The Thermochemical Conversion of Forestry Residues from Silver Fir (Abies alba Mill.) by Torrefaction and Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Torrefaction and Pyrolysis Experiments
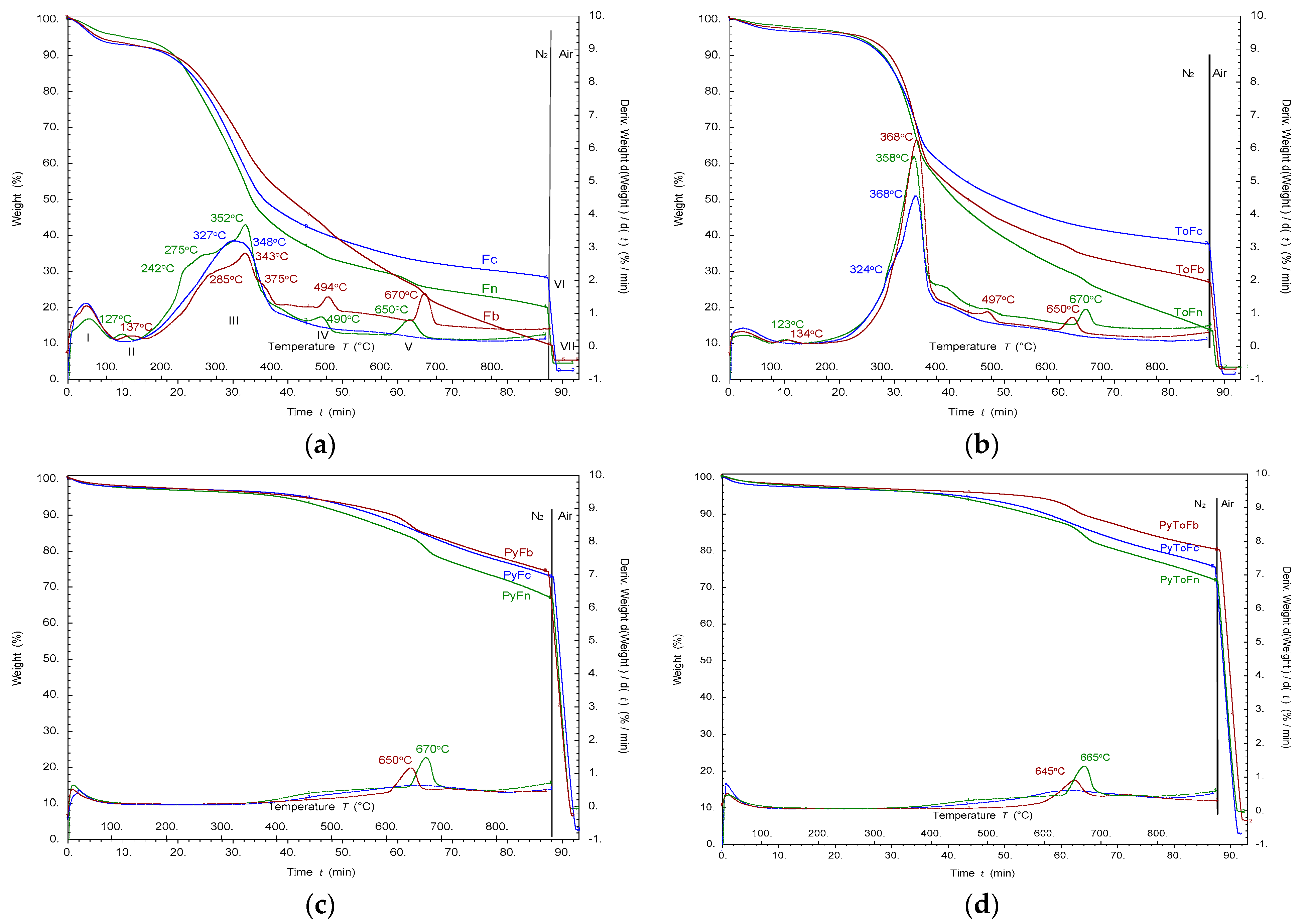
2.3. Proximate/Ultimate and Thermal Analysis
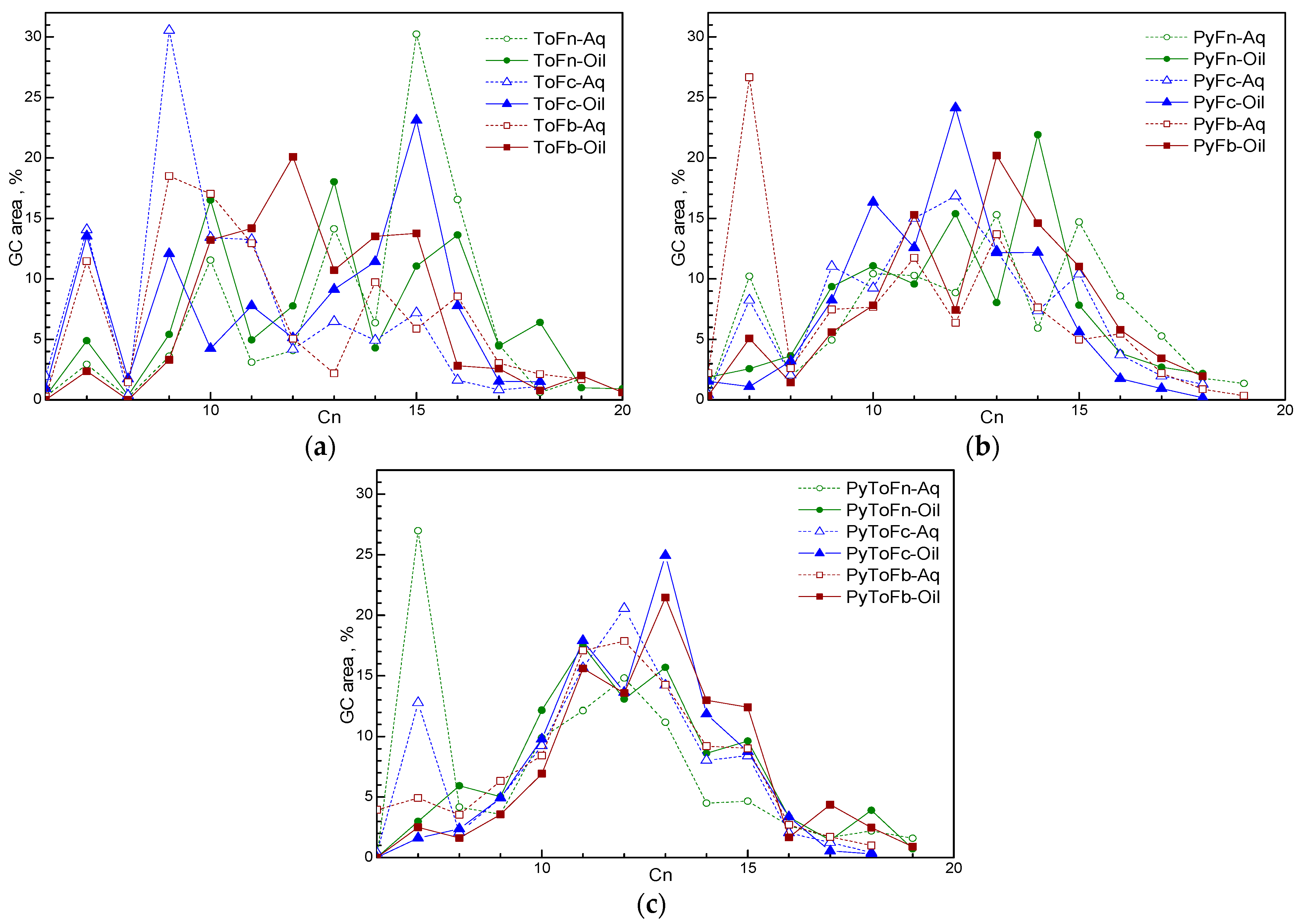
2.4. GC–MSD Analysis
3. Results and Discussion
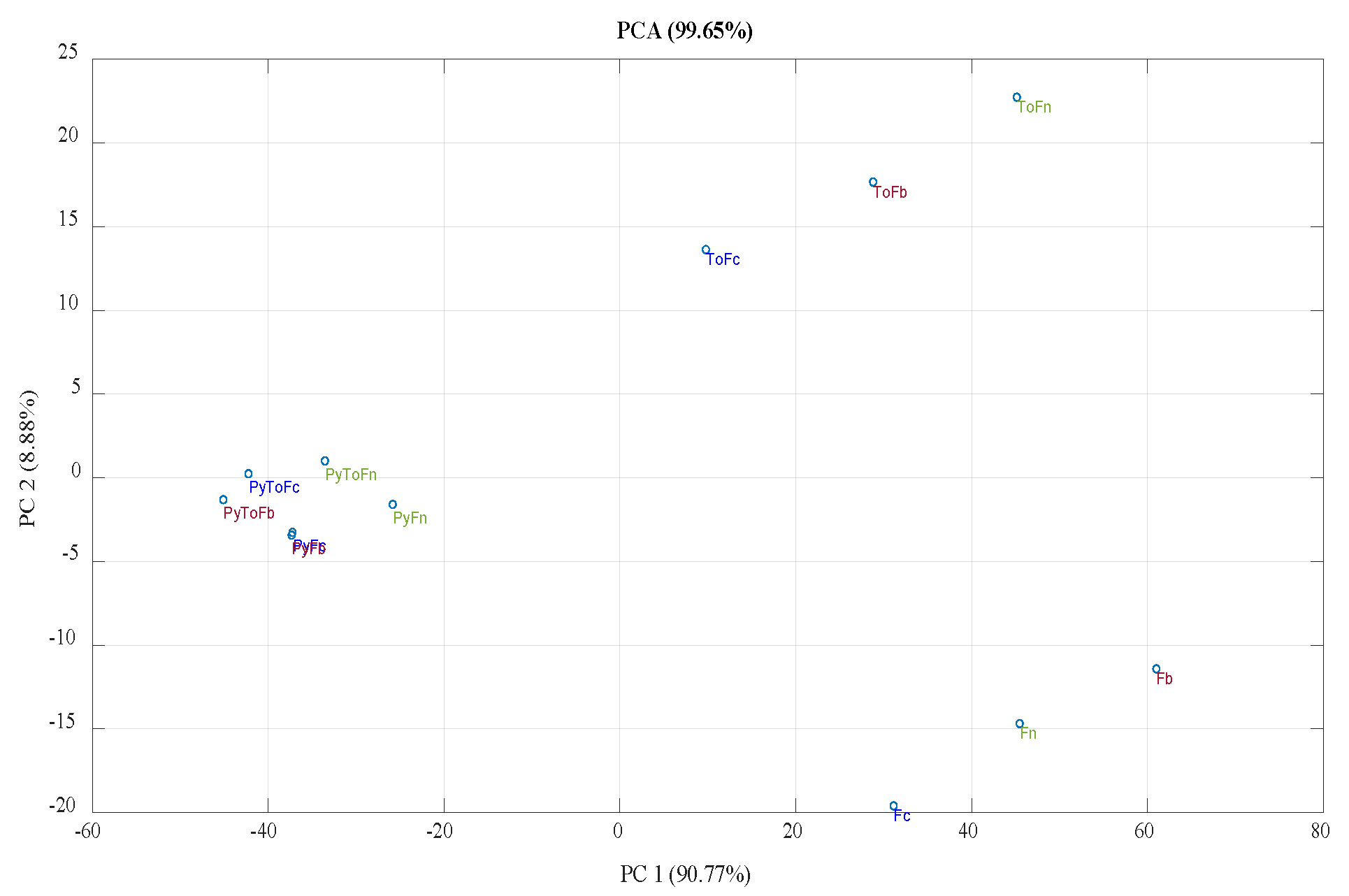
3.1. Product Yield and Characterization of Solid Materials
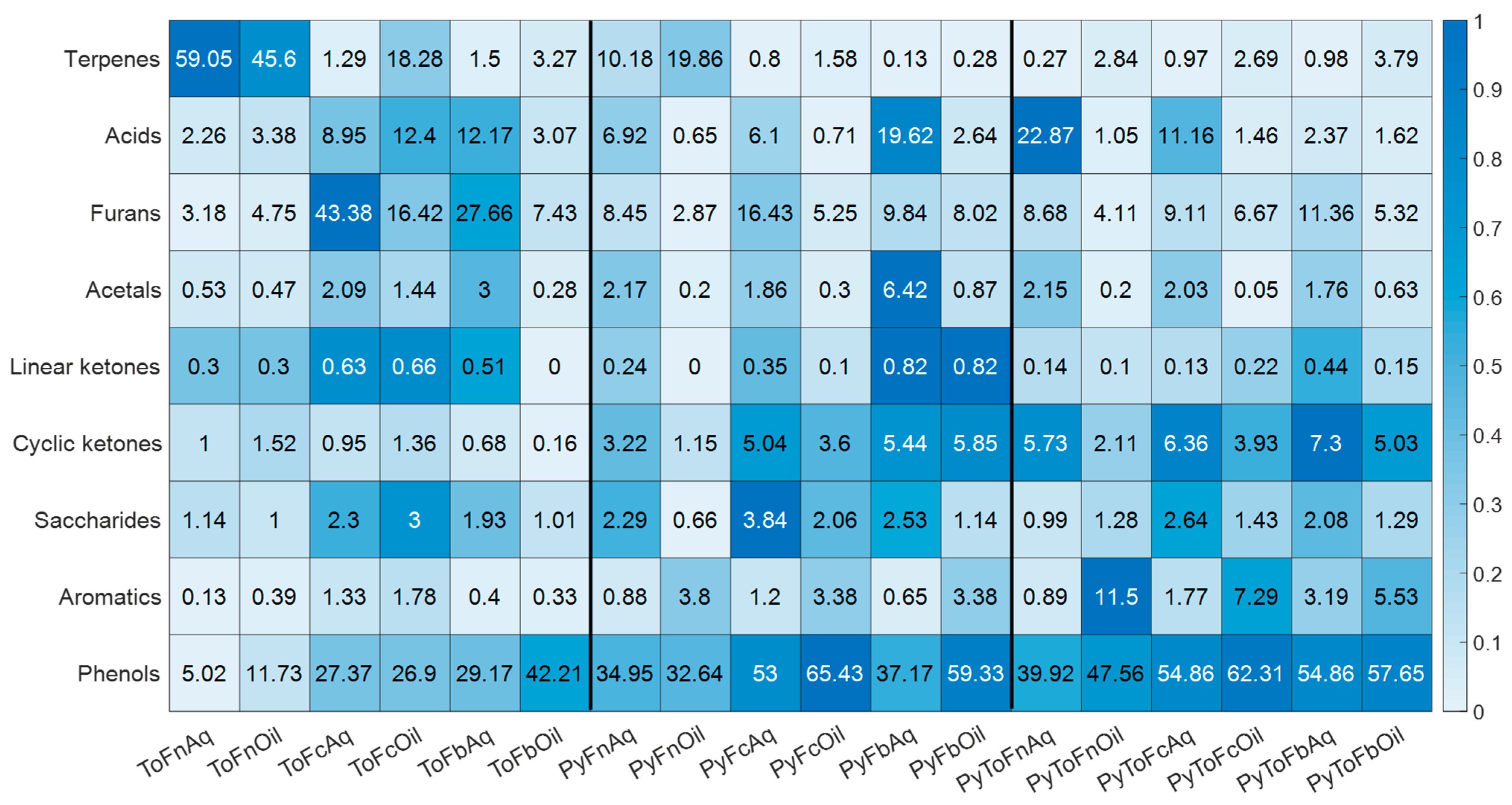
3.2. Characterization of Liquid Products
| No | Rt | Ri | Name | Type | CAS | RiNIST | ToFn-Aq | ToFn-Oil | ToFc-Aq | ToFc-Oil | ToFb-Aq | ToFb-Oil | PyFn-Aq | PyFn-Oil | PyFc-Aq | PyFc-Oil | PyFb-Aq | PyFb-Oil | PyToFn-Aq | PyToFn-Oil | PyToFc-Aq | PyToFc-Oil | PyToFb-Aq | PyToFb-Oil |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.21 | 598 | 2,3-Butanedione | Lk | 431-03-8 | 558 | 0.23 | 0.20 | 0.37 | 0.44 | 0.27 | 0.24 | 0.19 | 0.56 | 0.35 | |||||||||
| 2 | 2.30 | 604 | 2-Methylfuran | F | 534-22-5 | 610 | 0.07 | 0.41 | 1.17 | 1.46 | 0.75 | 0.81 | 0.60 | 1.72 | 0.56 | |||||||||
| 3 | 2.40 | 615 | Acetic acid | Ac | 64-19-7 | 594 | 2.26 | 3.38 | 8.29 | 11.81 | 9.33 | 6.92 | 0.65 | 6.10 | 0.34 | 19.62 | 2.64 | 22.87 | 1.05 | 11.16 | 0.87 | 2.37 | 1.11 | |
| 4 | 2.74 | 649 | 3-Methylbutanal | Al | 590-86-3 | 643 | 0.12 | 0.37 | 1.67 | 0.29 | 0.18 | 0.19 | 0.02 | 0.20 | ||||||||||
| 5 | 2.81 | 657 | Benzene | Ar | 71-43-2 | 680 | 0.10 | 0.16 | 0.17 | 0.13 | 0.12 | 0.1 | 0.32 | 0.52 | 0.06 | 0.52 | 0.52 | 0.54 | ||||||
| 6 | 2.87 | 663 | 2-Methylbutanal | Al | 96-17-3 | 643 | 0.06 | 0.27 | 1.05 | |||||||||||||||
| 7 | 2.91 | 667 | Acetol | Al | 116-09-6 | 681 | 0.36 | 0.26 | 1.73 | 1.13 | 1.96 | 1.64 | 0.1 | 1.44 | 0.22 | 5.89 | 0.63 | 1.83 | 0.2 | 1.61 | 0.05 | 1.33 | 0.52 | |
| 8 | 2.95 | 671 | 3-Penten-2-one | Lk | 625-33-2 | 697 | 0.10 | 0.18 | 0.14 | 0.1 | 0.13 | 0.22 | 0.44 | 0.15 | ||||||||||
| 9 | 3.22 | 699 | 2,3-Pentanedione | Lk | 600-14-6 | 666 | 0.07 | 0.10 | 0.26 | 0.22 | 0.24 | 0.16 | ||||||||||||
| 10 | 3.35 | 708 | 2,5-Dimethylfuran/Propanoic acid | F/Ac | 625-86-5/79-09-4 | 667/676 | 0.11 | 1.06 | 0.09 | 0.55 | 0.20 | 2.83 | 0.27 | 1.72 | 0.19 | 1.44 | 0.16 | |||||||
| 11 | 4.22 | 765 | Toluene | Ar | 108-88-3 | 755 | 0.02 | 0.09 | 1.22 | 0.53 | 2.13 | 0.27 | 0.62 | 0.12 | 0.86 | 3.98 | 0.03 | 1.60 | 1.00 | 1.23 | ||||
| 12 | 4.70 | 796 | Cyclopentanone | Ck | 120-92-3 | 771 | 0.35 | 0.40 | 0.44 | 0.17 | 0.06 | 0.29 | 0.87 | 0.23 | ||||||||||
| 13 | 5.46 | 839 | Furfural | F | 98-01-1 | 831 | 1.21 | 1.33 | 27.09 | 8.56 | 14.89 | 1.66 | 1.71 | 0.11 | 7.98 | 1.13 | 2.59 | 1.51 | 0.22 | 1.67 | 1.10 | 1.84 | 0.50 | |
| 14 | 5.49 | 840 | Cyclopentenone | Ck | 930-30-3 | 793 | 0.55 | 0.12 | 1.48 | 0.47 | 0.77 | 0.78 | 1.04 | 0.24 | 1.35 | 0.5 | 1.06 | 0.52 | ||||||
| 15 | 5.91 | 863 | Furfuryl alcohol | F | 98-00-0 | 885 | 0.50 | 0.57 | 1.11 | 1.03 | 1.93 | 0.67 | 1.70 | 0.33 | 1.16 | 0.49 | 2.10 | 1.11 | 1.09 | 0.43 | 1.19 | 0.50 | 1.99 | 0.63 |
| 16 | 5.87 | 862 | Ethylbenzene | Ar | 100-41-4 | 893 | 0.50 | 0.20 | 0.24 | 0.84 | 0.29 | 0.46 | 0.2 | 0.31 | ||||||||||
| 17 | 6.01 | 869 | p-Xylene | Ar | 106-42-3 | 855 | 0.19 | 0.78 | 0.3 | 0.45 | 0.10 | 0.69 | 1.53 | 1.41 | 0.38 | 0.66 | ||||||||
| 18 | 6.17 | 878 | 5-Methyl-2(3H)-furanone | F | 591-12-8 | 897 | 0.22 | 0.19 | ||||||||||||||||
| 19 | 6.18 | 879 | Acetol acetate | Al | 592-20-1 | 822 | 0.16 | 0.21 | 0.36 | 0.31 | 1.04 | 0.28 | 0.53 | 0.1 | 0.43 | 0.08 | 0.53 | 0.25 | 0.32 | 0.42 | 0.42 | 0.11 | ||
| 20 | 6.30 | 885 | Santene | T | 529-16-8 | 880 | 1.59 | 2.50 | 0.27 | 0.90 | 0.50 | 0.78 | ||||||||||||
| 21 | 6.44 | 893 | o-Xylene | Ar | 95-47-6 | 881 | 0.32 | 0.33 | 0.60 | 0.45 | 0.26 | 0.16 | ||||||||||||
| 22 | 6.79 | 913 | 2-Methyl-2-cyclopentenone | Ck | 1120-73-6 | 881 | 0.26 | 0.18 | 0.31 | 0.39 | 0.68 | 1.07 | 0.41 | 0.33 | 0.46 | 0.53 | 0.73 | 0.56 | ||||||
