Evolution of Biomarker Maturity Parameters and Feedback to the Pyrolysis Process for In Situ Conversion of Nongan Oil Shale in Songliao Basin
Abstract
1. Introduction
2. Samples and Methods
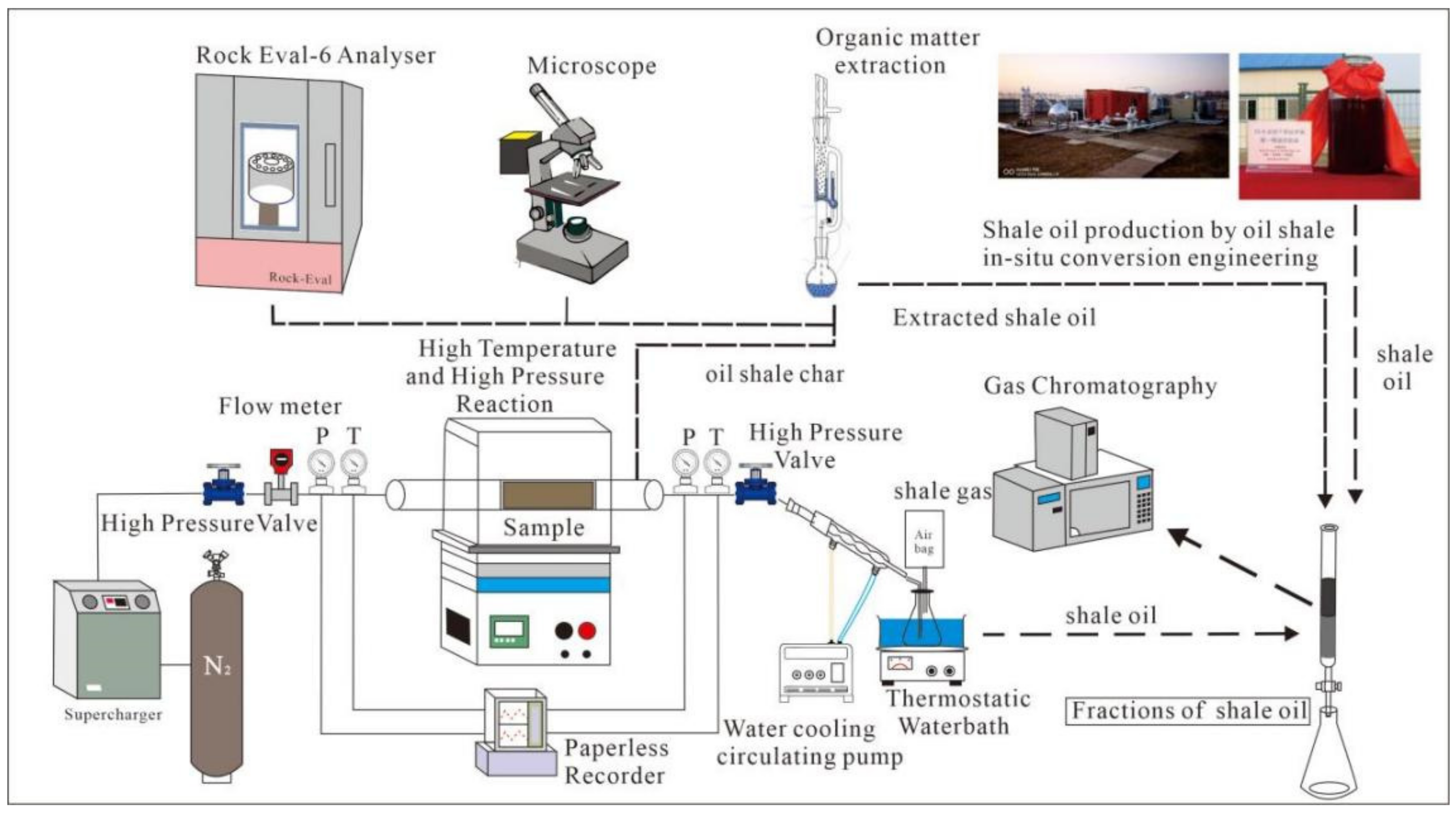
2.1. Research Methodology
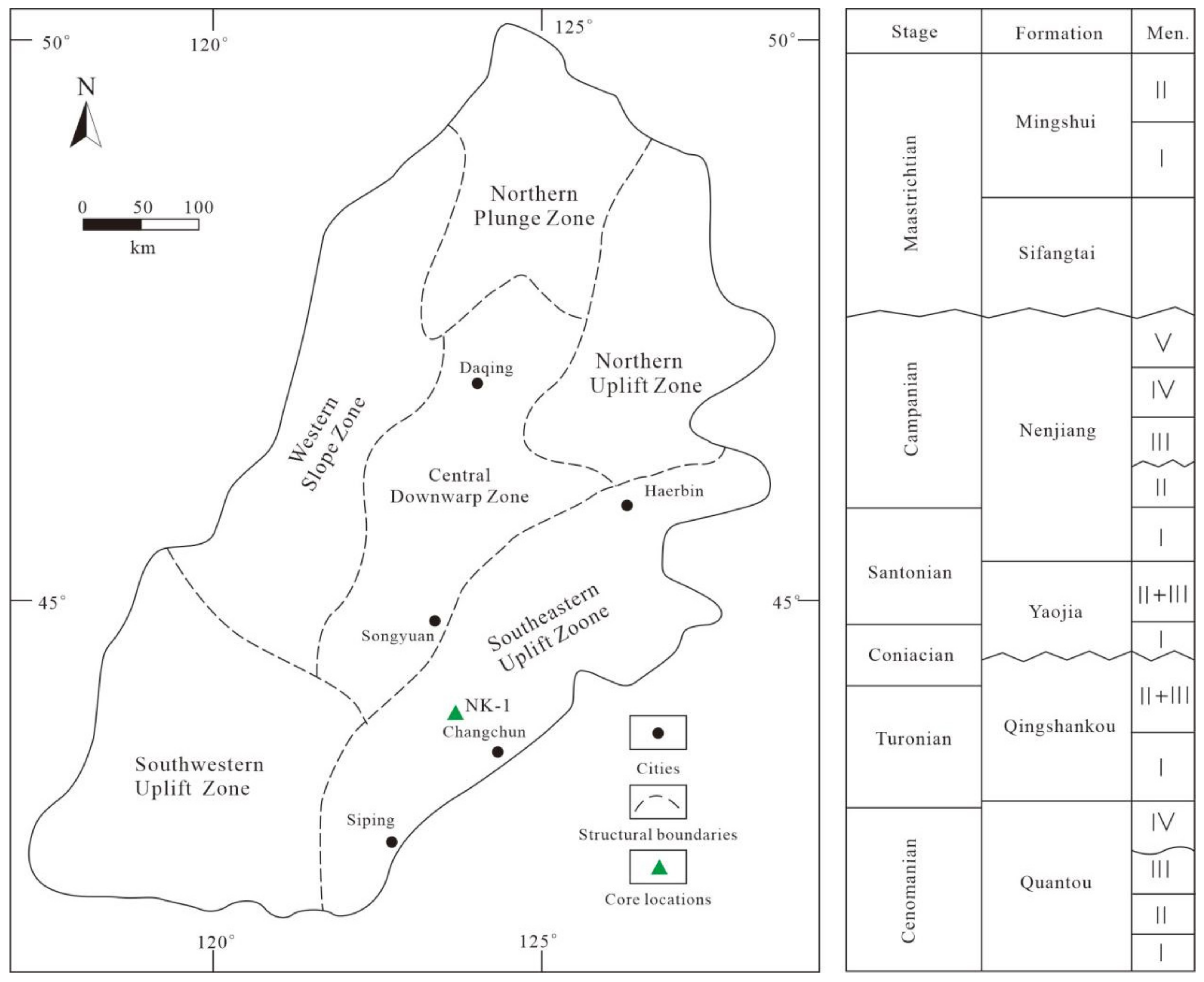
2.2. Selection of Oil Shale Samples
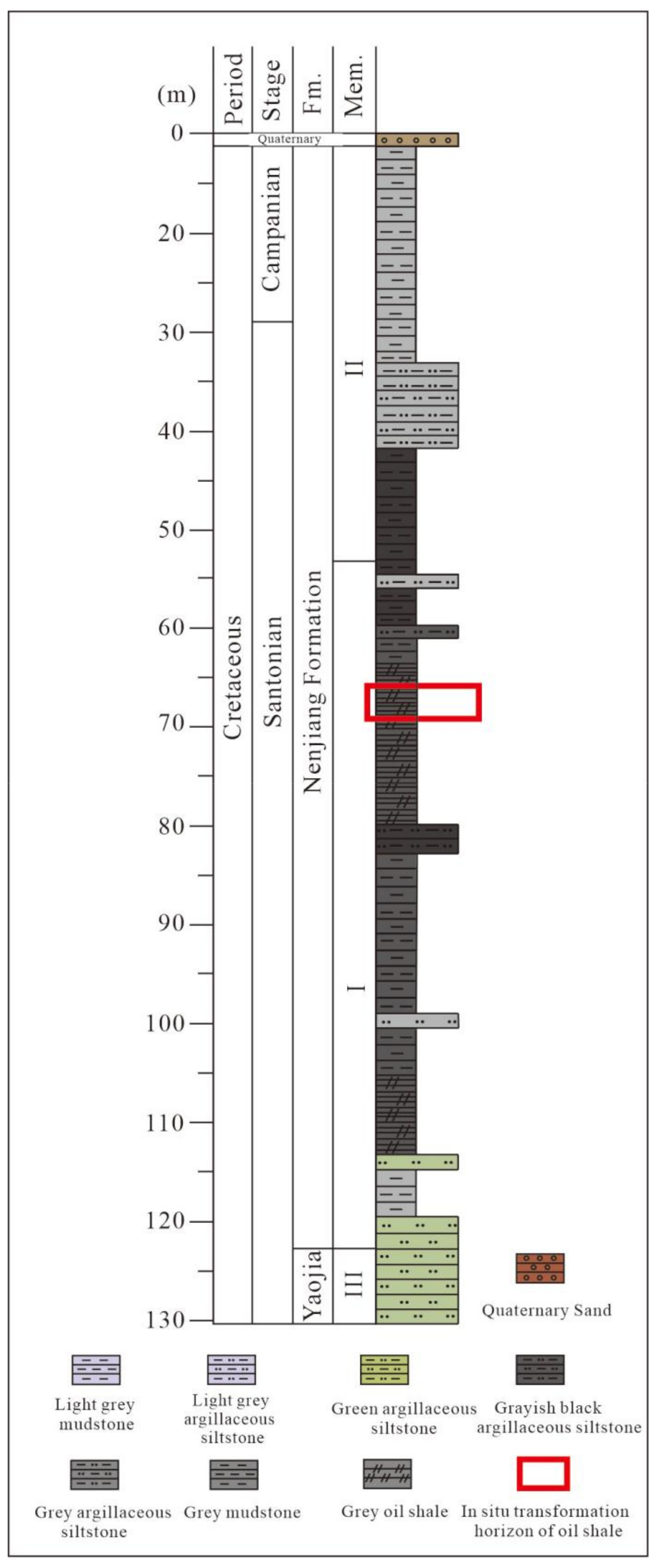
2.2.1. Stratigraphy of the Nenjiang Formation
2.2.2. Sampling of Shale Oil Samples for In Situ Conversion Project
2.3. Experimental Methods
- (1)
- High-pressure pyrolysis experiment
- (2)
- Rock pyrolysis evaluation experiment with oil shale semicoke
- (3)
- Total organic carbon
- (4)
- Separation of pyrolysis oil components
- (5)
- Gas chromatography-mass spectrometry
- (6)
- Organic lithofacies observations and vitrinite reflectance (Ro) test
3. Results and Discussion
3.1. Evolutionary Characteristics of Organic Matter at Different Pyrolysis Temperatures
3.1.1. Analysis of the High-Pressure Pyrolysis of Oil Shale
3.1.2. TOC Content
3.1.3. The Evolutionary Trend of Ro% and Tmax
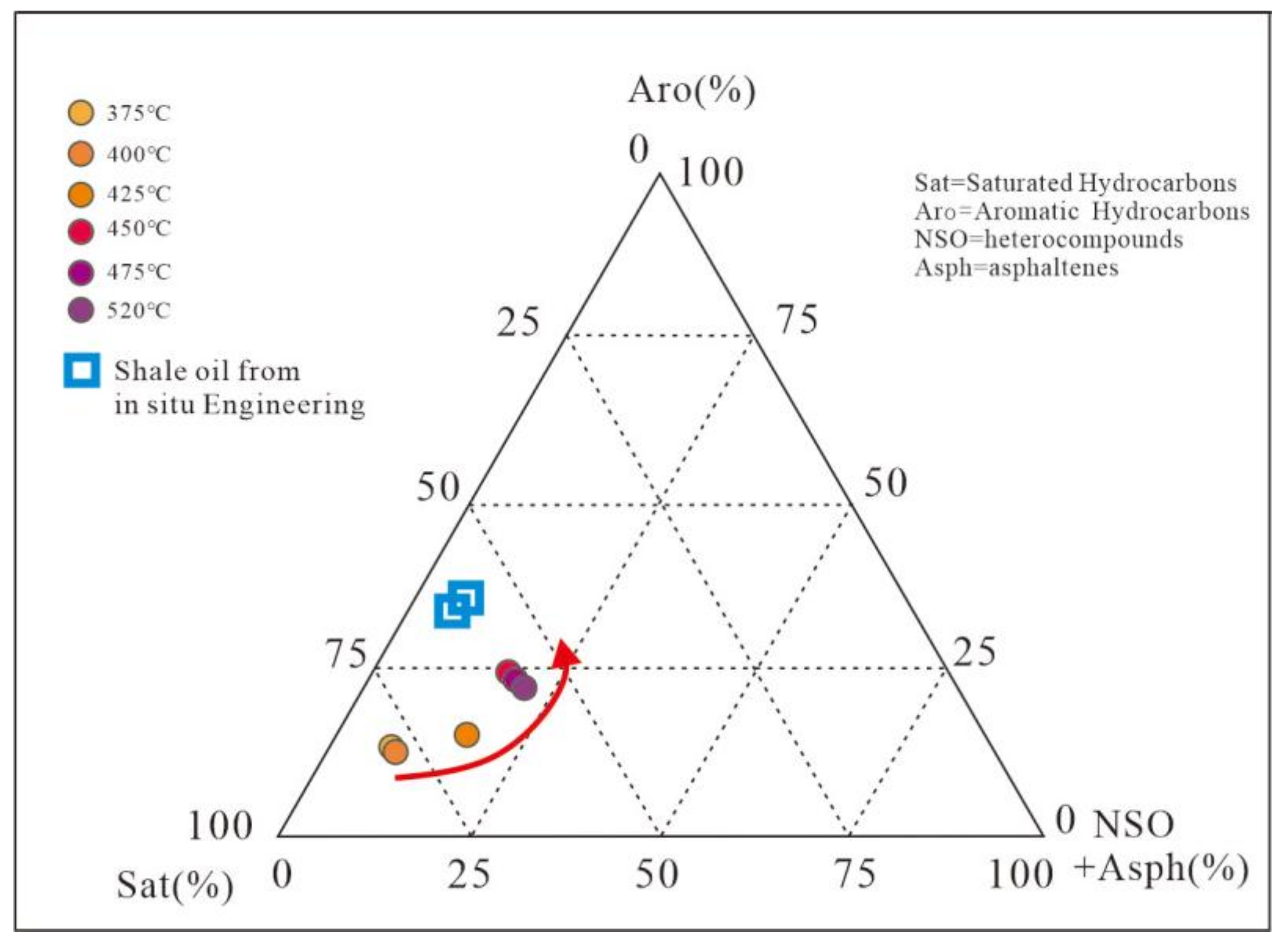
3.1.4. Changes in Pyrolysis Oil Composition
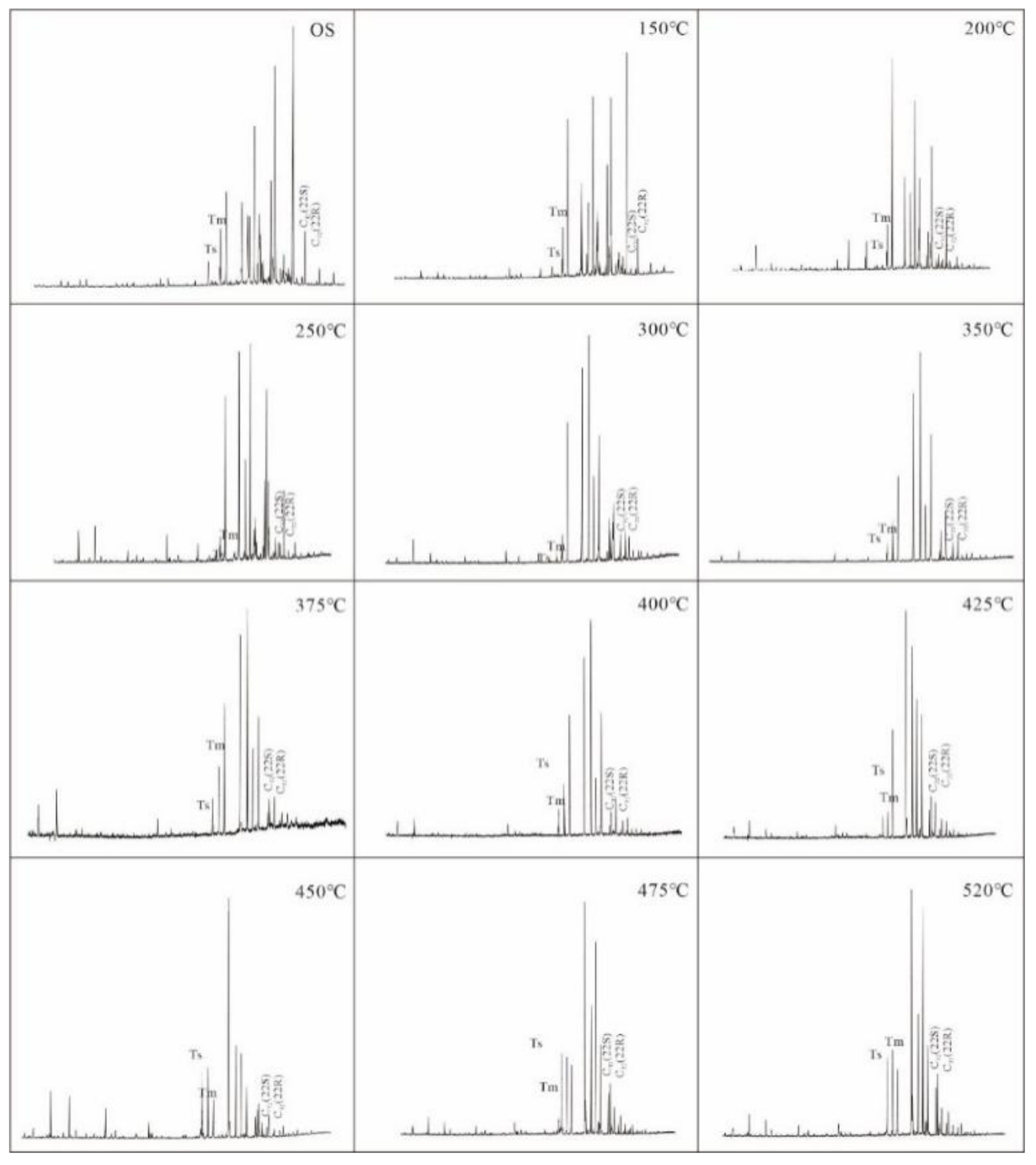
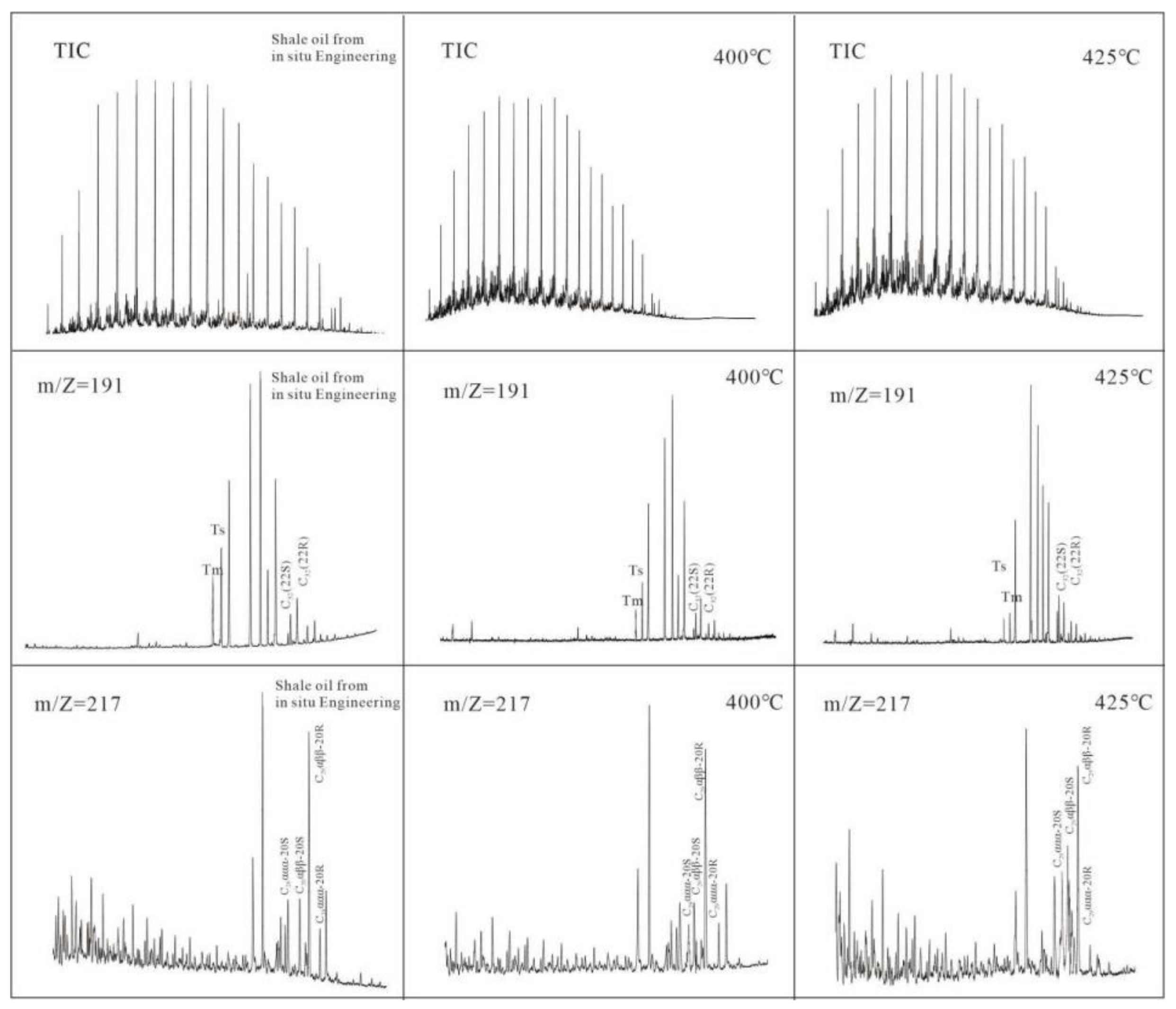
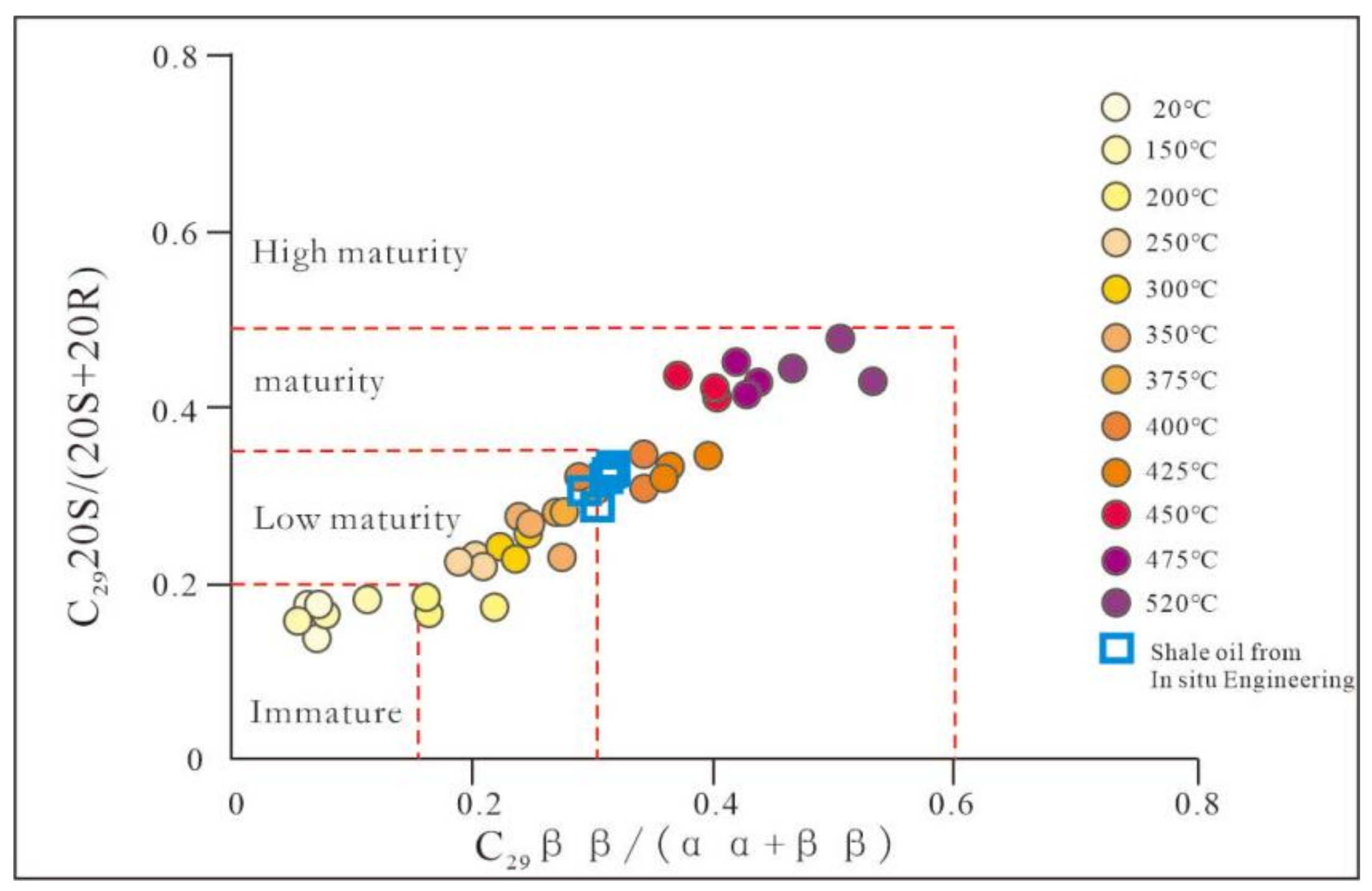
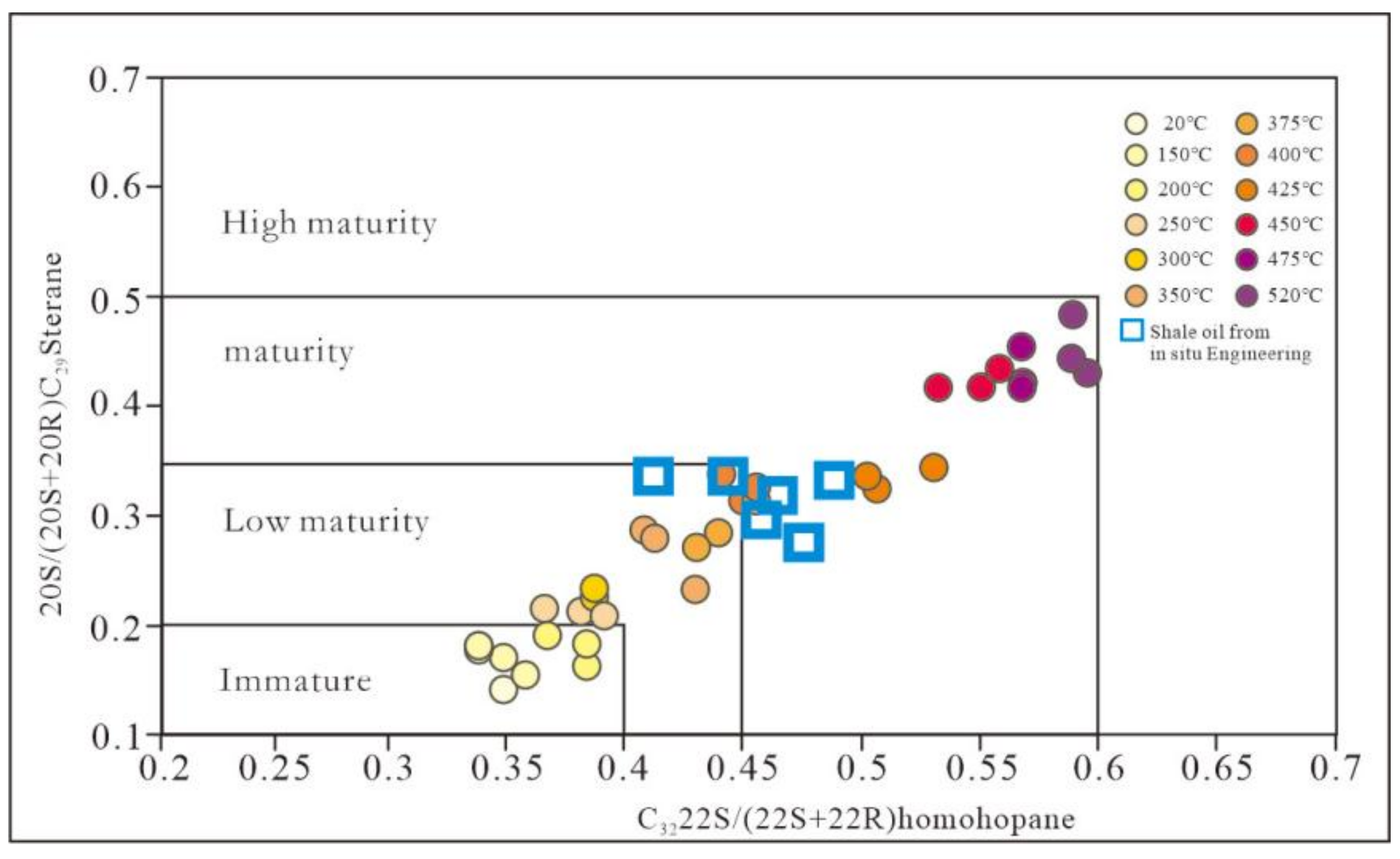
3.2. Biomarker Maturity Parameter of Pyrolysis Oil
3.3. Feedback of Maturity Parameters on the Progress of the Pyrolysis Reaction
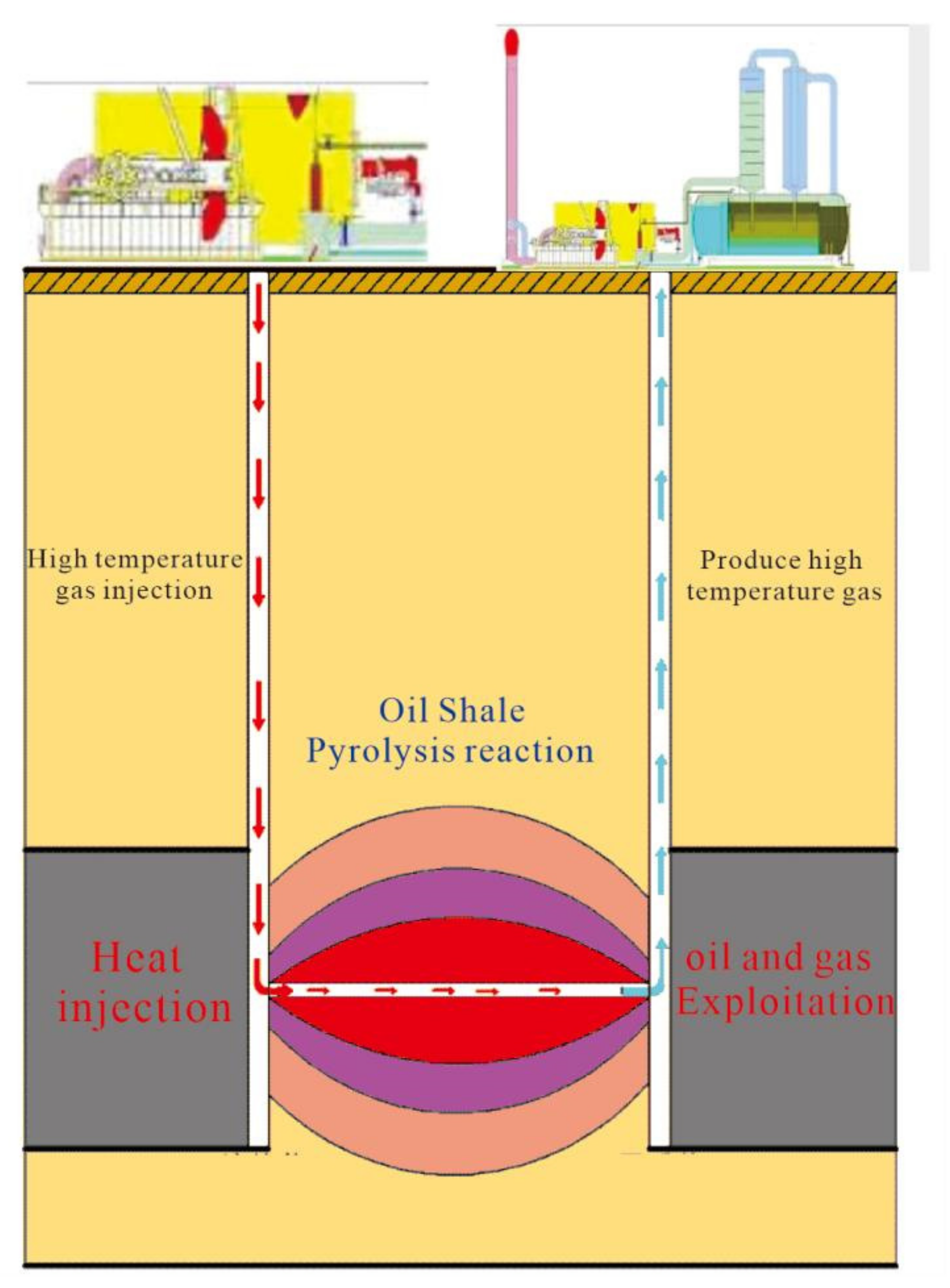
3.4. Analysis of Pyrolysis Process of Nong’an Oil Shale In Situ Conversion Project
3.5. Calculation and Application Feasibility of the In Situ Conversion Degree of Nongan Oil Shale
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dyni, J.R. Geology and Resources of Some World Oil Shale Deposits; US Geological Survey Scientific Investigations: Reston, VA, USA, 2006. [Google Scholar]
- Kok, M.V. Oil shale resources in Turkey. Oil Shale 2006, 23, 209–210. [Google Scholar]
- Taciuk, W. Does oil shale have a significant future? Oil Shale 2013, 30, 1–5. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, X.M.; Han, X.X.; Tong, J.H. Investigation of Chinese oil shale resources comprehensive utilization performance. Energy 2012, 42, 224–232. [Google Scholar] [CrossRef]
- Jiang, X.M.; Han, X.X.; Cui, Z.G. New technology for the comprehensive utilization of Chinese oil shale resources. Energy 2007, 32, 772–777. [Google Scholar] [CrossRef]
- Han, X.X.; Niu, M.T.; Jiang, X.M. Combined fluidized bed retorting and circulating fluidized bed combustion system of oil shale: 2. Energy and economic analysis. Energy 2014, 74, 788–794. [Google Scholar] [CrossRef]
- Altun, N.E.; Hicyilmaz, C.; Wang, J.Y.; Baggi, A.S.; Kok, M.V. Oil shales in the world and Turkey; reserves, current situation and future prospects: A review. Oil Shale 2006, 23, 211–227. [Google Scholar]
