Synthesis of High-Quality Two-Dimensional V2C MXene for Supercapacitor Application †
Abstract
:1. Introduction
2. Materials and Preparation Methods
2.1. Materials
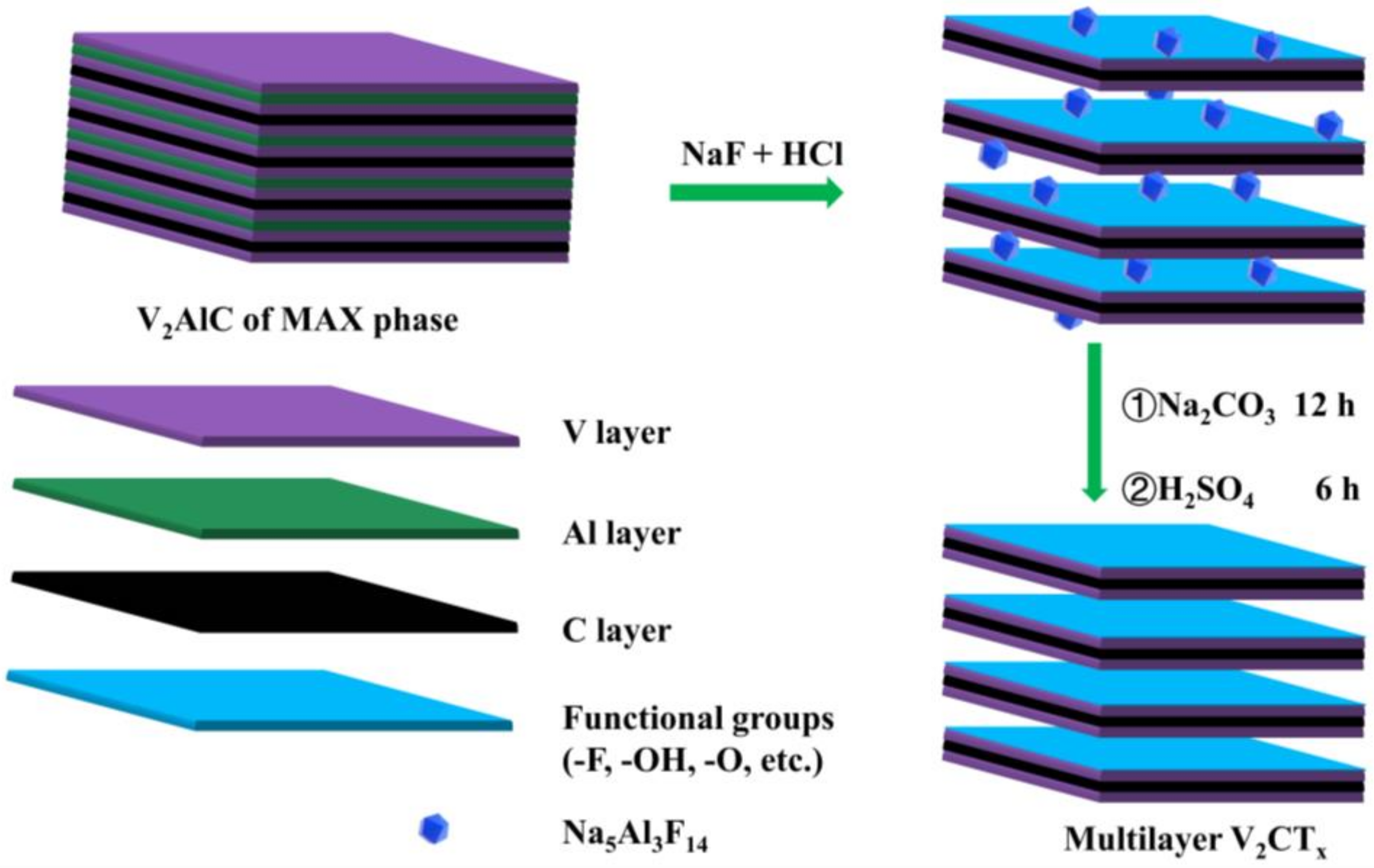
2.2. Synthesis of V2C
2.3. Preparation of the V2C Flexible Electrodes
2.4. Characterization and Electrochemical Analysis
3. Results and Discussion
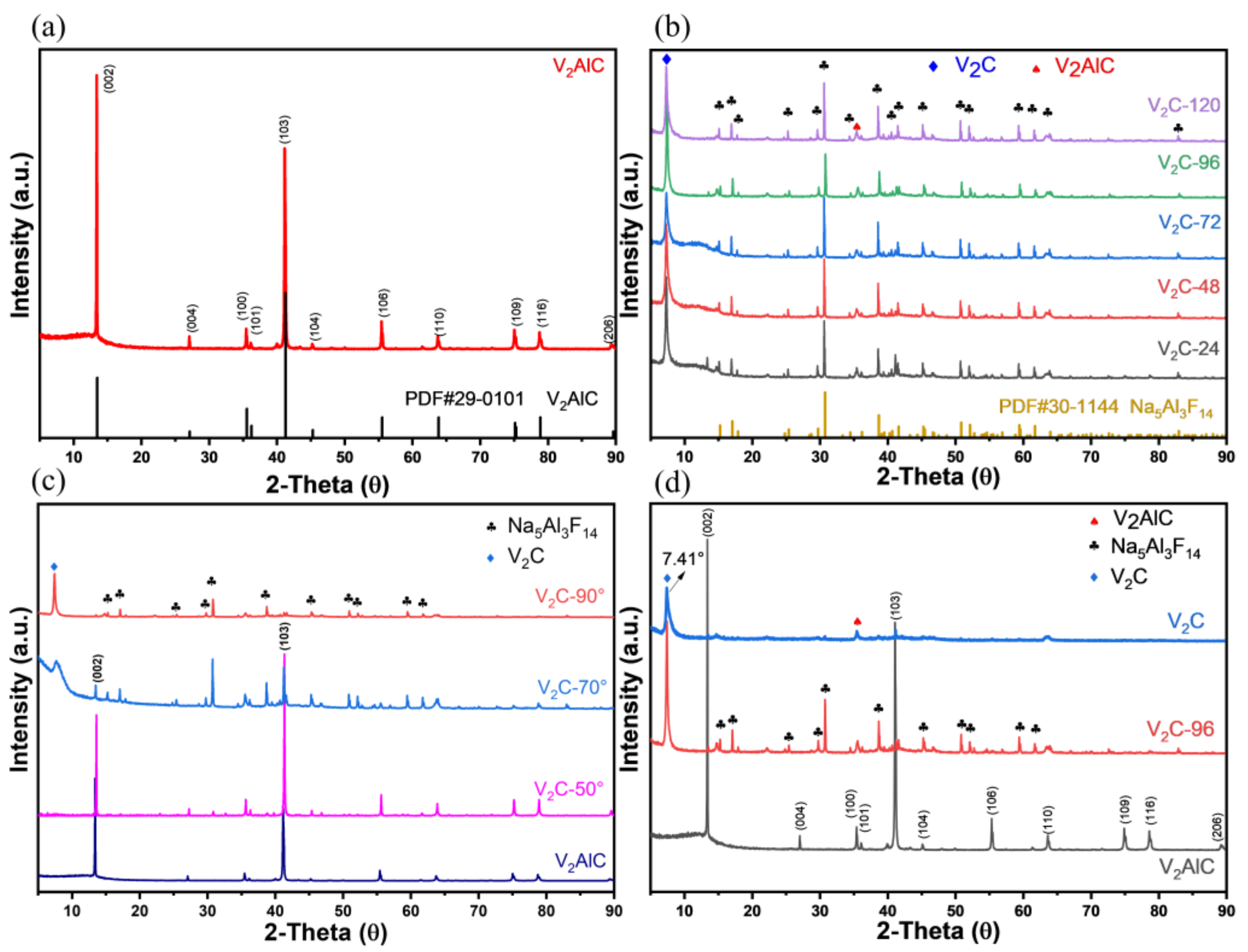
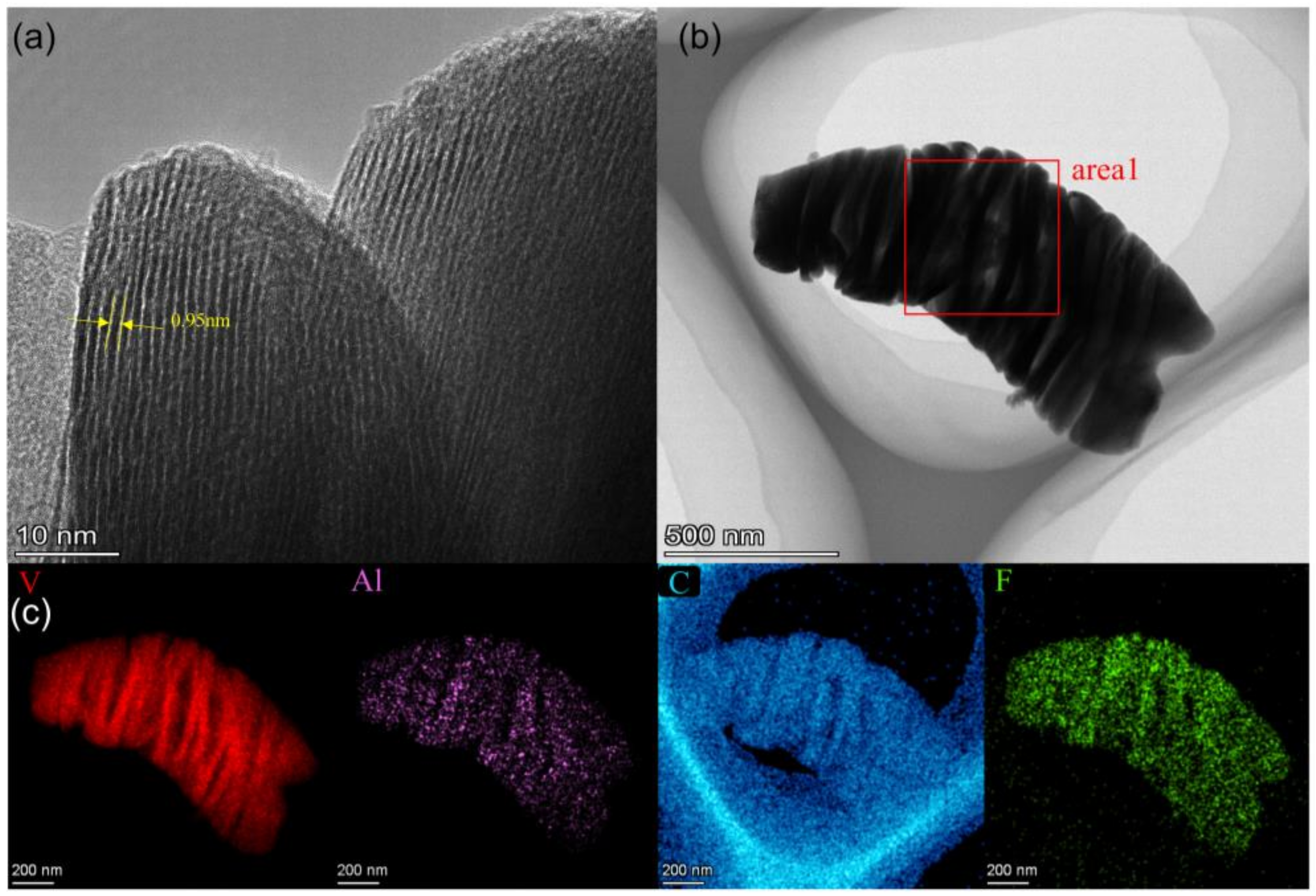
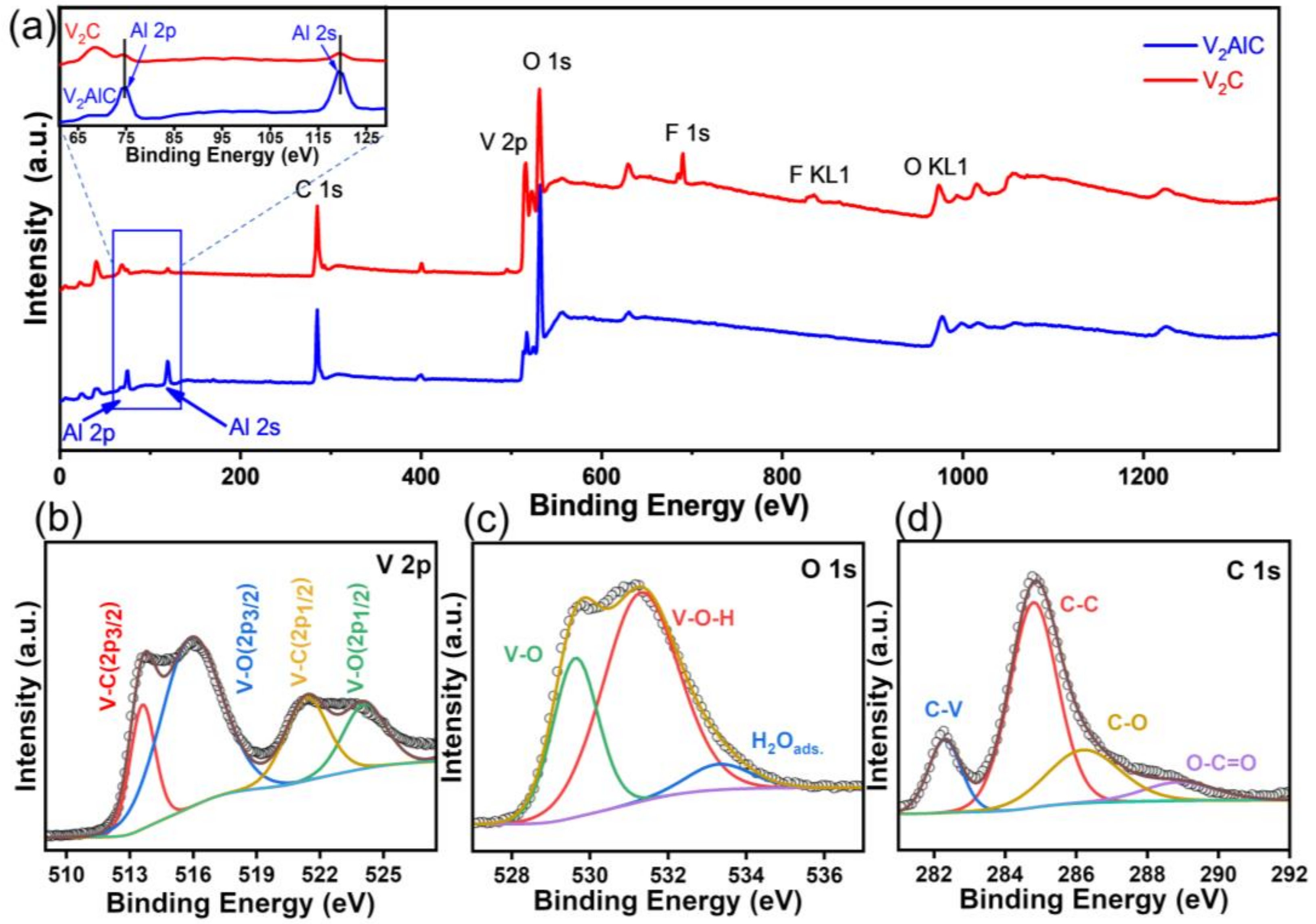
3.1. Material Characterization
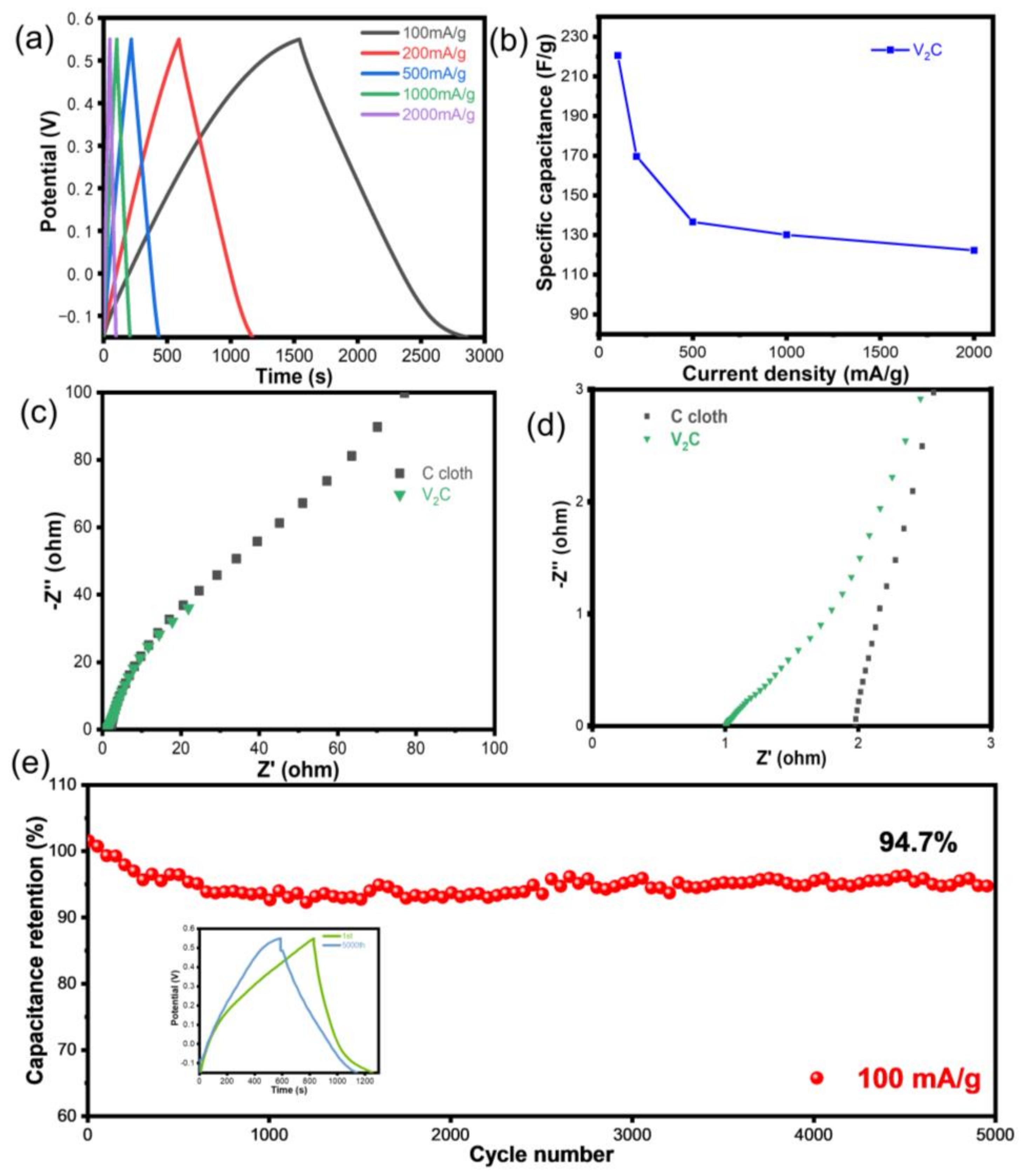
3.2. Electrochemical Performance of Flexible V2C Electrodes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, M.; Zhai, T.; Lu, X.; Chen, X.; Xie, S.; Li, W.; Liang, C.; Zhao, W.; Zhang, L.; Tong, Y. Manganese dioxide nanorod arrays on carbon fabric for flexible solid-state supercapacitors. J. Power Sources 2013, 239, 64–71. [Google Scholar] [CrossRef]
- Liu, L.; Niu, Z.; Chen, J. Unconventional supercapacitors from nanocarbon-based electrode materials to device configurations. Chem. Soc. Rev. 2016, 45, 4340–4363. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Sathiya, M.; Prakash, A.S.; Ramesha, K.; Tarascon, J.M.; Shukla, A.K. V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J. Am. Chem. Soc. 2011, 133, 16291–16299. [Google Scholar] [CrossRef]
- Zhai, T.; Xie, S.; Yu, M.; Fang, P.; Liang, C.; Lu, X.; Tong, Y. Oxygen vacancies enhancing capacitive properties of MnO2 nanorods for wearable asymmetric supercapacitors. Nano Energy 2014, 8, 255–263. [Google Scholar] [CrossRef]