| 23 | 6.90 | 919 | Butyrolactone | F | 96-48-0 | 870 | 0.67 | 0.12 | 0.82 | 0.39 | 1.33 | 0.61 | 1.39 | 0.14 | 0.93 | 0.33 | 1.15 | 0.47 | ||||||
| 24 | 6.94 | 921 | Acetylfuran | F | 1192-62-7 | 879 | 0.92 | 0.28 | 0.48 | 0.27 | 0.36 | 0.15 | 0.37 | 0.32 | 0.56 | 0.34 | ||||||||
| 25 | 6.98 | 923 | Tricyclene | T | 508-32-7 | 922 | 0.40 | 0.39 | ||||||||||||||||
| 26 | 7.14 | 932 | 2-Hydroxy-2-cyclopentenone | Ck | 10493-98-8 | 919 | 0.53 | 0.06 | 0.78 | 0.17 | 1.65 | 0.86 | 0.20 | 0.1 | 0.68 | 0.18 | 0.94 | 0.22 | ||||||
| 27 | 7.19 | 935 | α-Pinene | T | 7785-70-8 | 948 | 1.55 | 1.90 | 0.31 | |||||||||||||||
| 28 | 7.31 | 943 | 1,2-Cyclopentanedione | Ck | 3008-40-0 | 942 | 0.06 | 0.07 | 0.41 | 0.57 | 0.68 | 0.16 | ||||||||||||
| 29 | 7.56 | 956 | Propylbenzene | Ar | 103-65-1 | 992 | 0.16 | 0.20 | 0.11 | 0.16 | ||||||||||||||
| 30 | 7.47 | 951 | Camphene | T | 79-92-5 | 943 | 3.27 | 4.40 | 0.37 | 0.68 | ||||||||||||||
| 31 | 7.70 | 964 | m-Ethyltoluene | Ar | 620-14-4 | 1006 | 0.22 | 0.46 | 0.92 | 0.61 | 0.52 | |||||||||||||
| 32 | 7.82 | 971 | 5-Methylfurfural | F | 620-02-0 | 920 | 0.74 | 1.01 | 9.55 | 2.67 | 10.00 | 5.10 | 1.08 | 3.67 | 1.35 | 1.29 | 1.09 | 1.28 | 1.06 | 1.32 | 0.46 | |||
| 33 | 7.84 | 972 | 3-Methyl-2-cyclopentenone | Ck | 2758-18-1 | 923 | 0.34 | 0.54 | 0.62 | 0.55 | 0.46 | 0.64 | 0.53 | |||||||||||
| 34 | 7.94 | 977 | 1-Acetoxy-2-butanone | Lk | 1575-57-1 | 933 | 0.26 | 0.28 | ||||||||||||||||
| 35 | 7.94 | 978 | Methyl 2-furoate | F | 611-13-2 | 956 | 0.19 | 0.15 | ||||||||||||||||
| 36 | 8.12 | 988 | Phenol | Ph | 108-95-2 | 955 | 1.55 | 1.47 | 0.70 | 0.41 | 1.90 | 4.01 | 6.46 | 5.73 | 2.13 | 4.34 | 1.84 | 2.16 | 6.37 | 7.96 | 4.57 | 5.21 | 3.11 | 2.84 |
| 37 | 8.29 | 997 | Hexanoic acid | Ac | 142-62-1 | 974 | 0.67 | 0.59 | 2.84 | 3.07 | 0.37 | 0.59 | 0.51 | |||||||||||
| 38 | 8.42 | 1005 | 2-Furanone, 2,5-dihydro-3,5-dimethyl | F | 196881 | 958 | 0.16 | 0.71 | 0.35 | 0.22 | 0.21 | 0.17 | 0.22 | 0.18 | ||||||||||
| 39 | 8.43 | 1006 | 1,2-Cyclohexanedione | Ck | 765-87-7 | 1062 | 0.28 | 0.42 | ||||||||||||||||
| 40 | 8.73 | 1023 | 1,2,3-Trimethylbenzene | Ar | 526-73-8 | 1020 | 0.56 | 0.20 | 0.91 | 0.41 | ||||||||||||||
| 41 | 8.78 | 1026 | β-Cymene | T | 527-84-4 | 1042 | 0.13 | 0.19 | 0.92 | 1.09 | ||||||||||||||
| 42 | 8.85 | 1031 | Limonene/β-Phellandrene | T | 138-86-3/555-10-2 | 1018/1030 | 1.39 | 1.61 | 0.53 | 0.37 | 0.35 | 1.75 | 1.91 | |||||||||||
| 43 | 8.97 | 1038 | 3-Methyl-1,2-cyclopentanedione | Ck | 765-70-8 | 1043 | 1.02 | 0.62 | 1.44 | 0.97 | 1.83 | 1.37 | 1.56 | 0.80 | 1.57 | 0.85 | 1.72 | 1.16 | ||||||
| 44 | 9.07 | 1043 | 2,3-Dimethyl-2-cyclopentenone | Ck | 1121-05-7 | 999 | 0.56 | 0.29 | 0.24 | 0.89 | ||||||||||||||
| 45 | 9.17 | 1050 | Benzeneacetaldehyde | Ar | 122-78-1 | 1081 | 0.11 | 0.20 | 1.33 | 0.56 | 0.40 | 0.33 | ||||||||||||
| 46 | 9.27 | 1056 | 2-Methylphenol | Ph | 95-48-7 | 1014 | 0.45 | 0.39 | 0.42 | 1.09 | 0.88 | 2.61 | 1.04 | 1.43 | 0.66 | 2.00 | 1.94 | 3.58 | 1.78 | 2.54 | ||||
| 47 | 9.61 | 1077 | Acetophenone | F | 1679-47-6 | 941 | 0.30 | 0.12 | 0.11 | 0.23 | 0.25 | 0.18 | 0.22 | 0.32 | 0.37 | 0.1 | 0.31 | |||||||
| 48 | 9.62 | 1077 | 3-Methylphenol | Ph | 108-39-4 | 1053 | 0.45 | 1.26 | 1.80 | 2.79 | 2.02 | 5.58 | 1.17 | 2.13 | 2.52 | 4.90 | 4.52 | 6.50 | 3.08 | 4.23 | ||||
| 49 | 9.84 | 1090 | Terpinolene | T | 586-62-9 | 1052 | 0.32 | 0.41 | ||||||||||||||||
| 50 | 9.90 | 1094 | Guaiacol | Ph | 90-05-1 | 1090 | 0.47 | 0.97 | 10.59 | 4.97 | 7.41 | 6.58 | 3.49 | 2.57 | 8.72 | 4.66 | 5.26 | 7.20 | 2.99 | 3.08 | 5.31 | 3.87 | 6.05 | 4.49 |
| 51 | 10.08 | 1105 | 7-Methylbenzofuran | F | 17059-52-8 | 1131 | 0.19 | 0.33 | 1.50 | 0.26 | 0.50 | 0.39 | ||||||||||||
| 52 | 10.07 | 1105 | 6-Camphenol | T | 3570-04-5 | 1082 | 0.37 | 0.58 | ||||||||||||||||
