A Critical Review of Alkaline Flooding: Mechanism, Hybrid Flooding Methods, Laboratory Work, Pilot Projects, and Field Applications
Abstract
:1. Introduction
- -
- Lowering the interfacial tension between oil and water;
- -
- Emulsification of oil and water;
- -
- Solubilization of oil in the micelles;
- -
- Alteration of rock wettability (oil-wet to water-wet);
- -
- Mobility and sweep efficiency enhancement.
2. Alkali Flooding
- -
- Emulsification and entrainment;
- -
- Wettability reversal from oil-wet to water-wet;
- -
- Wettability reversal from water-wet to oil-wet;
- -
- Emulsification and entrapment;
- -
- Reduction in the oil–water interfacial tension.
3. Hybrid Modes of Injection
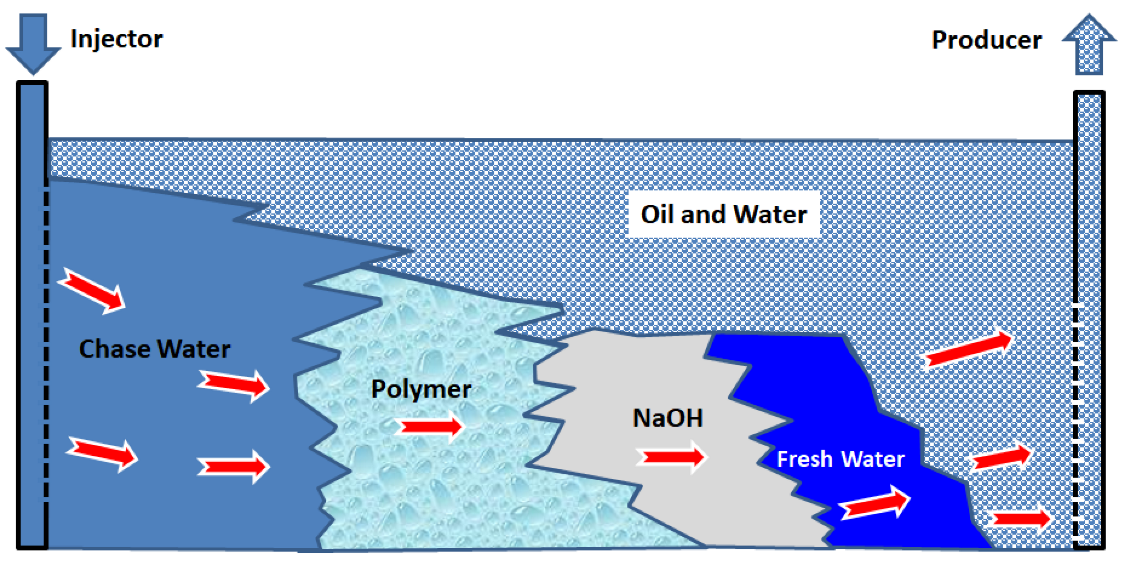
3.1. Alkaline–Polymer (AP) Flooding
3.2. Alkaline–Surfactant (AS) Flooding
3.3. Alkaline–Surfactant–Polymer (ASP) Flooding
3.4. Alkaline–Low Salinity Brine Flooding
3.5. Alkaline–Nanoparticle Flooding
4. Alkaline Flooding Experimental Work
5. Alkaline Flooding—Pilot Project
6. Alkaline Flooding—Screening Criteria
7. Alkaline Flooding—Field Applications
7.1. Field Case Study—Wilmington Field, USA
7.2. Field Case Study—Shengli Oil Field, China
8. Conclusions
- -
- AP flooding reduces polymer viscosity and helps to reduce alkali consumption.
- -
- AS flooding has a considerable impact on nonthermal flooding. When alkali is added to the surfactant solution, it increases the salinity of the solution and reduces the surfactant adsorption.
- -
- ASP flooding has been effective in terms of reducing IFT and mobility ratio, and its efficiency is improved by the addition of cosolvents and cosurfactants.
- -
- Alkaline–LSW flooding has a considerable impact on oil recovery; the addition of LSW to the alkaline also makes it a favorable candidate for carbonate reservoirs.
