Water Weakening of Artificially Fractured Chalk, Fracture Modification and Mineral Precipitation during Water Injection—An Experimental Study
Abstract
:1. Introduction
2. Samples, Sample Preparation and Methodology
2.1. Axial Strain, Yield Strength, and Bulk Modulus
2.2. Porosity and Permeability
2.3. Ion Chromatography (IC)
2.4. Optical Light Microscopy (OLM) and Fracture Modification
2.5. Geochemistry
2.6. Field Emission Gun Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS)
2.7. Specific Surface Area (SSA)
3. Results
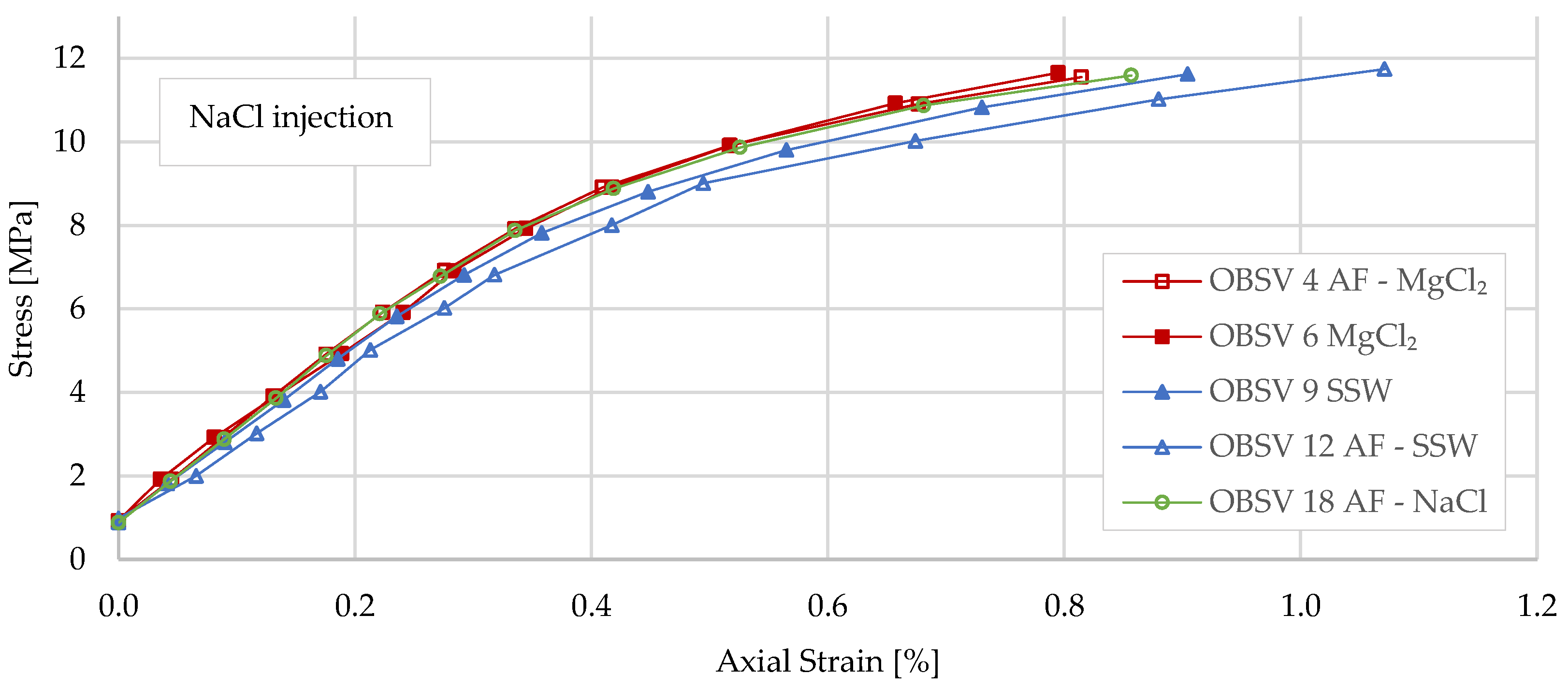
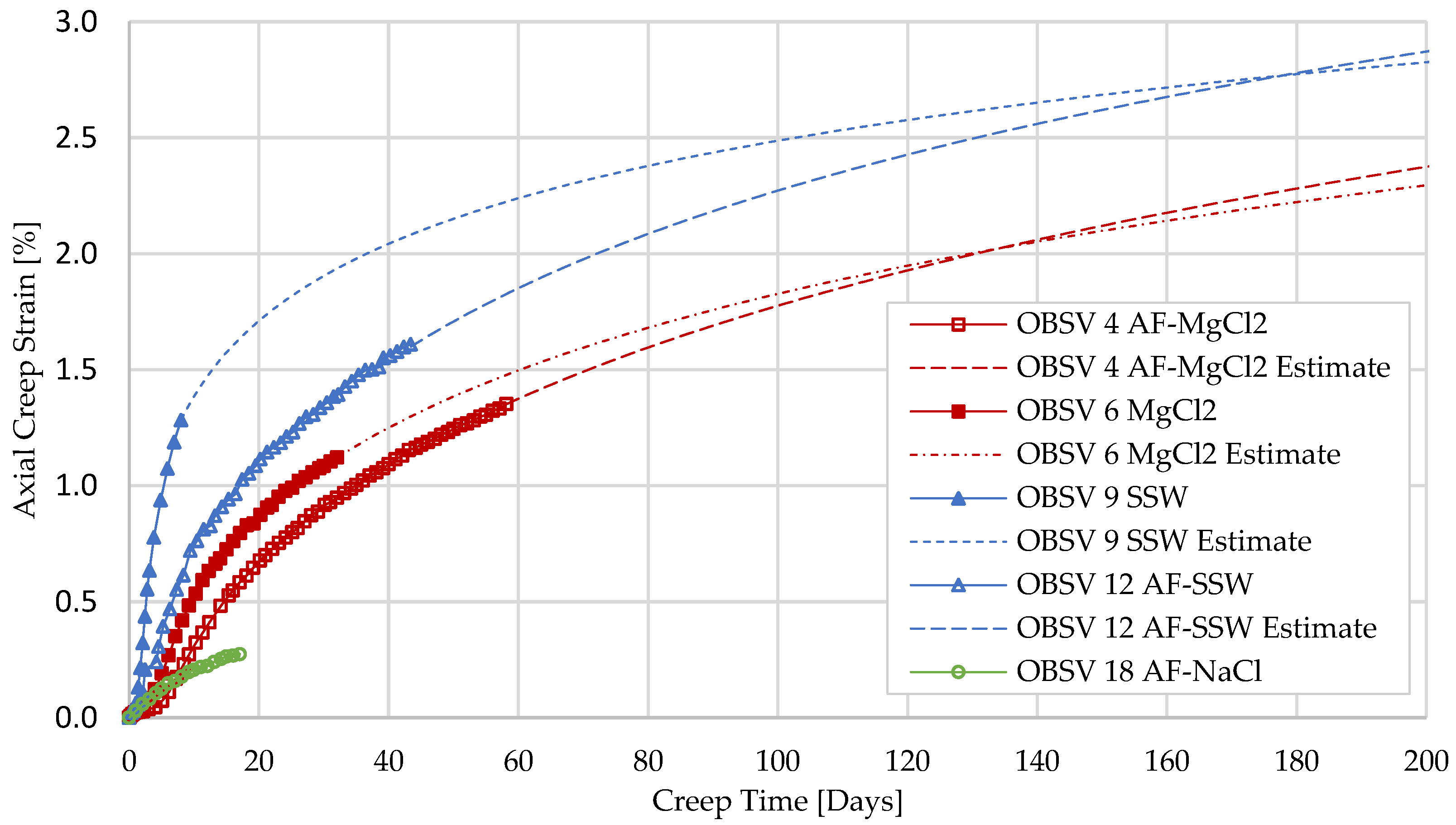
3.1. Geo-Mechanical Responses
3.2. Porosity, Permeability, and Mechanical Strength Data
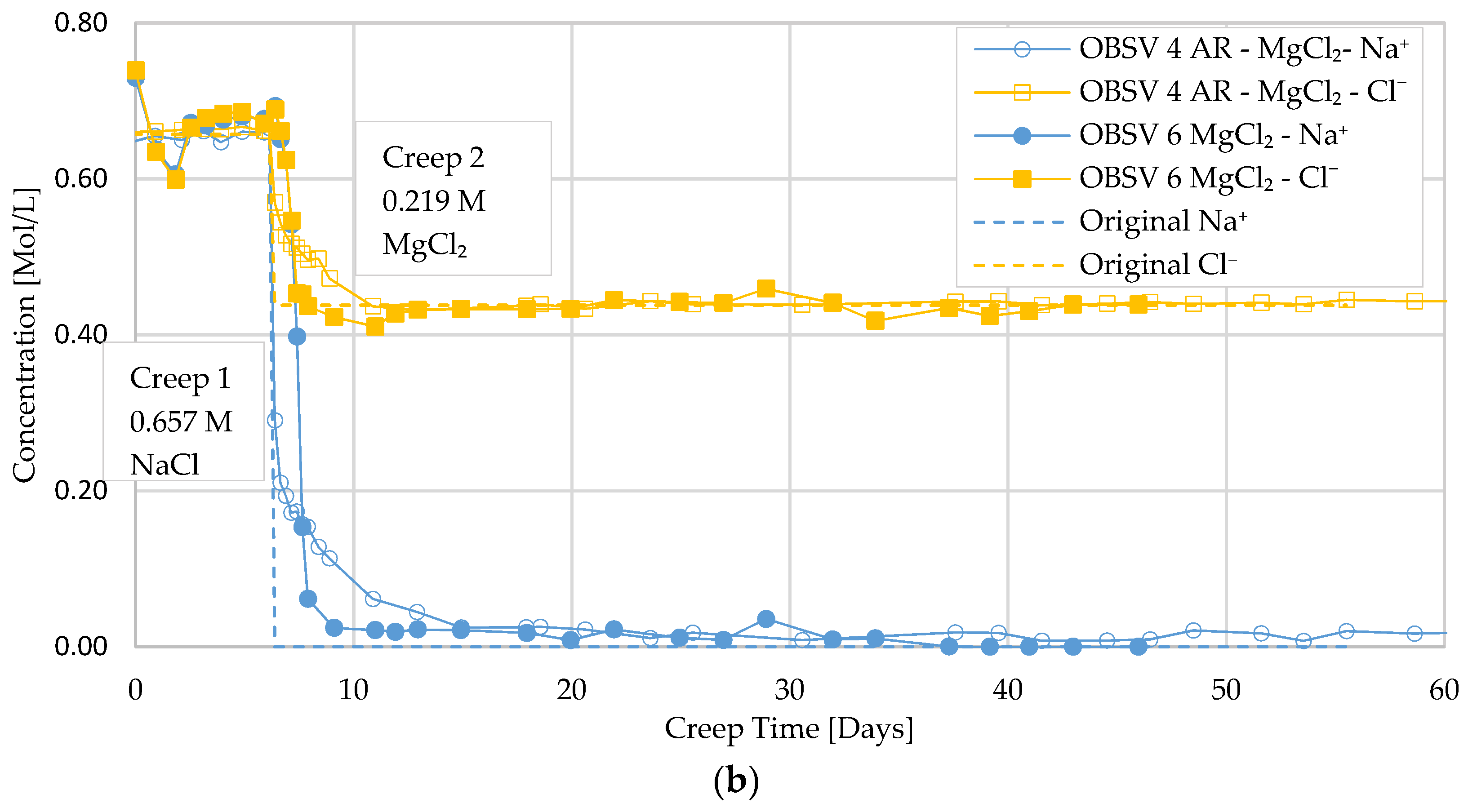
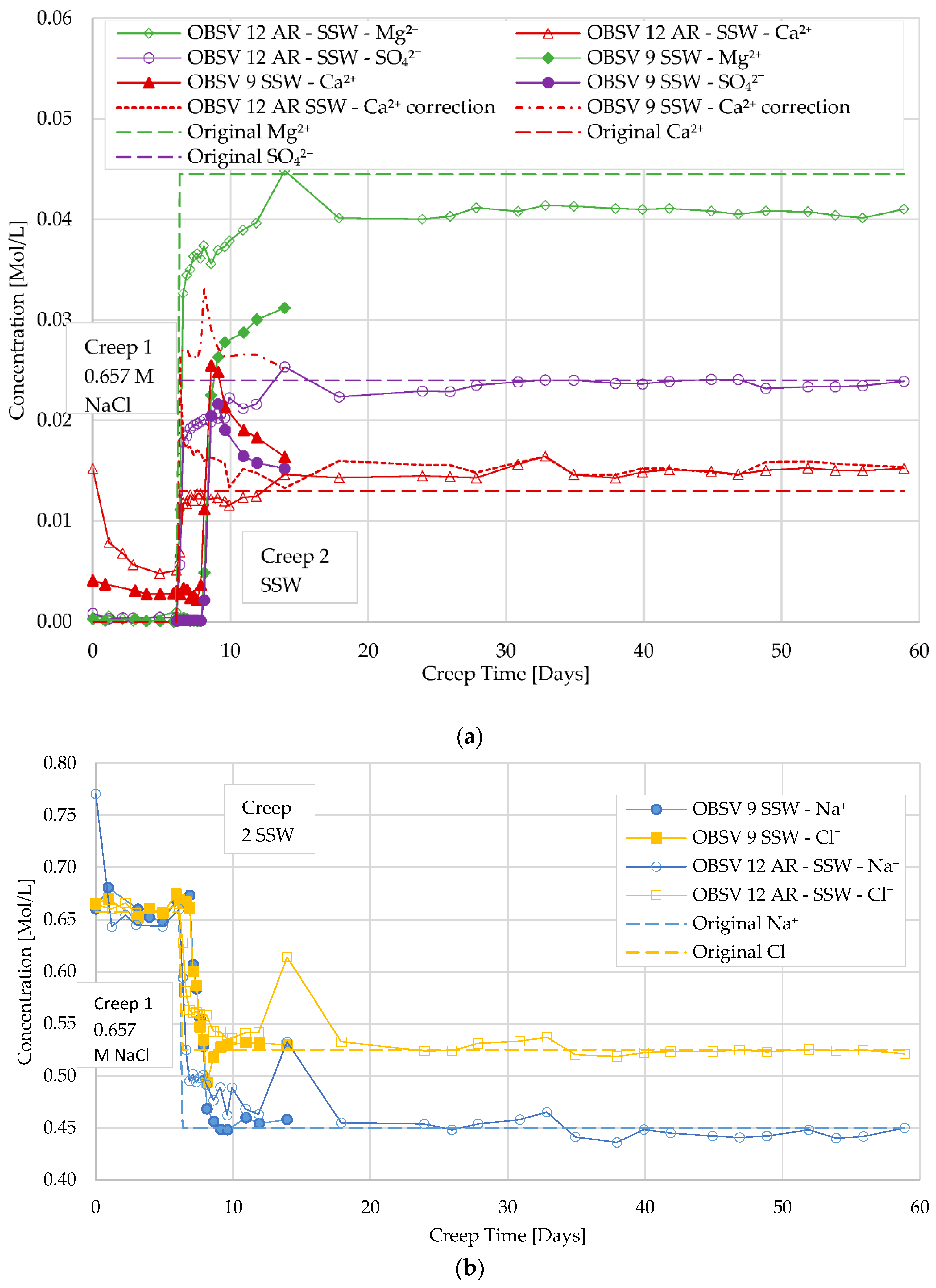
3.3. Ion Chromatography and Analysis of Effluent
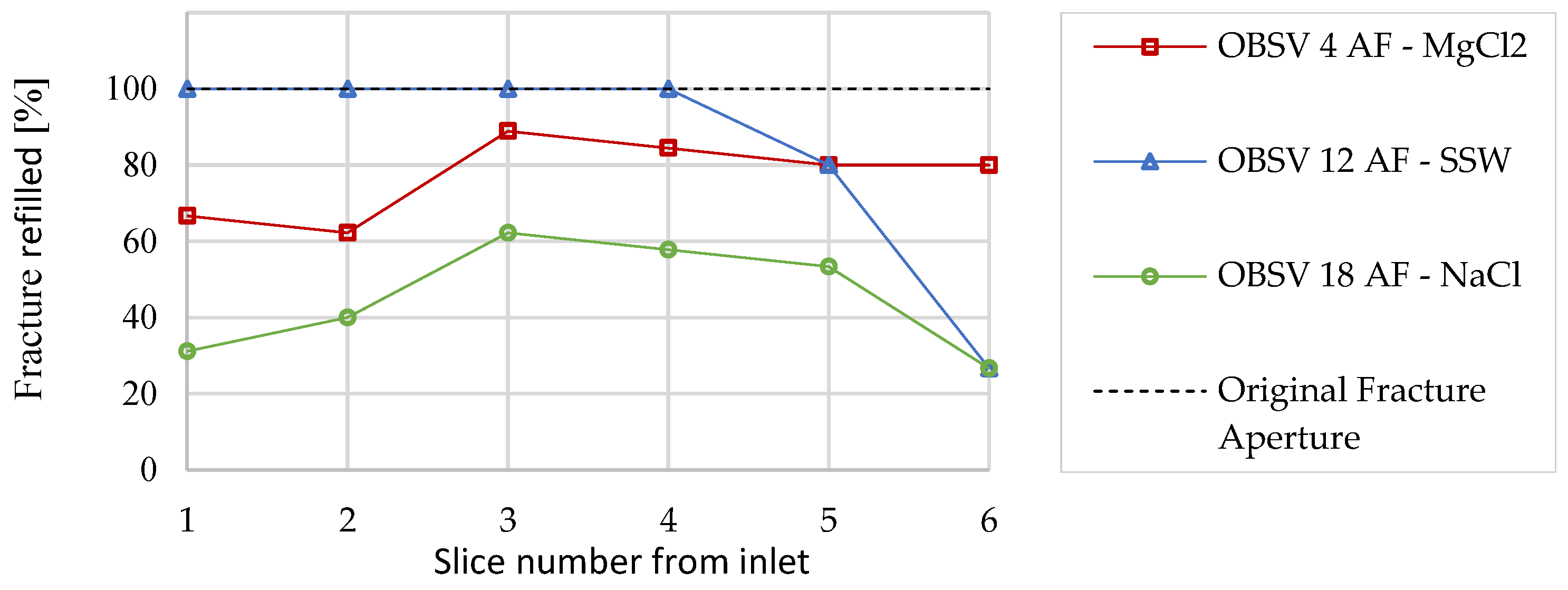
3.4. Textural and Morphological Observation OLM
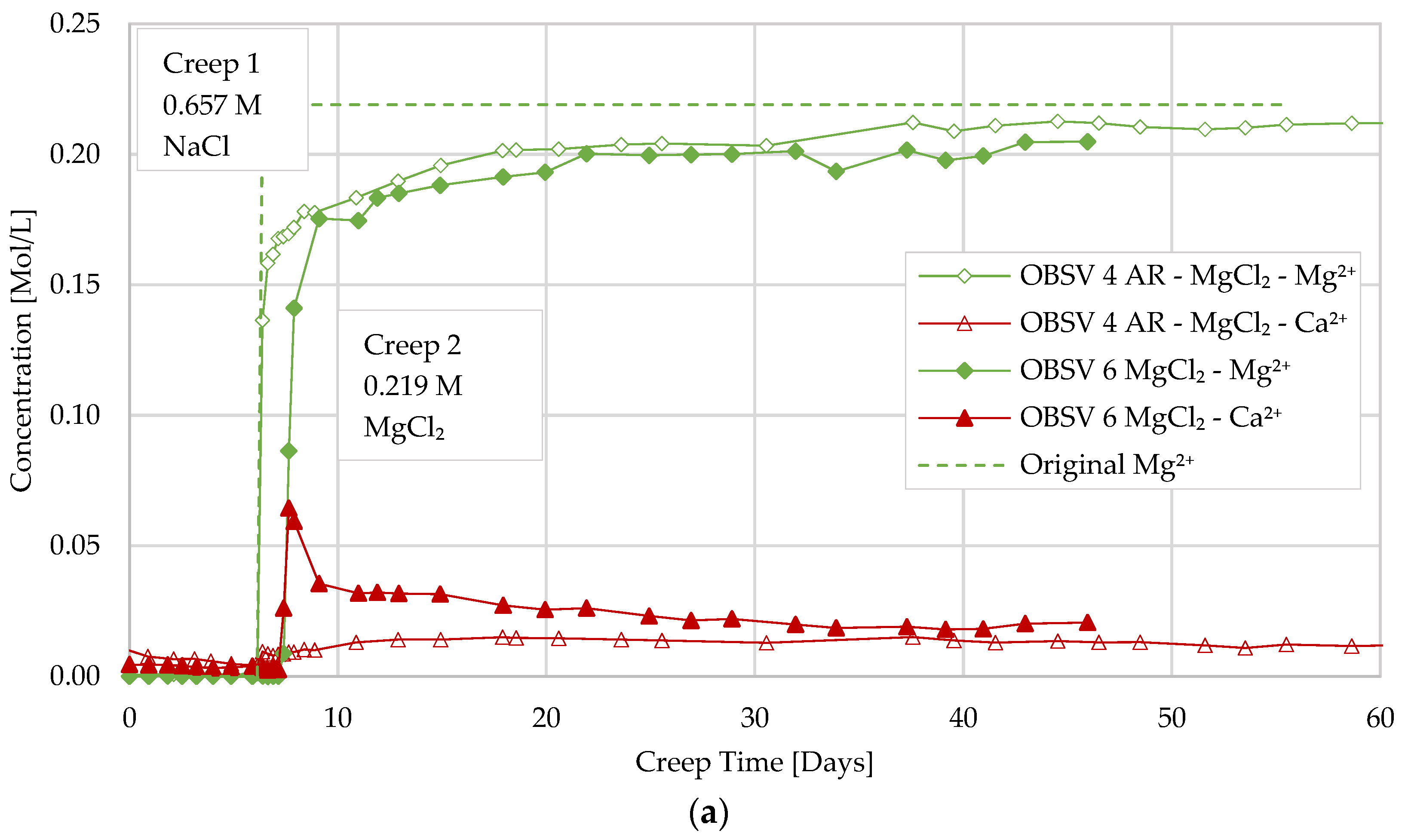