| 53 | 10.12 | 1108 | 2,5-Dimethylphenol | Ph | 95-87-4 | 1127 | 0.36 | 0.53 | 0.48 | 0.46 | ||||||||||||||
| 54 | 10.16 | 1110 | 2-Methylbenzofuran | F | 4265-25-2 | 1131 | 0.34 | 0.42 | 0.55 | 0.35 | ||||||||||||||
| 55 | 10.37 | 1124 | 3-Ethyl-2-hydroxy-2-cyclopentenone | Ck | 21835-01-8 | 1072 | 0.31 | 0.38 | 0.52 | |||||||||||||||
| 56 | 10.50 | 1132 | Maltol | Ck | 118-71-8 | 1092 | 0.54 | 0.79 | 0.52 | 0.17 | 0.69 | 1.06 | 0.52 | 1.38 | 0.59 | 0.48 | 1.03 | 1.12 | 0.58 | 0.91 | ||||
| 57 | 10.75 | 1149 | L-Pinocarveol | T | 547-61-5 | 1143 | 0.43 | 0.49 | 0.83 | 0.69 | ||||||||||||||
| 58 | 10.75 | 1149 | Ethyl/Dimethylphenols | Ph | 0.78 | 2.14 | 0.63 | 3.15 | 0.56 | 1.40 | 1.09 | 2.15 | 2.31 | 4.16 | 2.09 | 3.45 | ||||||||
| 59 | 11.03 | 1167 | Ethyl/Dimethylphenols | Ph | 0.25 | 0.57 | 0.32 | 0.2 | 1.19 | 2.39 | 1.25 | 2.16 | 0.62 | 1.24 | 1.61 | 2.98 | 1.62 | 2.4 | 1.52 | 1.85 | ||||
| 60 | 11.17 | 1176 | Borneol | T | 10385-78-1 | 1088 | 0.72 | 1.24 | 1.46 | |||||||||||||||
| 61 | 11.36 | 1189 | Naphthalene | Ar | 91-20-3 | 1170 | 0.50 | 0.56 | 1.61 | 0.46 | 0.62 | 0.51 | 0.68 | |||||||||||
| 62 | 11.47 | 1195 | 4,4,6-Trimethyl-2-cyclohexen-1-one | Ck | 13395-73-8 | 1069 | 0.66 | 1.04 | ||||||||||||||||
| 63 | 11.50 | 1197 | p-Methylguaiacol | Ph | 93-51-6 | 1162 | 1.92 | 1.81 | 2.54 | 3.53 | 1.53 | 1.77 | 4.01 | 3.41 | 3.94 | 6.48 | 1.30 | 2.31 | 2.48 | 2.67 | 3.86 | 4.20 | ||
| 64 | 11.63 | 1206 | Pyrocatechol | Ph | 120-80-9 | 1199 | 0.80 | 1.85 | 1.58 | 2.72 | 1.79 | 5.37 | 3.36 | 8.71 | 8.48 | 3.98 | 4.02 | 7.59 | 3.71 | 10.80 | 7.77 | 7.53 | 4.87 | |
| 65 | 11.76 | 1215 | 1,4:3,6-Dianhydro-α-D-glucopyranose | S | 98148 | 1198 | 0.16 | 0.72 | 0.42 | 0.59 | 0.18 | 0.36 | 0.34 | |||||||||||
| 66 | 11.81 | 1218 | 4,7-Dimethylbenzofuran | F | 28715-26-6 | 1244 | 0.18 | 0.31 | 0.37 | 0.32 | 0.83 | 0.34 | 1.18 | 0.92 | ||||||||||
| 67 | 11.87 | 1223 | Dihydrobenzofuran | F | 496-16-2 | 1188 | 0.66 | 1.43 | 2.32 | 2.96 | 1.44 | 0.95 | 1.46 | 0.86 | 0.74 | |||||||||
| 68 | 12.05 | 1236 | 5-Hydroxymethylfurfural | F | 67-47-0 | 1176 | 0.92 | 1.20 | 0.84 | 0.83 | 0.16 | 0.73 | 0.42 | 0.34 | 0.42 | 0.44 | 0.46 | 0.37 | 0.39 | |||||
| 69 | 12.11 | 1240 | 2-Ethyl-5-methylphenol | Ph | 1687-64-5 | 1236 | 0.33 | 0.71 | 0.21 | 0.16 | 0.62 | 0.42 | 0.93 | 0.43 | 0.97 | |||||||||
| 70 | 12.39 | 1259 | 3-Ethyl-5-methylphenol | Ph | 698-71-5 | 1260 | 0.40 | 0.35 | 0.40 | 0.65 | 0.60 | 0.52 | ||||||||||||
| 71 | 12.44 | 1263 | 3-Methylpyrocatechol | Ph | 488-17-5 | 1235 | 0.36 | 1.11 | 1.29 | 2.14 | 0.94 | 1.53 | 0.91 | 1.90 | 1.67 | 2.39 | 2.11 | 2.28 | ||||||
| 72 | 12.49 | 1266 | 3-Methoxycatechol | Ph | 934-00-9 | 1269 | 0.40 | 0.5 | 0.27 | 0.23 | 0.59 | 0.43 | 0.30 | |||||||||||
| 73 | 12.58 | 1273 | Hydroquinone | Ph | 123-31-9 | 1334 | 1.55 | 0.84 | 0.53 | 0.34 | 2.95 | 1.30 | 1.07 | |||||||||||
| 74 | 12.76 | 1285 | p-Ethylguaiacol | Ph | 2785-89-9 | 1250 | 0.17 | 0.37 | 0.94 | 1.02 | 2.03 | 3.62 | 2.39 | 2.19 | 2.80 | 5.52 | 1.61 | 1.26 | 1.62 | 1.58 | 2.49 | 2.95 | ||
| 75 | 12.90 | 1295 | Homocatechol | Ph | 452-86-8 | 1235 | 0.72 | 1.25 | 0.40 | 5.14 | 7.00 | 1.91 | 3.09 | 2.51 | 3.32 | 6.67 | 7.94 | 6.24 | 6.15 | |||||
| 76 | 12.91 | 1295 | Bornyl acetate | T | 76-49-3 | 1277 | 11.86 | 12.93 | ||||||||||||||||
| 77 | 13.25 | 1320 | p-Vinylguaiacol | Ph | 7786-61-0 | 1293 | 0.30 | 0.61 | 3.47 | 3.14 | 2.61 | 4.99 | 1.27 | 1.07 | 2.46 | 3.34 | 2.12 | 3.71 | 0.43 | 1.26 | 1.24 | 2.73 | 1.20 | 1.77 |
| 78 | 13.55 | 1342 | Methyl-p-hydroquinone | Ph | 95-71-6 | 1235 | 0.30 | 0.53 | 0.31 | 0.89 | 0.86 | 0.39 | ||||||||||||
| 79 | 13.71 | 1354 | 2,4-Dimethoxyphenol | Ph | 13330-65-9 | 1318 | 0.36 | 0.44 | 0.84 | 0.48 | 0.76 | 0.59 | 0.67 | |||||||||||
| 80 | 13.80 | 1361 | α-Longipinene | T | 5989-08-2 | 1358 | 2.09 | 1.15 | 2.56 | 0.38 | 1.03 | 0.39 | 0.96 | 0.27 | 0.74 | 0.75 | 1.05 | 0.98 | 1.37 | |||||