- -
- Alkaline–nanoparticle flooding helps recover oil in the complex reservoir because nanoparticles can withstand high temperature and pressure conditions. Nanoparticles, owing to their small size, can interact easily with reservoir fluids in tight formations.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EOR | Enhanced Oil Recovery |
| TAN | Total Acid Number |
| IFT | Interfacial Tension |
| MR | Mobility Ratio |
| CEOR | Chemical Enhanced Oil Recovery |
| ASP | Alkali–Surfactant–Polymer |
| AP | Alkaline–Polymer |
| AS | Alkali–Surfactant |
| LSW | Low Salinity Water |
| OIP | Oil In Place |
| PV | Pore Volume |
References
- Abidin, A.Z.; Puspasari, T.; Nugroho, W.A. Polymers for Enhanced Oil Recovery Technology. Proc. Chem. 2012, 4, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Brantson, E.T.; Ju, B.; Opoku Appau, P.; Akwensi, P.H.; Peprah, G.A.; Liu, N.; Aphu, E.S.; Annan Boah, E.; Aidoo Borsah, A. Development of Hybrid Low Salinity Water Polymer Flooding Numerical Reservoir Simulator and Smart Proxy Model for Chemical Enhanced Oil Recovery (CEOR). J. Pet. Sci. Eng. 2020, 187, 106751. [Google Scholar] [CrossRef]
- Akpoturi, P.; Ofesi, S.F. Enhanced Oil Recovery Using Local Alkaline. Nig. J. Technol. 2017, 36, 515. [Google Scholar] [CrossRef] [Green Version]
- Schumi, B.; Clemens, T.; Wegner, J.; Ganzer, L.; Kaiser, A.; Hincapie, R.E.; Leitenmüller, V. Alkali/Cosolvent/Polymer Flooding of High-TAN Oil: Using Phase Experiments, Micromodels, and Corefloods for Injection-Agent Selection. In SPE Reservoir Evaluation and Engineering; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2020; Volume 23, pp. 463–478. [Google Scholar] [CrossRef]
- Manrique, E.; Thomas, C.; Ravikiran, R.; Izadi, M.; Lantz, M.; Romero, J.; Alvarado, V. EOR: Current Status and Opportunities. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 24–28 April 2010; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2010; Volume 2, pp. 1584–1604. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Y.; Wang, Q.; Guo, Y.; Gao, M.; Wang, Z.; Liu, W. Status and Progress of Worldwide EOR Field Applications. J. Pet. Sci. Eng. 2020, 193, 107449. [Google Scholar] [CrossRef]
- Speight, J.G. General Methods of Oil Recovery. In Introduction to Enhanced Recovery Methods for Heavy Oil and Tar Sands; Elsevier: Amsterdam, The Netherlands, 2016; pp. 253–322. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced Oil Recovery Concepts. In Enhanced Oil Recovery; Elsevier: Amsterdam, The Netherlands, 2010; pp. 7–16. [Google Scholar] [CrossRef]
- Gbadamosi, A.O.; Kiwalabye, J.; Junin, R.; Augustine, A. A Review of Gas Enhanced Oil Recovery Schemes Used in the North Sea. J. Petrol. Explor. Prod. Technol. 2018, 8, 1373–1387. [Google Scholar] [CrossRef] [Green Version]
- Shafiai, S.H.; Gohari, A. Conventional and Electrical EOR Review: The Development Trend of Ultrasonic Application in EOR. J. Petrol. Explor. Prod. Technol. 2020, 10, 2923–2945. [Google Scholar] [CrossRef]
- Druetta, P.; Raffa, P.; Picchioni, F. Chemical Enhanced Oil Recovery and the Role of Chemical Product Design. Appl. Energy 2019, 252, 113480. [Google Scholar] [CrossRef]
- Guo, H.; Li, Y.; Wang, F.; Gu, Y. Comparison of Strong-Alkali and Weak-Alkali ASP-Flooding Field Tests in Daqing Oil Field. In SPE Production and Operations; Society of Petroleum Engineers: Richardson, TX, USA, 2018; Volume 33, pp. 353–362. [Google Scholar] [CrossRef]
- Deng, X.; Tariq, Z.; Murtaza, M.; Patil, S.; Mahmoud, M.; Kamal, M.S. Relative Contribution of Wettability Alteration and Interfacial Tension Reduction in EOR: A Critical Review. J. Mol. Liq. 2021, 325, 115175. [Google Scholar] [CrossRef]