- Pan, Y.; Zhang, X.M.; Liu, S.H.; Yang, S.C.; Ren, N. A review on technologies for oil shale surface retort. J. Chem. Soc. Pak. 2012, 34, 1331–1338. [Google Scholar]
- Pan, S.; Wang, Q.; Bai, J.; Liu, H.; Chi, M.; Cui, D.; Xu, F. Investigation of Behavior of Sulfur in Oil Fractions During Oil Shale Pyrolysis. Energy Fuels 2019, 33, 10622–10637. [Google Scholar] [CrossRef]
- Gavrilova, O.; Vilu, R.; Vallner, L. A life cycle environmental impact assessment of oil shale produced and consumed in Estonia. Resour. Conserv. Recycl. 2010, 55, 232–245. [Google Scholar] [CrossRef]
- Velts, O.; Uibu, M.; Rudjak, I.; Kallas, J.; Kuusik, R. Utilization of oil shale ash to prepare PCC: Leachibility dynamics and equilibrium in the ash-water system. Energy Procedia 2009, 1, 4843–4850. [Google Scholar] [CrossRef]
- Trikkela, A.; Kuusik, R.; Martins, A.; Pihu, T.; Stencel, J.M. Utilization of Estonian oil shale semicoke. Fuel Process. Technol. 2008, 89, 756–763. [Google Scholar] [CrossRef]
- Bai, F.T.; Sun, Y.H.; Liu, Y.M.; Guo, M.Y. Evaluation of the porous structure of Huadian oil shale during pyrolysis using multiple approaches. Fuel 2016, 187, 1–8. [Google Scholar] [CrossRef]
- Burnham, A.K. Porosity and permeability of Green River oil shale and their changes during retorting. Fuel 2017, 203, 208–213. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Sun, P.; Li, Y.; George, S.C. Sedimentary basin evolution, gravity flows, volcanism, and their impacts on the formation of the Lower Cretaceous oil shales in the Chaoyang Basin, northeastern China. Mar. Pet. Geol. 2020, 119, 104472. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Guo, W.; Shan, X. Controlling the in-situ conversion process of oil shale via geochemical methods: A case study on the Fuyu oil shale, China. Fuel Process. Technol. 2021, 219, 106876. [Google Scholar] [CrossRef]
- Cao, H.; Guo, W.; Shan, X.; Ma, L.; Sun, P. Paleolimnological environments and organic accumulation of the Nenjiang formation in the southeastern Songliao basin, China. Oil Shale 2015, 32, 5–24. [Google Scholar] [CrossRef]
- Bansal, V.R.; Kumar, R.; Sastry, M.I.S.; Badhe, R.M.; Kapur, G.S.; Saxena, D. Direct estimation of shale oil potential by the structural insight of Indian origin kerogen. Fuel 2019, 241, 410–416. [Google Scholar] [CrossRef]
- Burnham, A.K.; Mcconaghy, J.R. Semi-open pyrolysis of oil shale from the garden gulch member of the green river formation. Energy Fuels 2014, 28, 7426–7439. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, R.; Liu, Z.; Han, X.; Gao, Y. Organic Matter-Driven Electrical Resistivity of Immature Lacustrine Oil-Prone Shales. Geophysics 2021, 86, 165–178. [Google Scholar] [CrossRef]