| 81 | 13.83 | 1363 | Eugenol | Ph | 97-53-0 | 1392 | 0.69 | 0.86 | 1.14 | 2.75 | ||||||||||||||
| 82 | 13.86 | 1366 | 3-Allyl-2-methoxyphenol | Ph | 1941-12-4 | 1392 | 1.08 | 1.18 | 1.15 | 2.41 | 0.48 | 1.11 | ||||||||||||
| 83 | 13.92 | 1370 | p-Propylguaiacol | Ph | 2785-87-7 | 1392 | 0.32 | 0.54 | 0.58 | 0.39 | 0.59 | 0.56 | 1.54 | 0.27 | 0.62 | 0.51 | 0.50 | 0.61 | 0.74 | |||||
| 84 | 14.12 | 1385 | 4-Ethylcatechol | Ph | 1124-39-6 | 1334 | 0.89 | 1.32 | 1.42 | 2.79 | 1.39 | 2.21 | 1.15 | 2.18 | 2.39 | 3.09 | 3.26 | 4.07 | ||||||
| 85 | 14.45 | 1410 | Vanillin | Ph | 121-33-5 | 1403 | 0.87 | 1.49 | 1.13 | 1.11 | 1.12 | 0.53 | 1.03 | |||||||||||
| 86 | 14.46 | 1410 | 3-Allyl-2-methoxyphenol | Ph | 15314 | 1392 | 0.78 | 1.23 | 0.68 | 0.63 | 0.37 | 1.01 | 0.20 | 1.30 | ||||||||||
| 87 | 14.58 | 1420 | Junipene | T | 475-20-7 | 1398 | 1.54 | 1.08 | 7.70 | |||||||||||||||
| 88 | 14.74 | 1432 | β-Caryophyllene | T | 87-44-5 | 1424 | 9.85 | 4.52 | 1.62 | 4.97 | ||||||||||||||
| 89 | 14.81 | 1438 | Himachala-2,4-diene | T | 60909-27-5 | 1499 | 7.19 | 3.08 | 1.21 | 2.74 | ||||||||||||||
| 90 | 14.85 | 1441 | 2,4-Dimethoxyphenol | Ph | 13330-65-9 | 1318 | 0.46 | 0.90 | ||||||||||||||||
| 91 | 14.95 | 1449 | D-Mannose/D-Allose | S | 3458-28-4/2595-97-3 | 1698 | 1.14 | 0.84 | 0.87 | 1.98 | 1.40 | 0.72 | 1.57 | 0.66 | 1.90 | 0.16 | 0.20 | 0.99 | 0.50 | 1.61 | 1.26 | |||
| 92 | 15.05 | 1457 | Isoeugenol | Ph | 97-54-1 | 1410 | 1.97 | 2.91 | 4.41 | 4.67 | 11.76 | 1.96 | 1.67 | 2.98 | 3.44 | 2.82 | 5.48 | 0.49 | 0.92 | 1.30 | 1.81 | 1.70 | 2.59 | |
| 93 | 15.11 | 1462 | 2,3,4,5-Tetramethylbenzaldehyde | Ar | 29344-95-4 | 1435 | 0.50 | 0.60 | 0.65 | 0.34 | 1.10 | 0.93 | 0.58 | 0.31 | 0.86 | |||||||||
| 94 | 15.13 | 1463 | α-Himachalene | T | 3853-83-6 | 1447 | 1.58 | 0.87 | 0.78 | 1.33 | ||||||||||||||
| 95 | 15.14 | 1464 | α-Caryophyllene | T | 6753-98-6 | 1456 | 3.25 | 1.58 | 1.27 | 2.57 | ||||||||||||||
| 96 | 15.30 | 1477 | 4-Propyl-1,3-benzenediol | Ph | 18979-60-7 | 1434 | 0.81 | 0.26 | 0.97 | 1.19 | ||||||||||||||
| 97 | 15.48 | 1490 | Levoglucosan | S | 498-07-7 | 1426 | 1.42 | 1.02 | 0.53 | 0.28 | 1.52 | 1.90 | 1.74 | 1.14 | 0.60 | 1.02 | 1.07 | 0.82 | 0.95 | |||||
| 98 | 15.69 | 1508 | γ-Gurjunene | T | 22567-17-5 | 1469 | 2.06 | 0.91 | 0.55 | 3.05 | 0.30 | 0.85 | 0.41 | 0.62 | 0.13 | 0.28 | 0.19 | 0.22 | 0.37 | 0.31 | ||||
| 99 | 15.76 | 1513 | β-Himachalene | T | 1461-03-6 | 1497 | 2.82 | 0.90 | 1.38 | 0.27 | 0.56 | 0.90 | ||||||||||||
| 100 | 15.77 | 1514 | β-Cadinene | T | 523-47-7 | 1440 | 0.35 | 2.63 | ||||||||||||||||
| 101 | 15.89 | 1524 | γ-Cadinene | T | 39029-41-9 | 1505 | 1.46 | 0.73 | 0.78 | 0.59 | 0.73 | |||||||||||||
| 102 | 15.98 | 1532 | δ-Cadinene | T | 483-76-1 | 1514 | 3.09 | 1.52 | 0.30 | 0.87 | 1.11 | 1.67 | ||||||||||||
| 103 | 16.11 | 1543 | Guaiacylacetone | Ph | 2503-46-0 | 1538 | 0.36 | 0.46 | 0.71 | 1.58 | 1.36 | 1.12 | 0.94 | 0.59 | 1.31 | 1.02 | 0.86 | 1.42 | 0.61 | 0.48 | 0.69 | 0.85 | 1.35 | 1.18 |
| 104 | 16.17 | 1547 | α-Calacorene | T | 21391-99-1 | 1547 | 0.49 | 0.30 | ||||||||||||||||
| 105 | 16.34 | 1562 | 4-Hydroxybenzylacetone | Ph | 5471-51-2 | 1498 | 0.49 | 1.38 | 1.79 | 0.84 | 0.31 | 0.44 | 0.16 | 0.50 | 1.05 | 0.82 | 0.40 | 0.35 | 0.38 | 0.68 | ||||
| 106 | 16.46 | 1571 | 4-(3-Hydroxybutyl)phenol | Ph | 501-96-2 | 1475 | 0.24 | 1.04 | 1.03 | 0.44 | 0.46 | 0.43 | 0.50 | 0.73 | 0.43 | 0.99 | 0.39 | 0.45 | 0.62 | 0.51 | ||||
| 107 | 17.01 | 1618 | Longiborneol | T | 465-24-7 | 1588 | 0.59 | 1.11 | ||||||||||||||||
| 108 | 17.22 | 1637 | Juniper camphor | T | 473-04-1 | 1647 | 1.47 | 1.69 | ||||||||||||||||
| 109 | 17.48 | 1659 | Homovanillic acid | Ph | 306-08-1 | 1659 | 0.39 | 1.04 | 0.83 | 1.53 | 1.30 | 0.77 | 2.04 | 0.84 | 1.56 | 0.89 | 1.34 | 1.91 | 0.73 | 0.94 | 0.75 | 0.54 | 1.41 | 0.84 |
| 110 | 18.16 | 1720 | 3-Phenoxyphenol | Ph | 713-68-8 | 1664 | 0.52 | 0.28 | 0.21 | 0.19 | 0.14 | 0.24 | 0.33 | 0.59 | 0.28 | 0.32 | 0.43 | 0.38 | ||||||