- Sheng, J.J. Alkaline-Polymer Flooding. In Enhanced Oil Recovery Field Case Studies; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 169–178. [Google Scholar] [CrossRef]
- Speight, J.G. Asphalt Materials Science and Technology; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Campbell, T.C. Role of Alkaline Chemicals in the Recovery of Low-Gravity Crude Oils. J. Pet. Technol. 1982, 34, 2510–2516. [Google Scholar] [CrossRef]
- Nelson, R.C.; Lawson, J.B.; Thigpen, D.R.; Stegemeier, G.L. Cosurfactant-Enhanced Alkaline Flooding; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 1984. [Google Scholar] [CrossRef]
- Buijse, M.A.; Prelicz, R.M.; Barnes, J.R.; Cosmo, C. Application of Internal Olefin Sulfonates and Other Surfactants to EOR. Part 2: The Design and Execution of an ASP Field Test. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 24–28 April 2010; pp. 706–717. [Google Scholar] [CrossRef]
- Olajire, A.A. Review of ASP EOR (Alkaline Surfactant Polymer Enhanced Oil Recovery) Technology in the Petroleum Industry: Prospects and Challenges. Energy 2014, 77, 963–982. [Google Scholar] [CrossRef]
- Foedisch, H.; Abdullah, H.; Hincapie, R.E.; Ganzer, L. Optimizing Laboratory CEOR Flooding Evaluations to Assess Initial Oil Saturation and Mobility Ratio. In Society of Petroleum Engineers—SPE Europec Featured at 80th EAGE Conference and Exhibition 2018, Copenhagen, Denmark, 11–14 June 2018; Society of Petroleum Engineers: Richardson, TX, USA, 2018. [Google Scholar] [CrossRef]
- Krumrine, P.H.; Falcone, J.S. Beyond Alkaline Flooding: Design of Complete Chemical Systems. In Society of Petroleum Engineers of AIME, (Paper) SPE; Society of Petroleum Engineers of AIME, USA SPE: Richardson, TX, USA, 1987; pp. 415–426. [Google Scholar] [CrossRef]
- Ghadami, N.; Das, A.K.; Tunio, K.H.; Sabzabadi, A. Sensitivity Analysis and Optimization of Alkaline-Surfactant Flooding in a Thin Clastic Reservoir; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2015. [Google Scholar] [CrossRef]
- Sheng, J.J. Alkaline-Surfactant Flooding. In Enhanced Oil Recovery Field Case Studies; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 179–188. [Google Scholar] [CrossRef]
- DeZabala, E.F.; Vislocky, J.M.; Rubin, E.; Radke, C.J. Chemical Theory for Linear Alkaline Flooding. Soc. Pet. Eng. J. 1982, 22, 245–258. [Google Scholar] [CrossRef]
- Novosad, Z.; Novosad, J. Determination of Alkalinity Losses Resulting from Hydrogen Ion Exchange in Alkaline Flooding. Soc. Pet. Eng. J. 1984, 24, 49–52. [Google Scholar] [CrossRef]
- Forth, R.; Slevinsky, B.; Lee, D.; Fedenczuk, L. Application of Statistical Analysis to Optimize Reservoir Performance. J. Can. Pet. Technol. 1997, 36. [Google Scholar] [CrossRef]
- Dong, M.; Ma, S.; Li, A. Sweep Efficiency Improvement by Alkaline Flooding for Pelican Lake Heavy Oil. In Proceedings of the Society of Petroleum Engineers—Canadian Unconventional Resources Conference 2011, CURC 2011, Calgary, AL, Canada, 15–17 November 2011; Volume 2, pp. 1384–1398. [Google Scholar] [CrossRef]
- Liu, S.; Li, R.F.; Miller, C.A.; Hirasaki, G.J. ASP Processes: Wide Range of Conditions for Good Recovery. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008; Volume 3, pp. 1242–1259. [Google Scholar] [CrossRef]
- Ghorpade, T.S.; Patil, S.L.; Dandekar, A.Y.; Khataniar, S. Application of Alkali-Surfactant-Polymer ASP Flooding for Improving Viscous Oil Recovery from Alaskan North Slope Reservoir. In SPE Western Regional Meeting Proceedings; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2016; Volume 2016-January. [Google Scholar] [CrossRef]