- Campbell, J.H.; Koskinas, G.H.; Stout, N.D.; Coburn, T.T. Oil shale retorting—Effects of particle size and heating rate on oil evolution and intraparticle oil degradation. Situ 1978, 2, 1–47. [Google Scholar]
- Wang, Z.; Lü, X.; Li, Q.; Sun, Y.; Wang, Y.; Deng, S.; Guo, W. Downhole electric heater with high heating efficiency for oil shale exploitation based on a double-shell structure. Energy 2020, 211, 118539. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Z.; Sun, Z.; Sun, Y.; Lü, X.; Deng, S.; Qu, L.; Yuan, W.; Li, Q. Experimental investigation on performance of downhole electric heaters with continuous helical baffles used in oil shale in-situ pyrolysis. Appl. Therm. Eng. 2019, 147, 1024–1035. [Google Scholar] [CrossRef]
- Burnham, A.K.; Ethan, B.H.; Mary, F.S. Pyrolysis kinetics for Green River oil shale from the saline zone. Fuel 1983, 62, 1199–1204. [Google Scholar] [CrossRef]
- Jaber, J.O.; Probert, S.D. Non-isothermal thermogravimetry and decomposition kinetics of two Jordanian oil shales under different processing conditions. Fuel Process. Technol. 2000, 63, 57–70. [Google Scholar] [CrossRef]
- Jiang, X.M.; Han, X.X.; Cui, Z.G. Progress and recent utilization trends in combustion of Chinese oil shale. Prog. Energy Combust. Sci. 2007, 33, 552–579. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, Y.; Wang, H.; Li, Q.; Guo, W. Modeling and field testing of fracturing fluid back-flow after acid fracturing in unconventional reservoirs. J. Pet. Sci. Eng. 2019, 176, 494–501. [Google Scholar] [CrossRef]
- Kök, M.V. Heating rate effect on the DSC kinetics of oil shale. J. Anal. Calorim. 2007, 90, 817–821. [Google Scholar] [CrossRef]
- He, W.T.; Sun, Y.H.; Shan, X.L. Geochemical characteristics of the Lower Cretaceous Hengtongshan Formation in the Tonghua Basin, Northeast China: Implications for Depositional Environment and Shale Oil Potential Evaluation. Appl. Sci. 2021, 11, 23. [Google Scholar] [CrossRef]
- Cao, H.; He, W.; Chen, F.; Shan, X.; Kong, D.; Hou, Q.; Pu, X. Integrated chemostratigraphy (δ13C-δ34S-δ15N) constrains cretaceous lacustrine anoxic events triggered by marine sulfate input. Chem. Geol. 2021, 559, 119912. [Google Scholar] [CrossRef]
- Wang, L.; Yang, D.; Zhao, Y.; Wang, G. Evolution of pore characteristics in oil shale during pyrolysis under convection and conduction heating modes. Oil Shale 2020, 37, 224–241. [Google Scholar] [CrossRef]
- Staplin, F.L. Interpretation of thermal history from color of particulate organic matter—A review. Palynology 1977, 1, 9–18. [Google Scholar] [CrossRef]
- Wei, G.; Xie, Z.Y.; Bai, G.L.; Li, J.; Wang, Z.H.; Li, A.G.; Li, Z.S. Organic geochemical characteristics and origin of natural gas in Sinian-Lower Paleozoic reservoirs, Sichuan Basin. Nat. Gas Ind. 2014, 34, 44–49. [Google Scholar]