3.5. Geochemistry
3.6. Field Emission Gun Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS)
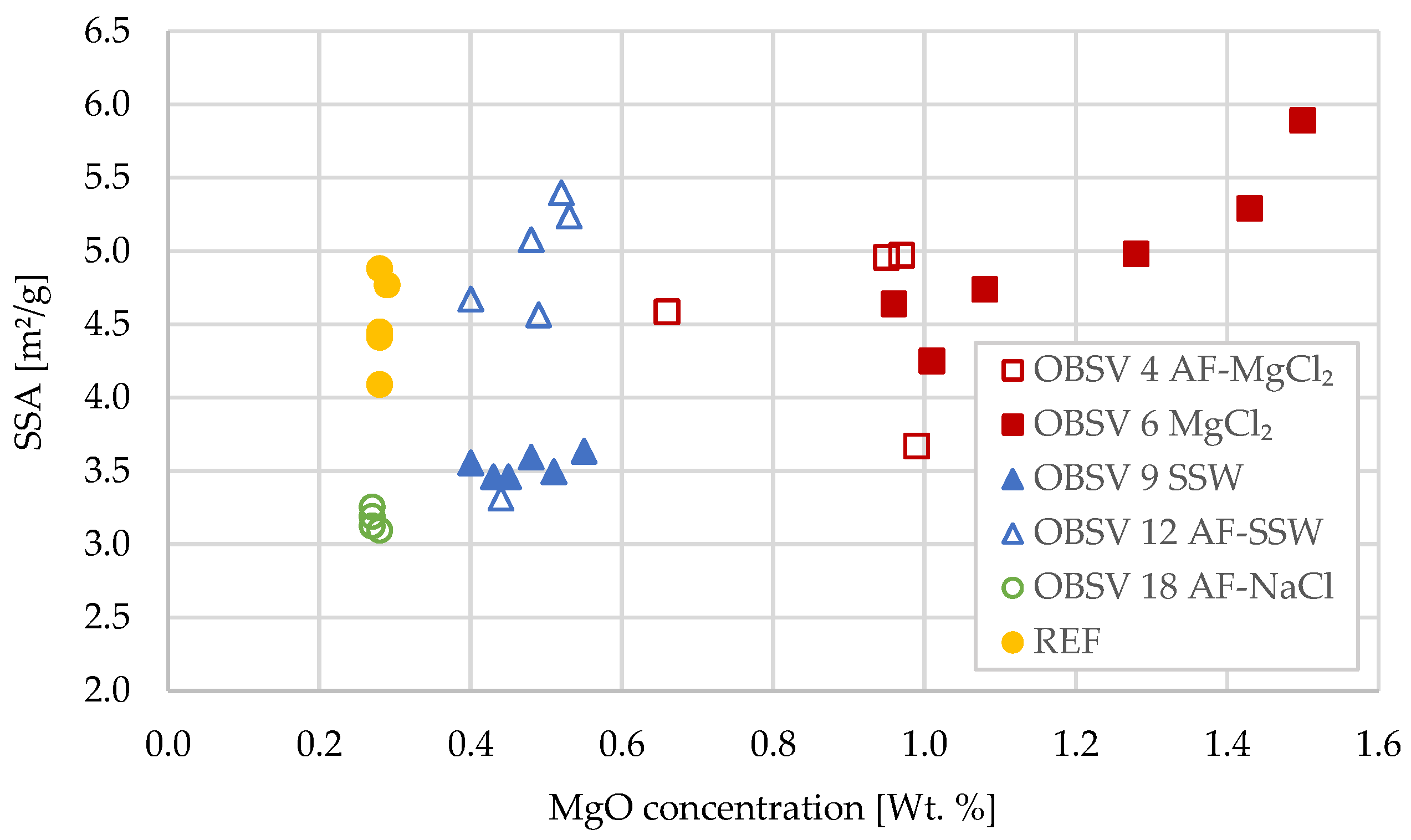
3.7. Specific Surface Area (SSA)
4. Discussion
4.1. Water-Weakening Effect versus Composition of Brine Injected
4.2. Compaction Rate in Artificially Fractured Samples versus Unfractured
4.3. Extrapolation of Compaction
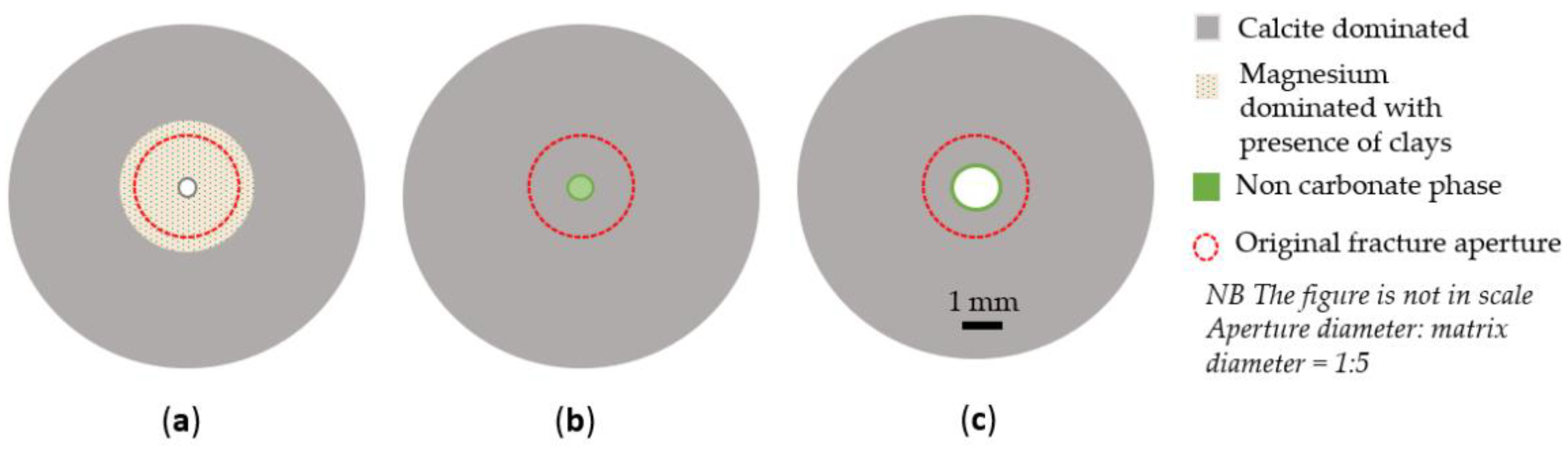
4.4. Textural Alterations versus Mineral Precipitation
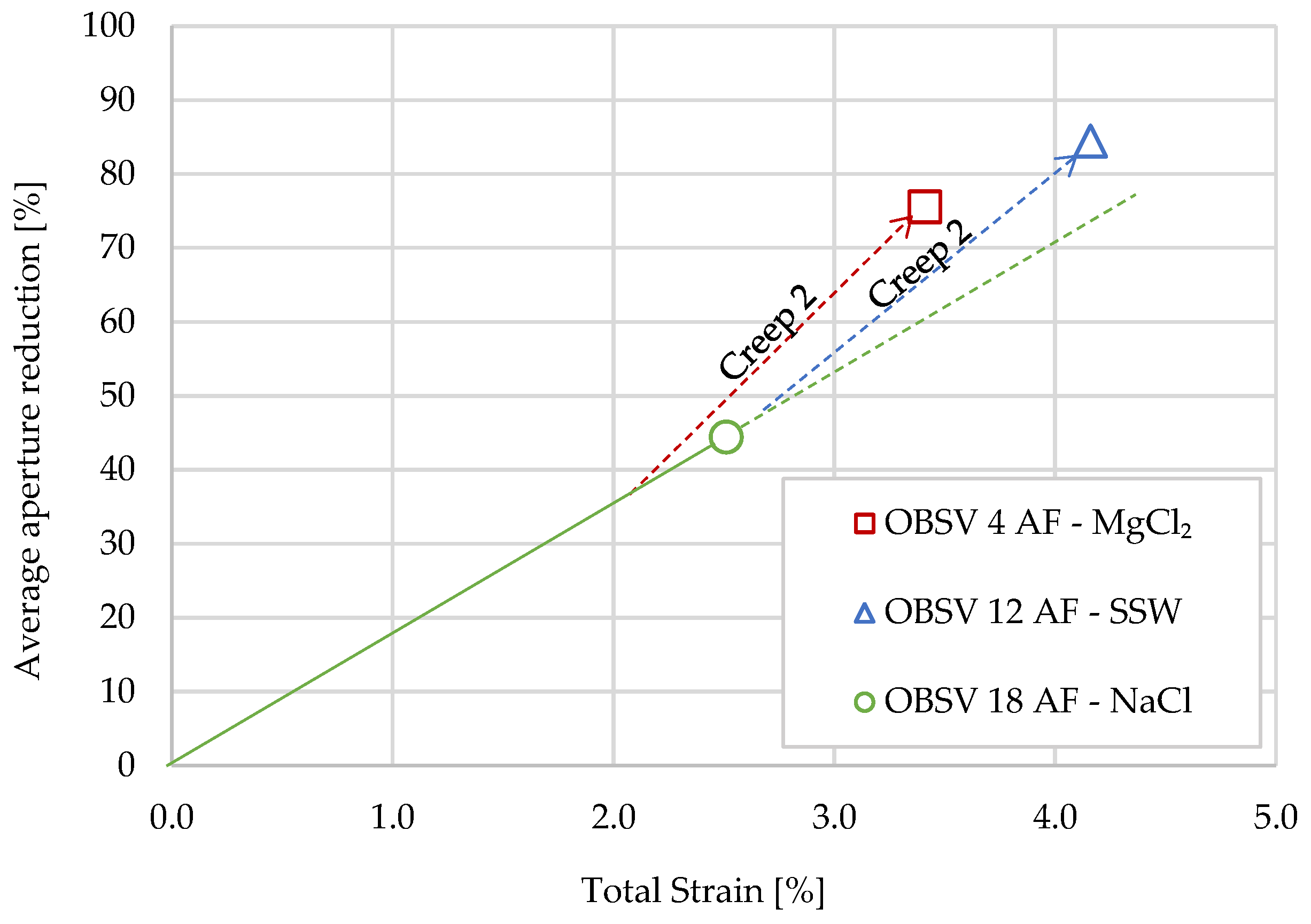
4.5. Aperture Modification versus Strain
5. Conclusions
- Flooded samples showed no significant difference in yield strength during loading when comparing unfractured with artificially fractured samples;
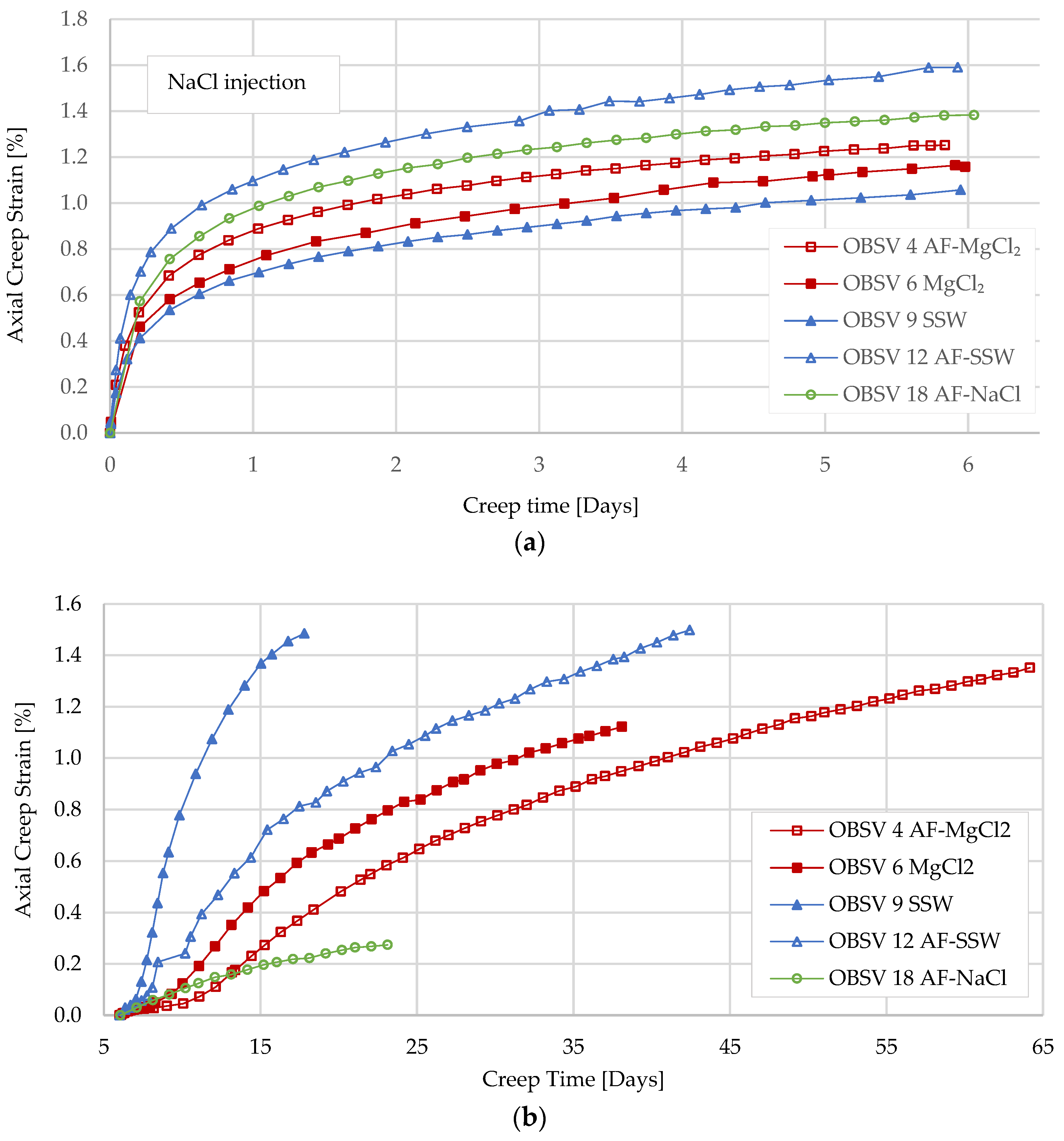
- Compaction during NaCl injection showed that artificially fractured cores experienced significantly higher axial strain compared to unfractured samples;