- Southwick, J.; Brewer, M.; van Batenburg, D.; Pieterse, S.; Bouwmeester, R.; Mahruqi, D.; Alkindi, A.; Mjeni, R. Ethanolamine as Alkali for Alkali Surfactant Polymer Flooding—Development of a Low-Complexity Field Implementation Concept. In Proceedings of the SPE Symposium on Improved Oil Recovery, Virtual, 31 August–4 September 2020; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2020; Volume 2020. [Google Scholar] [CrossRef]
- Sheng, J.J. Alkaline Flooding. In Enhanced Oil Recovery Field Case Studies; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 143–167. [Google Scholar] [CrossRef]
- Johnson, C.E. Status of Caustic and Emulsion Methods. JPT J. Pet. Technol. 1976, 28, 85–92. [Google Scholar] [CrossRef]
- Stoll, W.M.; Al Shureqi, H.; Finol, J.; Al-Harthy, S.A.A.; Oyemade, S.; de Kruijf, A.; van Wunnik, J.; Arkesteijn, F.; Bouwmeester, R.; Faber, M.J. Alkaline/Surfactant/Polymer Flood: From the Laboratory to the Field. SPE Reserv. Eval. Eng. 2011, 14, 702–712. [Google Scholar] [CrossRef]
- Panthi, K.; Sharma, H.; Mohanty, K.K. ASP Flood of a Viscous Oil in a Carbonate Rock. Fuel 2016, 164, 18–27. [Google Scholar] [CrossRef]
- Burk, J.H. Comparison of Sodium Carbonate, Sodium Hydroxide, and Sodium Orthosilicate for EOR. SPE Reserv. Eng. 1987, 2, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Berger, P.D.; Lee, C.H. Improved ASP Process Using Organic Alkali. In Proceedings of the SPE Symposium on Improved Oil Recovery; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2006; Volume 1, pp. 340–348. [Google Scholar] [CrossRef]
- Adibhatla, B.; Mohanty, K.K. Oil Recovery from Fractured Carbonates by Surfactant-Aided Gravity Drainage: Laboratory Experiments and Mechanistic Simulations. SPE Reserv. Eval. Eng. 2008, 11, 119–130. [Google Scholar] [CrossRef]
- Lu, J.; Goudarzi, A.; Chen, P.; Kim, D.H.; Delshad, M.; Mohanty, K.K.; Sepehrnoori, K.; Weerasooriya, U.P.; Pope, G.A. Enhanced Oil Recovery from High-Temperature, High-Salinity Naturally Fractured Carbonate Reservoirs by Surfactant Flood. J. Pet. Sci. Eng. 2014, 124, 122–131. [Google Scholar] [CrossRef]
- Leslie Zhang, D.; Liu, S.; Puerto, M.; Miller, C.A.; Hirasaki, G.J. Wettability Alteration and Spontaneous Imbibition in Oil-Wet Carbonate Formations. J. Pet. Sci. Eng. 2006, 52, 213–226. [Google Scholar] [CrossRef]
- Levitt, D.; Jackson, A.; Heinson, C.; Britton, L.N.; Malik, T.; Dwarakanath, V.; Pope, G.A. Identification and Evaluation of High-Performance EOR Surfactants. SPE Reserv. Eval. Eng. 2009, 12, 243–253. [Google Scholar] [CrossRef]
- Sheng, J.J. A Comprehensive Review of Alkaline-Surfactant-Polymer (ASP) Flooding: A Comprehensive Review of ASP Flooding. Asia-Pac. J. Chem. Eng. 2014. [Google Scholar] [CrossRef]
- Al-Saedi, H.N.; Flori, R.E.; Al-Jaberi, S.K.; Al-Bazzaz, W. Low-Salinity Water, C02, Alkaline, and Surfactant EOR Methods Applied to Heavy Oil in Sandstone Cores. SPE J. 2020, 25, 1729–1744. [Google Scholar] [CrossRef]
- Masalmeh, S.; Al-Hammadi, M.; Farzaneh, A.; Sohrabi, M. Low Salinity Water Flooding in Carbonate: Screening, Laboratory Quantification and Field Implementation. In Society of Petroleum Engineers—Abu Dhabi International Petroleum Exhibition and Conference 2019, ADIP 2019; Society of Petroleum Engineers: Richardson, TX, USA, 2019. [Google Scholar] [CrossRef]
- Assef, Y.; Arab, D.; Pourafshary, P. Application of Nanofluid to Control Fines Migration to Improve the Performance of Low Salinity Water Flooding and Alkaline Flooding. J. Pet. Sci. Eng. 2014, 124, 331–340. [Google Scholar] [CrossRef]