- Dhaundiyal, A.; Singh, S.B. Study of Distributed Activation Energy Model using Various Probability Distribution Functions for the Isothermal Pyrolysis Problem. Rud.-Geol.-Naft. Zb. 2017, 32, 1–15. [Google Scholar] [CrossRef][Green Version]
- Peters, K.E.; Moldowan, J.M. The biomarker guide: Interpreting molecular fossils in petroleum and ancient sediments. Choice Rev. Online 1993, 30, 30–2690. [Google Scholar]
- Peters, K.E.; Walters, C.C.; Moldowan, J.M. The Biomarker Guide; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Liu, Z.; Sun, P.; Jia, J.; Liu, R.; Meng, Q. Distinguishing features and their genetic interpretation of stratigraphic se-quences in continental deep water setting:A case from Qingshankou Formation in Songliao Basin. Earth Sci. Front. 2011, 18, 171–180, (In Chinese with English abstract). [Google Scholar]
- Liu, Z.; Wang, D.; Liu, L.; Liu, W.; Wang, P.; Du, X.; Yang, G. Sedimentary Characteristics of the Cretaceous Songliao Basin. Acta Geol. Sin. 1992, 66, 327–338. [Google Scholar]
- He, W.; Sun, Y.; Shan, X.; Cao, H.; Zheng, S.; Su, S. The oil shale formation mechanism of the Songliao Basin Nenjiang Formation triggered by marine transgression and OAE3. Oil Shale 2021, 38, 89–118. [Google Scholar]
- He, W.; Sun, Y.; Shan, X. Organic matter evolution in pyrolysis experiments of oil shale under high pressure: Guidance for in-situ conversion of oil shale in the Songliao Basin. J. Anal. Appl. Pyrolysis 2021, 155, 105091. [Google Scholar] [CrossRef]
- Caricchi, C.; Corrado, S.; Di Paolo, L.; Aldega, L.; Grigo, D. Thermal maturity of Silurian deposits in the Baltic Syneclise (on-shore Polish Baltic Basin): Contribution to unconventional resources assessment. Ital. J. Geosci. 2016, 135, 383–393. [Google Scholar] [CrossRef]
- Li, C.; Huang, W.; Zhou, C.; Chen, Y. Advances on the transition-metal based catalysts for aqua thermolysis upgrading of heavy crude oil. Fuel 2019, 257, 115779. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Jiang, L.; George, S.C. Organic petrology and geochemistry of Lower Cretaceous lacustrine sediments in the Chaoyang Basin (Liaoning Province, northeast China): Influence of volcanic ash on algal productivity and oil shale formation. Int. J. Coal Geol. 2021, 233, 103653. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Fernandez-Lopez, M.; Sánchez, P.; Valverde, J.L.; Sanchez-Silva, L. Pyrolysis process using a bench scale high pressure thermobalance. Fuel Process. Technol. 2017, 167, dd345–dd354. [Google Scholar] [CrossRef]
- Bai, F.; Sun, Y.; Liu, Y.; Li, Q.; Guo, M. Thermal and kinetic characteristics of pyrolysis and combustion of three oil shales. Energy Convers. Manag. 2015, 97, 374–381. [Google Scholar] [CrossRef]