- Water weakening was identified during injection of SSW and MgCl2 for unfractured and artificially fractured cores. Geo-mechanical response indicated that water weakening is activated shortly after injecting brines with surface-reactive ions (SSW and MgCl2) for unfractured samples. The artificially fractured samples showed a later onset of the increased compaction rate compared to the unfractured samples. Effluent data supported the water-weakening effect;
- A delayed water-weakening effect was observed for artificially fractured cores and is suggested to be linked to a later onset of brine–rock interaction processes in peripheral matrix locations caused by the diffusion of surface-active ions from the fracture despite a low permeable matrix. Geochemistry data identified MgO precipitation in unfractured samples besides the peripheral locations of artificially fractured samples;
- SSW-flooded samples experienced axial strain beyond MgCl2-flooded cores during the test phase when different brines were injected (Creep 2). Unfractured samples compacted 1.12% and 1.49%, caused by MgCl2 and SSW, respectively. The artificially fractured SSW-flooded core experienced a total strain of 1.50%, MgCl2 exhibited 1.35%, and NaCl showed the least compaction, at 0.27% (samples had different test duration);
- Extrapolation of the geo-mechanical response indicated that unfractured samples over time will be mechanically weaker than artificial fractured samples; despite that, the results obtained in this time-limited experiment showed that unfractured samples experienced the highest strain. The extrapolation suggests that the water-weakening effect will stagnate for intact cores and be delayed to the peripheral matrix in artificially fractured cores due to diffusion. A longer test duration of a similar setup is suggested to confirm the theory;
- The original fracture aperture of 2.25 (±0.05) mm was reduced in all three artificially fractured samples. The average aperture diameter reduction throughout the samples were 84%, 76%, and 44% for SSW, MgCl2, and NaCl, respectively;
- A relationship between strain and aperture reduction was identified. The artificial aperture reduction is believed to be caused by a combination of mechanical forces, where axial and radial strains drive the matrix towards void spaces besides mineral precipitation, causing additional aperture reduction, which is dependent on the brine composition and mineral present in the sample.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tanner, R.S.; Udegbunam, E.O.; McInerney, M.J.; Knapp, R.M. Microbially enhanced oil recovery from carbonate reservoirs. Geomicrobiol. J. 1991, 9, 169–195. [Google Scholar] [CrossRef]
- Sulak, R.; Danielsen, J. Reservoir aspects of Ekofisk subsidence. J. Pet. Technol. 1989, 41, 709–716. [Google Scholar] [CrossRef]
- NPD. Available online: https://factpages.npd.no/en/field/pageview/all/43506 (accessed on 13 July 2018).