- Lin, J.; Jia, H.; Liang, H.; Chen, S.; Cai, Y.; Qi, J.; Qu, C.; Cao, J.; Fei, W.; Feng, J. In Situ Synthesis of Vertical Standing Nanosized NiO Encapsulated in Graphene as Electrodes for High-Performance Supercapacitors. Adv. Sci. 2018, 5, 1700687. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Cheng, C.; Li, H.; Zhang, J.; Gong, H.; Fan, H.J. Co3O4 Nanowire@MnO2 ultrathin nanosheet core/shell arrays: A new class of high-performance pseudocapacitive materials. Adv. Mater. 2011, 23, 2076–2081. [Google Scholar] [CrossRef]
- Jiang, Q.; Kurra, N.; Alhabeb, M.; Gogotsi, Y.; Alshareef, H.N. All Pseudocapacitive MXene-RuO2 Asymmetric Supercapacitors. Adv. Energy Mater. 2018, 8, 1703043. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Goh, K.; Wang, H.; Wei, L.; Jiang, W.; Zhang, Q.; Dai, L.; Chen, Y. Scalable synthesis of hierarchically structured carbon nanotube–graphene fibres for capacitive energy storage. Nat. Nanotechnol. 2014, 9, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, X.; Yu, H.; Ding, T.; Yan, Y.; Yao, B.; Yao, X.; Chen, D.; Liu, M.; Huang, L. A Scalable Free-Standing V2O5/CNT Film Electrode for Supercapacitors with a Wide Operation Voltage (1.6 V) in an Aqueous Electrolyte. Adv. Funct. Mater. 2016, 26, 6114–6120. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Niu, S.; Wang, Z.; Yu, M.; Yu, M.; Xiu, L.; Wang, S.; Wu, X.; Qiu, J. MXene-Based Electrode with Enhanced Pseudocapacitance and Volumetric Capacity for Power-Type and Ultra-Long Life Lithium Storage. ACS Nano 2018, 12, 3928–3937. [Google Scholar] [CrossRef]