- Cao, H.; He, W.; Chen, F.; Kong, D. Superheavy pyrite in the upper cretaceous mudstone of the Songliao basin, NE China and its implication for paleolimnological environments. J. Asian Earth Sci. 2020, 189, 104156. [Google Scholar] [CrossRef]
- Burnham, A.K. Thermomechanical properties of the Garden Gulch Member of the Green River Formation. Fuel 2018, 219, 477–491. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, X.; Han, X.; Tong, J.H. Effect of residence time on products yield and characteristics of shale oil and gases produced by low-temperature retorting of Dachengzi oil shale. Oil Shale 2013, 30, 501–516. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, X.; Han, X.; Tong, J.H. Effect of retorting temperature on product yield and characteristics of non-condensable gases and shale oil obtained by retorting Huadian oil shales. Fuel Process. Technol. 2014, 121, 9–15. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Yang, D.; Kang, Z.; Zhao, J. Effect of pyrolysis on oil shale using superheated steam: A case study on the Fushun oil shale, China. Fuel 2019, 253, 1490–1498. [Google Scholar] [CrossRef]
- He, W.; Sun, Y.; Shan, X.; Cao, H.; Li, J. Geochemical characteristics and oil generation potential evaluation of lower cretaceous Xiahuapidianzi formation shale in the southeastern sankeyushu depression, Tonghua basin: Evidence from shale pyrolysis experiments and biomarkers. ACS Earth Space Chem. 2021, 5, 409–423. [Google Scholar] [CrossRef]
- Burnham, A.K.; Sweeney, J.J. A chemical kinetic model of vitrinite maturation and reflectance. Geochim. Cosmochim. Acta 1989, 53, 2649–2657. [Google Scholar] [CrossRef]
- Sweeney, J.J.; Burnham, A.K. Evaluation of a simple model of vitrinite reflectance based on chemical kinetics. Am. Assoc. Pet. Geol. Bull. 1990, 74, 1559–1570. [Google Scholar]
- Cao, H.; He, W. Correlation of carbon isotope stratigraphy and paleoenvironmental conditions in the cretaceous Jehol group, northeastern China. Int. Geol. Rev. 2020, 62, 113–128. [Google Scholar] [CrossRef]
- Kalkreuth, W.; Sherwood, N.; Cioccari, G.; da Silva, Z.C.; Silva, M.; Zhong, N.; Zufa, L. The application of famm™ (fluorescence alteration of multiples macerals) analyses for evaluating rank of parana basin coals, brazil. Int. J. Coal Geol. 2004, 57, 167–185. [Google Scholar] [CrossRef]
- Cheshire, S.; Craddock, P.R.; Xu, G.; Sauerer, B.; Pomerantz, A.E.; McCormick, D.; Abdallah, W. Assessing thermal maturity beyond the reaches of vitrinite reflectance and Rock-Eval pyrolysis: A case study from the Silurian Qusaiba Formation. Int. J. Coal Geol. 2017, 180, 29–45. [Google Scholar] [CrossRef]
- Burnham, A.K.; Schmidt, B.J.; Braun, R.L. A test of the parallel reaction model using kinetic measurements on hydrous pyrolysis residues. Org. Geochem. 1995, 23, 931–939. [Google Scholar] [CrossRef]