| 111 | 18.44 | 1746 | Coniferyl aldehyde | Ph | 458-36-6 | 1676 | 1.10 | 1.51 | ||||||||||||||||
| Total | 72.77 | 69.79 | 90.40 | 83.12 | 77.21 | 57.76 | 69.50 | 61.86 | 88.81 | 82.41 | 82.63 | 82.33 | 81.66 | 70.75 | 89.03 | 86.04 | 84.33 | 81.01 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scarlat, N.; Dallemand, J.-F.; Monforti-Ferrario, F.; Banja, M.; Motola, V. Renewable energy policy framework and bioenergy contribution in the European Union—An overview from National Renewable Energy Action Plans and Progress Reports. Renew. Sustain. Energy Rev. 2015, 51, 969–985. [Google Scholar] [CrossRef]
- Titus, B.D.; Brown, K.; Helmisaari, H.-S.; Vanguelova, E.; Stupak, I.; Evans, A.; Clarke, N.; Guidi, C.; Bruckman, V.J.; Varnagiryte-Kabasinskiene, I.; et al. Sustainable forest biomass: A review of current residue harvesting guidelines. Energy Sustain. Soc. 2021, 11, 10. [Google Scholar] [CrossRef]
- Balaman, Ş.Y. Introduction to biomass-resources, production, harvesting, collection, and storage. In Decision-Making for Biomass-Based Production Chains. The Basic Concepts and Methodologies; Balaman, Ş.Y., Ed.; Academic Press: London, UK, 2019; Chapter 1; pp. 1–23. [Google Scholar] [CrossRef]
- Barbati, A.; Marchetti, M.; Chirici, G.; Corona, P. European Forest Types and Forest Europe SFM indicators: Tools for monitoring progress on forest biodiversity conservation. For. Ecol. Manag. 2014, 321, 145–157. [Google Scholar] [CrossRef]
- Scarlat, N.; Blujdea, V.; Dallemand, J.-F. Assessment of the availability of agricultural and forest residues for bioenergy production in Romania. Biomass Bioenergy 2011, 35, 1995–2005. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, P. Chemical valorization of cellulose from lignocellulosic biomass: A step towards sustainable development. Cellul. Chem. Technol. 2021, 55, 207–222. [Google Scholar] [CrossRef]
- Galić, M.; Stajić, M.; Ćilerdžić, J. Lignocellulose waste valorization by an enzymatic cocktail of P. eryngii and P. pulmonarius. Cellul. Chem. Technol. 2021, 55, 1043–1050. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent insights into lignocellulosic biomass pyrolysis: A critical review on pretreatment, characterization, and products upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis—A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, M.; Jiang, E.; Wang, D.; Zhang, K.; Ren, Y.; Jiang, Y. Pyrolysis of torrefied biomass. Trends Biotechnol. 2018, 36, 1287–1298. [Google Scholar] [CrossRef]
- Basu, P. Torrefaction. In Biomass Gasification, Pyrolysis and Torrefaction—Practical Design and Theory, 3rd ed.; Basu, P., Ed.; Academic Press: San Diego, CA, USA, 2018; pp. 93–154. [Google Scholar] [CrossRef]
- Gent, S.; Twedt, M.; Gerometta, C.; Almberg, E. Introduction to Thermochemical Conversion Processes. In Theoretical and Applied Aspects of Biomass Torrefaction. For Biofuels and Value-Added Products; Gent, S., Twedt, M., Gerometta, C., Almberg, E., Eds.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 1–16. [Google Scholar] [CrossRef]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubiera, F.; Pis, J.J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T.; Mostek, A. Mechanical durability and grindability of pellets after torrefaction. Process. Energies 2021, 14, 6772. [Google Scholar] [CrossRef]
- Chew, J.J.; Doshi, V. Recent advances in biomass pretreatment—Torrefaction fundamentals and technology. Renew. Sustain. Energy Rev. 2011, 15, 4212–4222. [Google Scholar] [CrossRef]
- Rowell, R.M.; Pettersen, R.; Tshabalala, M.A. Cell Wall Chemistry. In Handbook of Wood Chemistry and Wood Composites, 2nd ed.; Rowell, R.M., Ed.; CRC Press: London, UK, 2012; pp. 33–72. Available online: https://www.routledgehandbooks.com/doi/10.1201/b12487-5 (accessed on 11 April 2020).