- Luo, J.; Tao, X.; Zhang, J.; Xia, Y.; Huang, H.; Zhang, L.; Gan, Y.; Liang, C.; Zhang, W. Sn4+ Ion Decorated Highly Conductive Ti3C2 MXene: Promising Lithium-Ion Anodes with Enhanced Volumetric Capacity and Cyclic Performance. ACS Nano 2016, 10, 2491–2499. [Google Scholar] [CrossRef]
- Hu, L.; Wu, L.; Liao, M.; Fang, X. High-performance NiCo2O4 nanofilm photodetectors fabricated by an interfacial self-assembly strategy. Adv. Mater. 2011, 23, 1988–1992. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Ahmed, B.; Hedhili, M.N.; Anjum, D.H.; Alshareef, H.N. Effect of Postetch Annealing Gas Composition on the Structural and Electrochemical Properties of Ti2CTx MXene Electrodes for Supercapacitor Applications. Chem. Mater. 2015, 27, 5314–5323. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lin, S.; Tong, H.; Huang, Y.; Tong, P.; Zhao, B.; Dai, J.; Liang, C.; Wang, H.; Zhu, X.; et al. Two-dimensional V4C3 MXene as high performance electrode materials for supercapacitors. Electrochim. Acta 2019, 307, 414–421. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Hedhili, M.N.; Gogotsi, Y.; Alshareef, H.N. H2O2 assisted room temperature oxidation of Ti2C MXene for Li-ion battery anodes. Nanoscale 2016, 8, 7580–7587. [Google Scholar] [CrossRef] [Green Version]
- Mashtalir, O.; Lukatskaya, M.R.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Amine-Assisted Delamination of Nb2C MXene for Li-Ion Energy Storage Devices. Adv. Mater. 2015, 27, 3501–3506. [Google Scholar] [CrossRef]
- Wang, S.; Guan, C.; Zhao, Z.; Wang, R.; Tian, Y.; Du, Y. Density Functional Theory Analysis of Electronic and Optical Properties of Two-Dimensional Tantalum Carbides Tan+1Cn (n = 1, 2, 3). Phys. Status Solidi B Basic Solid State Phys. 2019, 256, 1800457. [Google Scholar] [CrossRef]