- Panchal, H.; Patel, H.; Patel, J.; Shah, M. A Systematic Review on Nanotechnology in Enhanced Oil Recovery. In Petroleum Research; KeAi Publishing Communications Ltd.: Beijing, China, 2021. [Google Scholar] [CrossRef]
- Squires, F. Method of Recovering Oil and Gas. US Patent No. 1,238,355, 28 August 1917. [Google Scholar]
- Subkow, P. Process for Removal of Bitumen from Bituminous Deposits. U.S. Patent #2,288,857, 11 August 1942. [Google Scholar]
- Van, S.L.; Chon, B.H. Chemical Flooding in Heavy-Oil Reservoirs: From Technical Investigation to Optimization Using Response Surface Methodology. Energies 2016, 9, 711. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, R.; Wygal, R.J. Interrelation of Crude Oil and Rock Properties with the Recovery of Oil by Caustic Waterflooding. Soc. Pet. Eng. AIME J. 1977, 17, 263–270. [Google Scholar] [CrossRef]
- Larrondo, L.E.; Urness, C.M. Laboratory Evaluation of Sodium Hydroxide, Sodium Orthosilicate, and Sodium Metasilicate as Alkaline Flooding Agents for a Western Canada Reservoir. In SPE Oilfield and Geothermal Chemistry Symposium; Society of Petroleum Engineers: Richardson, TX, USA, 1985. [Google Scholar] [CrossRef]
- Mayer, E.H.; Berg, R.L.; Carmichael, J.D.; Weinbrandt, R.M. Alkaline Injection for Enhanced Oil Recovery—A Status Report. J. Pet. Technol. 1983, 35, 209–221. [Google Scholar] [CrossRef]
- Cockcroft, P.; Anli, J.; Duignan, J. EOR Potential of Indonesian Reservoirs. Annu. Conv. Proc. 1988, 2, 73–108. [Google Scholar]
- Hammershaimb, E.C.; Kuuskraa, V.A.; Stosur, G. Recovery Efficiency of Enhanced Oil Recovery Methods: A Review of Significant Field Tests. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Francisco, CA, USA, 5–8 October 1983. [Google Scholar]
- Lau, H.C.; Borchardt, J.K. Improved Steam Foam Formulations: Concepts and Laboratory ResuIts. In Proceedings of the SPE California Regional Meeting, Bakersfield, CA, USA, 5–6 April 1989. [Google Scholar]
- Gael, B.T.; Gross, S.J.; McNaboe, G.J. Development Planning and Reservoir Management in the Duri Steam Flood. In Proceedings of the SPE Western Regional Meeting, Bakersfield, CA, USA, 8–10 March 1995; SPE-29668-MS. [Google Scholar] [CrossRef]
- French, T.R.; Burchfield, T.E. Design and Optimization of Alkaline Flooding Formulations; Society of Petroleum Engineers of AIME: Richardson, TX, USA, 1990. [Google Scholar] [CrossRef]
- Taber, J.J.; Martin, F.D.; Seright, R.S. EOR Screening Criteria Revisited—Part 2: Applications and Impact of Oil Prices. SPE Reserv. Eng. 1997, 12, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Collins, A. Geochemistry of Oilfield Waters; Elsevier: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Shedid, S.A. Experimental Investigation of Alkaline/Surfactant/Polymer (ASP) Flooding in Low Permeability Heterogeneous Carbonate Reservoirs. In Proceedings of the Society of Petroleum Engineers—SPE North Africa Technical Conference and Exhibition 2015, NATC 2015, Cairo, Egypt, 14–16 September 2015; Society of Petroleum Engineers: Richardson, TX, USA, 2015; pp. 194–209. [Google Scholar] [CrossRef]
- Sheng, J. Status of Alkaline Flooding Technology. J. Pet. Eng. Technol. 2015, 5, 44–50. [Google Scholar]
- Raimondi, P.; Gallagher, B.J.; Ehrlich, R.; Messmer, J.H.; Bennett, G.S. Alkaline Waterflooding: Design and Implementation of a Field Pilot. J. Pet. Technol. 1977, 29, 1359–1368. [Google Scholar] [CrossRef]
- Kumar, S.; Yen, T.F.; Chilingarian, G.V.; Donaldson, E.C. Chapter 9 Alkaline Flooding. In Enhanced Oil Recovery, II; Donaldson, E.C., Chilingarian, G.V., Yen, T.F., Eds.; Developments in Petroleum Science; Elsevier: Amsterdam, The Netherlands, 1989; Volume 17, pp. 219–254. [Google Scholar] [CrossRef]
