitors. Energies. 2022; 15(11):3877. https://doi.org/10.3390/en15113877
Chicago/Turabian StyleLi, Chenliang, Ruizhi Li, and Yingke Zhou. 2022. "Preparation of a Honeycomb-like FeNi(OH/P) Nanosheet Array as a High-Performance Cathode for Hybrid Supercapacitors" Energies 15, no. 11: 3877. https://doi.org/10.3390/en15113877
APA StyleLi, C., Li, R., & Zhou, Y. (2022). Preparation of a Honeycomb-like FeNi(OH/P) Nanosheet Array as a High-Performance Cathode for Hybrid Supercapacitors. Energies, 15(11), 3877. https://doi.org/10.3390/en15113877