- Sun, D.; Hu, Q.; Chen, J.; Zhang, X.; Wang, L.; Wu, Q.; Zhou, A. Structural Transformation of MXene (V2C, Cr2C, and Ta2C) with O Groups during Lithiation: A First-Principles Investigation. ACS Appl. Mater. Interfaces 2016, 8, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Rufford, T.E.; Chen, X.; Li, N.; Lyu, M.; Dai, L.; Wang, L. Nitrogen-doped Ti3C2Tx MXene electrodes for high-performance supercapacitors. Nano Energy 2017, 38, 368–376. [Google Scholar] [CrossRef]
- Zhou, J.; Zha, X.; Zhou, X.; Chen, F.; Gao, G.; Wang, S.; Shen, C.; Chen, T.; Zhi, C.; Eklund, P.; et al. Synthesis and Electrochemical Properties of Two-Dimensional Hafnium Carbide. ACS Nano 2017, 11, 3841–3850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Chroneos, A.; Eppinger, J.; Schwingenschlögl, U. S-functionalized MXenes as electrode materials for Li-ion batteries. Appl. Mater. Today 2016, 5, 19–24. [Google Scholar] [CrossRef]
- Yadav, A.; Dashora, A.; Patel, N.; Miotello, A.; Press, M.; Kothari, D.C. Study of 2D MXene Cr2C material for hydrogen storage using density functional theory. Appl. Surf. Sci. 2016, 389, 88–95. [Google Scholar] [CrossRef]
- He, H.; Xia, Q.; Wang, B.; Wang, L.; Hu, Q.; Zhou, A. Two-dimensional vanadium carbide (V2CTx ) MXene as supercapacitor electrode in seawater electrolyte. Chin. Chem. Lett. 2020, 31, 984–987. [Google Scholar] [CrossRef]