- Zhang, L.; Liu, J.; Zhang, Z.; Yang, Z.; Wang, X.; Li, D.; Lin, R. Research of the two-step pyrolysis of lignocellulosic biomass based on the cross-coupling of components by Py-GC/MS. Biomass Conv. Biorefin. 2021. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Z. Pyrolysis of hemicellulose. In Pyrolysis of Biomass; De Gruyter: Berlin, Germany; Boston, MA, USA, 2016; Chapter 3; pp. 81–102. [Google Scholar] [CrossRef]
- Mettler, M.S.; Mushrif, S.H.; Paulsen, A.D.; Javadekar, A.D.; Vlachos, D.G.; Dauenhauer, P.J. Revealing pyrolysis chemistry for biofuels production: Conversion of cellulose to furans and small oxygenates. Energy Environ. Sci. 2012, 5, 5414–5424. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Z. Pyrolysis of lignin. In Pyrolysis of Biomass; De Gruyter: Berlin, Germany; Boston, MA, USA, 2016; Chapter 4; pp. 103–140. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Borella, M.; Casazza, A.A.; Garbarino, G.; Riani, P.; Busca, G. A study of the pyrolysis products of Kraft lignin. Energies 2022, 15, 991. [Google Scholar] [CrossRef]
- Părpăriță, E.; Brebu, M.; Uddin, M.A.; Yanik, J.; Vasile, C. Pyrolysis behaviors of various biomasses. Polym. Degrad. Stab. 2014, 100, 1–9. [Google Scholar] [CrossRef]
- Harun, N.Y.; Afzal, M.T. Thermal Decomposition Kinetics of Forest Residue. J. Appl. Sci. 2010, 10, 1122–1127. [Google Scholar] [CrossRef][Green Version]
- Enes, T.; Aranha, J.; Fonseca, T.; Lopes, D.; Alves, A.; Lousada, J. Thermal properties of residual agroforestry biomass of Northern Portugal. Energies 2019, 12, 1418. [Google Scholar] [CrossRef]
- Dyjakon, A.; Noszczyk, T. Alternative fuels from forestry biomass residue: Torrefaction process of horse chestnuts, oak acorns, and spruce cones. Energies 2020, 13, 2468. [Google Scholar] [CrossRef]
- Negahdar, L.; Gonzalez-Quiroga, A.; Otyuskaya, D.; Toraman, H.E.; Liu, L.; Jastrzebski, J.T.; Van Geem, K.M.; Marin, G.B.; Thybaut, J.W.; Weckhuysen, B.M. Characterization and comparison of fast pyrolysis bio-oils from pinewood, rapeseed cake, and wheat straw using 13C NMR and comprehensive GC × GC. ACS Sustain. Chem. Eng. 2016, 4, 4974–4985. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.; Renato, C.V.; Marco, A.C.; Rosangela, D.S. Separation of phenol from bio-oil produced from pyrolysis of agricultural wastes. Mod. Chem. Appl. 2017, 5, 1000199. [Google Scholar] [CrossRef]