- Shuai, Z.; Xiaoshu, L.; Qiang, L.; Youhong, S. Thermal-fluid coupling analysis of oil shale pyrolysis and displacement by heat-carrying supercritical carbon dioxide. Chem. Eng. J. 2020, 394, 125037. [Google Scholar] [CrossRef]
- Seifert, W.K.; Moldowan, J.M. Applications of steranes, terpanes and monoaromatics to the maturation, migration and source of crude oils. Geochim. Cosmochim. Acta 1978, 42, 77–95. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, R.; Ding, W.; Li, L.; Liu, Z. The influence of Early Cretaceous paleoclimate warming event on sedimentary environment evolution and organic matter sources in Yin’e Basin: Evidence from petrology and molecular geochemistry. Int. J. Coal Geol. 2022, 254, 103972. [Google Scholar] [CrossRef]
- Jia, J.; Liu, Z. Particle-Size Fractionation and Thermal Variation of Oil Shales in the Songliao Basin, NE China: Implication for Hydrocarbon-Generated Process. Energies 2021, 14, 7191. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, Y.; Lü, X.; Li, Q. Energy consumption and product release characteristics evaluation of oil shale non-isothermal pyrolysis based on TG-DSC. J. Pet. Sci. Eng. 2020, 187, 494–501. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Slougui, N.-E.; Watt, D.S.; Marcura, R.E.; Moldwan, J.M. Thermodynamic stability of various alkylated, dealkylated, and rearranged 17α and 17β hopane isomers using molecular mechanics calculations. Org. Geochem. 1990, 16, 1033–1038. [Google Scholar] [CrossRef]
- Ensminger, A.; Albrecht, P.; Ourisson, G.; Tissot, B. Evolution of polycyclic alkanes under the effect of burial (Early Toarcian shales, Paris Basin). In Advances in Organic Geochemistry 1975; Enadimsa: Madrid, Spain, 1977; pp. 45–52. [Google Scholar]
- Wang, L.; Yang, D.; Kang, Z. Evolution of permeability and mesostructure of oil shale exposed to high-temperature water vapor. Fuel 2021, 290, 119786. [Google Scholar] [CrossRef]
- Mackenzie, A.S. Application of Biological Markers in Petroleum Geochemistry; Academic Press: London, UK, 1984. [Google Scholar]
- Song, Y.; Li, S.; Hu, S. Warm-humid paleoclimate control of salinized lacustrine organic-rich shale deposition in the Oligocene Hetaoyuan Formation of the Biyang Depression, East China. Int. J. Coal Geol. 2019, 202, 69–84. [Google Scholar] [CrossRef]
- Peters, K.E.; Moldowan, J.M. The biomarker guide: Interpreting molecular fossils. In Petroleum and Ancient Sediments; Prentice Hall: Hoboken, NJ, USA, 1993; Volume 363. [Google Scholar]
- Peters, K.E. Guidelines for evaluating petroleum source rock using programmed pyrolysis. Am. Assoc. Pet. Geol. Bull. 1986, 70, 318–329. [Google Scholar]




| Pyrolysis Temperature (°C) | Oil Yield (wt%) | Moisture Content (wt%) | Semicoke Yield (wt%) | Gas and Loss (wt%) |
|---|---|---|---|---|
| 150 | 0 | 0.78 | 98.83 | 0.39 |