- Wu, M.; Wang, B.; Hu, Q.; Wang, L.; Zhou, A. The Synthesis Process and Thermal Stability of V2C MXene. Materials 2018, 11, 2112. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; He, Y.; Wang, L.; Xia, Q.; Zhou, A. Synthesis and electrochemical properties of V2C MXene by etching in opened/closed environments. J. Adv. Ceram. 2020, 9, 749–758. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Ahmed, B.; Anjum, D.; Alshareef, H.N. Direct Chemical Synthesis of MnO2 Nanowhiskers on Transition-Metal Carbide Surfaces for Supercapacitor Applications. ACS Appl. Mater. Interfaces 2016, 8, 18806–18814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Q.X.; Shinde, N.M.; Yun, J.M.; Zhang, T.; Mane, R.S.; Mathur, S.; Kim, K.H. Bismuth Oxychloride/MXene symmetric supercapacitor with high volumetric energy density. Electrochim. Acta 2018, 271, 351–360. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Fu, Z.; Lu, Z.; Zhang, X.; Wang, Y.; Yang, Z.; Wu, R. Tailoring the Electronic Structure of Transition Metals by the V2C MXene Support: Excellent Oxygen Reduction Performance Triggered by Metal-Support Interactions. ACS Appl. Mater. Interfaces 2020, 12, 28206–28216. [Google Scholar] [CrossRef] [PubMed]
- Le, T.A.; Tran, N.Q.; Hong, Y.; Lee, H. Intertwined Titanium Carbide MXene within a 3 D Tangled Polypyrrole Nanowires Matrix for Enhanced Supercapacitor Performances. Chemistry 2019, 25, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, L.; Jiao, Y.; Li, Z.; Gao, Y. Controllable fabrication of ZnCo2O4 ultra-thin curved sheets on Ni foam for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 299, 388–394. [Google Scholar] [CrossRef]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.J.; Shao-Horn, Y.; Dinca, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater 2017, 16, 220–224. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, M.; Kim, S.K.; Bae, G.; Song, W.; Myung, S.; Lim, J.; Lee, S.S.; Zyung, T.; An, K.-S. A Strategy for Synthesis of Carbon Nitride Induced Chemically Doped 2D MXene for High-Performance Supercapacitor Electrodes. Adv. Energy Mater. 2018, 8, 1703173. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, B.; Guo, A.; Sun, Z.; Zhao, J.; Cui, F.; Yang, X. MnO2 nanoshells/Ti3C2Tx MXene hybrid film as supercapacitor electrode. Appl. Surf. Sci. 2021, 560, 150040. [Google Scholar] [CrossRef]
- Venkatkarthick, R.; Rodthongkum, N.; Zhang, X.; Wang, S.; Pattananuwat, P.; Zhao, Y.; Liu, R.; Qin, J. Vanadium-Based Oxide on Two-Dimensional Vanadium Carbide MXene (V2Ox@V2CTx) as Cathode for Rechargeable Aqueous Zinc-Ion Batteries. Acs Appl. Energy Mater. 2020, 3, 4677–4689. [Google Scholar] [CrossRef]
- Matthews, K.; Zhang, T.; Shuck, C.E.; VahidMohammadi, A.; Gogotsi, Y. Guidelines for Synthesis and Processing of Chemically Stable Two-Dimensional V2CTx MXene. Chem. Mater. 2021, 34, 499–509. [Google Scholar] [CrossRef]
- VahidMohammadi, A.; Hadjikhani, A.; Shahbazmohamadi, S.; Beidaghi, M. Two-Dimensional Vanadium Carbide (MXene) as a High-Capacity Cathode Material for Rechargeable Aluminum Batteries. ACS Nano 2017, 11, 11135–11144. [Google Scholar] [CrossRef]
- Guan, Y.; Jiang, S.; Cong, Y.; Wang, J.; Dong, Z.; Zhang, Q.; Yuan, G.; Li, Y.; Li, X. A hydrofluoric acid-free synthesis of 2D vanadium carbide (V2C) MXene for supercapacitor electrodes. 2D Mater. 2020, 7, 025010. [Google Scholar] [CrossRef]