- Motte, J.-C.; Sambusiti, C.; Dumas, C.; Barakat, A. Combination of dry dark fermentation and mechanical pretreatment for lignocellulosic deconstruction: An innovative strategy for biofuels and volatile fatty acids recovery. Appl. Energy 2015, 147, 67–73. [Google Scholar] [CrossRef]
- Granados, D.A.; Basu, P.; Chejne, F.; Nhuchhen, D.R. Detailed investigation into torrefaction of wood in a two-stageinclined rotary torrefier. Energy Fuels 2017, 31, 647–658. [Google Scholar] [CrossRef]
- Butnaru, E.; Pamfil, D.; Stoleru, E.; Brebu, M. Characterization of bark, needles and cones from silver fir (Abies alba Mill.) towards valorization of biomass forestry residues. Biomass Bioenergy 2022, 159, 106413. [Google Scholar] [CrossRef]
- Pereira, B.L.C.; Carneiro, A.; de Cássia Oliveira Carneiro, A.; Carvalho, A.M.M.L.; Colodette, J.L.; Oliveira, A.C.; Fontes, M.P.F. Influence of chemical composition of Eucalyptus wood on gravimetric yield and charcoal properties. BioResources 2013, 8, 4574–4592. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Chen, W.-H.; Peng, J.; Bi, X.T. A state-of-the-art review of biomass torrefaction, densification and applications. Renew. Sustain. Energy Rev. 2015, 44, 847–866. [Google Scholar] [CrossRef]
- Murata, K.; Hirano, Y.; Sakata, Y.; Uddin, M.A. Basic study on a continuous flow reactor for thermal degradation of polymers. J. Anal. Appl. Pyrolysis 2002, 65, 71–90. [Google Scholar] [CrossRef]
- Brebu, M.; Tamminen, T.; Hannevold, L.; Stöcker, M.; Spiridon, I. Catalytic upgrading of co-pyrolysis oils from bisphenol a polycarbonate and lignins. Polym. Degrad. Stab. 2014, 102, 88–94. [Google Scholar] [CrossRef]
- Brebu, M.; Cazacu, G.; Chirilă, O. Pyrolysis of lignin—A potential method for obtaining chemicals and/or fuels. Cellul. Chem. Technol. 2011, 45, 43–50. [Google Scholar]
- Febrero, L.; Granada, E.; Pérez, C.; Patiño, D.; Arce, E. Characterisation and comparison of biomass ashes with different thermal histories using TG-DSC. J. Therm. Anal. Calorim. 2014, 118, 669–680. [Google Scholar] [CrossRef]
- Wise, M.L.; Croteau, R. Monoterpene Biosynthesis. In Comprehensive Natural Products Chemistry; Barton, D., Nakanishi, K., Meth-Cohn, O., Eds.; Pergamon: Pullman, WA, USA, 1999; Volume 2, pp. 97–153. [Google Scholar] [CrossRef]
- Cates, R.G.; Henderson, C.B.; Redak, R.A. Responses of the western spruce budworm to varying levels of nitrogen and terpenes. Oecologia 1987, 73, 312. Available online: https://www.jstor.org/stable/4218368 (accessed on 11 April 2020). [CrossRef] [PubMed]
- Brebu, M.; Tamminen, T.; Spiridon, I. Thermal degradation of various lignins by TG-MS/FTIR and Py-GC-MS. J. Anal. Appl. Pyrolysis 2013, 104, 531–539. [Google Scholar] [CrossRef]