- Andersen, P.; Wang, W.; Madland, M.V.; Zimmermann, U.; Korsnes, R.I.; Bertolino, S.R.A.; Minde, M.; Schulz, B.; Gilbricht, S. Comparative Study of Five Outcrop Chalks Flooded at Reservoir Conditions: Chemo-mechanical Behaviour and Profiles of Compositional Alteration. Transp. Porous Media 2017, 121, 135–181. [Google Scholar] [CrossRef]
- Korsnes, R.; Madland, M.; Austad, T. Impact of brine composition on the mechanical strength of chalk at high Temperature. In Eurock 2006; CRC Press: Boca Raton, FL, USA, 2006; pp. 133–140. [Google Scholar] [CrossRef]
- Korsnes, R.I.; Madland, M.V.; Austad, T.; Haver, S.; Røsland, G. The effects of temperature on the water weakening of chalk by seawater. J. Pet. Sci. Eng. 2008, 60, 183–193. [Google Scholar] [CrossRef]
- Nermoen, A.; Korsnes, R.I.; Aursjø, O.; Madland, M.V.; Kjørslevik, T.A.; Østensen, G. How stress and temperature conditions affect rock-fluid chemistry and mechanical deformation. Front. Phys. 2016, 4, 2. [Google Scholar] [CrossRef]
- Strand, S.; Standnes, A.D.C.; Austad, T. Spontaneous imbibition of aqueous surfactant solutions into neutral to oil-wet carbonate cores: Effects of brine salinity and composition. Energy Fuels 2003, 17, 1133–1144. [Google Scholar] [CrossRef]
- Bjørlykke, K.; Høeg, K. Effects of burial diagenesis on stresses, compaction and fluid flow in sedimentary basins. Mar. Pet. Geol. 1997, 14, 267–276. [Google Scholar] [CrossRef]
- Minde, M.W.; Wang, W.; Madland, M.V.; Zimmermann, U.; Korsnes, R.I.; Bertolino, S.R.; Andersen, P. Temperature effects on rock engineering properties and rock-fluid chemistry in opal-CT-bearing chalk. J. Pet. Sci. Eng. 2018, 169, 454–470. [Google Scholar] [CrossRef]
- Madland, M.V.; Hiorth, A.; Omdal, E.; Megawati, M.; Hildebrand-Habel, T.; Korsnes, R.I.; Evje, S.; Cathles, L.M. Chemical alterations induced by rock–fluid interactions when injecting brines in high porosity chalks. Transp. Porous Media 2011, 87, 679–702. [Google Scholar] [CrossRef]
- Megawati, M.; Hiorth, A.; Madland, M.V. The impact of surface charge on the mechanical behavior of high-porosity chalk. Rock Mech. Rock Eng. 2012, 46, 1073–1090. [Google Scholar] [CrossRef]
- Megawati, M.; Madland, M.; Hiorth, A. Mechanical and physical behavior of high-porosity chalks exposed to chemical perturbation. J. Pet. Sci. Eng. 2015, 133, 313–327. [Google Scholar] [CrossRef] [Green Version]
- Megawati, M.; Andersen, P.Ø.; Korsnes, R.I.; Evje, S.; Hiorth, A.; Madland, M.V. The effect of aqueous chemistry pH on the time-dependent deformation behaviour of chalk- experimental and modelling study. Les Rencontres scientifiques d’IFP Energies Nouvelles. Flows and mechanics in natural porous media from pore to field scale. In Proceedings of the Pore2Fluid IFP Energies Nouvelles Paris, Aussois, France, 16–18 November 2011. [Google Scholar]
- Mukherjee, H.; Poe, B.J.; Heidt, J.H.; Watson, T.B.; Barree, R.D. Effect of Pressure Depletion on Fracture-Geometry Evolution and Production Performance. SPE Prod. Facil. 2000, 15, 144–150. [Google Scholar] [CrossRef]
- Snow, S.E.; Brownlee, M.H. Practical and theoretical aspects of well testing in the Ekofisk area chalk fields. In SPE Annual Technical Conference and Exibition; OnePetro: Richardson, TX, USA, 1989. [Google Scholar]
- Michie, E.; Haines, T.; Healy, D.; Neilson, J.; Timms, N.; Wibberley, C. Influence of carbonate facies on fault zone architecture. J. Struct. Geol. 2014, 65, 82–99. [Google Scholar] [CrossRef]
- Philip, Z.G.; Jennings, J.W.; Olson, J.E.; Laubach, S.E.; Holder, J. Modeling coupled fracture-matrix fluid flow in geomechanically simulated fracture networks. SPE Reserv. Eval. Eng. 2005, 8, 300–309. [Google Scholar] [CrossRef]
- Chester, F.M.; Evans, J.P.; Biegel, R.L. Internal structure and weakening mechanisms of the San Andreas Fault. J. Geophys. Res. Earth Surf. 1993, 98, 771–786. [Google Scholar] [CrossRef]
- Caine, J.S.; Evans, J.P.; Forster, C.B. Fault zone architecture and permeability structure. Geology 1996, 24, 1025–1028. [Google Scholar] [CrossRef]
- Goddard, J.V.; Evans, J.P. Chemical changes and fluid-rock interaction in faults of crystalline thrust sheets, northwestern Wyoming, U.S.A. J. Struct. Geol. 1995, 17, 533–547. [Google Scholar] [CrossRef]
- Laubach, S.E. Practical approaches to identifying sealed and open fractures. AAPG Bull. 2003, 87, 561–579. [Google Scholar] [CrossRef]
- Wibberley, C.A.; Shimamoto, T. Internal structure and permeability of major strike-slip fault zones: The Median Tectonic Line in Mie Prefecture, Southwest Japan. J. Struct. Geol. 2003, 25, 59–78. [Google Scholar] [CrossRef]
- Agosta, F.; Prasad, M.; Aydin, A. Physical properties of carbonate fault rocks, fucino basin (Central Italy): Implications for fault seal in platform carbonates. Geofluids 2007, 7, 19–32. [Google Scholar] [CrossRef]
- Hjuler, M.; Fabricius, I. Engineering properties of chalk related to diagenetic variations of Upper Cretaceous onshore and offshore chalk in the North Sea area. J. Pet. Sci. Eng. 2009, 68, 151–170. [Google Scholar] [CrossRef]
- Geitle, K. Chemically Induced Compaction in Fractured and Intact Chalk Cores in Institute for Energy and Resources (IER). Master’s Thesis, University of Stavanger, Stavanger, Norway, 2013; p. 78. Available online: https://uis.brage.unit.no/uis (accessed on 13 December 2021).
- Abubeker, E. Water weakening of chalks-comparsion of intact and fractured cores, in Faculty of Science and Technology. Master’s Thesis, University of Stavanger, Stavanger, Norway, 2013; p. 88. Available online: https://uis.brage.unit.no/ (accessed on 13 December 2021).