- Naguib, M.; Halim, J.; Lu, J.; Cook, K.M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. New two-dimensional niobium and vanadium carbides as promising materials for Li-ion batteries. J. Am. Chem. Soc. 2013, 135, 15966–15969. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Gao, S.; Chen, Y.; Shi, J. Theranostic 2D Tantalum Carbide (MXene). Adv. Mater. 2018, 30, 1703284. [Google Scholar] [CrossRef]
- Shan, Q.; Mu, X.; Alhabeb, M.; Shuck, C.E.; Pang, D.; Zhao, X.; Chu, X.-F.; Wei, Y.; Du, F.; Chen, G.; et al. Two-dimensional vanadium carbide (V2C) MXene as electrode for supercapacitors with aqueous electrolytes. Electrochem. Commun. 2018, 96, 103–107. [Google Scholar] [CrossRef]
- Dall’Agnese, Y.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Two-Dimensional Vanadium Carbide (MXene) as Positive Electrode for Sodium-Ion Capacitors. J. Phys. Chem. Lett. 2015, 6, 2305–2309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- VahidMohammadi, A.; Mojtabavi, M.; Caffrey, N.M.; Wanunu, M.; Beidaghi, M. Assembling 2D MXenes into Highly Stable Pseudocapacitive Electrodes with High Power and Energy Densities. Adv. Mater. 2019, 31, e1806931. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Q.; Zhang, H.; Passerini, S.; Qian, X. Two-Dimensional Titanium Carbide/RGO Composite for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 15661–15667. [Google Scholar] [CrossRef]


| Materials | Chemical Formula | Molecular Mass |
|---|---|---|
| Vanadium Carbide Aluminide | V2AlC | 140.8 |
| Hydrochloric Acid | HCl | 36.46 |
| Sulfuric Acid | H2SO4 | 98.08 |
| Sodium Fluoride | NaF | 41.99 |
| Potassium Hydroxide | KOH | 56 |
| Sodium Carbonate | Na2CO3 | 105.99 |
| Polyvinylidene Fluoride | -(C2H2F2)n- | ---- |