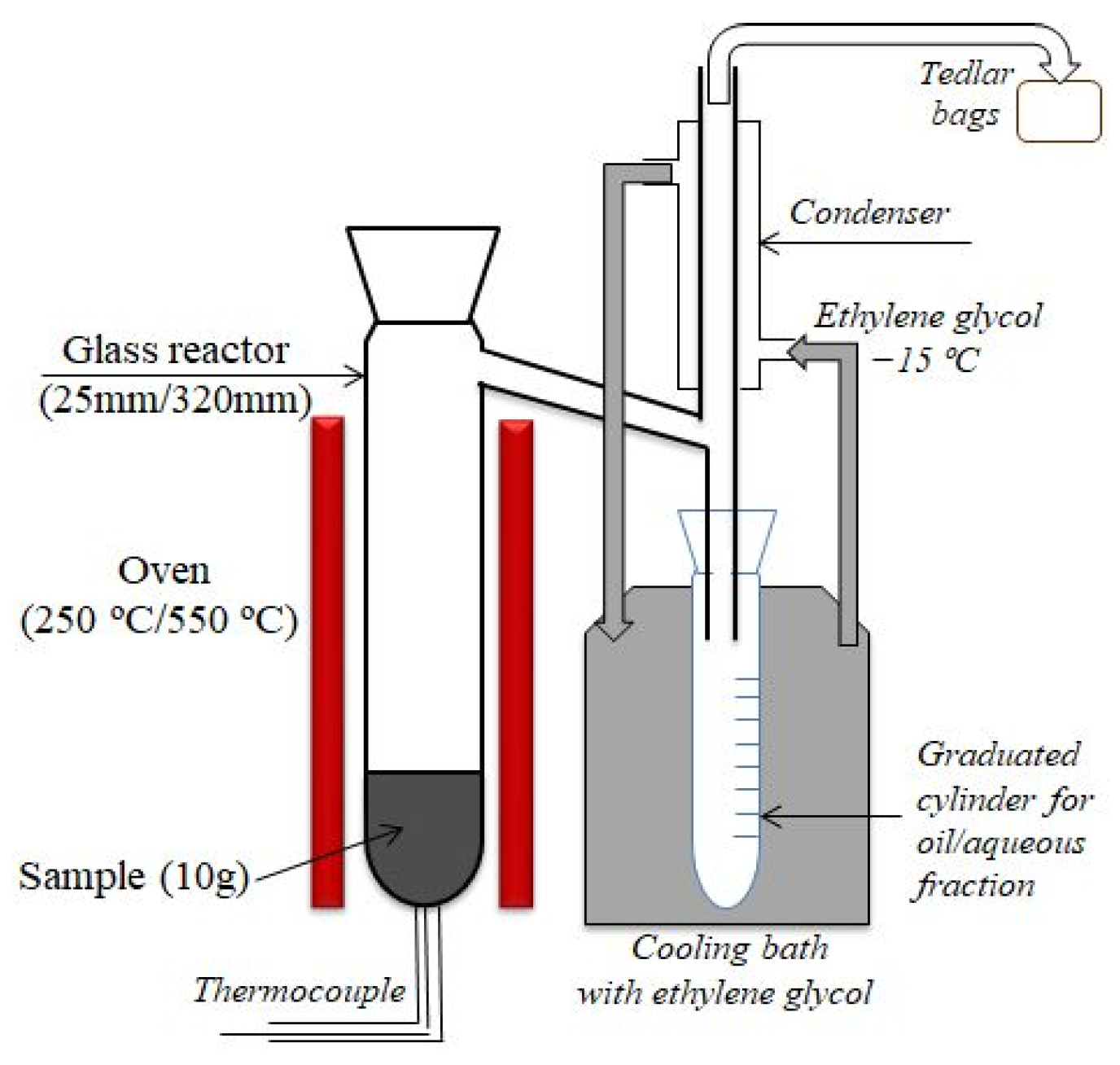
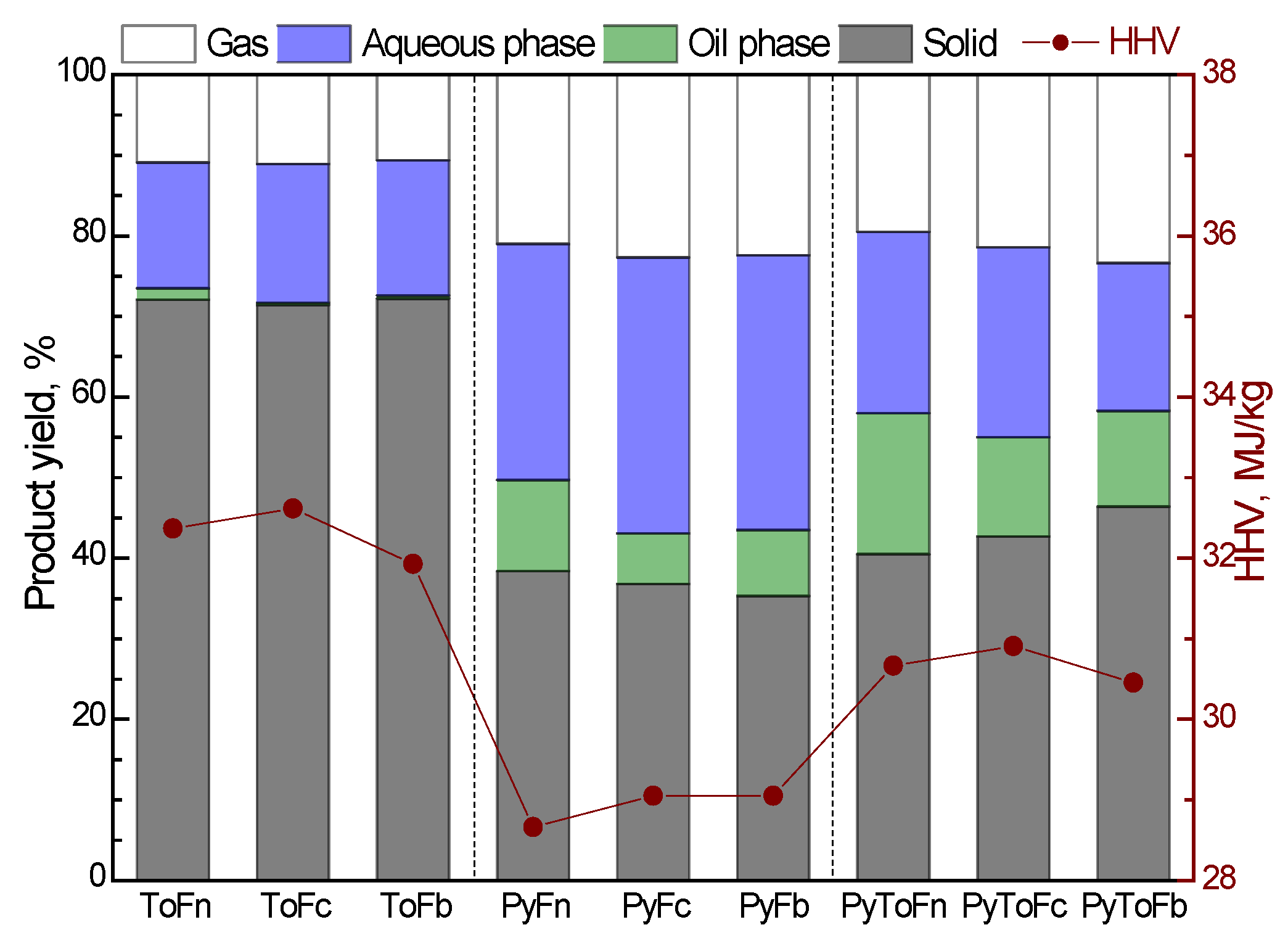
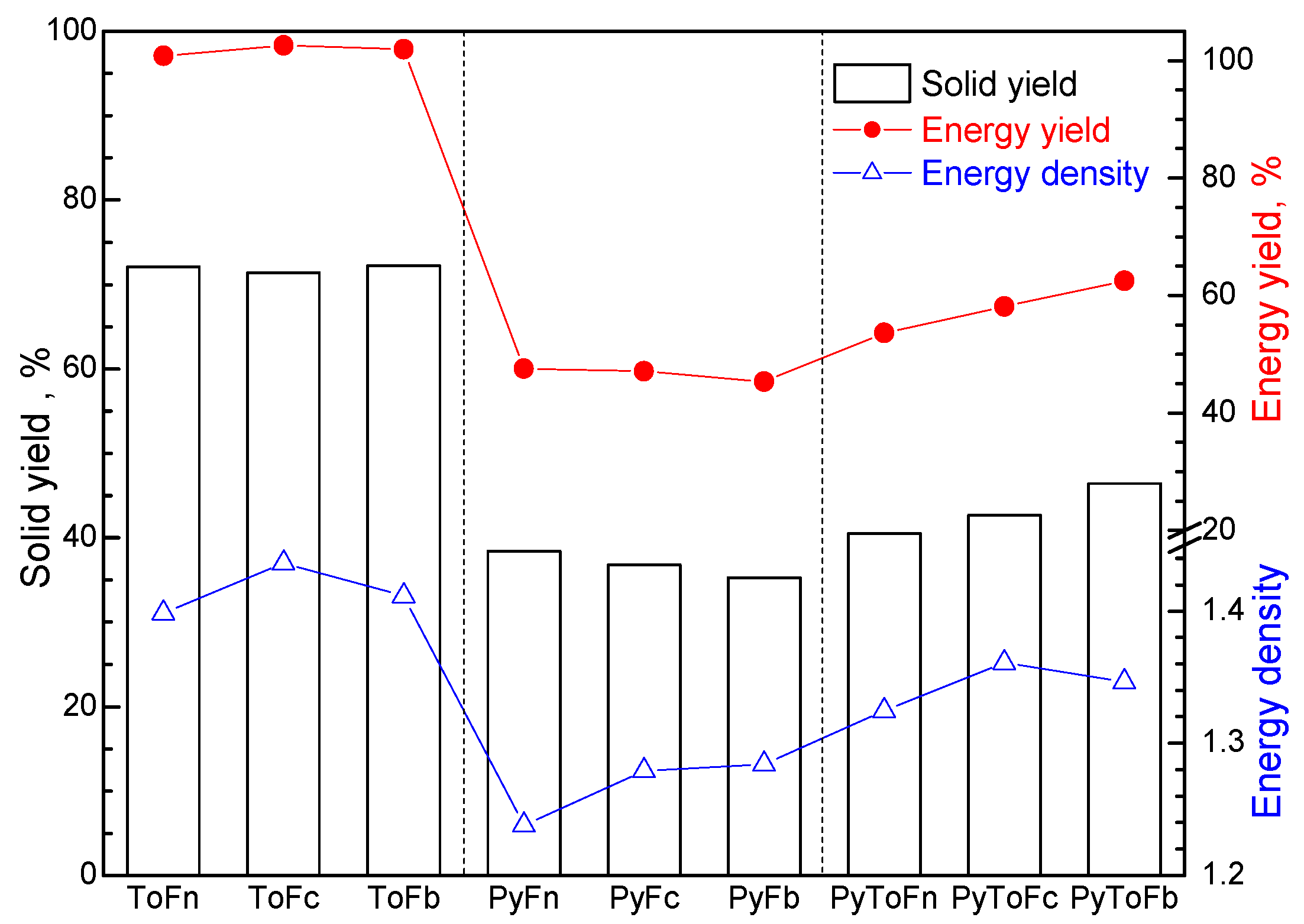
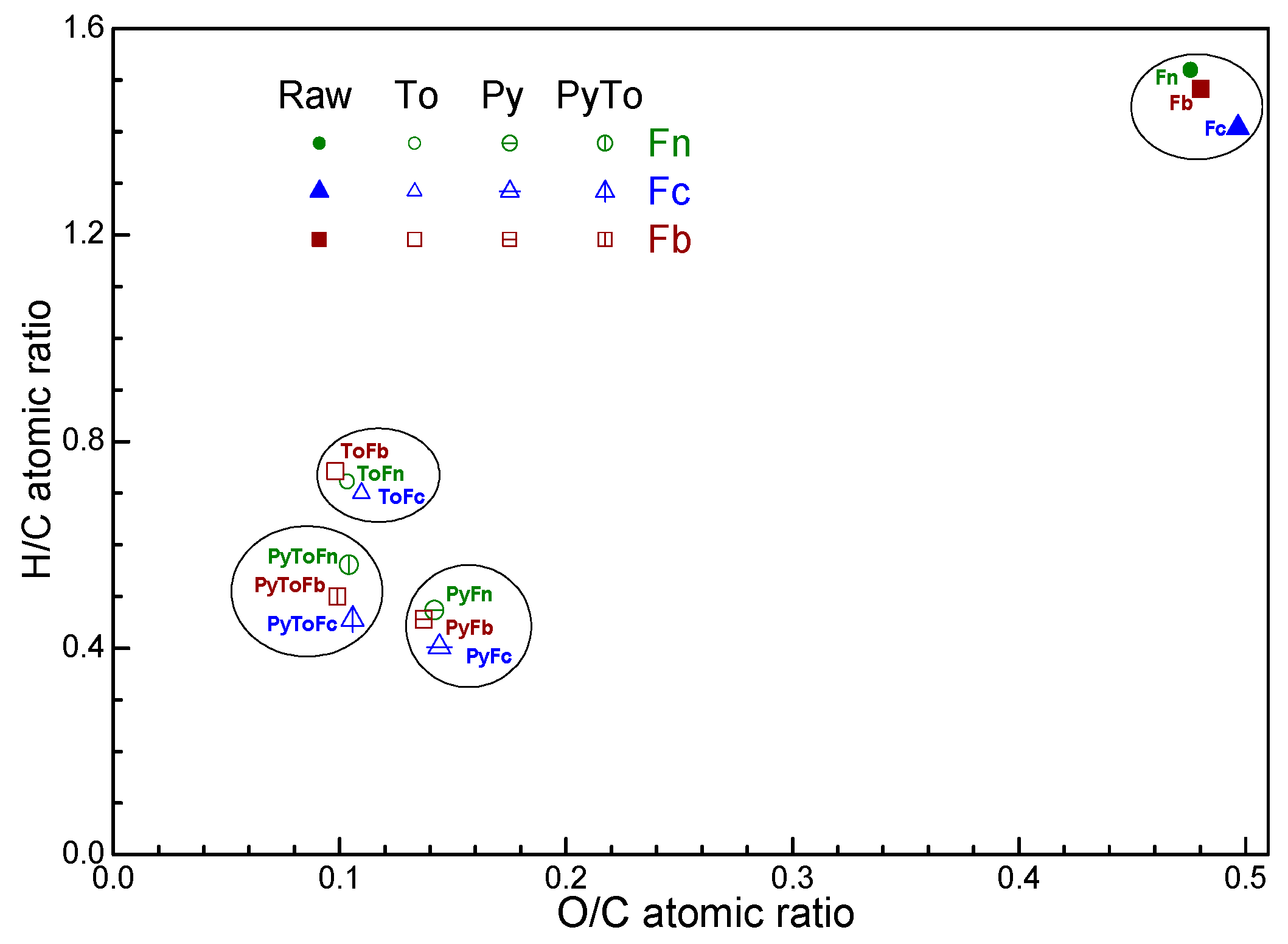

| Sample | Moisture | Volatile Matter | Fixed Carbon | Ash | C | H | N | S | O * | Atomic H/C | Atomic O/C | HHV MJ/kg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fn | 4.49 | 78.99 | 16.22 | 4.79 | 53.58 | 6.78 | 0.14 | 0.73 | 33.98 | 0.48 | 1.52 | 23.15 |
| Fc | 7.28 | 69.38 | 27.98 | 2.64 | 54.26 | 6.36 | 0.37 | 0.48 | 35.89 | 0.50 | 1.41 | 22.72 |
| Fb | 6.70 | 89.71 | 4.50 | 5.79 | 52.90 | 6.54 | 0.14 | 0.75 | 33.88 | 0.48 | 1.48 | 22.62 |
| ToFn | 1.84 | 86.13 | 10.33 | 3.54 | 79.89 | 4.81 | 0.41 | 0.34 | 11.01 | 0.10 | 0.72 | 32.37 |
| ToFc | 3.01 | 61.27 | 37.15 | 1.58 | 80.94 | 4.72 | 0.32 | 0.60 | 11.84 | 0.11 | 0.70 | 32.62 |
| ToFb | 2.50 | 72.72 | 21.13 | 6.15 | 78.46 | 4.85 | 0.10 | 0.15 | 10.29 | 0.10 | 0.74 | 31.93 |
| PyFn | 1.60 | 32.23 | 62.15 | 5.62 | 76.52 | 3.02 | 0.23 | 0.12 | 14.49 | 0.14 | 0.47 | 28.66 |
| PyFc | 2.44 | 25.53 | 71.62 | 2.85 | 78.86 | 2.64 | 0.11 | 0.39 | 15.15 | 0.14 | 0.40 | 29.05 |
| PyFb | 1.60 | 24.49 | 70.80 | 4.71 | 77.73 | 2.95 | 0.21 | 0.18 | 14.22 | 0.14 | 0.45 | 29.05 |
| PyToFn | 1.69 | 27.16 | 66.78 | 6.06 | 78.94 | 3.69 | 0.12 | 0.22 | 10.97 | 0.10 | 0.56 | 30.67 |
| PyToFc | 2.39 | 22.64 | 74.40 | 2.96 | 81.44 | 3.09 | 0.18 | 0.83 | 11.50 | 0.11 | 0.46 | 30.91 |
| PyToFb | 1.50 | 18.68 | 74.92 | 6.40 | 79.52 | 3.31 | 0.10 | 0.17 | 10.50 | 0.10 | 0.50 | 30.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butnaru, E.; Brebu, M. The Thermochemical Conversion of Forestry Residues from Silver Fir (Abies alba Mill.) by Torrefaction and Pyrolysis. Energies 2022, 15, 3483. https://doi.org/10.3390/en15103483
Butnaru E, Brebu M. The Thermochemical Conversion of Forestry Residues from Silver Fir (Abies alba Mill.) by Torrefaction and Pyrolysis. Energies. 2022; 15(10):3483. https://doi.org/10.3390/en15103483
Chicago/Turabian StyleButnaru, Elena, and Mihai Brebu. 2022. "The Thermochemical Conversion of Forestry Residues from Silver Fir (Abies alba Mill.) by Torrefaction and Pyrolysis" Energies 15, no. 10: 3483. https://doi.org/10.3390/en15103483
APA StyleButnaru, E., & Brebu, M. (2022). The Thermochemical Conversion of Forestry Residues from Silver Fir (Abies alba Mill.) by Torrefaction and Pyrolysis. Energies, 15(10), 3483. https://doi.org/10.3390/en15103483