- Zainal, S.; Manap, A.A.A.; Hamid, P.A.; Othman, M.; Chong, M.B.O.; Yahaya, A.W.; Darman, N.B.; Mat Sai, R. Offshore Chemical EOR: The Role of an Innovative Laboratory Program in Managing Result Uncertainty to Ensure the Success of a Pilot Field Implementation. In Proceedings of the Europec/EAGE Conference and Exhibition, Rome, Italy, 9–12 June 2008. [Google Scholar]
- Ibrahim, Z.B.; Manap, A.A.A.; Hamid, P.A.; Hon, V.Y.; Lim, P.H.; Wyatt, K. Laboratory Aspect of Chemical EOR Processes Evaluation for Malaysian Oilfields. In Proceedings of the SPE Asia Pacific Oil & Gas Conference and Exhibition, Adelaide, Australia, 11–13 September 2006. [Google Scholar]
- Mayer, E.H.; Breit, V.S. Alkaline Flood Prediction Studies: Ranger VII Pilot, Wilmington Field, California. SPE Reserv. Eng. 1986, 1, 9–22. [Google Scholar] [CrossRef]
- Pei, H.H.; Zhang, G.C.; Ge, J.J.; Ding, L.; Tang, M.G.; Zheng, Y.F. A Comparative Study of Alkaline Flooding and Alkaline/Surfactant Flooding for Zhuangxi Heavy Oil. In Proceedings of the Society of Petroleum Engineers—SPE Heavy Oil Conference Canada, Calgary, AL, Canada, 12–14 June 2012; Society of Petroleum Engineers: Richardson, TX, USA, 2012; Volume 1, pp. 144–153. [Google Scholar] [CrossRef]
- Guan, W.; Wu, S.; Wang, S.; Cao, J.; Chen, Y. Physical Simulation of In-Situ Combustion of Sensitive Heavy Oil Reservoir. In Proceedings of the SPE—Asia Pacific Oil and Gas Conference, Jakarta, Indonesia, 20 October–1 November 2007; pp. 981–988. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, H.; Lin, C. Source Rocks and Petroleum Reservoirs in the Chexi Sunken of the Jiyang Depression, China. Energy, Explor. Exploit. 2006, 24, 151–159. [Google Scholar] [CrossRef]
- Almubarak, T.; Ng, J.H.; Ramanathan, R.; Nasr-El-Din, H.A. Chelating Agents for Oilfield Stimulation: Lessons Learned and Future Outlook. J. Pet. Sci. Eng. 2021, 205, 108832. [Google Scholar] [CrossRef]
| Country | Name | Description | Properties | Values | References |
|---|---|---|---|---|---|
| China | Daqing field | It was found that the oil production rate using weak alkali was 20% more than that of strong alkali. Similarly, in weak alkali, the scaling problem was less severe than in strong alkali | Alkaline used Average incremental oil recovery (%) Viscosity (mPa.s) Temperature (°F) Oil formation volume factor (RB/STB) Formation net thickness (ft) Permeability (mD) | Sodium hydroxide and sodium carbonate 22 8.7 109 1.12 Sandstone 27 650 | |
| Canada | Epping field | The pilot project was carried out using a five-spot injection well. | Alkaline used Average incremental oil recovery (%) Viscosity (mPa.s) Temperature (°F) Oil formation volume factor (RB/STB) Formation net thickness (ft) Permeability (mD) | Sodium hydroxide <1 150 72 - Sparky 20 1000 | [51] |
| Indonesia | Duri field | Incremental oil recovery was found to be 5% of oil in place | Alkaline used Average incremental oil recovery (%) Viscosity (mPa.s) Temperature (°F) Oil formation volume factor (RB/STB) Formation net thickness (ft) Permeability (mD) | Sodium hydroxide 5 >120 100 1.02 Sandstone 120 1500 | [52] |
| United States | Kern River field | A combination of alkaline and steam flooding was carried out. | Alkaline used Average incremental oil recovery (%) Viscosity (mPa.s) Temperature (°F) Oil formation volume factor (RB/STB) Formation net thickness (ft) Permeability (mD) | Sodium hydroxide <0.5 >1000 90 1.03 Kern River 88 2000 | [51,53,54,55] |
| Properties | Values |