| 200 | 0 | 0.96 | 98.39 | 0.65 |
| 250 | 0.16 | 1.73 | 97.68 | 0.43 |
| 300 | 0.76 | 2.7 | 96.16 | 0.38 |
| 350 | 1.25 | 3.46 | 93.89 | 1.4 |
| 375 | 1.53 | 3.86 | 93.43 | 1.18 |
| 400 | 1.76 | 4.36 | 92.91 | 0.97 |
| 425 | 2.55 | 4.83 | 90.88 | 1.74 |
| 450 | 6.24 | 5.31 | 87.02 | 1.43 |
| 475 | 6.37 | 6.56 | 82.35 | 4.72 |
| 520 | 6.58 | 6.55 | 82.46 | 4.41 |
| Pyrolysis Temperature, °C | TOC, wt% | Ro, % | Tmax, °C |
|---|---|---|---|
| original sample | 8.09 | 0.266 | 430 |
| 150 | 7.46 | 0.284 | 433 |
| 200 | 6.84 | 0.455 | 437 |
| 250 | 6.59 | 0.584 | 439 |
| 300 | 6.22 | 0.643 | 440 |
| 350 | 4.55 | 0.846 | 440 |
| 375 | 3.78 | 0.864 | 441 |
| 400 | 3.41 | 0.887 | 443 |
| 425 | 2.94 | 0.946 | 445 |
| 450 | 2.88 | 1.143 | 566 |
| 475 | 2.65 | 1.257 | 590 |
| 520 | 2.63 | 1.376 | 600 |
| Area-Pyrolysis Temperature | Sample Properties | Oil Content | Pyrolysis Oil Group Components | N-Alkanes and Isoprenoids | Terpanes (m/z = 191) | Steranes and Rearranged Steranes (m/z = 217) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saturated Hydrocarbon % | Aromatic Hydrocarbon % | Non Hydrocarbon + Asphaltene% | CPI | Ts/(Ts + Tm) | C32 22S/(22S + 22R) | C29 20S/(20S + 20R) | C29 ββ/(αα + ββ) | ||||||||||||||||||
| 1 | 2 | 3 | Average | 1 | 2 | 3 | Average | 1 | 2 | 3 | ave | 1 | 2 | 3 | Average | 1 | 2 | 3 | Average | ||||||
| NA-0 | Oil shale semi coke | 0.16 | 0.06 | 0.15 | 0.79 | 1.59 | 1.95 | 1.86 | 1.8 | 0.15 | 0.09 | 0.11 | 0.12 | 0.34 | 0.34 | 0.35 | 0.34 | 0.18 | 0.18 | 0.14 | 0.17 | 0.08 | 0.07 | 0.07 | 0.07 |
| NA-150 | 0.11 | 0.08 | 0.02 | 0.9 | 1.54 | 1.73 | 1.78 | 1.68 | 0.15 | 0.15 | 0.17 | 0.15 | 0.35 | 0.34 | 0.36 | 0.35 | 0.17 | 0.18 | 0.16 | 0.17 | 0.06 | 0.11 | 0.08 | 0.08 | |
| NA-200 | 0.46 | 0.16 | 0.22 | 0.62 | 1.43 | 1.37 | 1.46 | 1.42 | 0.17 | 0.2 | 0.17 | 0.18 | 0.37 | 0.38 | 0.38 | 0.37 | 0.19 | 0.16 | 0.18 | 0.18 | 0.16 | 0.16 | 0.22 | 0.18 | |
| NA-250 | 0.47 | 0.25 | 0.124 | 0.626 | 1.31 | 1.29 | 1.38 | 1.32 | 0.26 | 0.24 | 0.27 | 0.26 | 0.37 | 0.38 | 0.39 | 0.38 | 0.22 | 0.22 | 0.21 | 0.21 | 0.19 | 0.21 | 0.21 | 0.2 | |
| NA-300 | 0.55 | 0.24 | 0.14 | 0.62 | 1.28 | 1.13 | 1.19 | 1.2 | 0.28 | 0.31 | 0.29 | 0.29 | 0.38 | 0.38 | 0.39 | 0.38 | 0.23 | 0.24 | 0.26 | 0.24 | 0.23 | 0.22 | 0.25 | 0.23 | |
| NA-350 | 0.39 | 0.26 | 0.23 | 0.51 | 1.16 | 1.06 | 1.03 | 1.08 | 0.3 | 0.29 | 0.31 | 0.3 | 0.41 | 0.41 | 0.43 | 0.42 | 0.27 | 0.27 | 0.23 | 0.26 | 0.25 | 0.24 | 0.27 | 0.25 | |
| NA-375 | Pyrolysis oil | 0.63 | 0.2 | 0.17 | 0.94 | 0.98 | 1.16 | 1.03 | 0.35 | 0.32 | 0.32 | 0.33 | 0.44 | 0.43 | 0.46 | 0.44 | 0.29 | 0.28 | 0.31 | 0.29 | 0.28 | 0.27 | 0.31 | 0.28 | |
| NA-400 | 0.63 | 0.28 | 0.09 | 0.85 | 0.77 | 1.05 | 0.89 | 0.44 | 0.43 | 0.43 | 0.43 | 0.45 | 0.44 | 0.46 | 0.45 | 0.32 | 0.34 | 0.31 | 0.32 | 0.29 | 0.34 | 0.34 | 0.32 | ||