| N-Methylpyrrolidone | C5H9NO | 99.13 |
| Element | Atomic Fraction (%) | Mass Fraction (%) |
|---|---|---|
| C | 29.82 | 10.09 |
| F | 11.24 | 6.01 |
| Al | 1.01 | 0.77 |
| V | 57.93 | 83.13 |
| Electrode Materials | Specific Capacitance (F/g) | Scan Rates (mV/s) | Electrolyte | References |
|---|---|---|---|---|
| V2C (NaF + HCl) | 556.7 | 2 | Na2SO4 | This work |
| V2C (NaF + HCl) | 113.4 | 2 | H2SO4 | This work |
| V2C (NaF + HCl) | 96.4 | 2 | KOH | This work |
| V2C (NaF + HCl) | 361 | 10 | Na2SO4 | This work |
| V2C (KF + HCl) | 164 | 2 | Na2SO4 | [42] |
| V2C (49 wt% HF) | 225 | 2 | Mg2SO4 | [45] |
| V2C (49 wt% HF) | 487 | 2 | H2SO4 | [45] |
| V2C (50 wt% HF) | 100 | 0.2 | NaPF6 | [46] |
| V2C (50 wt% HF) | 120 | 10 | Na2SO4 | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ai, W.; Zhang, C.; Xia, L.; Miao, H.; Yuan, J. Synthesis of High-Quality Two-Dimensional V2C MXene for Supercapacitor Application. Energies 2022, 15, 3696. https://doi.org/10.3390/en15103696
Ai W, Zhang C, Xia L, Miao H, Yuan J. Synthesis of High-Quality Two-Dimensional V2C MXene for Supercapacitor Application. Energies. 2022; 15(10):3696. https://doi.org/10.3390/en15103696
Chicago/Turabian StyleAi, Wangsheng, Chunfei Zhang, Lan Xia, He Miao, and Jinliang Yuan. 2022. "Synthesis of High-Quality Two-Dimensional V2C MXene for Supercapacitor Application" Energies 15, no. 10: 3696. https://doi.org/10.3390/en15103696
APA StyleAi, W., Zhang, C., Xia, L., Miao, H., & Yuan, J. (2022). Synthesis of High-Quality Two-Dimensional V2C MXene for Supercapacitor Application. Energies, 15(10), 3696. https://doi.org/10.3390/en15103696