|---|---|
| Gravity (°API) | 20–30 |
| Viscosity (cp) | 11–200 |
| Temperature (°F) | 68–203 |
| Average permeability (md) | 10–1500 |
| Composition | Organic acids are required to achieve low IFT |
| Oil saturation (% PV) | 35–74.8 |
| CO2 content | Reservoirs with high content of CO2 (>0.01) and low pH of formation water (pH < 6.5) are not good candidates for alkaline flooding |
| Formation preferred | Sandstone is preferred, but alkaline flooding has also generated good results in limestone formation (alkalis with high pH react with carbonates) |
| Gypsum (%) | <0.1 (because of high adsorption of alkali from the alkaline solution, any reservoir with layered anhydrite content of more than 0.1% should be rejected as a candidate) |
| Divalent ion exchange capacity (meq/kg) | <5 (high content of montmorillonite in the field results in absorbing most of the injected alkali due to its high surface area and cation-exchange ability that result in adverse precipitation reactions) |
| In situ pH | >6.5 (in case the of a high content of kaolinite in the reservoir, alkali flooding can be carried out with a low pH value (8.2–10) |
| Depth (ft) | 900–3000 |
| Country | Name | Description | References |
|---|---|---|---|
| United States (USA) | Bison Basin field | The test was conducted on 6 injection and 8 production wells | [51,60] |
| United States (USA) | North Ward Estes field | Sodium hydroxide was used as an alkali with a concentration of 5 wt% | [51,61] |
| Hungary | Nagylengyel field | A limestone reservoir, so ammonium hydroxide was used | [51,62] |
| United States (USA) | Bradford field | Sodium carbonate was used as an alkali | [32,51] |
| Malaysia | Angsi field | Alkaline surfactant and some other chemicals were used | [63,64] |
| Properties | Values |
|---|---|
| Initial OIP (bbl/acre-ft) | 1260 |
| Gravity (°API) | 12.92 |
| Total acid number (mg/gm) | 3.40 |
| Kinematic viscosity (cSt) | 413 |
| Avg. formation volume factor (RB/STB) | 1.1 |
| Pilot area (Acres) | 193 |
| Formation type | Shaly sand |
| Net thickness (ft) | 320 |
| Average permeability (md) | 240 |
| Average porosity (%) | 25 |
| Depth (ft) | 2225 |
| Temperature (°F) | 125.06 |
| Properties | Values |
|---|---|
| Gravity (0API) | 20.6 |
| Total acid number (mg/gm) | 0.8 |
| Kinematic viscosity (cSt) | 325 |
| Initial oil saturation (%) | 57 |
| Formation type | Sandstone |
| Net thickness (ft) | 43.6 |
| Average permeability (md) | 560 |
| Average porosity (%) | 26 |
| Depth (ft) | 4260 |
| Temperature (°F) | 131 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khlaifat, A.L.; Dakhlallah, D.; Sufyan, F. A Critical Review of Alkaline Flooding: Mechanism, Hybrid Flooding Methods, Laboratory Work, Pilot Projects, and Field Applications. Energies 2022, 15, 3820. https://doi.org/10.3390/en15103820
Khlaifat AL, Dakhlallah D, Sufyan F. A Critical Review of Alkaline Flooding: Mechanism, Hybrid Flooding Methods, Laboratory Work, Pilot Projects, and Field Applications. Energies. 2022; 15(10):3820. https://doi.org/10.3390/en15103820
Chicago/Turabian StyleKhlaifat, Abdelaziz L., Duaa Dakhlallah, and Faraz Sufyan. 2022. "A Critical Review of Alkaline Flooding: Mechanism, Hybrid Flooding Methods, Laboratory Work, Pilot Projects, and Field Applications" Energies 15, no. 10: 3820. https://doi.org/10.3390/en15103820
APA StyleKhlaifat, A. L., Dakhlallah, D., & Sufyan, F. (2022). A Critical Review of Alkaline Flooding: Mechanism, Hybrid Flooding Methods, Laboratory Work, Pilot Projects, and Field Applications. Energies, 15(10), 3820. https://doi.org/10.3390/en15103820