- Madland, M.V.; Finsnes, A.; Alkafadgi, A.; Risnes, R.; Austad, T. The influence of CO2 gas and carbonate water on the mechanical stability of chalk. J. Pet. Sci. Eng. 2006, 51, 149–168. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Wen, S.T.S. Systematic Specific Surface Area Analysis on Rocks to Implement as a Necessary, Quick, and Informative Method to Understand Geo-Mechanical Parameter in IOR Experiments, in Faculty of Science and Technology. Master’s Thesis, University of Stavanger, Stavanger, Norway, 2018; p. 253. Available online: https://uis.brage.unit.no/uis-xmlui/handle/11250/2570740 (accessed on 3 February 2022).
- Minde, M.W.; Zimmermann, U.; Madland, M.V.; Korsnes, R.I.; Schulz, B.; Gilbricht, S. Mineral replacement in long-term flooded porous carbonate rocks. Geochim. Cosmochim. Acta 2019, 268, 485–508. [Google Scholar] [CrossRef]
- Kallesten, E.; Zimmermann, U.; Madland, M.; Bertolino, S.; Omdal, E.; Andersen, P. Mineralogy and geochemistry of reservoir and non-reservoir chalk from the Norwegian continental shelf. J. Pet. Sci. Eng. 2021, 205, 108914. [Google Scholar] [CrossRef]
- Madland, M.V.; Midtgarden, K.; Manafov, R.; Korsnes, R.I.; Kristiansen, T.; Hiorth, A. The effect of temperature and brine composition on the mechanical strength of Kansas chalk. In Proceedings of the International Symposium of the Society of Core Analysts, Abu Dhabi, United Arab Emirates, 29 October–2 November 2008; p. 6. Available online: scaweb.org (accessed on 16 April 2022).
- Kallesten, E.; Andersen, P.; Berawala, D.S.; Korsnes, R.I.; Madland, M.V.; Omdal, E.; Zimmermann, U. Modeling of Permeability and Strain Evolution in Chemical Creep Compaction Experiments with Fractured and Unfractured Chalk Cores Conducted at Reservoir Conditions. SPE J. 2020, 25, 2710–2728. [Google Scholar] [CrossRef]
- Shahbazi, A.; Monfared, M.S.; Thiruchelvam, V.; Fei, T.K.; Babasafari, A.A. Integration of knowledge-based seismic inversion and sedimentological investigations for heterogeneous reservoir. J. Southeast Asian Earth Sci. 2020, 202, 104541. [Google Scholar] [CrossRef]
- Soleimani, M. Naturally fractured hydrocarbon reservoir simulation by elastic fracture modeling. Pet. Sci. 2017, 14, 286–301. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, U.; Madland, M.V.; Nermoen, A.; Hildebrand-Habel, T.; Bertolino, S.A.; Hiorth, A.; Korsnes, R.I.; Audinot, J.-N.; Grysan, P. Evaluation of the compositional changes during flooding of reactive fluids using scanning electron microscopy, nano-secondary ion mass spectrometry, X-ray diffraction, and whole-rock geochemistry Compositional Changes during Flooding. AAPG Bull. 2015, 99, 791–805. [Google Scholar] [CrossRef] [Green Version]
- Gale, J.F.; Lander, R.H.; Reed, R.M.; Laubach, S. Modeling fracture porosity evolution in dolostone. J. Struct. Geol. 2010, 32, 1201–1211. [Google Scholar] [CrossRef]


| Brine/mol/L | Cl− | Na+ | K+ | Mg2+ | Ca2+ | SO42− |
|---|---|---|---|---|---|---|
| MgCl2 | 0.438 | * | * | 0.219 | * | * |
| SSW | 0.525 | 0.450 | 0.010 | 0.0445 | 0.013 | 0.024 |
| NaCl | 0.657 | 0.657 | * | * | * | * |
| Sample | Porosity | Permeability | Strain | Yield Strength | Bulk Modulus | Duration | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| [%] | [mD] | [%] | [MPa] | [GPa] | [Days] | ||||||
| Pre-Test | Post-Test | Start | Stop | Loading | Creep 1 | Creep 2 | Total | ||||
| OBSV 4 AF-MgCl2 | 40.49 | 37.95 | * | * | 0.81 | 1.25 | 1.35 | 3.41 | 9.4 | 0.750 | 62 |
| OBSV 6 MgCl2 | 40.81 | 38.60 | 0.53 | 0.17 | 0.80 | 1.16 | 1.12 | 3.08 | 9.9 | 0.685 | 46 |
| OBSV 9 SSW | 41.34 | 37.02 | 0.59 | 0.00 | 0.90 | 1.06 | 1.49 | 3.45 | 9.1 | 0.696 | 18 |
| OBSV 12 AF-SSW | 41.34 | 37.15 | * | * | 1.07 | 1.59 | 1.50 | 4.16 | 9.4 | 0.625 | 53 |
| OBSV 18 AF-NaCl | 40.18 | * | * | * | 0.86 | 1.38 | 0.27 | 2.51 | 9.4 | 0.730 | 27 |
| Element wt.% | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | P2O5 | LOI | Sum |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Detection limit LF 200 | 0.01 | 0.01 | 0.04 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | ||
| OBSV 4 REF IN | 3.32 | 0.97 | 0.34 | 0.29 | 52.06 | 0.04 | 0.22 | 0.04 | 0.06 | 42.5 | 99.84 |
| OBSV 4 AF-MgCl2 #1 | 3.38 | 0.99 | 0.31 | 0.66 | 51.81 | 0.03 | 0.18 | 0.04 | 0.05 | 42.4 | 99.85 |
| OBSV 4 AF-MgCl2 #3 | 3.39 | 1.00 | 0.36 | 0.97 | 51.37 | 0.03 | 0.16 | 0.05 | 0.07 | 42.4 | 99.80 |
| OBSV 4 AF-MgCl2 #4 | 3.32 | 0.98 | 0.34 | 0.99 | 51.73 | 0.03 | 0.15 | 0.04 | 0.09 | 42.1 | 99.77 |
| OBSV 4 AF-MgCl2 #6 | 3.37 | 0.98 | 0.34 | 0.98 | 51.67 | 0.03 | 0.16 | 0.04 | 0.06 | 42.2 | 99.83 |
| OBSV 4 AF-MgCl2 #7 | 3.32 | 0.98 | 0.35 | 0.95 | 51.68 | 0.03 | 0.15 | 0.04 | 0.06 | 42.2 | 99.76 |
| OBSV 4 REF OUT | 3.50 | 1.03 | 0.37 | 0.28 | 52.01 | 0.04 | 0.23 | 0.05 | 0.05 | 42.3 | 99.86 |
| OBSV 6 REF IN | 3.38 | 1.00 | 0.34 | 0.28 | 52.29 | 0.04 | 0.22 | 0.05 | 0.06 | 42.2 | 99.86 |