| NA-425 | 0.63 | 0.25 | 0.12 | 0.94 | 1.13 | 0.99 | 1.02 | 0.48 | 0.45 | 0.47 | 0.46 | 0.52 | 0.51 | 0.53 | 0.52 | 0.32 | 0.34 | 0.34 | 0.33 | 0.36 | 0.39 | 0.36 | 0.37 | ||
| NA-450 | 0.66 | 0.21 | 0.13 | 1.08 | 1.05 | 0.91 | 1.02 | 0.57 | 0.55 | 0.54 | 0.55 | 0.53 | 0.55 | 0.56 | 0.55 | 0.42 | 0.41 | 0.44 | 0.42 | 0.4 | 0.4 | 0.37 | 0.39 | ||
| NA-475 | 0.67 | 0.22 | 0.11 | 1.07 | 0.97 | 0.86 | 0.97 | 0.62 | 0.59 | 0.63 | 0.61 | 0.57 | 0.57 | 0.57 | 0.57 | 0.45 | 0.42 | 0.42 | 0.43 | 0.41 | 0.43 | 0.43 | 0.42 | ||
| NA-520 | 0.67 | 0.14 | 0.19 | 0.95 | 1.08 | 1.16 | 1.06 | 0.63 | 0.63 | 0.61 | 0.62 | 0.58 | 0.58 | 0.59 | 0.58 | 0.49 | 0.44 | 0.43 | 0.45 | 0.5 | 0.46 | 0.53 | 0.5 | ||
| Sample | Asphaltene, % | Saturated Hydrocarbon, % | Aromatic Hydrocarbon, % | Non-Hydrocarbon, % |
|---|---|---|---|---|
| NA 1 | 0.51 | 60.26 | 30.42 | 8.80 |
| NA 2 | 0.82 | 54.70 | 35.21 | 9.25 |
| Sample | N-Alkanes and Isoprenoids | Terpanes (m/z = 191) | Steranes and Rearranged Steranes (m/z = 217) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPI | Ts/(Ts + Tm) | C32 22S/(22S + 22R) | C29 20S/(20S + 20R) | C29ββ/(αα + ββ) | ||||||||||||||||
| 1 | 2 | 3 | Average | 1 | 2 | 3 | Average | 1 | 2 | 3 | Average | 1 | 2 | 3 | Average | 1 | 2 | 3 | Average | |
| NA-1 | 1.07 | 1.04 | 1.05 | 1.05 | 0.46 | 0.41 | 0.46 | 0.44 | 0.46 | 0.47 | 0.41 | 0.45 | 0.31 | 0.33 | 0.33 | 0.32 | 0.28 | 0.31 | 0.33 | 0.31 |
| NA-2 | 1.02 | 0.95 | 1.06 | 1.01 | 0.42 | 0.46 | 0.41 | 0.43 | 0.48 | 0.44 | 0.49 | 0.47 | 0.29 | 0.34 | 0.34 | 0.32 | 0.30 | 0.32 | 0.34 | 0.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, H.; He, W.; Yang, L.; Su, J.; Meng, X.; Cen, X.; Guo, W. Evolution of Biomarker Maturity Parameters and Feedback to the Pyrolysis Process for In Situ Conversion of Nongan Oil Shale in Songliao Basin. Energies 2022, 15, 3715. https://doi.org/10.3390/en15103715
Zeng H, He W, Yang L, Su J, Meng X, Cen X, Guo W. Evolution of Biomarker Maturity Parameters and Feedback to the Pyrolysis Process for In Situ Conversion of Nongan Oil Shale in Songliao Basin. Energies. 2022; 15(10):3715. https://doi.org/10.3390/en15103715
Chicago/Turabian StyleZeng, Hao, Wentong He, Lihong Yang, Jianzheng Su, Xianglong Meng, Xueqi Cen, and Wei Guo. 2022. "Evolution of Biomarker Maturity Parameters and Feedback to the Pyrolysis Process for In Situ Conversion of Nongan Oil Shale in Songliao Basin" Energies 15, no. 10: 3715. https://doi.org/10.3390/en15103715
APA StyleZeng, H., He, W., Yang, L., Su, J., Meng, X., Cen, X., & Guo, W. (2022). Evolution of Biomarker Maturity Parameters and Feedback to the Pyrolysis Process for In Situ Conversion of Nongan Oil Shale in Songliao Basin. Energies, 15(10), 3715. https://doi.org/10.3390/en15103715