| OBSV 6 MgCl2 #2 | 3.19 | 1.00 | 0.34 | 1.50 | 51.24 | 0.03 | 0.15 | 0.05 | 0.07 | 42.2 | 99.77 |
| OBSV 6 MgCl2 #3 | 3.37 | 1.02 | 0.36 | 1.43 | 51.21 | 0.03 | 0.15 | 0.05 | 0.08 | 42.1 | 99.80 |
| OBSV 6 MgCl2 #4 | 3.27 | 1.00 | 0.34 | 1.28 | 51.38 | 0.04 | 0.16 | 0.05 | 0.09 | 42.2 | 99.81 |
| OBSV 6 MgCl2 #5 | 3.31 | 1.02 | 0.41 | 1.08 | 51.68 | 0.03 | 0.17 | 0.05 | 0.08 | 42.0 | 99.83 |
| OBSV 6 MgCl2 #6 | 3.52 | 1.05 | 0.38 | 0.96 | 51.50 | 0.04 | 0.17 | 0.05 | 0.10 | 42.0 | 99.77 |
| OBSV 6 MgCl2 #7 | 3.54 | 1.03 | 0.40 | 1.01 | 51.86 | 0.04 | 0.18 | 0.05 | 0.08 | 41.6 | 99.79 |
| OBSV6 REF OUT | 3.40 | 1.03 | 0.33 | 0.30 | 51.89 | 0.04 | 0.23 | 0.05 | 0.09 | 42.5 | 99.86 |
| OBSV9 REF IN | 3.44 | 1.01 | 0.39 | 0.29 | 51.97 | 0.04 | 0.22 | 0.05 | 0.10 | 42.3 | 99.81 |
| OBSV 9 SSW #1 | 3.37 | 1.04 | 0.36 | 0.55 | 51.88 | 0.04 | 0.21 | 0.05 | 0.10 | 42.2 | 99.80 |
| OBSV 9 SSW #2 | 3.43 | 1.03 | 0.40 | 0.51 | 51.64 | 0.04 | 0.22 | 0.05 | 0.09 | 42.4 | 99.81 |
| OBSV 9 SSW #3 | 3.52 | 1.04 | 0.38 | 0.48 | 51.50 | 0.04 | 0.23 | 0.05 | 0.08 | 42.5 | 99.82 |
| OBSV9 SSW #4 | 3.45 | 1.04 | 0.39 | 0.45 | 51.65 | 0.05 | 0.22 | 0.05 | 0.08 | 42.5 | 99.88 |
| OBSV 9 SSW #5 | 3.49 | 1.02 | 0.42 | 0.43 | 51.80 | 0.05 | 0.23 | 0.05 | 0.07 | 42.3 | 99.86 |
| OBSV 9 SSW #6 | 3.55 | 1.04 | 0.42 | 0.40 | 51.60 | 0.05 | 0.24 | 0.05 | 0.06 | 42.4 | 99.81 |
| OBSV9 REF OUT | 3.59 | 1.05 | 0.47 | 0.28 | 52.00 | 0.04 | 0.23 | 0.05 | 0.07 | 42.0 | 99.78 |
| OBSV 12 REF IN | 3.58 | 1.05 | 0.40 | 0.28 | 51.89 | 0.04 | 0.24 | 0.05 | 0.06 | 42.2 | 99.79 |
| OBSV 12 AF-SSW #1 | 3.61 | 1.07 | 0.47 | 0.44 | 51.93 | 0.04 | 0.24 | 0.05 | 0.08 | 41.9 | 99.83 |
| OBSV 12 AF-SSW #2 | 3.52 | 1.05 | 0.38 | 0.48 | 52.05 | 0.04 | 0.22 | 0.05 | 0.07 | 42.0 | 99.86 |
| OBSV 12 AF-SSW #3 | 3.56 | 1.07 | 0.40 | 0.53 | 51.93 | 0.03 | 0.23 | 0.05 | 0.08 | 41.9 | 99.78 |
| OBSV 12 AF-SSW #4 | 3.64 | 1.08 | 0.43 | 0.52 | 51.88 | 0.04 | 0.22 | 0.05 | 0.06 | 41.9 | 99.82 |
| OBSV 12 AF-SSW #5 | 3.60 | 1.06 | 0.39 | 0.49 | 51.67 | 0.04 | 0.23 | 0.05 | 0.07 | 42.2 | 99.80 |
| OBSV 12 AF-SSW #6 | 3.62 | 1.08 | 0.43 | 0.40 | 51.94 | 0.04 | 0.24 | 0.05 | 0.08 | 42.0 | 99.88 |
| OBSV 18 AF-NaCl #1 | 3.49 | 1.02 | 0.39 | 0.28 | 52.21 | 0.06 | 0.22 | 0.05 | 0.09 | 42.0 | 99.81 |
| OBSV 18 AF-NaCl #2 | 3.30 | 0.98 | 0.38 | 0.27 | 52.52 | 0.06 | 0.21 | 0.04 | 0.10 | 42.0 | 99.86 |
| OBSV 18 AF-NaCl #3 | 3.21 | 0.98 | 0.36 | 0.27 | 52.46 | 0.06 | 0.21 | 0.04 | 0.09 | 42.1 | 99.78 |
| OBSV 18 AF-NaCl #4 | 3.30 | 1.00 | 0.38 | 0.27 | 52.46 | 0.07 | 0.21 | 0.04 | 0.07 | 42.0 | 99.80 |
| OBSV 18 AF-NaCl #5 | 3.28 | 0.99 | 0.39 | 0.27 | 52.38 | 0.07 | 0.21 | 0.04 | 0.07 | 42.1 | 99.80 |
| OBSV 18 AF-NaCl #6 | 3.39 | 1.09 | 0.43 | 0.28 | 52.94 | 0.06 | 0.22 | 0.05 | 0.11 | 41.6 | 100.16 |
| OBSV 18 REF IN | 3.39 | 1.00 | 0.54 | 0.27 | 51.90 | 0.04 | 0.22 | 0.05 | 0.10 | 42.3 | 99.81 |
| Detection limit FUS-ICP | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.001 | 0.01 | ||
| OBSV 4 AF-MgCl2 CF #1 | 8.77 | 1.55 | 0.77 | 41.05 | 1.95 | <0.01 | 0.24 | 0.074 | 0.12 | 54.55 | |
| OBSV 4 AF-MgCl2 CF #7 | 4.96 | 1.14 | 0.44 | 42.68 | 4.11 | <0.01 | 0.16 | 0.05 | 0.1 | 53.69 |
| OBSV 4 AF-MgCl2 | OBSV 6 MgCl2 | OBSV 9 SSW | OBSV 12 AF-SSW | OBSV 18 AF-NaCl | |
|---|---|---|---|---|---|
| m2/g | m2/g | m2/g | m2/g | m2/g | |
| Slice 1 | 4.58 | 5.49 | 3.64 | 3.32 | 3.09 |
| Slice 2 | * | 5.89 | 3.49 | 5.08 | 3.25 |
| Slice 3 | 4.97 | 5.29 | 3.60 | * | 3.19 |
| Slice 4 | 3.67 | 4.98 | 3.46 | 5.40 | 3.12 |
| Slice 5 | * | 4.74 | 3.46 | * | 3.13 |
| Slice 6 | 4.95 | * | 3.56 | 4.67 | * |
| Slice 7 | * | 4.25 | * | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bredal, T.V.; Korsnes, R.I.; Zimmermann, U.; Minde, M.W.; Madland, M.V. Water Weakening of Artificially Fractured Chalk, Fracture Modification and Mineral Precipitation during Water Injection—An Experimental Study. Energies 2022, 15, 3817. https://doi.org/10.3390/en15103817
Bredal TV, Korsnes RI, Zimmermann U, Minde MW, Madland MV. Water Weakening of Artificially Fractured Chalk, Fracture Modification and Mineral Precipitation during Water Injection—An Experimental Study. Energies. 2022; 15(10):3817. https://doi.org/10.3390/en15103817
Chicago/Turabian StyleBredal, Tine Vigdel, Reidar Inge Korsnes, Udo Zimmermann, Mona Wetrhus Minde, and Merete Vadla Madland. 2022. "Water Weakening of Artificially Fractured Chalk, Fracture Modification and Mineral Precipitation during Water Injection—An Experimental Study" Energies 15, no. 10: 3817. https://doi.org/10.3390/en15103817
APA StyleBredal, T. V., Korsnes, R. I., Zimmermann, U., Minde, M. W., & Madland, M. V. (2022). Water Weakening of Artificially Fractured Chalk, Fracture Modification and Mineral Precipitation during Water Injection—An Experimental Study. Energies, 15(10), 3817. https://doi.org/10.3390/en15103817





